Abstract
Introduction
Despite increasing evidence of its efficacy in advanced age or in mild or severe strokes, intravenous thrombolysis remains underused for acute ischaemic stroke (AIS). Our aim was to obtain an updated view of reasons for non-thrombolysis and to identify its changing patterns over time.
Methods
This is a retrospective study of prospectively collected data from the Acute Stroke Registry and Analysis of Lausanne (ASTRAL) from the years 2003–2011. Patients admitted with acute stroke in the past 24 hours who had not had thrombolysis were identified; reasons for non-thrombolysis documented in the prospectively entered data were tabulated and analysed for the group as a whole. Data were analysed for the years 2003–2006 and 2007 forward because of changes in contraindications. A subgroup of patients who arrived within the treatment window ≤180 min was separately analysed for reasons for non-thrombolysis. Predictors of non-thrombolysis were investigated via multivariate regression analyses.
Results
In the 2019 non-thrombolysed patients the most frequent reasons for non-thrombolysis were admission delays (66.3%), stroke severity (mostly mild) (47.9%) and advanced age (14.1%); 55.9% had more than one exclusion criterion. Among patients arriving ≤180 min after onset, the main reasons were stroke severity and advanced age. After 2006, significantly fewer patients were excluded because of age (OR 2.65, p<0.001) or (mostly mild) stroke severity (OR 10.56, p=0.029). Retrospectively, 18.7% of all non-thrombolysed patients could have been treated because they only had relative contraindications.
Conclusion
Onset-to-admission delays remain the main exclusion criterion for thrombolysis. Among early arrivals, relative contraindications such as minor stroke severity and advanced age were frequent. Thrombolysis rate increased with the reduction of thrombolysis restrictions (eg, age and stroke severity).
Keywords: neurology, stroke; research, clinical; stroke; thrombolysis; urgent care
Key messages.
What is already known on this subject?
Thrombolysis with recombinant tissue plasminogen activator (rt-PA) is standard therapy for patients with acute ischaemic stroke in the first 4.5 hours after stroke onset. Some contraindications defined at the introduction of thrombolysis with rt-PA for ischaemic stroke have not shown be increase the risk for patients. Therefore, most such patients should also be treated with thrombolysis and reasons for not being thrombolysed are of major interest in improving thrombolysis rates.
What this study adds?
In this study using prospectively collected data from a large, single centre Swiss stroke database, we found that the primary reason for non-thrombolysis was delay in presentation. About 20% of patients who arrived within the appropriate timeframe were not thrombolysed due to only relative contraindications. Growing evidence about safety of thrombolysis in those certain patient groups may lead to higher thrombolysis rates. Finally, given that onset-to-admission delays remain the main exclusion criterion for thrombolysis, we could corroborate the urgent need for further optimising stroke recognition and pre-hospital systems of care by educating the population and (para)medical personnel about the paramount importance of time in suspected stroke.
Introduction
Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) administered within 4.5 hours from onset of stroke symptoms improves the clinical outcome of patients with acute ischaemic stroke (AIS).1–4 Some early arrival patients are not treated with rt-PA because of exclusion criteria that were previously based on exclusions from the initial thrombolysis trials.5 Further randomised trials6 and analyses from subgroups of trials7 and large case series have since suggested early thrombolysis is indicated even in advanced age,8 9 in both mild and high stroke severity and with rapidly improving symptoms.10–13 One prominent reason for non-thrombolysis is time delay.14 15 Although very early thrombolysis remains a major goal in acute stroke care, there is now scientific evidence that intravenous thrombolysis is effective up to 4.5 hours.2 4 With respect to relative and absolute contraindications for intravenous thrombolysis, our aim was to obtain the frequency and reasons for non-thrombolysis and how these have changes over time.
Methods
This was a retrospective analysis of the Acute STroke Registry and Analysis of Lausanne (ASTRAL). As described previously,16 data of all patients with AIS admitted to the stroke unit and/or intensive care unit at the Centre Hospitalier Univeristaire Vaudois (CHUV), Switzerland, between 2003 and 2011 were collected in a pre-specified manner at the time of patient presentation. Demographic data, onset-to-door-time, known or newly diagnosed vascular risk factors (arterial hypertension, atrial fibrillation, diabetes mellitus, valve replacements, coronary artery disease, smoking, etc) and previous cerebrovascular events were recorded. Stroke pathophysiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment17 classification, with four classes added (cervical artery dissections, likely atherosclerosis without significant stenosis,18 multiple mechanisms and probable relation to a patent foramen ovale). Stroke severity on arrival was recorded with the National Institute of Health Stroke Scale (NIHSS) score performed or supervised by certified medical personnel. Relevant data such as demographics, initial NIHSS score, onset-to-admission delays, acute recanalisation treatment and reasons for non-thrombolysis were completely available because the registry forces the user to complete these fields.
Thrombolysis and stroke management of ASTRAL patients and the written in-hospital thrombolysis guidelines are in line with European Stroke Organisation (ESO)19 and Swiss recommendations20 and are adapted regularly to take account of evidence-based publications.2 21 22 Detailed reasons for non-thrombolysis are pre-specified in ASTRAL and collected in six domains: time delays, initial stroke severity, age, imaging, high bleeding risk and other reasons. The detailed reasons for each domain with modifications over time are listed in table 1. In the whole observation period, thrombolysis contraindications were classified as absolute or relative (table 1). Further absolute contraindications such as acute pancreatitis, bacterial endocarditis, pericarditis, oesophageal varices, ulcerative gastrointestinal disease or neoplasms with acute bleeding risk are not explicitly listed but were considered in the database. Reasons for non-thrombolysis could be singular or multiple. The absence of any good reasons against intravenous thrombolysis according to the current hospital recommendations (a ‘missed’ thrombolysis opportunity) was explicitly documented at the time of entering the data in ASTRAL, that is, during the acute hospital stay of the patient. Using the ASTRAL registry, consecutive patients with AIS admitted to the stroke unit and/or intensive care unit at the CHUV between 2003 and 2011 within 24 hours of the last proof of good health were retrospectively analysed for the reasons why they did not receive intravenous rt-PA treatment. Since delayed presentation was likely to be an important factor in those presenting outside the treatment window, we also compared the reasons for non-thrombolysis between those presenting within the early treatment window and those presenting later. Because the response to thrombolysis becomes minor beyond 3 hours and even less beyond 4.5 hours,23 we chose 180 min as the latest cut-off, considering that patients could not be thrombolysed beyond this delay before 2008 and allowing for some in-hospital time thereafter. Additionally, we compared the frequency and reasons for non-thrombolysis during the first (2003–2006) versus the second (2007–2011) half of the observation period to identify changes in thrombolysis implementation and decision-making over time. The 2006 cut-off was chosen in order to obtain two cohorts of comparable size which maximised the power of the statistical analysis to identify true differences between the two time periods. Sample size was based on the number of patients in the registry. The anonymised use of ASTRAL data for scientific purposes without the need for individual consent was approved by the Ethics Committee for Research on Humans of the canton of Vaud, sub-commission III.
Table 1.
Contraindications for thrombolysis in our centre, with changes over time (some further softening of contraindications took place since end of data collection for this analysis)
| Domain | Contraindication | Change over time |
|---|---|---|
| Time delays | Thrombolysis time window >180 min | Thrombolysis time window >270 min since November 2008 |
| Thrombolysis time window >180 min and no indication for intra-arterial treatment within 6 hours (‘too late intravenous and no indication intra-arterial’) | – | |
| Unknown stroke onset and inability to treat within recommended time limits since last proof of good health | – | |
| Initial stroke severity | * Too mild: NIHSS <6 | NIHSS <4, unless hemianopia or aphasia since October 2006 |
| * Too severe: NIHSS >25 | No upper NIHSS-limit since October 2006 | |
| * Any ‘rapid improvement’ (not quantified) | Rapid improvement reaching NIHSS <4 since September 2006 | |
| Age limit | * >80 years | No age limit since October 2006, unless significant pre-existing disability |
| Imaging contraindications | Plain cCT: >30% hypo-attenuation of MCA territory | – |
| In borderline indications: large core on acute perfusion CT and/or little salvageable tissue | – | |
| Large subacute (silent) infarction on imaging, defined as a poorly demarcated, hypodense territorial lesion with mild local swelling or absence of the usual atrophy of chronic stroke lesions | – | |
| Acute intracranial haemorrhage | – | |
| High bleeding risk | INR >1.2 | INR >1.5 since October 2006 |
| Thrombocytopenia <100 000/mm3 | – | |
| Recent surgical intervention <14 days | – | |
| Previous intracranial haemorrhage | – | |
| Intracranial vascular malformation (known or suspected on plain cCT) | – | |
| Full dose heparin or LMWH | – | |
| Other bleeding risk | – | |
| Other reasons | No good reason (thrombolysis opportunity missed) | – |
| Recent ischaemic stroke or brain trauma <3 months | – | |
| Stroke diagnosis uncertain | – | |
| * Concomitant epileptic seizure | Thrombolysis indicated, if imaging confirmed acute ischaemic stroke since 9/2006 | |
| * Comorbidity, severely limiting life expectancy, or pre-stroke dependency, defined as mRS >2 | – |
Asterisk (*) indicates a relative contraindication.
cCT, cranial CT; INR, international normalised ratio; LMWH, low molecular weight heparin; MCA, middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale score.
Statistical analysis
We calculated the proportion of patients not receiving thrombolysis for the entire period and for the time periods between 2003–2006 and 2007–2011. Descriptive statistics of non-thrombolysed and thrombolysed patients (baseline characteristics/mechanisms of stroke) and of the most common reasons for non-thrombolysis are presented. Patients arriving before and after 180 min and patients with admission during the first (2003–2006) versus the second (2007–2011) half of the observation period were compared using a logistic regression model that included potential predictor variables (age, sex, NIHSS score at admission, risk factors, stroke mechanism, time/severity/imaging/bleeding/other reasons for non-thrombolysis). The OR and its 95% CI or its associated p values were given to quantify and test the significance of the strength of the association. Predictors with p<10% in bivariate analysis were used to fit a multivariate logistic model. P Values of 0.683 (table 2) and 0.469 (table 3) in Hosmer-Lemeshow goodness-of-fit test suggested that each model fitted reasonably well. Analyses were conducted with STATA/IC (V.13.0; College Station, Texas, USA).
Table 2.
Reason for non-thrombolysis. Multivariate analysis of reasons for non-thrombolysis divided by onset-to-admission delay (≤180 min vs >180 min)
| Patients (n=2019) | ≤180 min (n=659) | >180 min (n=1360) | OR | p Value | 95% CI |
|---|---|---|---|---|---|
| Too mild stroke | 323 (49%) | 567 (42%) | 3.35* | 0.000 | 1.82 to 6.18 |
| Age >80 years | 112 (17%) | 164 (12%) | 2.98* | 0.007 | 1.36 to 6.56 |
| Other bleeding reasons | 15 (2%) | 9 (1%) | 10.12* | 0.026 | 1.33 to 77.23 |
| Recent stroke (clinically or radiologically) <3 months | 36 (6%) | 13 (1%) | 4.50 | 0.071 | 0.88 to 23.01 |
| Other reasons | 16 (2%) | 8 (1%) | 13.77* | 0.033 | 1.23 to 153.68 |
| Microangiopathic stroke mechanism | 75 (11%) | 233 (17%) | 0.28* | 0.010 | 0.11 to 0.74 |
| Other determined or rare stroke mechanism | 54 (8%) | 32 (2%) | 3.48 | 0.096 | 0.80 to 15.08 |
| Atrial fibrillation | 179 (27%) | 287 (21%) | 1.94* | 0.034 | 1.05 to 3.58 |
| Mechanical or biological heart valves | 23 (4%) | 40 (3%) | 3.62* | 0.049 | 1.01 to 13.01 |
OR >1 means more likely ≤180 min and <1 means more likely >180 min.
Asterisk (*), significant on p<0.05 level. Definitions of reasons for non-thrombolysis see text/table 5.
Table 3.
Multivariate analysis of reasons for non-thrombolysis comparing the first (n=959) with the second (n=1060) half of the observation period
| Patients (n=2019) | 2003–2006 | 2006–2011 | OR | p Value | 95% CI |
|---|---|---|---|---|---|
| Too late intravenous and no indication intra-arterial | 127 (13%) | 76 (7%) | 1.79* | 0.010 | 1.15 to 2.78 |
| Unknown onset | 268 (28%) | 399 (38%) | 0.69* | 0.008 | 0.53 to 0.91 |
| Too mild stroke | 442 (46%) | 497 (47%) | 0.66* | 0.002 | 0.51 to 0.86 |
| Too severe stroke | 20 (2%) | 5 (1%) | 10.56* | 0.029 | 1.28 to 87.42 |
| Rapid improvement to below threshold | 16 (2%) | 2 (0.2%) | 5.43 | 0.109 | 0.69 to 43.01 |
| Age >80 years | 183 (19%) | 102 (10%) | 2.65* | 0.000 | 1.76 to 3.99 |
| Intracranial haemorrhage | 3 (0.3%) | 20 (2%) | 0.11* | 0.004 | 0.02 to 0.50 |
| Other bleeding reasons | 3 (0.3%) | 21 (2%) | 0.18* | 0.020 | 0.04 to 0.77 |
| Stroke uncertain | 10 (1%) | 48 (5%) | 0.19* | 0.000 | 0.08 to 0.44 |
| Comorbidity/dependency | 21 (2%) | 64 (6%) | 0.16* | 0.000 | 0.09 to 0.31 |
| Diabetes mellitus | 132 (14%) | 200 (19%) | 0.53* | 0.000 | 0.39 to 0.72 |
| Hyperlipidaemia | 589 (61%) | 707 (67%) | 0.53* | 0.000 | 0.40 to 0.70 |
| Probable atherosclerotic stroke mechanism (<50% stenosis) | 151 (16%) | 152 (14%) | 1.50* | 0.023 | 1.06 to 2.11 |
| Microangiopathic stroke mechanism | 171 (18%) | 149 (14%) | 1.72* | 0.002 | 1.21 to 2.43 |
OR >1 means more likely in first half and <1 means more likely in second half of observation period.
Asterisk (*), significant on p<0.05 level. Definitions of reasons for non-thrombolysis see text/table 5.
Results
Patient population
Over the 9-year observation period, 599 of 2618 patients with AIS (22.9%) in ASTRAL received thrombolysis. The annual thrombolysis rate increased from 9.7% in 2003 to 33.6% in 2011. Among all thrombolysed patients, 27.5% were thrombolysed between 2003 and 2006 and 72.5% were thrombolysed between 2007 and 2011. The median age of non-thrombolysed patients was 73 (IQR 61, 82); admission NIHSS score was 4.5 (IQR 2, 10), whereas the median age of those thrombolysed was 69 (IQR 58, 78) and median NIHSS score was 13 (IQR 8, 19, table 4). Among both thrombolysed and non-thrombolysed patients, cardioembolism was the most common aetiology of the stroke. Non-thrombolysed patients had less cardioembolic strokes respectively more microangiopathic aetiology than thrombolysed patients (see online supplementary table S1). Further baseline characteristics of thrombolysed and non-thrombolysed patients are listed in table 4. During the study period, the number of endovascular recanalisation treatments (mostly combined intravenous and mechanical thrombectomy) remained minor thrombolysis and reached 5/98 patients (5.1%) in 2011. Yearly thrombolysis rates are set out in figure 1. Stroke aetiology of the 2019 non-thrombolysed and 599 thrombolysed patients is shown in online supplementary table S1.
Table 4.
Baseline characteristics of non-thrombolysed and thrombolysed patients. Continuous variables are given as medians with IQR (lower and upper quartiles) and as n (%) for categorical variables
| Non-thrombolysed patients (n=2019) |
Thrombolysed patients (n=599) |
|||
|---|---|---|---|---|
| n or median | % or quartiles | n or median | % or quartiles | |
| Age (years) | 73 | 61, 82 | 69 | 58, 78 |
| Sex (male) | 1131 | 56.0% | 349 | 58.3% |
| Onset-to-door time (min) | 337 | 125, 774 | 93 | 58, 135.5 |
| Onset to admission ≤180 min | 659 | 32.6% | 523 | 87.3% |
| Admission NIHSS | 4.5 | 2, 10 | 13 | 8, 19 |
| Treated in first observation period (2003–2006) | 959 | 47.5% | 173 | 28.9% |
| Hypertension | 1317 | 65.2% | 346 | 57.8% |
| Hyperlipidaemia | 1296 | 64.2% | 362 | 60.4% |
| Atrial fibrillation | 488 | 24.2% | 156 | 26.0% |
| Active smoking | 434 | 21.5% | 136 | 22.7% |
| Diabetes mellitus | 332 | 16.4% | 97 | 16.2% |
| Symptomatic coronary artery disease* | 295 | 14.6% | 85 | 14.2% |
| Symptomatic peripheral artery disease | 106 | 5.3% | 23 | 3.8% |
| Low ejection fraction (<35%) | 84 | 4.2% | 34 | 5.7% |
| Cancer not in remission | 77 | 3.8% | 14 | 2.3% |
| Heart valves | 64 | 3.2% | 10 | 1.7% |
*Documented by myocardial infarct diagnosis, coronarography or stress test.
NIHSS, National Institute of Health Stroke Scale score.
Figure 1.
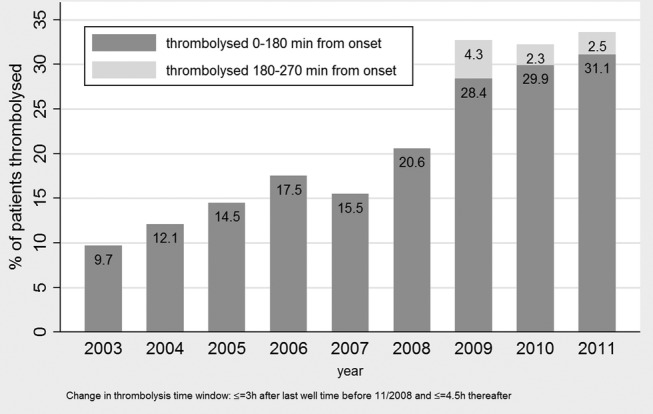
Rates of thrombolysed patients per year (n=2618).
Mechanisms of stroke of non-thrombolysed and thrombolysed patients1
emermed-2015-205140supp001.pdf (156.1KB, pdf)
Relative contraindications
A total of 321 of all non-thrombolysed patients (15.9%) had only relative contraindications to thrombolysis as defined in table 1. Of these, 283 patients (14.0%) had only one and 38 (1.9%) had multiple relative contraindications. About 57 patients (2.8%) had clearly been overlooked as candidates for thrombolysis by the physician not performing the thrombolysis when compared with the current hospital recommendations at the time.
Reasons for non-thrombolysis of patients
The most frequent causes for exclusion of thrombolysis among all patients were onset-to-admission delays (often including wake-up strokes), which were found in 66.3% of non-thrombolysed patients followed by mild strokes (45.8%), according to the hospital guidelines at the time (table 5). A total of 1128 patients (55.9%) had more than only one reason for exclusion.
Table 5.
Reasons for not being thrombolysed (n=2019)
| Reason: time | ||
| Too late intravenous | 51 | 2.5% |
| Too late intravenous and no indication intra-arterial | 203 | 10.1% |
| Too late intravenous and intra-arterial | 418 | 20.7% |
| Unknown onset* | 667 | 33.0% |
| Total | 1339 | 66.3% |
| Reason: severity | ||
| Too mild† | 924 | 45.8% |
| Too severe‡ | 25 | 1.2% |
| Rapid improvement to below threshold | 18 | 0.9% |
| Total | 967 | 47.9% |
| Reason: age | ||
| >80 years till 2006§ | 285 | 14.1% |
| Reason: imaging | ||
| Too large infarct¶ | 32 | 1.6% |
| Too little penumbra | 12 | 0.6% |
| Too large infarct and too little penumbra | 7 | 0.3% |
| Large subacute infarct on imaging** | 18 | 0.9% |
| Other/intracranial haemorrhage | 23 | 1.1% |
| Total | 92 | 4.6% |
| Reason: high bleeding risk | ||
| INR elevated†† | 174 | 8.6% |
| Thrombocytopenia‡‡ | 6 | 0.3% |
| Recent intervention | 19 | 0.9% |
| Previous intracranial haemorrhage | 17 | 0.8% |
| Intracranial vascular malformation | 14 | 0.7% |
| Full dose heparin or LMWH | 20 | 1.0% |
| Other bleeding risk | 24 | 1.2% |
| Total | 274 | 13.6% |
| Reason: other | ||
| No good reason according to hospital recommendations (thrombolysis missed) | 57 | 2.8% |
| Recent stroke§§ | 51 | 2.5% |
| Stroke uncertain | 58 | 2.9% |
| Epileptic seizure | 13 | 0.6% |
| Comorbidity/dependency | 85 | 4.2% |
| Total | 290 | 14.4% |
*>60 min uncertainty and too late for thrombolysis.
†NIHSS <6 until September 2006, NIHSS <4 from October 2006 without isolated hemianopia or aphasia thereafter.
‡NIHSS>25 till September 2006, no limit thereafter.
§Since October 2006: >80 and significant comorbidity or dependency.
¶Non-contrast image or perfusion image.
**Defined as a poorly demarcated, hypodense territorial lesion with mild local swelling or absence of the usual atrophy of chronic stroke lesions.
††>1.2 before October 2006 and >1.5 thereafter.
‡‡<100 000/mm3.
§§Clinically or radiologically <3 months.
INR, international normalised ratio; LMWH, low molecular weight heparin; NIHSS, National Institute of Health Stroke Scale score.
Reasons for non-thrombolysis for those within the early therapeutic window
The median age of non-thrombolysed patients treated ≤180 min was 73 (IQR 61, 82), admission NIHSS score was 4.5 (IQR 2, 11) and main stroke aetiology was cardioembolic (30.8%) followed by macroangiopathic stroke without significant stenosis18 (13.5%) and macroangiopathic stroke with ≥50% stenosis (13.2%), whereas the median age of those treated >180 min was 73 (IQR 60, 82), median NIHSS score was 5 (IQR 2, 10) and main stroke aetiology was cardioembolic (26.1%) followed by microangiopathic stroke (17.1%) and macroangiopathic stroke without significant stenosis (14.9%). The bivariate analysis of patients treated ≤180 min versus >180 min after stroke onset is shown in table 6; significantly more patients in the early group had severity reasons for exclusion (mostly too mild strokes), were aged > 80 years, had higher bleeding risks and had other reasons like recent stroke or pre-stroke comorbidity/dependency. In the multivariate analysis of early (≤180 min) versus late arriving non-thrombolysed patients, those in the early group significantly had more mild strokes, were aged >80 years, had high bleeding risk and had atrial fibrillation (table 2). Among patients admitted early, reasons for not being thrombolysed were more often a combination of different reasons rather than one single reason alone.
Table 6.
Reasons for non-thrombolysis divided by onset-to-admission delay (≤180 min vs >180 min)
| Arrival |
OR | 95% CI | ||||
|---|---|---|---|---|---|---|
| ≤180 min (n=659) |
>180 min (n=1360) |
|||||
| Patient characteristics | ||||||
| Age (median quartiles) | 73 | 61, 82 | 73 | 60, 82 | 1.00 | 0.99 to 1.01 |
| Sex (male) | 393 | 59.6% | 699 | 51.4% | 1.24* | 1.03 to 1.50 |
| Admission NIHSS (median quartiles) | 4.5 | 2, 11 | 5 | 2, 10 | 1.00 | 0.99 to 1.00 |
| Stroke mechanism (TOAST)† | ||||||
| Atherosclerosis with ≥50% (NASCET) stenosis | 87 | 13.2% | 158 | 11.6% | 1.08 | 0.82 to 1.43 |
| Likely atherosclerosis/aortic, without significant stenosis‡ | 89 | 13.5% | 203 | 14.9% | 0.83 | 0.63 to 1.08 |
| Cardioembolism | 203 | 30.8% | 355 | 26.1% | 1.16 | 0.95 to 1.43 |
| Small vessel occlusion | 75 | 11.4% | 233 | 17.1% | 0.58* | 0.44 to 0.76 |
| Dissections | 24 | 3.6% | 49 | 3.6% | 0.95 | 0.58 to 1.56 |
| Other determined | 54 | 8.2% | 32 | 2.4% | 3.49* | 2.23 to 5.46 |
| Undetermined mechanism | 57 | 8.6% | 121 | 8.9% | 0.91 | 0.65 to 1.26 |
| Multiple mechanisms | 39 | 5.9% | 56 | 4.1% | 1.38 | 0.90 to 2.10 |
| PFO as likely cause | 19 | 2.9% | 50 | 3.7% | 0.73 | 0.43 to 1.25 |
| Reason: severity | ||||||
| Too mild | 323 | 49.0% | 567 | 41.7% | 1.22* | 1.01 to 1.47 |
| Too severe | 12 | 1.8% | 12 | 0.9% | 1.97 | 0.88 to 4.40 |
| Rapid improvement to below threshold | 15 | 2.3% | 3 | 0.2% | 9.94* | 2.87 to 34.48 |
| Total | 350 | 53.1% | 582 | 42.8% | 1.37* | 1.13 to 1.66 |
| Reason: age | ||||||
| >80 years | 112 | 17.0% | 164 | 12.1% | 1.40* | 1.08 to 1.82 |
| Reason: imaging | ||||||
| Too large infarct | 3 | 0.5% | 27 | 2.0% | 0.21* | 0.06 to 0.70 |
| Too little penumbra | 6 | 0.9% | 6 | 0.4% | 1.96 | 0.63 to 6.09 |
| Too large infarct and too little penumbra | 3 | 0.5% | 3 | 0.2% | 1.95 | 0.39 to 9.70 |
| Subacute infarct on imaging | 12 | 1.8% | 5 | 0.4% | 4.74* | 1.66 to 13.52 |
| Other/intracranial haemorrhage | 10 | 1.5% | 13 | 1.0% | 1.51 | 0.66 to 3.45 |
| Total | 34 | 5.2% | 54 | 4.0% | 1.24 | 0.80 to 1.92 |
| Reason: high bleeding risk | ||||||
| INR elevated | 71 | 10.8% | 96 | 7.1% | 1.49* | 1.08 to 2.06 |
| Thrombocytopenia | 2 | 0.3% | 4 | 0.3% | 0.97 | 0.18 to 5.33 |
| Recent intervention | 16 | 2.4% | 3 | 0.2% | 10.62* | 3.08 to 36.60 |
| Previous intracranial haemorrhage | 10 | 1.5% | 6 | 0.4% | 3.28* | 1.19 to 9.07 |
| Intracranial vascular malformation | 5 | 0.8% | 9 | 0.7% | 1.08 | 0.36 to 3.24 |
| Other | 15 | 2.3% | 9 | 0.7% | 3.30* | 1.44 to 7.58 |
| Full dose heparin or LMWH | 17 | 2.6% | 3 | 0.2% | 11.31* | 3.30 to 38.72 |
| Total | 136 | 20.6% | 130 | 9.6% | 2.31* | 1.78 to 3.00 |
| Reason: other | ||||||
| No good reason according to hospital recommendations | 45 | 6.8% | 10 | 0.7% | 9.34* | 4.68 to 18.66 |
| Recent stroke | 36 | 5.5% | 13 | 1.0% | 5.65* | 2.98 to 10.73 |
| Stroke uncertain | 26 | 3.9% | 29 | 2.1% | 1.78* | 1.04 to 3.04 |
| Epileptic seizure | 7 | 1.1% | 4 | 0.3% | 3.43* | 1.00 to 11.78 |
| Comorbidity/dependency | 38 | 5.8% | 43 | 3.2% | 1.77* | 1.13 to 2.76 |
| Total | 152 | 23.1% | 99 | 7.3% | 3.97* | 1.69 to 9.32 |
Asterisk (*), significant on p<0.05 level. Definitions of reasons for non-thrombolysis see text/table 5.
†TOAST17 classification.
‡Ipsilateral internal carotid stenosis <50%(NASCET)/risk factors for atherosclerotic disease, for details see PERFORM definition.18
INR, International Normalised Ratio; LMWH, low molecular weight heparin; NIHSS, National Institute of Health Stroke Scale score; PFO, patent foramen ovale; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Change over time
The median age of non-thrombolysed patients treated in 2003–2006 was 73 (IQR 60, 81); admission NIHSS score was 5 (IQR 3, 12) and main stroke aetiology was cardioembolic (28.9%) followed by microangiopathic stroke (17.8%) and macroangiopathic stroke without significant stenosis (15.7%), whereas the median age of those treated in 2007–2011 was 74 (IQR 61, 83); median NIHSS score was 4 (IQR 2, 9) and main stroke aetiology was cardioembolic (28.4%) followed by macroangiopathic stroke without significant stenosis (14.3%) and microangiopathic stroke (14.1%). Most frequently, time reasons were a cause for not being thrombolysed, showing significantly more patients with unknown stroke onset in the group treated 2007–2011 followed by severity reasons, showing significantly more patients with too severe stroke or rapid improvement of stroke symptoms in the group treated 2003–2006 (table 7). More patients treated in 2003–2006 were excluded from thrombolysis than in 2007–2011 (86.7% vs 73.5%). Of those 378 non-thrombolysed patients who had no (or only relative) contraindications, 204 (54%) were treated in the earlier period and 174 (46%) in the later period. After distraction of patients thrombolysed in the extended time window of 3–4.5 hours after October 2008, there was still an increase in the number of thrombolysed patients in the later time period (figure 1). Compared with the 2007 onward group (multivariate analysis, table 3), in the 2003–2006 group, significantly more patients were excluded because they were thought to be too severely affected, their age was >80 years, they had rapid neurological improvement or they were too late arriving. After 2006 (when older patients and later-arriving patients could be thrombolysed), significantly more patients were excluded because of unknown stroke onset, too mild stroke, comorbidity or dependency, unrecognised stroke, increased bleeding risk, imaging reasons and intracranial haemorrhage. Also more patients had diabetes and hyperlipidaemia, while fewer patients had microangiopathic strokes.
Table 7.
Reasons for non-thrombolysis comparing the first with the second half of the observation period
| Year |
||||||
|---|---|---|---|---|---|---|
| Patients (n=2019) | 2003–2006 (n=959) |
2007–2011 (n=1060) |
OR | 95% CI | ||
| Patient characteristics | ||||||
| Age (median quartiles) | 73 | 60, 81 | 74 | 61, 83 | 1.00 | 0.99 to 1.00 |
| Sex (male) | 530 | 55.3% | 601 | 56.7% | 0.94 | 0.79 to 1.13 |
| Admission NIHSS (median quartiles) | 5 | 3, 12 | 4 | 2, 9 | 1.00* | 1.00 to 1.01 |
| Stroke mechanism (TOAST)† | ||||||
| Atherosclerosis with ≥50% (NASCET) stenosis | 106 | 11.1% | 147 | 13.9% | 0.73* | 0.56 to 0.95 |
| Likely atherosclerosis/aortic, without significant stenosis‡ | 151 | 15.7% | 152 | 14.3% | 1.06 | 0.83 to 1.35 |
| Cardioembolism | 277 | 28.9% | 301 | 28.4% | 1.02 | 0.84 to 1.25 |
| Small vessel occlusion | 171 | 17.8% | 149 | 14.1% | 1.26 | 0.99 to 1.60 |
| Dissections | 45 | 4.7% | 30 | 2.8% | 1.61* | 1.01 to 2.58 |
| Other determined | 38 | 4.0% | 48 | 4.5% | 0.83 | 0.54 to 1.28 |
| Undetermined mechanism | 89 | 9.3% | 93 | 8.8% | 1.01 | 0.74 to 1.37 |
| Multiple mechanisms | 46 | 4.8% | 54 | 5.1% | 0.89 | 0.60 to 1.34 |
| PFO as likely cause | 35 | 3.6% | 36 | 3.4% | 1.03 | 0.64 to 1.65 |
| Reason: time | ||||||
| Too late intravenous | 25 | 2.6% | 26 | 2.5% | 1.04 | 0.59 to 1.81 |
| Too late intravenous and no indication intra-arterial | 127 | 13.2% | 76 | 7.2% | 1.92* | 1.42 to 2.59 |
| Too late intravenous and intra-arterial | 209 | 21.8% | 209 | 19.7% | 1.10 | 0.88 to 1.36 |
| Unknown onset | 268 | 27.9% | 399 | 37.6% | 0.62* | 0.51 to 0.74 |
| Total | 629 | 65.6% | 710 | 67.0% | 0.86 | 0.72 to 1.04 |
| Reason: severity | ||||||
| Too mild | 442 | 46.1% | 497 | 46.9% | 0.97 | 0.82 to 1.16 |
| Too severe | 20 | 2.1% | 5 | 0.5% | 4.37* | 1.63 to 11.70 |
| Rapid improvement to below threshold | 16 | 1.7% | 2 | 0.2% | 8.73* | 2.00 to 38.08 |
| Total | 478 | 49.8% | 489 | 46.1% | 1.10 | 0.92 to 1.32 |
| Reason: age | ||||||
| >80 years | 183 | 19.1% | 102 | 9.6% | 2.15* | 1.66 to 2.79 |
| Reason: imaging | ||||||
| Too large infarct | 0 | 0.0% | 32 | 3.0% | 30.24*§, p=0.000 | |
| Too little penumbra | 6 | 0.6% | 6 | 0.6% | 1.08 | 0.35 to 3.35 |
| Too large infarct and too little penumbra | 0 | 0.0% | 7 | 0.7% | 6.53*¶ , p=0.016 | |
| Subacute infarct on imaging | 6 | 0.6% | 12 | 1.1% | 0.53 | 0.20 to 1.43 |
| Other/intracranial haemorrhage | 3 | 0.3% | 20 | 1.9% | 0.16* | 0.05 to 0.54 |
| Total | 15 | 1.6% | 77 | 7.3% | 0.20* | 0.11 to 0.34 |
| Reason: high bleeding risk | ||||||
| INR elevated | 98 | 10.2% | 76 | 7.2% | 1.43* | 1.05 to 1.96 |
| Thrombocytopenia | 1 | 0.1% | 5 | 0.5% | 0.21 | 0.02 to 1.84 |
| Recent intervention | 5 | 0.5% | 14 | 1.3% | 0.38 | 0.14 to 1.06 |
| Previous intracranial haemorrhage | 5 | 0.5% | 12 | 1.1% | 0.45 | 0.16 to 1.27 |
| Intracranial vascular malformation | 6 | 0.6% | 8 | 0.8% | 0.81 | 0.28 to 2.33 |
| Other | 3 | 0.3% | 21 | 2.0% | 0.15* | 0.04 to 0.51 |
| Full dose heparin or LMWH | 5 | 0.5% | 15 | 1.4% | 0.36* | 0.13 to 0.98 |
| Total | 123 | 12.8% | 151 | 14.2% | 0.86 | 0.66 to 1.11 |
| Reason: other | ||||||
| No good reason according to hospital recommendations | 32 | 3.3% | 25 | 2.4% | 1.39 | 0.82 to 2.36 |
| Recent stroke | 25 | 2.6% | 26 | 2.5% | 1.04 | 0.59 to 1.81 |
| Stroke uncertain | 10 | 1.0% | 48 | 4.5% | 0.22* | 0.11 to 0.43 |
| Epileptic seizure | 5 | 0.5% | 8 | 0.8% | 0.67 | 0.22 to 2.06 |
| Comorbidity/dependency | 21 | 2.2% | 64 | 6.0% | 0.34* | 0.20 to 0.56 |
| Other | 13 | 1.4% | 13 | 1.2% | 1.08 | 0.50 to 2.33 |
| Total | 106 | 11.1% | 184 | 17.4% | 0.57* | 0.44 to 0.74 |
Asterisk (*), significant on p<0.05 level. Definitions of reasons for non-thrombolysis see text/table 5.
†TOAST17 classification.
‡Ipsilateral internal carotid stenosis <50%(NASCET)/risk factors for atherosclerotic disease, for details see PERFORM definition.18
§χ2 test.
¶Fisher's exact test (expected cell frequency <5).
INR, international normalised ratio; LMWH, low molecular weight heparin; NIHSS, National Institute of Health Stroke Scale score; PFO, patent foramen ovale; TOAST, TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Discussion
Using a consecutive single-centre series of patients with AIS having detailed pre-specified recording of reasons for non-thrombolysis over a period of 9 years, we found time delays to be the main reason. We also found a remarkable number of patients excluded from thrombolysis on account of one single relative contraindication—mild stroke symptoms being the most frequent cause in all patients and in early arrivals.
The thrombolysis rate increased over time because fewer restrictions related to age, stroke severity, comorbidities or other relative contraindications were applied and the time window was increased from 3 to 4.5 hours in November 2008, leading to fewer patients excluded because of arriving too late. Long pre-hospital time delays underline the importance of improving stroke recognition via continuous public awareness campaigns and use of simplified pre-hospital stroke scales (eg, FASTER protocol24) by dispatchers and paramedics. Furthermore, triage, routines of pre-notification of specialised hospitals and diagnosis by telemedicine approaches could optimise pre-hospital patient flow.25–29 A significant number of patients (especially those with wake-up strokes) would also benefit from a further extension of the time window: several such late revascularisation trials are now in progress.30–32 The relative frequency of reasons for non-thrombolysis was similar to previously published data,15 33–35 but we found more patients excluded because of advanced age or unknown stroke onset. The large number of patients excluded because of unknown stroke onset, especially in the second observation period, may be due to an increase of such patients referred to us after our randomised pilot trial on thrombolysis for unknown stroke onset.36
In the first half of the observation period, age >80 years was a main reason for non-thrombolysis. ‘Too severe stroke’ or rapid improvement was also found as a reason but, because of low frequencies, did not contribute to the failure of thrombolysis in a substantial way. In the second half of the observation period, after the age restriction was removed, ‘too mild stroke’ became a relatively more frequent reason for non-thrombolysis, although we lowered our threshold NIHSS score from 6 to 4 and recommended thrombolysis for patients with isolated aphasia or hemianopia. Significantly more patients in the second observation period were not thrombolysed because of comorbidities, pre-stroke dependency or bleeding risks, probably reflecting an increasingly fragile stroke population over time. Many trials now confirm the safety and efficacy of thrombolytic therapy in patients with too mild stroke symptoms and in those aged >80 years.9–11 22 23 However, only one of these trials (International Stroke Trial-36) had a randomised controlled design with pre-specified subgroup analysis. The main benefit in this study was seen within the first 3 hours. Another randomised trial focusing on the elderly is in progress (Thrombolysis in Elderly Stroke Patients in Italy37). Thrombolysis is also effective in patients with mild stroke symptoms38–43 and can be improved by multimodal imaging.44
The strengths of our study are pre-specified and detailed documentation of exclusion criteria for thrombolysis. Its limitation is its monocentric character with specialised stroke care, where a subset of patients with AIS was specifically referred for acute recanalisation therapy. Still, 77.8% of the population examined came from our primary catchment area and most non-thrombolysed patients came from this area.
Conclusions
Liberalising criteria for thrombolysis were associated with an increase in thrombolysis of stroke patients at our centre. Onset-to-admission delays remain the main exclusion criteria for thrombolysis, emphasising the need for better pre-hospital stroke identification and patient delivery. However, in patients arriving early, relative contraindications prevented thrombolysis in about 20% of otherwise eligible patients.
Footnotes
Contributors: PM: designing data collection tools, monitoring data collection, conception and design of the study, interpretation of data, manuscript writing, final approval of the version to be published and responsible for the overall content as guarantor. TR: conception and design of the study, cleaning and statistical analysis of data, interpretation of data, manuscript writing, submitting and responsible for the overall content as guarantor.
Funding: Swiss Heart Foundation.
Competing interests: PM: speaker fees from Bayer, Pfizer, Medtronic, St Jude Medical and Boehringer Ingelheim; consulting fees from Pierre-Fabre and Amgen; honoraria from scientific advisory boards of Bayer, Pfizer and Boehringer Ingelheim.
Ethics approval: Ethics Committee for Research on Humans of the canton of Vaud, sub-commission III.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All the data of this study are only available with the authors.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–7. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703. 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 5.Tong D. Are all IV thrombolysis exclusion criteria necessary? Being SMART about evidence-based medicine. Neurology 2011;76:1780–1. [DOI] [PubMed] [Google Scholar]
- 6.Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–63. 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA stroke trial. Stroke 1997;28:2119–25. 10.1161/01.STR.28.11.2119 [DOI] [PubMed] [Google Scholar]
- 8.Asdaghi N, Butcher KS, Hill MD. Risks and benefits of thrombolysis in the elderly. Int J Stroke 2012;7:142–9. 10.1111/j.1747-4949.2011.00744.x [DOI] [PubMed] [Google Scholar]
- 9.Berrouschot J, Röther J, Glahn J, et al. Outcome and severe hemorrhagic complications of intravenous thrombolysis with tissue plasminogen activator in very old (> or =80 years) stroke patients. Stroke 2005;36:2421–5. 10.1161/01.STR.0000185696.73938.e0 [DOI] [PubMed] [Google Scholar]
- 10.Breuer L, Blinzler C, Huttner HB, et al. Off-label thrombolysis for acute ischemic stroke: rate, clinical outcome and safety are influenced by the definition of ‘minor stroke’. Cerebrovasc Dis 2011;32:177–85. 10.1159/000328811 [DOI] [PubMed] [Google Scholar]
- 11.Karliński M, Kobayashi A, Litwin T, et al. Intravenous thrombolysis for acute ischaemic stroke in patients not fully adhering to the European licence in Poland. Neurol Neurochir Pol 2012;46:3–14. [DOI] [PubMed] [Google Scholar]
- 12.De Keyser J, Gdovinová Z, Uyttenboogaart M, et al. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke 2007;38:2612–18. 10.1161/STROKEAHA.106.480566 [DOI] [PubMed] [Google Scholar]
- 13.Levine SR, Khatri P, Broderick JP, et al. Review, historical context, and clarifications of the NINDS rt-PA stroke trials exclusion criteria: part 1: rapidly improving stroke symptoms. Stroke 2013;44:2500–5. 10.1161/STROKEAHA.113.000878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber PA, Zhang J, Demchuk AM, et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001;56:1015–20. [DOI] [PubMed] [Google Scholar]
- 15.García-Moncó JC, Pinedo A, Escalza I, et al. Analysis of the reasons for exclusion from tPA therapy after early arrival in acute stroke patients. Clin Neurol Neurosurg 2007;109:50–3. 10.1016/j.clineuro.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Michel P, Odier C, Rutgers M, et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke 2010;41:2491–8. 10.1161/STROKEAHA.110.596189 [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 18.Bousser MG, Amarenco P, Chamorro A, et al. Rationale and design of a randomized, double-blind, parallel-group study of terutroban 30 mg/day versus aspirin 100 mg/day in stroke patients: the prevention of cerebrovascular and cardiovascular events of ischemic origin with terutroban in patients with a history of ischemic stroke or transient ischemic attack (PERFORM) study. Cerebrovasc Dis 2009;27:509–18. 10.1159/000216835 [DOI] [PubMed] [Google Scholar]
- 19.Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 20.Michel P, Arnold M, Hungerbühler H, et al. Thrombolyse beim ischämischen Hirnschlag—Aktualisierte Leitlinien. Schweiz Med Forum 2009;49:892–6. [Google Scholar]
- 21.Mishra NK, Lyden P, Grotta JC, et al. Thrombolysis is associated with consistent functional improvement across baseline stroke severity: a comparison of outcomes in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010;41:2612–17. 10.1161/STROKEAHA.110.589317 [DOI] [PubMed] [Google Scholar]
- 22.Mishra NK, Diener HC, Lyden PD, et al. Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 2010;41:2840–8. 10.1161/STROKEAHA.110.586206 [DOI] [PubMed] [Google Scholar]
- 23.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–35. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien W, Crimmins D, Donaldson W, et al. FASTER (Face, Arm, Speech, Time, Emergency Response): experience of Central Coast Stroke Services implementation of a pre-hospital notification system for expedient management of acute stroke. J Clin Neurosci 2012;19:241–5. 10.1016/j.jocn.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 25.Engelter ST, Gostynski M, Papa S, et al. Barriers to stroke thrombolysis in a geographically defined population. Cerebrovasc Dis 2007;23:211–15. 10.1159/000097643 [DOI] [PubMed] [Google Scholar]
- 26.Donnan GA, Davis SM, Parsons MW, et al. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol 2011;7:400–9. 10.1038/nrneurol.2011.89 [DOI] [PubMed] [Google Scholar]
- 27.Fassbender K, Balucani C, Walter S, et al. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol 2013;12:585–96. 10.1016/S1474-4422(13)70100-5 [DOI] [PubMed] [Google Scholar]
- 28.Audebert HJ, Saver JL, Starkman S, et al. Prehospital stroke care: new prospects for treatment and clinical research. Neurology 2013;81:501–8. 10.1212/WNL.0b013e31829e0fdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinney JS, Mylavarapu K, Lane J, et al. Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis 2013;22:113–18. 10.1016/j.jstrokecerebrovasdis.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 30.Ma H, Parsons MW, Christensen S, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke 2012;7:74–80. 10.1111/j.1747-4949.2011.00730.x [DOI] [PubMed] [Google Scholar]
- 31.Thomalla G, Ebinger M, Fiehler J, et al. [EU-funded treatment study: WAKE-UP: A randomized, placebo-controlled MRI-based trial of thrombolysis in wake-up stroke]. Nervenarzt 2012;83:1241–51. 10.1007/s00115-012-3532-7 [DOI] [PubMed] [Google Scholar]
- 32.Amiri H, Bluhmki E, Bendszus M, et al. European cooperative acute stroke study-4: extending the time for thrombolysis in emergency neurological deficits ECASS-4: ExTEND. Int J Stroke 2016;11:260–7. 10.1177/1747493015620805 [DOI] [PubMed] [Google Scholar]
- 33.Cocho D, Belvís R, Martí-Fàbregas J, et al. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology 2005;64:719–20. 10.1212/01.WNL.0000152041.20486.2F [DOI] [PubMed] [Google Scholar]
- 34.Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med 2006;31Suppl 2):S210–16. 10.1016/j.amepre.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Laloux P, Thijs V, Peeters A, et al. Obstacles to the use of intravenous tissue plasminogen activator for acute ischemic stroke. Is time the only barrier? Acta Neurol Belg 2007;107:103–7. [PubMed] [Google Scholar]
- 36.Michel P, Ntaios G, Reichhart M, et al. Perfusion-CT guided intravenous thrombolysis in patients with unknown-onset stroke: a randomized, double-blind, placebo-controlled, pilot feasibility trial. Neuroradiology 2012;54:579–88. 10.1007/s00234-011-0944-1 [DOI] [PubMed] [Google Scholar]
- 37.Lorenzano S, Toni D. TESPI (Thrombolysis in Elderly Stroke Patients in Italy): a randomized controlled trial of alteplase (rt-PA) versus standard treatment in acute ischaemic stroke in patients aged more than 80 years where thrombolysis is initiated within three hours after stroke onset. Int J Stroke 2012;7:250–7. 10.1111/j.1747-4949.2011.00747.x [DOI] [PubMed] [Google Scholar]
- 38.Balucani C, Levine SR. Mild stroke and rapidly improving symptoms: it's not always a happy ending. Stroke 2011;42:3005–7. 10.1161/STROKEAHA.111.628701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke 2007;38:2531–5. 10.1161/STROKEAHA.107.482554 [DOI] [PubMed] [Google Scholar]
- 40.Baumann CR, Baumgartner RW, Gandjour J, et al. Good outcomes in ischemic stroke patients treated with intravenous thrombolysis despite regressing neurological symptoms. Stroke 2006;37:1332–3. 10.1161/01.STR.0000217272.38455.a2 [DOI] [PubMed] [Google Scholar]
- 41.Smith EE, Abdullah AR, Petkovska I, et al. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke 2005;36:2497–9. 10.1161/01.STR.0000185798.78817.f3 [DOI] [PubMed] [Google Scholar]
- 42.Leira EC, Ludwig BR, Gurol ME, et al. The types of neurological deficits might not justify withholding treatment in patients with low total National Institutes of Health Stroke Scale scores. Stroke 2012;43:782–6. 10.1161/STROKEAHA.111.620674 [DOI] [PubMed] [Google Scholar]
- 43.Hassan AE, Hassanzadeh B, Tohidi V, et al. Very mild stroke patients benefit from intravenous tissue plasminogen activator without increase of intracranial hemorrhage. South Med J 2010;103:398–402. 10.1097/SMJ.0b013e3181d7814a [DOI] [PubMed] [Google Scholar]
- 44.Coutts SB, O'Reilly C, Hill MD, et al. Computed tomography and computed tomography angiography findings predict functional impairment in patients with minor stroke and transient ischaemic attack. Int J Stroke 2009;4:448–53. 10.1111/j.1747-4949.2009.00346.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mechanisms of stroke of non-thrombolysed and thrombolysed patients1
emermed-2015-205140supp001.pdf (156.1KB, pdf)


