Abstract
Background:
Ventriculostomy-associated infection (VAI) is a major concern to physicians. Limited studies have looked at the outcomes of external ventricular drain (EVD) infection and predictors of unfavorable outcomes. In this study, we assessed the outcomes of EVD infection and predictors of unfavorable outcomes.
Methods:
This was a retrospective medical chart review, conducted at the Aga Khan University Hospital. All the patients irrespective of age and gender, fulfilling the diagnostic criteria of VAI were included. Patients with preexisting bacterial meningitis or ventriculitis were excluded from the study. Outcome assessment was based on Glasgow outcome scale (GOS) at 1 and 3 months after procedure. Other outcomes included 30-day mortality and total length of hospital stay.
Results:
We included 256 patients in the study. 66 patients (25.8%) developed VAI. EVD was the primary procedure in 21 (31.8%) cases. Most patients, 24 (36.4%), had EVD as a secondary procedure for tumor surgery. Median interval between EVD placement and diagnosis of infection was 3 days. Mean length of stay in VAI patients was 31.85 ± 20.53 days. Seven patients required ICU care. Ten patients (15.2%) expired during hospital stay or within 30 days of discharge and further four had GOS of 2 or 3. A total of 52 patients had a favorable outcome after 6 months.
Conclusions:
Rate of VAI in this cohort was high. VAI is associated with increased morbidity, mortality, and prolonged hospital stay.
Keywords: External ventricular drain, infection, ventriculostomy
INTRODUCTION
External ventricular drain (EVD) placement is one of the most commonly performed neurosurgical procedures.[6] Indications for EVD include acute hydrocephalus, intracranial pressure (ICP) monitoring, posterior fossa surgeries, and for administration of intraventricular antibiotics. Ventriculostomy-associated infection (VAI) is the major complication of this procedure.[4] Reported rates of VAI vary from 3–19%.[12] Numerous studies have shown that VAI is associated with an increase in overall morbidity, mortality, length of hospital, and intensive care unit (ICU) stay.[1,2,14]
Focus of the studies on VAI has been to determine its predictors and outline measures to minimize and prevent it. Few studies have looked at the outcomes of EVD infection and predictors of unfavorable outcomes. We decided to determine the impact of VAI on outcomes of these patients. The subject was of interest to us as we observed high rates of VAI in our setup.
MATERIALS AND METHODS
This was a retrospective observational study, conducted at the Aga Khan University Hospital, Karachi by Sections of Infectious disease and Neurosurgery. The Aga Khan University Hospital is a tertiary care facility and a referral center for neurosurgery.
Inclusion and exclusion criteria
All the patients irrespective of age and gender, fulfilling the diagnostic criteria of VAI were included. Patients with preexisting bacterial meningitis or ventriculitis were excluded from the study.
Operational definition
VAI was diagnosed if a patient has any three of the following within 2 months of EVD insertion:
Cerebrospinal fluid (CSF) positive cultures
CSF glucose <45 gm/dL
CSF white cell count >10/mm3
CSF protein >45 mg/dL
Diagnosis of meningitis by infectious diseases consultant.
External ventricular drain insertion and management
At our center EVD is inserted in the main operating room by a senior resident or instructor under supervision of a consultant. Common indications of the procedure include hydrocephalus due to, intracranial hemorrhage (ICH) with intraventricular extension, tuberculosis meningitis (TBM), subarachnoid hemorrhage (SAH). Other indications include posterior fossa surgeries. Most common site of insertion is Kocher's point and the right side is preferred. For posterior fossa surgeries, EVD is inserted through Keene's point. A BMI® EVD system is used for insertion and subsequent drainage and pressure monitoring. A minimum of 5 cm subcutaneous tunneling of catheter is performed. CSF cultures are routinely sent every 72 hours till the EVD is in place and whenever VAI is suspected clinically. EVD tip and CSF culture are also sent at the time of removal of drain. Impermeable sterile dressing is applied and changed on alternate days or whenever a stain is noticed.
Outcome measures
Outcomes were based on Glasgow outcome scale (GOS) at 3 months, post-procedure. Score of 1–3 was regarded as unfavorable outcome while score of 4–5 was grouped as favorable outcome. Other outcomes included 30-day mortality and total length of hospital stay.
Data collection
Data were collected according to a self-designed form. Same investigator was assigned to collect the data. Variables included age, gender, primary diagnosis, co-morbids, reason for EVD insertion, site and length of EVD tunnel, number of days with EVD, components of diagnostic criteria, and intervention done for VAI. Variables on outcomes were recorded as defined. We used medical records, Patient Care Inquiry (PCI – hospital online database for patient laboratory and radiology reports). For incomplete records, the patients/attendants were contacted through telephone. A formal introduction about the study was given and informed consent was taken before obtaining any information.
Approval from the Departmental Review Committee (DRC) and the Ethical Review Committee (ERC) was sought before commencement of the study. The data was handled by the principal investigator and his team only, making sure that the patient's identity and confidentiality is preserved during all stages.
Data analysis
Data from the forms was entered into Microsoft Excel, checked for missing variables and screened to remove duplicates. Then all data was entered via EpiData software version 3.1 and analyzed using Statistical Package for Social Sciences (SPSS) version 21 (IBM SPSS Statistics). Continuous variables with normal and non-normal distributions were represented as mean [standard deviation (SD)] and median (interquartile range) respectively. Categorical data was represented as percentage and proportions. We grouped the data according to the outcomes (favorable versus unfavorable). Means were compared with the help of independent sample t-test while Chi-square test was used to compare the categorical data.
Sample size
Sample size was calculated by considering the objectives of the study. We used Epi Info version 6 to calculate the sample size. The sample size calculation was based on the assumption that the prevalence of EVD infection was 16.9% per procedure. Therefore, taking the frequency of 17% with 95% confidence level and with 5% (0.05) bound on error of estimation, a sample of 217 participants was required.
To identify the factors associated with EVD infection the sample size for the most important factor (mortality) was estimated. Poor outcome was present in 40% of the unexposed group. By considering these figures together with 95% confidence interval, 80% power and relative risk of 1.39, the sample size came out to be 320. Sample size accommodating the risk factors was larger than the prevalence, so 320 study participants were required to cover the study objectives.
RESULTS
A total of 369 patients underwent the EVD placement over the study period. Two hundred and fifty six patients underwent the procedure in previously sterile CSF. Of the 256 patients, 66 (25.8%) developed EVD infection per our definition. Mean age of patients with EVD infection was 37.92 ± 22.47 years. Thirty-eight patients (57.6%) were male and 28 females (42.4%). In 63 patients, the diagnosis of VAI was based on reports of CSF Detailed Report and Culture and Sensitivity while another three had positive EVD tip culture as well. Frequency of organisms isolated on CSF culture shown in Table 1. About 17 patients (15.8%) had concurrent infection elsewhere in the body as well. Twenty-one patients had a GCS of 9 or less at the time of presentation.
Table 1.
Frequency of organisms isolated on cerebrospinal fluid (CSF) culture

EVD was the primary procedure in 21 (31.8%) cases. Most patients 24 (36.4%) had EVD as a secondary procedure for tumor surgery. ICH was present in another 24 (36.4%). Other procedures were done for CSF rhinorrhea and otorrhea, after traumatic brain injury (TBI), suspected TBM, for hydrocephalus or with endoscopic third ventriculostomy (ETV). Median interval between EVD placement and diagnosis of infection was 3 days with an interquartile range of 9.75 days. Twenty patients had a history of EVD in the past.
Twenty-nine patients had definitive CSF diversion procedure. Twenty-three patients had a VP shunt and six had long tunnel EVD. Mean length of stay was 31.85 ± 20.53 days. Seven patients required ICU care. Ten patients (15.2%) expired during hospital stay or within 30 days of discharge and further four had GOS of 2 or 3. Clinical characteristics of survivors and non-survivors are given in Table 2. A total of 52 patients had a favorable outcome after 6 months, defined as GOS 4–5. Of 66 patients with EVD infection 55 patients achieved CSF sterility.
Table 2.
Clinical characteristics of survivors and nonsurvivors
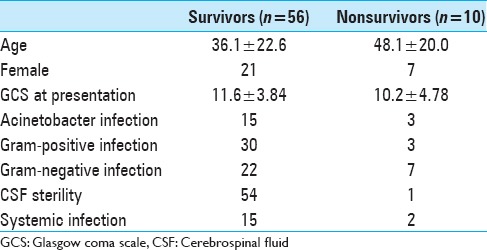
Using multivariate binary logistic regression analysis, a significant relation was found between age and gender of the patients with GOS at 1 month. In our data, female gender (P value: 0.019) and greater age of patients (P value: 0.018) was associated with worse outcome in VAI (odds ratio 0.212 and 1.039). Using logistic regression, we found no association between age/gender/GCS at presentation/primary or secondary EVD and length of hospital stay (P value: >0.05). There was a significant association between diagnosis and length of hospital stay. However, our patient numbers were not large enough to establish which conditions were associated with longer hospital stay.
DISCUSSION
The patients included in our study were selected after strict evaluation of their previous CSF sterility status. Strict inclusion criteria limited the sample size to 256 from the original 369 cases identified. Of these 256 subjects, 66 contracted EVD-related infection, so the incidence of infection was found to be 25.8%. This was a little higher compared to various other published studies conducted in similar tertiary care hospitals.[3,13] Some studies have reported significantly lower incidence rates of infection, the probable cause of these low rates is due to varying definitions of EVD infection and different inclusion and exclusion criteria.[5] Such differences in basic definition of infection lead to over or under estimation of similarly evaluated data.
Our study focused mainly on the mortality associated with EVD infection. It was seen that the mortality rate was significantly high at 15.1%. This included all the patients who did not survive after they were diagnosed with infection. Cause of mortality or differences in management protocols were however not considered. The data available on mortality associated with EVD infection is extremely limited. However, in our study, no significant correlation was found between patient demographics and mortality associated with EVD infection.
It was seen that the mean number of EVD days for patients who developed infection was 19.61 days in contrast to the patients who did not develop infection who had EVD for a mean of 7.09 days. This difference is significant and leads to a conclusion that the longer the duration of EVD the higher the rate of infection. These findings were consistent with another study done which showed that people who developed infection had EVD in place for twice as many days as those who did not develop infection with EVD.[8,9] Conversely, another study by Hagel et al. highlighted that the reason for prolongation of EVD is the development of infection and not vice versa.[5]
In the process of infection detection, the main tools used were Cerebro-Spinal Fluid Culture & Sensitivity (CSFCS), Cerebro-Spinal Fluid Detailed Report (CSFDR), and EVD tip culture. It was seen that 95.4% patients had positive CSFCS and CSFDR. EVD tip cultures were positive only in 4.5% of the cases indicating low sensitivity for this form of diagnosis. EVD tip culture is hence a very poor diagnostic method, and in most cases, was thought to be contaminated during the removal process and therefore did not give a true representation of the CSF. When identifying the causative agents in our study we identified that 51.5% of the subjects were infected with gram-negative organisms and the remaining 43.9% were infected with gram-positive organisms. In our data, the most commonly isolated organism was coagulase-positive staph. The main reason for this high staph count was the introduction of skin flora in the CSF after skin breakdown during the shunt insertion process. Skin flora has been found as main culprit behind EVD infection.[17]
We identified that gram-negative bacteria were associated with a higher mortality. Of all the nonsurvivors in our study, 63.6% were the ones infected by gram-negative bacteria. This link has been studied in the past with an established association of gram-negative infection with higher rates mortality in patients with nosocomial meningitis.[16]
It was identified that out of the gram-negative isolates, Acinetobacter was the most commonly isolated organism.[11,15] It was also the main organism responsible for significant mortality with 27.2% of all deaths associated with Acinetobacter.[7] This has been studied in the past where similar results have been obtained with Acinetobacter mortality being higher in most cases with mean ranging from 15% to 71%.[7]
There are several limitations to this study. The retrospective nature of data collection limits accurate data analysis. The indications for the placement of EVD were heterogenous. Due to relatively small sample sub group analysis could not be carried out separately for different diagnosis. Despite these limitations, we believe that the results of the study provide information of clinical significance.
CONCLUSION
We found a high incidence of VAI at out center. It is significantly associated with increased morbidity, mortality, prolonged hospital stay, and poor outcome. Gram-positive organisms were the most frequent pathogen whereas gram-negative infection particularly Acinetobacter is associated with higher mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Muhammad E. Bari, Email: ehsan.bari@aku.edu.
Ghani Haider, Email: ghanihaider@hotmail.com.
Komail Malik, Email: komailmalik26@gmail.com.
Muhammad Waqas, Email: shaiq_waqas@hotmail.com.
Syed F. Mahmood, Email: faisal.mahmood@aku.edu.
Mubbashira Siddiqui, Email: mubbashira_siddiqui@hotmail.com.
REFERENCES
- 1.Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, et al. Ventriculostomy-associated infections: Incidence and risk factors. Am J Infect Control. 2005;33:137–43. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent JL. Ventriculostomy-related infections in critically ill patients: A 6-year experience. J Neurosurg. 2005;103:468–72. doi: 10.3171/jns.2005.103.3.0468. [DOI] [PubMed] [Google Scholar]
- 3.Camacho E, Boszczowski I, Basso M, Jeng B, Freire M, Guimaraes T, et al. Infection rate and risk factors associated with infections related to external ventricular drain. Infection. 2011;39:47–51. doi: 10.1007/s15010-010-0073-5. [DOI] [PubMed] [Google Scholar]
- 4.Hader WJ, Steinbok P. The value of routine cultures of the cerebrospinal fluid in patients with external ventricular drains. Neurosurgery. 2000;46:1149–55. doi: 10.1097/00006123-200005000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Hagel S, Bruns T, Pletz M, Engel C, Kalff R, Ewald C. External ventricular drain infections: Risk factors and outcome. Interdiscip Perspect Infect Dis 2014. 2014:708531. doi: 10.1155/2014/708531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakarla UK, Chang SW, Theodore N, Spetzler RF, Kim LJ. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008;63:ONS162–7. doi: 10.1227/01.neu.0000335031.23521.d0. [DOI] [PubMed] [Google Scholar]
- 7.Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–55. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JH, Desai NS, Ricci J, Stieg PE, Rosengart AJ, Härtl R, et al. Factors contributing to ventriculostomy infection. World Neurosurg. 2012;77:135–40. doi: 10.1016/j.wneu.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr Ventriculostomy-related infections: A critical review of the literature. Neurosurgery. 2002;51:170–82. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Lu CH, Chang WN, Chuang YC, Chang HW. The prognostic factors of adult gram-negative bacillary meningitis. J Hosp Infect. 1998;40:27–34. doi: 10.1016/s0195-6701(98)90021-4. [DOI] [PubMed] [Google Scholar]
- 11.Metan G, Alp E, Aygen B, Sumerkan B. Carbapenem-resistant Acinetobacter baumannii: An emerging threat for patients with post-neurosurgical meningitis. Int J Antimicrob Agents. 2007;29:112–3. doi: 10.1016/j.ijantimicag.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Mikhaylov Y, Wilson TJ, Rajajee V, Thompson BG, Maher CO, Sullivan SE, et al. Efficacy of antibiotic-impregnated external ventricular drains in reducing ventriculostomy-associated infections. J Clin Neurosci. 2014;21:765–8. doi: 10.1016/j.jocn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Omar MA, Haspani MSM. Drainage-Related Infection at Hospital Kuala Lumpur: An Observational Study. Malays J Med Sci. 2010;17:48–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Rebuck JA, Murry KR, Rhoney DH, Michael DB, Coplin WM. Infection related to intracranial pressure monitors in adults: Analysis of risk factors and antibiotic prophylaxis. J Neurol Neurosurg Psychiatry. 2000;69:381–4. doi: 10.1136/jnnp.69.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacar S, Turgut H, Toprak S, Cirak B, Coskun E, Yilmaz O, et al. A retrospective study of central nervous system shunt infections diagnosed in a university hospital during a 4-year period. BMC Infect Dis. 2006;6:1. doi: 10.1186/1471-2334-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54. doi: 10.1056/NEJMra0804573. [DOI] [PubMed] [Google Scholar]
- 17.Zingale A, Ippolito S, Pappalardo P, Chibbaro S. Infections and re-infections in long-term external ventricular drainage: A variation upon a theme. J Neurosurg Sci. 1999;43:125. [PubMed] [Google Scholar]


