Abstract
Production of active TGF-β is regulated at a posttranslational level and implies release of the mature cytokine dimer from the inactive, latent TGF-β precursor. There are several cell-type specific mechanisms of TGF-β activation. We identified a new mechanism operating on the surface of human regulatory T cells and involving membrane protein GARP, which binds latent TGF-β1. The paracrine activity of regulatory T cell–derived TGF-β1 contributes to immunosuppression and can be inhibited with anti-GARP Abs. Whether other immune cell types use surface GARP to activate latent TGF-β1 was not known. We show in this study that stimulated, human B lymphocytes produce active TGF-β1 from surface GARP/latent TGF-β1 complexes with isotype switching to IgA production.
Introduction
Transforming growth factor–β1 is a potent immunosuppressive cytokine. Mice with a germline deletion of the Tgfb1 gene or a T cell–specific deletion of the Tfgbr1 or Tgfbr2 genes die early in life from severe multiorgan inflammation due to the uncontrolled activity of T lymphocytes (1–3). The production of TGF-β1 is a tightly regulated process, which occurs mostly at a posttranslational level. Most human and mouse cells express the Tgfb1 gene and produce the TGF-β1 precursor, prepro-TGF-β1. After signal peptide removal and homodimerization, the resulting pro-TGF-β1 is cleaved by furin to generate two dimeric fragments. The Cter dimer, or mature TGF-β1, remains noncovalently associated to the Nter dimer, or latency associated peptide (LAP), forming a complex called latent TGF-β1. Latent TGF-β1 is inactive because LAP prevents binding of mature TGF-β1 to its receptor. TGF-β1 bioactivity requires the release of mature TGF-β1 from LAP, a process referred to as TGF-β1 activation. Many cells, including most immune cells, secrete latent TGF-β1. However, TGF-β1 activation only occurs in a few cell types, via mechanisms that are cell-type specific. The best-described mechanisms implicate RGD-binding integrins, such as integrins αVβ1 in fibroblasts (4), αVβ6 in epithelial cells (5), and αVβ8 in dendritic cells, glial cell, or fibroblasts (6–8).
We and others recently demonstrated that in contrast to most other cells and in response to TCR stimulation, human regulatory T cells (Tregs) produce latent TGF-β1 in association with a transmembrane protein called GARP (9, 10). This association implies disulfide linkage between two cysteines in one GARP monomer and one cysteine in each monomer of the LAP homodimer (11, 12). This results in the display of GARP/latent TGF-β1 complexes on TCR-stimulated Tregs. We also showed that the activation of latent TGF-β1 by stimulated Tregs is GARP dependent, and that this active TGF-β1 exerts paracrine immunosuppressive actions at a short distance, when Treg to T effector cell contacts are allowed (13, 14). We derived mAbs against GARP/latent TGF-β1 complexes that block active TGF-β1 production by human Tregs. These blocking anti-GARP mAbs inhibited the immunosuppressive activity of human Tregs in vivo, in a xenogeneic graft-versus-host-disease induced by transfer of human PBMCs into immunodeficient mice (14).
Blocking anti-GARP mAbs is currently explored as a novel immunotherapeutic approach to inhibit Treg function and increase immune responses in the context of cancer or chronic infections. In contrast to Abs directed against TGF-β1 itself, anti-GARP mAbs are expected to prevent TGF-β1 activation by Tregs, but not by cells that activate TGF-β1 independently from GARP. This may prove important as TGF-β1 exerts many actions outside the immune system, such as tumor-suppressive effects on preneoplastic epithelial cells (15).
GARP is present on nonTreg cells. It was initially discovered in mouse and human megakaryocytes and platelets (16, 17), and was later shown to be expressed on mouse liver sinusoid endothelial cells (18), mouse and human hepatic stellate cells (19), and mouse and human mesenchymal stromal cells (20). Whether other, nonTreg immune cells also express GARP, and whether any nonTreg cell expressing GARP produces active TGF-β1 in a GARP-dependent manner, has not been completely elucidated to date. Addressing this may help predict potential undesired effects of therapeutic anti-GARP mAbs used to inhibit Treg immunosuppression, and improve our understanding of the mechanisms leading to TGF-β1 activation in various cell types.
We therefore sought to determine whether other, nonTreg human immune cells release active TGF-β1 from GARP/latent TGF-β1 complexes on their surface. We found that stimulated, but not resting B cells, express GARP/latent TGF-β1 complexes and produce active TGF-β1 in a GARP-dependent manner, which increases isotype switching to IgA.
Materials and Methods
Ethics statement
Experiments with human cells were approved by our institution’s ethics committee.
Cell purification
PBMCs were isolated from the blood of hemochromatosis donors. CD19+ B cells were purified from PBMCs using magnetic beads (Miltenyi Biotec), and CD20+CD27− and CD20+CD27+ B cells were sorted by FACS.
Reagents used for in vitro stimulation of B cells
B cells were stimulated with the indicated combinations of anti-human IgM F(ab′)2 fragment (25 μg/ml; Jackson ImmunoResearch), anti-human IgM/IgG F(ab′)2 fragment (25 μg/ml; eBioscience), megaCD40L (150 ng/ml; Enzo Life Sciences), CpG ODN2006 (2,5 μg/ml; InvivoGen), IL-21 (10 ng/ml; eBioscience), IL-2 (120 UI/ml; Proleukin), and IL-4 (4 ng/ml). Additional reagents were added as indicated in the figure legends: rTGF-β1 (R&D Systems), blocking anti-TGF-β1,2,3 mAb (1D11; R&D Systems), anti-TNP Ab (B8401H5.M, mIgG1), anti-GARP mAbs [MHG-8 (mIgG1), LHG-10, LHG-11, and LHG-14 (hIgG1) (14)].
Flow cytometry
Cells were labeled according to standard protocols. Viability dye (Molecular Probes) was used to exclude dead cells. Data were analyzed using FlowJo (TreeStar). Primary mAbs against GARP were described in (14). Other Abs were from R&D Systems (anti-LAP), BD Biosciences (anti-CD3, CD56, CD86, IgG), BioLegend (anti-CD4, CD14, CD20, CD27, IgD), and Southern Biotec (anti-IgA).
RT-PCR analysis
Quantitative RT-PCR was performed on cDNA using a Takyon for Probe Assay kit (Eurogentec) on an ABI StepOnePlus system (Applied Biosystems). Primers and probes for EF-1 and GARP are described in (10). Primers for IαCμ are described in (21).
Small interfering RNA electroporation
SilencerSelect small interfering RNAs (siGARP#2: s5577, siGARP#3: s5575, siScramble: 4390846) from Thermo Fischer Scientific were electroporated using a 4D-Nucleofector instrument (Lonza).
Western blot analysis
Cell lysates were submitted to SDS-PAGE under reducing conditions. Blots were incubated with primary Abs against SMAD2 phosphorylation (pSMAD2) (#3108; Cell Signaling Technology), GARP (Plato-1; Enzo Life Sciences), or β-actin (Sigma), then secondary HRP-coupled Abs, and revealed with an ECL substrate (Thermo Fisher Scientific) on a Fusion Solo 4S system (Vilber). Quantifications were performed with Bio1D (Vilber).
TGF-β1 ELISA
Supernatants from CD19+ B cells stimulated in X-VIVO-10 serum-free medium (Lonza) were used nonacidified or after acid-treatment to quantify TGF-β1 with the TGF-β1 Duoset ELISA (R&D Systems).
Results and Discussion
GARP/latent TGF-β1 complexes are expressed on the surface of human B cells after stimulation in vitro
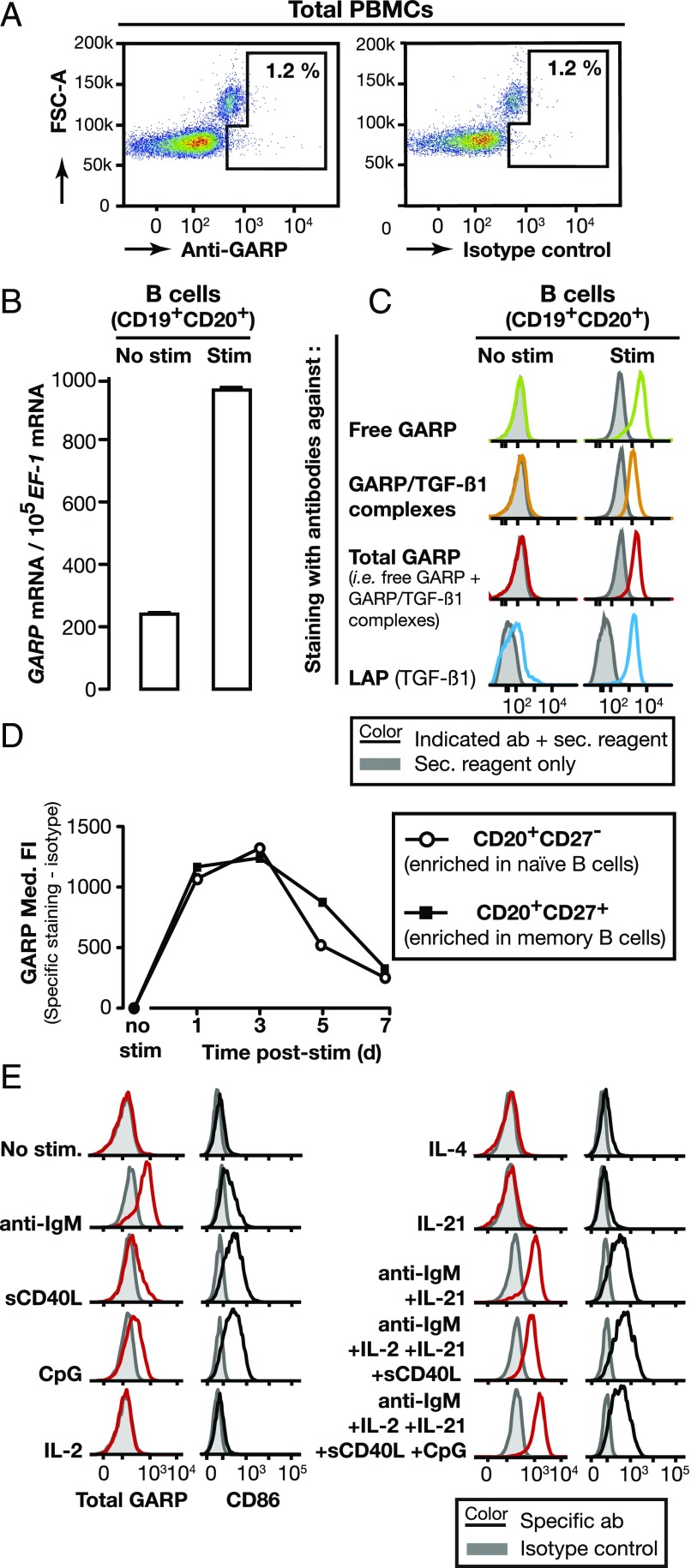
Protein GARP is not detected on the surface of unstimulated human PBMCs [(9) and Fig. 1A]. Human PBMCs comprise 1–2% Tregs and GARP was shown to appear on the surface of Tregs after stimulation of their TCR, e.g., in vitro with anti-CD3/anti-CD28 Abs (9, 10). We examined whether in vitro stimulation induced GARP expression on other subsets of human PBMCs. No GARP protein was detected on CD8+ cells stimulated with anti-CD3/CD28 Abs, on NK cells stimulated with IL-2 and IL-12, or on monocytes stimulated with LPS or CpG (Supplemental Fig. 1A). However, for purified B lymphocytes, GARP mRNA levels increased 5-fold, and surface GARP protein appeared after in vitro stimulation with a mixture containing anti-IgM/IgG Abs, soluble CD40L (sCD40L), IL-2, IL-21, and CpG (Fig. 1B, 1C). Stimulated but not unstimulated B cells were stained by mAbs that recognize free GARP, GARP/latent TGF-β1 complexes, both, or LAP (Fig. 1C, Supplemental Fig. 1B). This indicates that free GARP and GARP/latent TGF-β1 complexes appear on the surface of human B cells after stimulation in vitro, and thus that GARP binds and presents latent TGF-β1 on these cells. To determine whether GARP appears on stimulated naive, memory B cells or both, we isolated CD20+CD27− (enriched in naive) and CD20+CD27+ (enriched in memory) B cells from human PBMCs (Supplemental Fig. 1C) and stimulated them in vitro with the mixture described above. As shown in Fig. 1D, GARP appeared on the surface of the vast majority of both types of cells 1 d after stimulation, remained high until day 3 then decreased to return to resting levels on day 7.
FIGURE 1.
Human B cells express surface GARP/latent TGF-β1 complexes after in vitro stimulation. (A) Human PBMCs were labeled with anti-GARP (mAb MHG-6) or isotype control Abs and analyzed by flow cytometry. Dot plots are gated on live cells. (B) CD19+ B cells were stimulated or not with anti-IgM/IgG Abs, IL-2, IL-21, sCD40L, and CpG for 24 h. Bar graphs indicate mean GARP mRNA copy numbers normalized to 105 EF-1 mRNA copies (technical duplicates + SD) as determined by quantitative RT-PCR. (C) CD19+ B cells stimulated as in (B) were labeled with Abs against free GARP (mAb MHG-5), total GARP (mAb MHG-6), GARP/TGF-β1 complexes (mAb MHG-8), or LAP, then analyzed by flow cytometry. Histograms are gated on live cells. (D) CD20+CD27− and CD20+CD27+ B cells were stimulated or not as in (B) during the indicated number of days, labeled with Abs against total GARP (mAb MHG-6) and analyzed by flow cytometry. Med. FI: median fluorescence intensity. (E) CD20+CD27− B cells were exposed during 24 h to the indicated stimuli, then labeled with Abs against total GARP (mAb MHG-6) and analyzed by flow cytometry. Histograms are gated on live cells.
We then examined which stimuli induced GARP expression on naive B cells and used CD86 as an activation marker (Fig. 1E, Supplemental Fig. 1D). The most efficient stimulus was the anti-IgM Abs that stimulate the cells through the BCR. Triggering of CD40 with sCD40L, or of TLR9 with CpG also induced surface GARP, albeit to a lesser extent. Stimulation with IL2, IL-4, or IL-21 alone was not sufficient to induce surface CD86 and did not induce surface GARP either. As expected, maximal GARP expression was induced by stimulation with a combination of anti-IgM, sCD40L, CpG, and cytokines.
From the above, we concluded that surface GARP and GARP/latent TGF-β1 complexes are transiently induced by stimulation on naive and memory human B cells.
Stimulated B cells produce active TGF-β1 in a GARP-dependent manner
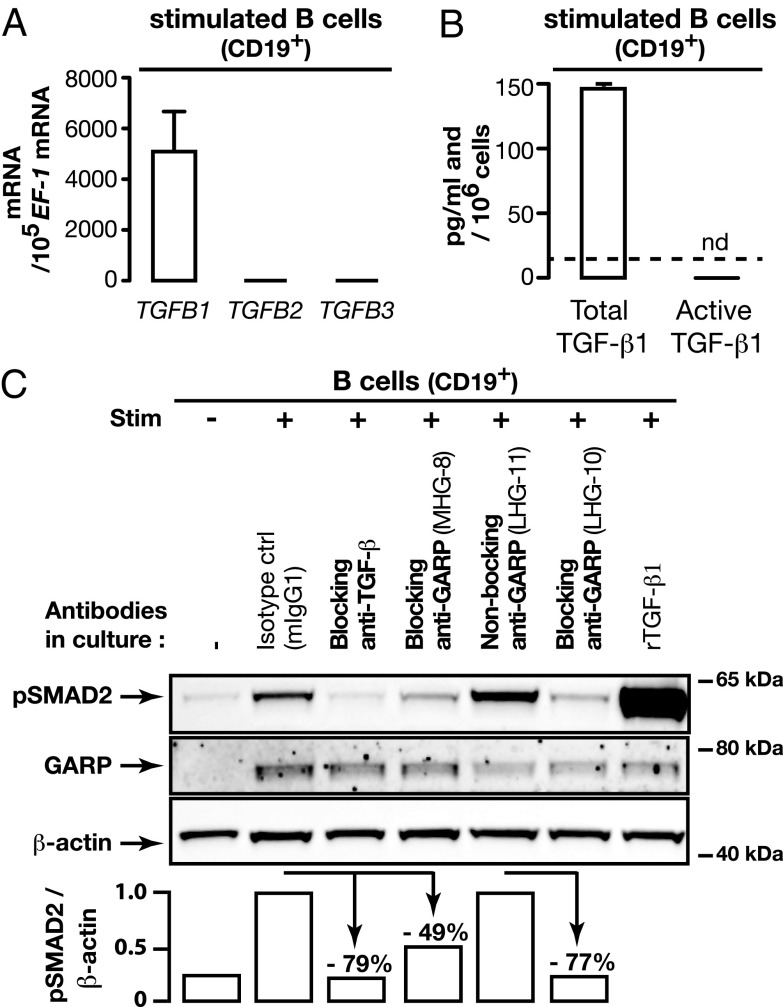
To date, stimulated Tregs were the only immune cells known to produce active TGF-β1 from GARP/latent TGF-β1 complexes (14, 22). Active TGF-β1 produced by Tregs is not detected as a soluble form in Treg supernatants. It is only released close to the Treg surface, where it exerts autocrine and paracrine actions that induce phosphorylation of SMAD2 and SMAD3 transcription factors in responding cells (13). Stimulated B cells express high levels of TGFB1, but no TGFB2 or TGFB3 mRNAs (Fig. 2A). They secrete latent TGF-β1 but no detectable active TGF-β1 (Fig. 2B). Thus, as in Tregs, active TGF-β1 is not released as a soluble form by these cells. To examine whether B cells produce active TGF-β1 from GARP/TGF-β1 complexes close to their surface, we measured pSMAD2 by Western blot in CD19+ B cells stimulated with the mixture used above, in the presence of various anti-GARP mAbs (Fig. 2C). No pSMAD2 was detected in unstimulated B cells. Stimulation induced GARP expression, as expected, and induced pSMAD2. It was blocked by a neutralizing anti-TGF-β Ab, indicating that stimulated B cells produce active TGF-β1. pSMAD2 was also reduced in the presence of blocking, but not nonblocking anti-GARP Abs. We conclude that stimulated B cells release active TGF-β1 from GARP/latent TGF-β1 complexes close to their cell surface.
FIGURE 2.
Human B cells produce active TGF-β1 in a GARP-dependent manner. (A) Levels of TGFB1, 2 and 3 mRNA were measured in CD19+ B cells stimulated for 24 h with the mixture used in Fig. 1B. Bar graphs indicate mRNA copy numbers normalized to 105 EF-1 mRNA copies (means of duplicates + SD) as determined by quantitative RT-PCR. (B) Total and active TGF-β1 were measured by ELISA in the supernatants of B cells stimulated with the mixture used in Fig. 1B during 4 d in serum-free medium. Means of duplicates + SD. Detection limit: 16 pg/ml. nd, not detected. (C) CD19+ B cells were stimulated as in (A). The indicated Abs (10 μg/ml) were added on day 0 and 3 of the cultures, and rTGF-β1 (0.1 ng/ml) on day 3. Cells collected on day 4 were analyzed by Western blot, and luminescence signals were quantified using Bio1D software. Reduction in pSMAD2/β-actin ratios in cells treated with anti-TGF-β or the blocking anti-GARP MHG-8 Ab (both mIgG1s) are shown relative to the cells stimulated in the presence of a mIgG1 isotype control. Reduction in cells treated with the blocking anti-GARP LHG-10 Ab is shown relative to cells treated with the nonblocking anti-GARP LHG-11 (both hIgG1s). Data are representative of three independent experiments.
Active TGF-β1 produced from GARP/latent TGF-β1 complexes by naive B cells does not reduce B cell proliferation
TGF-β1 inhibits the proliferation of many cells types, including T and B lymphocytes (23). To determine if the production of active TGF-β1 by human B cells inhibits their proliferation, we stimulated freshly isolated naive B cells in vitro and added blocking anti-TGF-β or anti-GARP mAbs. Surprisingly, we could not detect significant changes in B cell proliferation (Supplemental Fig. 2A, 2B). We then tested the sensitivity of B cells to recombinant active TGF-β1 (rTGF-β1, 0.1 ng/ml) on day 0, 1, 2, or 3 of a 4-d culture. rTGF-β1 inhibited B cell proliferation when added on day 0 or 1, but it had no effect when added at later time points (Supplemental Fig. 2A, 2B). Resistance to the cytostatic effect of TGF-β1 acquired at later time points could not be overcome by higher concentrations of rTGF-β1 (Supplemental Fig. 2C). The absence of effect of anti-TGF-β and anti-GARP on B cell proliferation could result from active TGF-β1 being released by B cells at a time point when they have become resistant to the anti-proliferative effect of the cytokine. In support of this hypothesis, pSMAD2, which is indicative of autocrine TGF-β activity, was induced to high levels only 2–4 d after stimulation of B cells in vitro (Supplemental Fig. 2D). This indicates that the autocrine activity of TGF-β1 released from GARP/latent TGF-β1 complexes by B cells does not reduce their proliferation.
Active TGF-β1 produced from GARP/latent TGF-β1 complexes by naive B cells induces isotype switching to IgA production
A nonredundant function of TGF-β1 signaling in B cells is to induce isotype switching to IgA (24–27). The cell type that produces the active TGF-β1 required for the IgA switch is not known. We thus tested whether active TGF-β1 produced by stimulated B cells themselves could induce IgA switching.
Noncoding IgA1 germline transcripts (IgA1 GLT) are required for the gene recombination events leading to IgA1 production (28). Next, recombination events generate circular molecules of excised DNA, which are transcribed into so-called switch circle transcripts, or IαCμ transcripts in the case of IgA switching. IgA1 GLT and IαCμ transcripts are induced early during the maturation process in the B cells that will eventually switch to IgA1 production, resulting in both IgA1 surface expression and secretion.
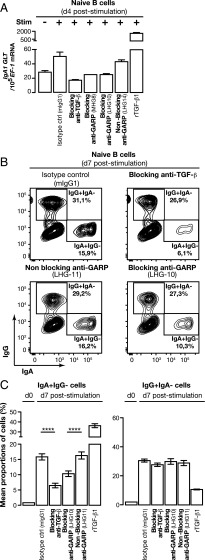
We measured IgA1 GLT transcripts 4 d after in vitro stimulation of human naive B cells (Fig. 3A). IgA1 GLT were increased ∼1.8-fold in stimulated versus nonstimulated B cells. This increase was abrogated in the presence of blocking anti-TGF-β or anti-GARP mAbs, but not of a nonblocking anti-GARP mAb. As expected, rTGF-β1, taken in this study as a positive control, increased IgA1 GLT to very high levels. Two small interfering RNAs, that efficiently reduced GARP mRNA and protein levels also reduced IgA1 GLT to a similar extent as blocking anti-GARP Abs (Supplemental Fig. 2E). IαCμ transcripts were also induced by stimulation of human naive B cells, and this induction was abrogated in the presence of anti-TGF-β or anti-GARP Abs (Supplemental Fig. 2F). Finally, we measured the proportions of cells expressing surface IgA or IgG before or 7 d after in vitro stimulation of naive B cells in the presence of anti-TGF-β or anti-GARP Abs (Fig. 3B, 3C, Supplemental Fig. 2G). As expected, more than 95% naive B cells expressed IgD but none or very few (<2%) expressed surface IgA or IgG before stimulation. Seven days after stimulation in the presence of a negative control Ab, ±16% B cells expressed surface IgA, ±30% B cells expressed surface IgG, and all IgA+ or IgG+ B cells had lost IgD expression. Mean proportions of IgA+ B cells were significantly reduced to ±6 and ±10% in the presence of a blocking anti-TGF-β1 or a blocking anti-GARP Ab, respectively, but were not reduced in the presence of a nonblocking anti-GARP Ab. Proportions of IgG+ B cells, in contrast, were not reduced in the presence of anti-TGF-β or anti-GARP Abs. Altogether, these data demonstrate that active TGF-β1 released from GARP/latent TGF-β1 complexes on the surface of BCR-stimulated naive B cells acts in an autocrine fashion to induce isotype switching to IgA production, but does not affect isotype switching to IgG.
FIGURE 3.
Blocking active TGF-β1 production in B cells with anti-GARP blocking Abs decreases IgA class switch. (A) CD20+CD27− B cells were stimulated or not with anti-IgM, sCD40L, IL-2, IL-21, and CpG for 4 d in the presence of the indicated Abs. IgA1 GLT were quantified by quantitative RT-PCR. rTGF-β1 (0.1 ng/ml) added on day 2 was used as positive control. Bar graphs indicate mean IgA1 GLT per 105 EF-1 copies (technical duplicates + SD). (B and C) Naive B lymphocytes were stimulated as above during 7 d, then labeled with anti-IgA, IgG, and IgD and analyzed by flow cytometry. IgD stainings on stimulated and nonstimulated cells are shown in Supplemental Fig. 2G. rTGF-β1 (0.1 ng/ml) added 3 d after stimulation was used as a positive control. Representative contour plots are gated on live cells (B) and bar graphs indicate the mean percentages of six technical replicates ± SD (C). Selected pairs were compared using one-way ANOVA followed by a Bonferroni multiple comparison test. Data are representative of at least three independent experiments. ****p < 0.0001.
Conclusions
IgA is the most abundantly produced Ab isotype in the body, and plays important roles in mucosal immunity. TGF-β signaling induces IgA isotype switching, as mice with a B cell–specific deletion of the Tgfbr2 gene display profoundly impaired IgA production (25). However, the cellular source of TGF-β inducing IgA switching in vivo has not been clearly identified. We show in this study that, after in vitro stimulation, human B cells release active TGF-β1 from surface GARP/TGF-β complexes, which increases IgA class switching. Whether in vivo B cell–derived TGF-β1 is the exclusive source of TGF-β1 for IgA switching cannot be inferred from our experiments. Of note, it was recently reported that IgA production in murine Peyer’s patches requires interaction of B cells with subepithelial dendritic cells expressing integrin αVβ8, which activates latent TGF-β produced by an undefined cellular source (29). Garp/latent TGF-β1 complexes on the surface of murine B cells themselves could represent a source of latent TGF-β1, available for activation in trans by integrin αVβ8 on the surface of dendritic cells. However, our data indicate that in humans at least, dendritic cells are not always required for activation of TGF-β1 presented on the surface of B cells, and that purified B cells alone can activate TGF-β1 without the intervention of another cell type. Mere expression of GARP/latent TGF-β1 complexes on the surface of B cells is probably not sufficient for TGF-β1 activation, as we previously demonstrated that lentiviral-mediated expression of GARP in nonTreg T cells was not sufficient to activate the cytokine (10). Release of active TGF-β1 from GARP/latent TGF-β1 complexes thus probably implicates an additional surface protein, which could be an RGD-binding integrin.
In addition to providing insight into the regulation of IgA isotype switching in human B cells, our data also indicate that the use of blocking anti-GARP mAbs to inhibit immunosuppression by Tregs in patients suffering from cancer or chronic infections may impair IgA class switch, and hence defense against infections at mucosal sites. Of note, however, most patients with a selective IgA deficiency, representing the most common primary immunodeficiency with a prevalence ranging from 1:200 to 1:3000 in the general Western world population, are asymptomatic (30). This suggests that transient inhibition of IgA class switch may represent an acceptable side effect of immunotherapies with anti-GARP mAbs.
Supplementary Material
Acknowledgments
We thank Maria Panagiotakopoulos, Stéphanie D’Hondt and Amandine Collignon for expert technical assistance. We are very grateful to Suzanne Depelchin for editorial help and Nicolas Dauguet for FACS sortings.
This work was supported by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programming, by the Actions de Recherche Concertées of the Communauté Française de Belgique, and by grants from the European Union’s Horizon 2020 Research and Innovation Programme (Grant 682818), and the Fonds J. Maisin (Belgium). S.L., O.D. and J.S. are supported by the Fonds National pour la Recherche Scientifique (Belgium).
The online version of this article contains supplemental material.
- LAP
- latency associated peptide
- pSMAD2
- SMAD2 phosphorylation
- Treg
- regulatory T cell.
Disclosures
B.v.d.W. is full-time employee of argenx. S.L., B.v.d.W., and P.G.C. are listed as inventors on the patent describing MHG and LHG Abs (Patent Cooperation Treaty, international publication number WO 2016/125017 A1). The other authors have no financial conflicts of interest.
References
- 1.Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D., et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. 1993. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marie J. C., Liggitt D., Rudensky A. Y. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 25: 441–454. [DOI] [PubMed] [Google Scholar]
- 4.Reed N. I., Jo H., Chen C., Tsujino K., Arnold T. D., DeGrado W. F., Sheppard D. 2015. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 7: 288ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A., et al. 1999. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. [DOI] [PubMed] [Google Scholar]
- 6.Travis M. A., Reizis B., Melton A. C., Masteller E., Tang Q., Proctor J. M., Wang Y., Bernstein X., Huang X., Reichardt L. F., et al. 2007. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold T. D., Ferrero G. M., Qiu H., Phan I. T., Akhurst R. J., Huang E. J., Reichardt L. F. 2012. Defective retinal vascular endothelial cell development as a consequence of impaired integrin αVβ8-mediated activation of transforming growth factor-β. J. Neurosci. 32: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura H., Cambier S., Somanath S., Barker T., Minagawa S., Markovics J., Goodsell A., Publicover J., Reichardt L., Jablons D., et al. 2011. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 121: 2863–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran D. Q., Andersson J., Wang R., Ramsey H., Unutmaz D., Shevach E. M. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 106: 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stockis J., Colau D., Coulie P. G., Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur. J. Immunol. 39: 3315–3322. [DOI] [PubMed] [Google Scholar]
- 11.Wang R., Zhu J., Dong X., Shi M., Lu C., Springer T. A. 2012. GARP regulates the bioavailability and activation of TGFβ. Mol. Biol. Cell 23: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthy E., Cuende J., Stockis J., Huygens C., Lethé B., Collet J. F., Bommer G., Coulie P. G., Lucas S. 2013. GARP is regulated by miRNAs and controls latent TGF-β1 production by human regulatory T cells. PLoS One 8: e76186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockis J., Fink W., François V., Connerotte T., de Smet C., Knoops L., van der Bruggen P., Boon T., Coulie P. G., Lucas S. 2009. Comparison of stable human Treg and Th clones by transcriptional profiling. Eur. J. Immunol. 39: 869–882. [DOI] [PubMed] [Google Scholar]
- 14.Cuende J., Liénart S., Dedobbeleer O., van der Woning B., De Boeck G., Stockis J., Huygens C., Colau D., Somja J., Delvenne P., et al. 2015. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci. Transl. Med. 7: 284ra56. [DOI] [PubMed] [Google Scholar]
- 15.Massagué J. 2008. TGFbeta in cancer. Cell 134: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macaulay I. C., Tijssen M. R., Thijssen-Timmer D. C., Gusnanto A., Steward M., Burns P., Langford C. F., Ellis P. D., Dudbridge F., Zwaginga J. J., et al. 2007. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood 109: 3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubin R., Pizette S., Ollendorff V., Planche J., Birnbaum D., Delapeyriere O. 1996. Structure and developmental expression of mouse Garp, a gene encoding a new leucine-rich repeat-containing protein. Int. J. Dev. Biol. 40: 545–555. [PubMed] [Google Scholar]
- 18.Carambia A., Freund B., Schwinge D., Heine M., Laschtowitz A., Huber S., Wraith D. C., Korn T., Schramm C., Lohse A. W., et al. 2014. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J. Hepatol. 61: 594–599. [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Kim B. G., Qian S., Letterio J. J., Fung J. J., Lu L., Lin F. 2015. Hepatic stellate cells inhibit T cells through active TGF-β1 from a cell surface-bound latent TGF-β1/GARP complex. J. Immunol. 195: 2648–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrillo-Galvez A. B., Cobo M., Cuevas-Ocaña S., Gutiérrez-Guerrero A., Sánchez-Gilabert A., Bongarzone P., García-Pérez A., Muñoz P., Benabdellah K., Toscano M. G., et al. 2015. Mesenchymal stromal cells express GARP/LRRC32 on their surface: effects on their biology and immunomodulatory capacity. Stem Cells 33: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerutti A., Zan H., Schaffer A., Bergsagel L., Harindranath N., Max E. E., Casali P. 1998. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J. Immunol. 160: 2145–2157. [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards J. P., Thornton A. M., Shevach E. M. 2014. Release of active TGF-β1 from the latent TGF-β1/GARP complex on T regulatory cells is mediated by integrin β8. J. Immunol. 193: 2843–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M. O., Wan Y. Y., Sanjabi S., Robertson A. K., Flavell R. A. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24: 99–146. [DOI] [PubMed] [Google Scholar]
- 24.Coffman R. L., Lebman D. A., Shrader B. 1989. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J. Exp. Med. 170: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazac B. B., Roes J. 2000. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13: 443–451. [DOI] [PubMed] [Google Scholar]
- 26.Cerutti A. 2008. The regulation of IgA class switching. Nat. Rev. Immunol. 8: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda E., Matsumoto R., Hitoshi Y., Ishii T., Sugimoto M., Araki S., Tominaga A., Yamaguchi N., Takatsu K. 1989. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J. Exp. Med. 170: 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stavnezer J., Guikema J. E., Schrader C. E. 2008. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26: 261–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reboldi A., Arnon T. I., Rodda L. B., Atakilit A., Sheppard D., Cyster J. G. 2016. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 352: aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh K., Chang C., Gershwin M. E. 2014. IgA deficiency and autoimmunity. Autoimmun. Rev. 13: 163–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.