Abstract
Human exposure to the neurotoxic methylmercury (MeHg) occurs primarily via the consumption of marine fish, but the processes underlying large-scale spatial variations in fish MeHg concentrations [MeHg], which influence human exposure, are not sufficiently understood. We used the Atlantic silverside (Menidia menidia), an extensively studied model species and important forage fish, to examine latitudinal patterns in total Hg [Hg] and [MeHg]. Both [Hg] and [MeHg] significantly increased with latitude (0.014 and 0.048 μg MeHg g−1 dw per degree of latitude in juveniles and adults, respectively). Four known latitudinal trends in silverside traits help explain these patterns: latitudinal increase in MeHg assimilation efficiency, latitudinal decrease in MeHg efflux, latitudinal increase in weight loss due to longer and more severe winters, and latitudinal increase in food consumption as an adaptation to decreasing length of the growing season. Given the absence of a latitudinal pattern in particulate MeHg, a diet proxy for zooplanktivorous fish, we conclude that large-scale spatial variation in growth is the primary control of Hg bioaccumulation in this and potentially other fish species.
Keywords: methylmercury, growth, condition, growth dilution hypothesis, bioaccumulation
Introduction
Consumption of marine fish comprises the leading route of human exposure to methylmercury (MeHg) (Sunderland 2007), which is the toxic form of mercury (Hg) produced by microorganisms and biomagnified in marine food webs (Gilmour et al. 2013, Hammerschmidt et al. 2013, Mason et al. 2012). Negative links between consumption of MeHg-laden fish and human health (e.g. Karagas et al. 2012, Oken et al. 2005) have inspired much of the Hg research in recent decades as well as the signing of the Minamata Convention on Mercury by 128 countries in 2013 (Sunderland et al. 2016). Atmospheric Hg concentrations over the North Atlantic and deposition have declined since the 1970s (Soerensen et al. 2012), as have MeHg concentrations in bluefish (Cross et al. 2015), while questions remain whether the response is linear or dependent on other factors besides Hg input.
Fish acquire most of their MeHg from diet (Hall et al. 1997, Mathews and Fisher 2009, Pickhardt et al. 2006), but additional factors including fish age, trophic level, prey type, and feeding depth (Atwell et al. 1998, Choy et al. 2009, Cutshall et al. 1978, Karimi et al. 2016) also affect MeHg bioaccumulation. Assimilation of inorganic forms of dietary Hg, on the other hand, is thought to be negligible (<15%) given the much faster turnover rate of assimilated inorganic Hg compared to MeHg (Dutton and Fisher 2010). Overall, MeHg bioaccumulation is the net result of interacting physiological processes and environmental factors. In particular the feedbacks between dietary intake, growth, and MeHg bioaccumulation are still insufficiently understood (Braune 1987, Doyon et al. 1998, Karimi et al. 2016). Such knowledge would arguably improve the interpretation of MeHg patterns in wild fish populations, particularly when patterns are compared across large geographic regions. Along the North-American Pacific coast, for example, total Hg concentrations in populations of Pacific hake Merluccius productus appear to increase with latitude (Cutshall et al. 1978), whereas sablefish (Anoplopoma fimbria) and Pacific halibut (Hippoglossus stenolepis) populations exhibit the opposite pattern (Hall et al. 1976a, Hall et al. 1976b). These findings might be reconciled if the interactions between population growth and MeHg bioaccumulation across environmental gradients were better understood.
Inhabited by diverse and often widely distributed fish species the North-American Atlantic coast exhibits one of the world's steepest latitudinal gradients, with average coastal sea surface temperatures declining by almost 1°C per degree of latitude (Baumann and Doherty 2013). This gradient has been shown to promote local within-species adaptation in traits such as growth, vertebral number, and environmental sex determination, and in no fish species has this been more extensively studied than in the Atlantic silverside Menidia menidia (Billerbeck et al. 1997, Conover 1992, Conover and Heins 1987, Conover and Present 1990, Munch and Conover 2003, Schultz and Conover 1997, Yamahira and Conover 2002). This abundant coastal forage fish inhabits nearshore areas between northern Florida (~28°N) and the Canadian Magdalen Islands (~46°N). Its broad distribution and ecological importance, along with its semelparous, annual life history and its extensive use in rearing experiments have made M. menidia a valuable model species in ecological and evolutionary research (Middaugh et al. 1987). It is also well suited for examining the geographic variability in MeHg bioaccumulation and its relationship to population growth patterns.
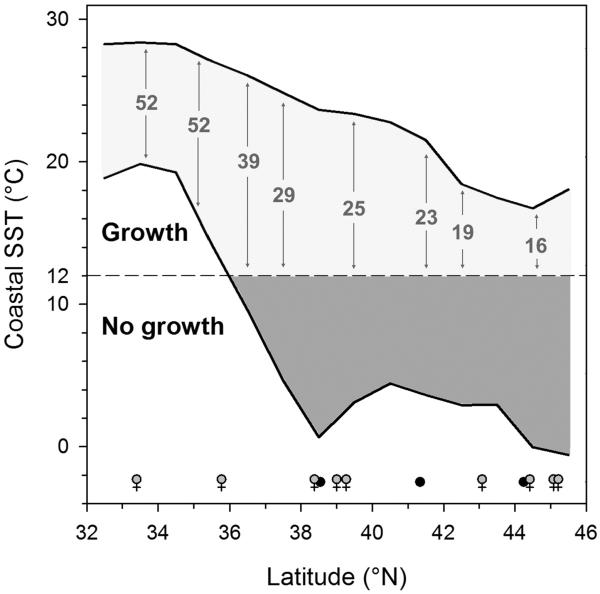
A central factor determining the selection for growth in M. menidia is the length of the growing season, which decreases with latitude from 52 to only 16 weeks for southern vs. northernmost populations, respectively (Fig. 1; Baumann and Conover 2011). Continuous natural selection results in countergradient growth variation, i.e., an increase in temperature-specific growth capacities with latitude that allows populations from the north to reach similar sizes to their southern conspecifics in less time (Conover and Present 1990). To support faster growth, fish need to be more efficient at ingesting and converting food to somatic tissue. Experiments that used a common garden approach have demonstrated that at constant food abundance M. menidia from northern populations ingest more food and grow more efficiently than their southern conspecifics (Present and Conover 1992).
Figure 1.
Latitudinal temperature gradient along the North-American Atlantic coast and length of the growing season in M. menidia. Shape and black lines depict long-term average minimum and maximum annual sea surface temperatures (SST) for each latitude (modified after Baumann and Conover 2011). Numbers refer to the number of weeks per year that SST is above the species 12°C thermal growth threshold (Conover and Present 1990; dashed line). Female symbols denote latitudes from which adult females were selected; solid black dots represent latitudes of juvenile samples.
These population differences likely influence the bioaccumulation of MeHg, which may therefore also show a latitudinal pattern. According to the growth dilution hypothesis (Trudel and Rasmussen, 2006) MeHg concentrations in fish are inversely related to growth efficiency, thus predicting declining MeHg concentrations with latitude in M. menidia populations. On the other hand, a higher assimilation efficiency of MeHg and slower loss in northern compared to southern M. menidia populations (Dutton and Fisher 2010) may attenuate or reverse this pattern. Given the counteracting influences of ingestion, growth efficiency, and MeHg assimilation, the goal of the present study was to measure and then examine latitudinal patterns in MeHg concentrations M. menidia in both the juveniles and adults.
Materials and methods
Juvenile and adult samples
Fish were collected during previous field campaigns across a broad geographical range (33.4°N – 45.2°N) (Chen et al. 2014, Hice et al. 2012). M. menidia juveniles were caught from July to September of 2013 in four locations (38 – 41°N) using a 6.1 × 1.2 m beach seine (mesh size = 6 mm) (Table 1). Adults were selected from archived (−20°C) specimens, which were collected with a 30 × 2m beach seine (mesh size = 5 mm) during a large spatial sampling campaign in 2006/07 (Hice et al. 2012) (Table 1). We focused on adult females sampled in 2006 to eliminate sex-related size differences (Middaugh et al. 1987) and potential inter-annual differences in bioaccumulation as confounding factors. To further constrain sources of uncertainty, only adults and juveniles of similar size were selected for analysis (Table 1). Hence, the mean total length (TL, nearest cm ± 0.05 cm) of juveniles was 5.8 cm (SD = 0.5 cm), and the TL of adults was 8.6 cm (SD = 0.7 cm). Wet mass (m; measurement error ± 0.001g) was individually recorded and used to calculate Fulton's condition factor (K = m × TL−3).
Table 1.
Overview of the latitudinal samples for K, Hg, and MeHg of Atlantic silverside juveniles and adults used in this study. nm = not measured.
| Sampling locations | Latitude °N | N | TL, mean ± SD (cm) | K, mean ± SD | Hg, mean ± SD (mg g−1 dw) | NMeHg | MeHg, mean ± SD (mg g−1 dw) | % MeHg ± SD | |
|---|---|---|---|---|---|---|---|---|---|
| Juveniles | JP Park, MD | 38.39 | 13 | 5.9±0.4 | 0.71±0.09 | 0.04±0.04 | 13 | 0.04±0.04 | 99±3 |
| Parker's Creek, MD | 38.54 | 9 | 6.2±0.4 | 0.77±0.10 | 0.04±0.03 | 9 | 0.04±0.03 | 95±3 | |
| Barn Island, CT | 41.34 | 3 | 6.1±0.7 | 0.60±0.05 | 0.16±0.02 | 3 | 0.16±0.02 | 97±2 | |
| Bass Harbor, ME | 44.25 | 19 | 5.8±0.5 | 0.51±0.05 | 0.12±0.03 | 19 | 0.12±0.03 | 99±2 | |
|
| |||||||||
| Adults | Pawley's Island, SC | 33.40 | 10 | 8.7±0.4 | 0.86±0.08 | 0.35±0.08 | 5 | 0.21±0.09 | 57±19 |
| Oregon Inlet, NC | 35.77 | 9 | 9.1±0.4 | 0.80±0.09 | 0.37±0.11 | nm | |||
| JP Park, MD | 38.38 | 10 | 9.1±0.2 | 0.89±0.09 | 0.13±0.01 | 10 | 0.08±0.03 | 64±25 | |
| Sandy Point, MD | 39.00 | 10 | 8.9±0.3 | 0.99±0.09 | 0.10±0.03 | 8 | 0.07±0.02 | 66±17 | |
| Elk Neck, MD | 39.27 | 10 | 9.2±0.4 | 0.83±0.06 | 0.14±0.02 | nm | |||
| Sandy Hook, NJ× | 40.40 | 10 | 9.1±0.3 | 0.75±0.08 | 1.05±0.39 | 10 | 0.82±0.40 | 77±18 | |
| Setauket Harbor, NY* | 40.95 | 10 | 8.9±1.7 | 0.55±0.05 | 0.50±0.16 | nm | |||
| Kittery Point, ME | 43.08 | 10 | 9.0±0.3 | 0.79±0.08 | 0.36±0.13 | 8 | 0.27±0.16 | 70±20 | |
| Mt. Desert Island, ME | 44.42 | 10 | 7.7±0.3 | 0.58±0.04 | 0.34±0.09 | nm | |||
| St. Andrews, NB | 45.08 | 10 | 8.4±0.6 | 0.61±0.08 | 0.28±0.04 | nm | |||
| St. John, NB | 45.22 | 10 | 7.6±0.5 | 0.56±0.06 | 0.65±0.12 | 4 | 0.43±0.10 | 67±18 | |
Value excluded from latitudinal trends, likely contaminated site;
fish were sampled in 2012 and have not been included in latitudinal trends
Hg and MeHg measurements in fish
We used three different analytical facilities to determine total Hg and MeHg. Each laboratory used the same standard reference material (DORM-2) to assure accuracy of the analysis. While juveniles were processed whole, a piece of white muscle from above the lateral line was sampled from the adults, frozen at −80°C, freeze-dried, and homogenized prior to measuring the concentration of total and methylmercury (hereafter [Hg] and [MeHg], respectively). Hg speciation for juveniles was determined at the Dartmouth Trace Element Analysis Core facility, while for adults [Hg] was determined at Stony Brook University. [MeHg] was determined for a subset of adult fish at the University of Connecticut. [Hg] was determined by direct mercury analyzers (DMA-80, Milestone, Bergamo, Italy). Methods to determine [MeHg] differed slightly for M. menidia juveniles and adults, however recoveries of Hg from standard reference material assured the quality of measurments. For juveniles, 40–60 mg of the homogenized dry tissue was digested with 4N HNO3 and analyzed for [MeHg] and inorganic Hg by species-specific isotope dilution with an automated MeHg system MERX-M (Brooks Rand Labs, Seattle, WA) interfaced with an Agilent 7500cx ICP-MS (Agilent, Santa Clara, CA) using a method similar to EPA 1630 (Taylor et al. 2011, Taylor et al. 2008). Standards, duplicates and blanks were analyzed for QA/QC with each sample set.
For adults, [MeHg] was determined for a subset of specimens from five locations (Table 1). Between 30 and 90 mg of homogenized dry muscle tissue was digested in 5 mL aliquots of trace metal grade 4N HNO3 overnight at ~60°C (Hammerschmidt and Fitzgerald 2005). A small volume (<0.2 mL) of digest was added to ~30 mL deionized water, and the acid was neutralized with 8N KOH. MeHg was determined by purge and trap (Tenax) gas chromatographic CVAFS (Tekran 2700 analyzer) following pH adjustment with 2M acetate buffer and ethylation with 1% sodium tetraethyl borate (Hammerschmidt and Fitzgerald 2001). [MeHg] was measured after calibration with aqueous standard solution (Brooks Rand). Duplicates and blanks were analyzed for QC/QA with each sample set.
Particulate [MeHg]
To characterize [MeHg] of particulate matter as a proxy for ingested food (nominal size of Quartz Fiber Filter or QFF = 0.45μm), we used newly generated or previously published values for a range of locations (Table S1) that were near or at the sites used for fish collections. Selected study sites were away from direct Hg pollution sources and all had similar regional inputs of Hg from the atmosphere and watershed.
Water was sampled with trace metal clean techniques using acid-washed Teflon or glass bottles. Water was filtered via QFF in a laminar flow hood to assure clean conditions and filters were stored as frozen (−20°C) prior to analysis. Particle-bound MeHg on filters immersed in ~25 mL deionized water were distilled with 1 mL of 50% trace metal grade sulfuric acid (H2SO4) and 0.5 mL of 20% potassium chloride (KCl). Similar to adult fish samples, particulate MeHg samples were analyzed using a Tekran 2700 automated analyzer. Sample results were corrected for reagent blanks, and the minimum detection limit (MDL) was 0.12 pM.
Statistical analyses
Pearson correlation analysis was used to first explore the associations between [MeHg], [Hg], latitude, life stage, and condition factor across the entire data set of analyzed specimens. Similarly, we correlated [MeHg] in particles to latitude in the assembled dataset of particulate [MeHg]. In a second step, we quantified the latitudinal relationships using stepwise multiple linear regression to model [MeHg] as a function of life stage, latitude, and condition factor, using forward stepwise inclusion of predictor variables (p < 0.05) and the lowest Akaike Information Criterion (AIC) to select the best model. Finally, we constructed a General Linear Model of the form
to test for main effects and their interaction. A significant interaction term (p < 0.05) would indicate that the slope of the tested relationships differed between juveniles and adults. T-tests were used to test for significant differences in [MeHg] between individual sites. All statistical analyses were done using SPSS (V.20, IBM).
Results
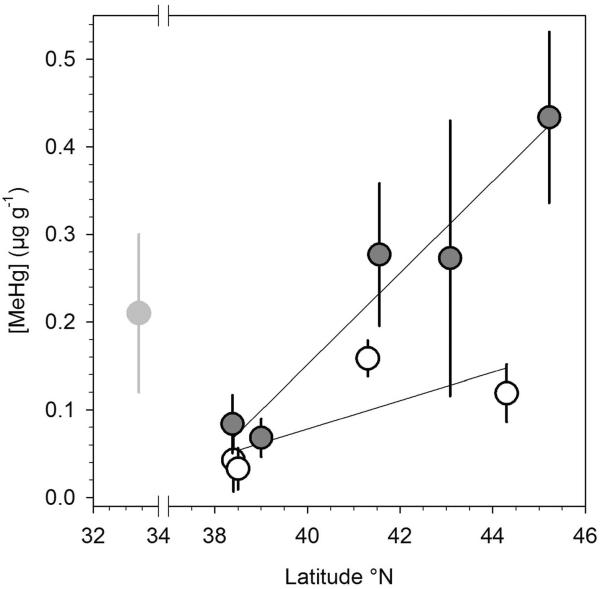
In juveniles, whole body MeHg comprised >90% of measured total Hg, with average±SD of [MeHg] ranging from 0.04±0.03 to 0.16±0.02 μg g−1 dw (Table1). Juveniles from JP Park and Parkers Creek (38.5°N) had significantly lower [MeHg] than their conspecifics from Bass Harbor (44.3°N, t-test, p < 0.001), suggesting a latitudinal trend (Fig. 2). In adults, the average±SD of [MeHg] ranged from 0.07±0.02 to 0.82±0.40 μg g−1 dw (Table 1). The %MeHg in juveniles were higher in comparison to adults (this study) and to values previously reported for the silversides (<80%; Chen et al. 2014). Unusually high [Hg] and [MeHg] values were found for fish sampled from Sandy Hook, NJ (40.4°N), an urban and likely polluted site, given that values were almost twice as high as measurements from adults from an undisturbed site at a similar latitude (Setauket, NY, 40.9°N, Table 1). The Sandy Hook values were therefore excluded from further analyses of latitudinal trends.
Figure 2.
Relationship between latitude and mean±SD [MeHg] in juvenile (white circles) and adult (grey circles) M. menidia from sampling locations along the North American Coast. The [MeHg] of adult females from the southernmost location (light grey circle) were not included into the regression.
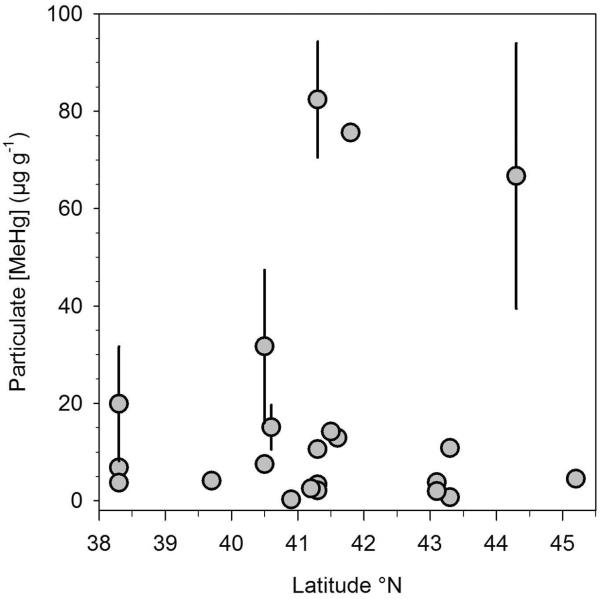
Pearson correlation analysis showed significant positive associations between [MeHg] and life stage, and [MeHg] and latitude, whereas condition factor was negatively correlated to [MeHg] and to latitude (Table 2). Average [MeHg] in particulate matter, as compiled from literature values and our additional recent measurements, was highly variable (Fig. 3) and ranged from 52.2±4.0 to 16.5×103 μg g−1 dry weight (Table S1). There was no correlation between particulate [MeHg] and latitude across all sites (Pearson correlation = 0.13, p = 0.58, Fig. 3, Table 2).
Table 2.
Pearson bivanate correlation analysis between [MeHg], [Hg] in Atlantic silversides and their life stage (juveniles vs. adult females), latitude of origin, and condition factor, as well as particulate [MeHg] and latitude.
| Life stage | Latitude | Condition factor | |
|---|---|---|---|
| [MeHg] (n = 85) | 0.480 *** | 0.552 *** | −0.147 |
| [Hg] (n = 124) | 0.566 *** | 0.604 *** | −0.275 ** |
| Condition factor (n = 124) | 0.333 *** | −0.701 *** | |
| Particulate [MeHg] (n = 22)a | 0.130 |
Significant correlations are in bold
p < 0.05,
p < 0.01,
p < 0.001
average values were used for the correlation.
Figure 3.
Mean±1.s.e. of particulate [MeHg] in coastal waters along the North American Coast. Data sources: this study, Benoit et al. (1998); Balcom et al. (2008); Balcom et al. (2013); Chen et al (2014); Gosnell et al. (2015); Guentzel et al. (2001); Sunderland et al. (2010); Hollweg et al. (2009); see Table S1 in supporting information.
Stepwise multiple regression identified life stage and latitude as significant predictor variables of [MeHg] (AIC = −417.5, df = 82, R2adj = 0.54), whereas including condition factor did not improve the model (AIC = −402.8, df = 81, R2adj = 0.54). The GLM confirmed that life stage and latitude both explained significant parts of variability in [MeHg], with significantly higher [MeHg] in adults than juveniles (F83,1 = 33.0, p < 0.001), significantly increasing [MeHg] with increasing latitude, and a significant interaction between the two factors, because the latitudinal [MeHg] increase was steeper in adults than juveniles (F1,83 = 39.2, p < 0.001). Hence for juveniles, the linear [MeHg]:latitude relationship predicted an average [MeHg] increase of 0.014 μg g−1 dw degree of lat−1 (R2 = 0.51, df = 42, p <0.001, Fig. 2) or an almost 3-fold increase in predicted [MeHg] between 38 – 44.5°N. For adults north of 38°N, the [MeHg]:latitude relationship was more than three times steeper, with an average increase of 0.048 μg g−1 dw degree of lat−1 (R2 = 0.58; df = 39, p < 0.001, Fig. 2) or a 7.5-fold increase between 38 – 46°N. [Hg] similarly increased with latitude in juveniles and adults (2-tailed Pearson correlation r = 0.74; p < 0.001; Fig. S1).
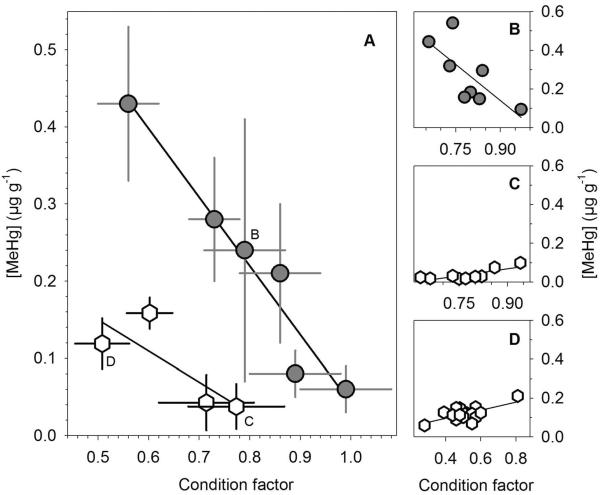
In both juveniles and adults, [MeHg] and condition factor were significantly negatively related (R2juv = 0.65; p = 0.008, R2adults =0.94; p < 0.001, Fig. 4A). Within locations, the relationship was either non-significant or weakly positive for juveniles or strongly negative or nonsignificant for adults (Fig. 4B–D).
Figure 4.
(A) Relationship between mean K and [MeHg] in juvenile (white hexagons) and adult Atlantic silversides (grey circles) across the US Atlantic coast. Error bars are 1 SD. (B–D) Within-location K:[MeHg] relationship for sites, where this relationship was significant (p < 0.05).
Discussion
We quantified total and methylmercury concentrations in juvenile and adult Atlantic silversides across most of their distributional range and found that both [Hg] and [MeHg] increased significantly with latitude. We further observed that [MeHg] and the slope of the [MeHg] : latitude relationship increased from the juvenile to the adult life stage. This suggests that a secondary process modifies [MeHg] between the juvenile and adult stage predominantly in higher compared to lower latitude populations. Adult silversides had comparatively low and highly variable %MeHg values that were similar to those reported for silversides in other estuaries (Greenfield and Jahn 2010) but notably lower than the generally assumed fraction of > 95% MeHg in fish (Bloom 1992).
While the ontogenetic increase in [MeHg] is consistent with the cumulative effect of dietary uptake and accumulation of MeHg in muscle tissue over the lifetime of fish (Cutshall et al. 1978), the mechanisms underlying the latitudinal trends are unknown. One immediate explanation would be if [MeHg] in the fish's diet (Chen et al. 2014) followed a similar latitudinal trend. However, we compiled published and self-measured water column particulate [MeHg] that covered the size fractions of zooplankton as a proxy for dietary [MeHg] for silversides. The absence of a latitudinal pattern in particulate [MeHg] indicates that a different process causes the latitudinal [MeHg] increase in this species.
Other studies have previously detected latitudinal patterns in mercury concentrations in wild fish populations. Consistent with our findings, Cutshall et al. (1978) reported that Hg concentrations in Pacific hake (Merluccius productus) increased with length and latitude, with the slope of the [Hg]:latitude relationship (0.024 μg g−1 degree of lat−1) falling between the slopes measured in this study for silverside juveniles and adults (0.014 and 0.051 μg g−1 degree of lat−1, respectively). However, the study did not attempt to explain the latitudinal trend, other than generally invoking potential differences in fish metabolism. Reverse latitudinal trends have also been reported, e.g., for Pacific halibut and sablefish (Hall et al. 1976a, Hall et al. 1976b), but again the mechanisms underlying these patterns remained unexplored or were possibly confounded by trends of decreasing fish size with latitude.
In Atlantic silversides, latitudinal growth differences due to local adaptation of populations (Fig. 1) have been extensively studied for decades (Billerbeck et al. 2000, Conover et al. 2009, Conover and Present 1990). Fish in high latitudes have evolved, and are continuously selected for, higher growth capacities, which allows them to reach similar sizes at the end of their shorter growing season compared to their lower latitude conspecifics (Conover and Present 1990). Conversely, slower growth is adaptive in populations at lower latitudes (longer growing seasons), because of known trade-offs between growth rate and metabolic scope, swimming performance, and thus the ability to escape predators (Munch and Conover 2003). These latitudinal differences in growth and associated traits have important implications for MeHg bioaccumulation and bioconcentration in silversides. Generally, fish acquire MeHg through their food at rates depending on dietary [MeHg], ingestion rate, growth- and assimilation efficiency. Meanwhile, the very low turnover rate of MeHg ensures that most of it remains in the fish's body (Cutshall et al. 1978).
While dietary [MeHg] appears to be independent of latitude (this study), previous studies have demonstrated clear latitudinal patterns in silverside ingestion rates, growth- and MeHg assimilation efficiencies. Using common garden experiments, Billerbeck et al. (2000) showed that ingestion rates in larval and early juvenile silversides increase with latitude, likely to sustain the higher growth rates required to compensate for shorter growing seasons. Increased ingestion rates could result in increased MeHg burdens (Trudel and Rasmussen 2006), which is consistent with the observed latitudinal [MeHg] increase in this study. Growth efficiency, on the other hand, also increases with latitude in silverside populations as these and other common garden experiments have revealed (Billerbeck et al. 2000, Present and Conover 1992). Higher growth efficiencies mean that fish can form more tissue per unit of ingested food, which would result in lower MeHg burdens (Harris and Bodaly 1998, Trudel and Rasmussen 2006, Ward et al. 2010) contrary to the direction of our observed latitudinal MeHg gradient. Finally, the latitudinal increase in food conversion efficiency likely has an important corollary, i.e., high latitude fish are also more efficient MeHg assimilators (Dutton and Fisher 2010), given that they retain more MeHg per unit food in their bodies. We conclude that higher ingestion rates and higher MeHg assimilation efficiencies outweigh higher growth efficiencies in higher vs. lower latitude silverside populations, thus producing the latitudinal increase in juvenile [MeHg] observed in this study. Further experimentation is warranted to test this hypothesis.
In adult silversides, the slope of this latitudinal [MeHg] increase was significantly steeper than in juveniles. Juveniles and adults of this semelparous, annual forage fish are separated by one winter, and the severity of winter increases with latitude. Hence, with increasing latitude, silversides encounter longer periods below their species-specific thermal growth threshold of 12°C (Conover and Present 1990). In combination with poor offshore feeding conditions (Cushing 1975), overwintering thus leads to increasing weight loss (Hurst 2007, Schultz and Conover 1997), decreasing body condition (Reimers 1963, Thompson et al. 1991), and therefore results in the observed strengthening of the latitudinal [MeHg] increase in adults, because the absolute body burden of MeHg remains unchanged (i.e., bioconcentration). We conclude that in M. menidia the interplay between local population growth patterns during the juvenile stage and latitudinal patterns of overwintering could explain the documented latitudinal increases in [MeHg] concentrations.
In addition, our study found a significant negative relationship between condition factor and [MeHg] in both juveniles and adults, which is consistent with the growth dilution hypothesis, because fish forming more tissue per unit length are bound to have lower [MeHg] if all else is equal (Harris and Bodaly 1998, Trudel and Rasmussen 2006, Ward et al. 2010). However, while within-population differences in condition may be interpreted in this way (e.g., Fig. 4), the value of condition factor as a proxy for energy density across populations and large spatial scales is limited. For example, most fish species including Atlantic silversides show a predictable increase in vertebral number with latitude known as Jordans Rule (Baumann et al. 2012, Hice et al. 2012, Yamahira et al. 2006), which results in slimmer body shapes and different proportions that confound inter-population comparisons in condition factor. We conclude that the negative [MeHg]:condition factor relationship in this study, while generally in the expected direction, could be coincidental and driven mostly by latitudinal differences in condition factor related to body shape.
In summary, it is likely that MeHg bioaccumulation is intricately linked to growth patterns and thus expected to change with interpopulation growth differences that evolved across environmental gradients. Furthermore, even at a given location, inter-annual food web and temperature variability influence growth and therefore MeHg bioaccumulation. As abundant forage fish, Atlantic silversides are important sources of [MeHg] for many marine piscivores, hence, future research should further explore the underlying mechanisms in this and other fish species, particularly in those species consumed by humans.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of A. Kenagy, V. Ortiz, B. Dimento and E. Seelen during lab measurements. We thank V.F. Taylor for Hg measurements in juvenile Atlantic silversides. We are grateful to the former members of the Conover and Chen labs for their efforts collecting Atlantic silversides. We are especially grateful to L. Hice for verifying the information pertaining to fish selected in the present study. Finally, we would like to thank the two anonymous reviewers for their constructive feedback, which had helped improving this manuscript. This work was partially funded by NSF-OCE #0425830, #1536165 and by NIH # P42 ES007373 from the National Institute of Environmental Health Sciences.
References
- Atwell L, Hobson KA, Welch HE. Biomagnification and bioaccumulation of mercury in an arctic marine food web: insights from stable nitrogen isotope analysis. Can J Fish Aquat Sci. 1998;55(5):1114–1121. [Google Scholar]
- Balcom PH, Hammerschmidt CR, Fitzgerald WF, Lamborg CH, O'Connor JS. Seasonal distributions and cycling of mercury and methylmercury in the waters of New York/New Jersey Harbor Estuary. Mar Chem. 2008;109(1–2):1–17. [Google Scholar]
- Balcom PH, Schartup AT, Mason RP, Chen CY. Pathways of methylmercury transfer to the water column across multiple estuaries. Mar Chem. 2015;177:721–730. doi: 10.1016/j.marchem.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Conover DO. Adaptation to climate change: contrasting patterns of thermal-reaction-norm evolution in Pacific versus Atlantic silversides. Proc R Soc B - Biol Sci. 2011;278:2265–2273. doi: 10.1098/rspb.2010.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Doherty O. Decadal changes in the world's coastal latitudinal temperature gradients. PloS one. 2013;8(6):e67596. doi: 10.1371/journal.pone.0067596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Rosales-Casian JA, Conover DO. Contrasting latitudinal variations in vertebral number and sex determination in Pacific vs. Atlantic silverside fishes. Copeia. 2012;2012:341–350. [Google Scholar]
- Benoit JM, Gilmour CC, Mason RP, Riedel GS, Riedel GF. Behavior of mercury in the Patuxent river estuary. Biogeochemistry. 1998;40(2/3):249–265. [Google Scholar]
- Billerbeck JM, Ortí G, Conover DO. Latitudinal variation in vertebral number has a genetic basis in the Atlantic silverside, Menidia menidia. Can J Fish Aquat Sci. 1997;54:1796–1801. [Google Scholar]
- Billerbeck JM, Schultz ET, Conover DO. Adaptive variation in energy acquisition and allocation among latitudinal populations of the Atlantic silverside. Oecologia. 2000;122(2):210–219. doi: 10.1007/PL00008848. [DOI] [PubMed] [Google Scholar]
- Bloom NS. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci. 1992;49(5):1010–1017. [Google Scholar]
- Bone SE, Charette MA, Lamborg CH, Gonneea ME. Has submarine groundwater discharge been overlooked as a source of mercury to coastal waters? Environ Sci Technol. 2007;41(9):3090–3095. doi: 10.1021/es0622453. [DOI] [PubMed] [Google Scholar]
- Braune BM. Mercury accumulation in relation to size and age of Atlantic herring (Clupea harengus harengus) from the southwestern Bay of Fundy, Canada. Arch Environ Contam Toxicol. 1987;16(3):311–320. doi: 10.1007/BF01054948. [DOI] [PubMed] [Google Scholar]
- Chen CY, Borsuk ME, Bugge DM, Hollweg T, Balcom PH, Ward DM, Williams J, Mason RP. Benthic and pelagic pathways of methylmercury bioaccumulation in estuarine food webs of the Northeast United States. PloS one. 2014;9(2):e89305. doi: 10.1371/journal.pone.0089305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy CA, Popp BN, Kaneko JJ, Drazen JC. The influence of depth on mercury levels in pelagic fishes and their prey. Proc Natl Acad Sci USA. 2009;106(33):13865–13869. doi: 10.1073/pnas.0900711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover DO. Seasonality and the scheduling of life history at different latitudes. Journal of Fish Biology. 1992;41:161–178. [Google Scholar]
- Conover DO, Duffy TA, Hice LA. The covariance between genetic and environmental influences across ecological gradients - reassessing the evolutionary significance of countergradient and cogradient variation. Ann NY Acad Sci. 2009;1168:100–129. doi: 10.1111/j.1749-6632.2009.04575.x. [DOI] [PubMed] [Google Scholar]
- Conover DO, Heins SW. Adaptive variation in environmental and genetic sex determination in a fish. Nature. 1987;326(6112):496–498. doi: 10.1038/326496a0. [DOI] [PubMed] [Google Scholar]
- Conover DO, Present TMC. Countergradient variation in growth rate: compensation for length of the growing season among Atlantic silversides from different latitudes. Oecologia. 1990;83:316–324. doi: 10.1007/BF00317554. [DOI] [PubMed] [Google Scholar]
- Cross FA, Evans DW, Barber RT. Decadal declines of mercury in adult bluefish (1972–2011) from the mid-Atlantic coast of the U.S.A. Environ Sci Technol. 2015;49(15):9064–9072. doi: 10.1021/acs.est.5b01953. [DOI] [PubMed] [Google Scholar]
- Cushing DH. Marine ecology and fisheries. Cambridge University Press; Cambridge: 1975. [Google Scholar]
- Cutshall NH, Naidu, Pearcy WG. Mercury concentrations in Pacific hake, Merluccius productus (Ayres), as a function of length and latitude. Science. 1978;200(4349):1489–1491. doi: 10.1126/science.663631. [DOI] [PubMed] [Google Scholar]
- Doyon J-F, Schetagne R, Verdon R. Different mercury bioaccumulation rates between sympatric populations of dwarf and normal lake whitefish (Coregonus clupeaformis) in the La Grande complex watershed, James Bay, Quebec. Biogeochemistry. 1998;40(2–3):203–216. [Google Scholar]
- Dutton J, Fisher N. Intraspecific comparisons of metal bioaccumulation in the juvenile Atlantic silverside Menidia menidia. Aquat Biol. 2010;10(3):211–226. [Google Scholar]
- Gilmour CC, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA, Bailey KL, Elias DA. Mercury methylation by novel microorganisms from new environments. Environ Sci Technol. 2013;47(20):11810–11820. doi: 10.1021/es403075t. [DOI] [PubMed] [Google Scholar]
- Gosnell K, Balcom P, Ortiz V, DiMento B, Schartup A, Greene R, Mason R. Seasonal cycling and transport of mercury and methylmercury in the turbidity maximum of the Delaware estuary. Aquat Geochem. 2015:1–24. [Google Scholar]
- Greenfield BK, Jahn A. Mercury in San Francisco Bay forage fish. Environ Pollut. 2010;158(8):2716–2724. doi: 10.1016/j.envpol.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Guentzel JL, Tsukamoto Y. Processes influencing mercury speciation and bioconcentration in the North Inlet-Winyah Bay estuary, South Carolina, USA. Mar Pollut Bull. 2001;42(7):615–619. doi: 10.1016/s0025-326x(01)00090-x. [DOI] [PubMed] [Google Scholar]
- Hall A, Teeny F, Gauglitz E., Jr Mercury in fish and shellfish of the northeast Pacific. II. Sablefish, Anoplopoma fimbria. Fish Bull. 1976;74(4):791–797. [Google Scholar]
- Hall A, Teeny F, Lewis L, Hardman W, Gauglitz E., Jr Mercury in fish and shellfish of the northeast Pacific. I. Pacific Halibut, Hippoglossus stenolepis. Fish Bull. 1976;74(4):783–789. [Google Scholar]
- Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the Dominant Pathway of Methylmercury Uptake by Fish. Water Air Soil Poll. 1997;100(1):13–24. [Google Scholar]
- Hammerschmidt CR, Finiguerra MB, Weller RL, Fitzgerald WF. Methylmercury accumulation in plankton on the continental margin of the Northwest Atlantic Ocean. Environ Sci Technol. 2013;47(8):3671–3677. doi: 10.1021/es3048619. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Formation of artifact methylmercury during extraction from a sediment reference material. Anal Chem. 2001;73(24):5930–5936. doi: 10.1021/ac010721w. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Methylmercury in mosquitoes related to atmospheric mercury deposition and contamination. Environ Sci Technol. 2005;39(9):3034–3039. doi: 10.1021/es0485107. [DOI] [PubMed] [Google Scholar]
- Harris RC, Bodaly RA. Temperature, growth and dietary effects on fish mercury dynamics in two Ontario lakes. Biogeochemistry. 1998;40(2/3):175–187. [Google Scholar]
- Hice LA, Duffy TA, Munch SB, Conover DO. Spatial scale and divergent patterns of variation in adapted traits in the ocean. Ecol Lett. 2012;15(6):568–575. doi: 10.1111/j.1461-0248.2012.01769.x. [DOI] [PubMed] [Google Scholar]
- Hollweg TA, Gilmour CC, Mason RP. Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar Chem. 2009;114(3–4):86–101. [Google Scholar]
- Hurst T. Causes and consequences of winter mortality in fishes. J Fish Biol. 2007;71(2):315–345. [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120(6):799. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Chen CY, Folt CL. Comparing nearshore benthic and pelagic prey as mercury sources to lake fish: the importance of prey quality and mercury content. Sci Total Environ. 2016;565:211–221. doi: 10.1016/j.scitotenv.2016.04.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Choi AL, Fitzgerald WF, Hammerschmidt CR, Lamborg CH, Soerensen AL, Sunderland EM. Mercury biogeochemical cycling in the ocean and policy implications. Environmental Research. 2012;119:101–117. doi: 10.1016/j.envres.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Lawson NM, Lawrence AL, Leaner JJ, Lee JG, Sheu G-R. Mercury in the Chesapeake Bay. Mar Chem. 1999;65(1–2):77–96. [Google Scholar]
- Mathews T, Fisher NS. Dominance of dietary intake of metals in marine elasmobranch and teleost fish. Sci Total Environ. 2009;407(18):5156–5161. doi: 10.1016/j.scitotenv.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Middaugh DP, Hemmer MJ, Goodman LR. Methods for spawning, culturing and conducting toxicity-tests with early life stages of four atherinid fishes. 1987. In 1987; Vol. EPA/600/8-87/004. [Google Scholar]
- Munch SB, Conover DO. Rapid growth results in increased susceptibility to predation in Menidia menidia. Evolution. 2003;57(9):2119–2127. doi: 10.1111/j.0014-3820.2003.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a US cohort. Environ Health Perspect. 2005:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PC, Stepanova M, Fisher NS. Contrasting uptake routes and tissue distributions of inorganic and methylmercury in mosquitofish (Gambusia affinis) and redear sunfish (Lepomis microlophus) Environ Toxicol Chem. 2006;25(8):2132–2142. doi: 10.1897/05-595r.1. [DOI] [PubMed] [Google Scholar]
- Present TMC, Conover DO. Physiological basis of latitudinal growth differences in Menidia menidia: variation in consumption or efficiency? Funct Ecol. 1992;6:23–31. [Google Scholar]
- Reimers N. Body condition, water temperature, and over-winter survival of hatchery-reared trout in Convict Creek, California. T Am Fish Soc. 1963;92(1):39–46. [Google Scholar]
- Schultz ET, Conover DO. Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia) Oecologia. 1997;109(4):516–529. doi: 10.1007/s004420050112. [DOI] [PubMed] [Google Scholar]
- Seelen ES, Mazrui NM, Balcom PH, Ortiz VL, Dimento B, K., G., Buckman KL, Taylor VF, Jackson B, Chen CY, Mason RP. Mercury sources and cycling in coastal systems along the upper Northeast coast of the United States. ICMGP; Jeju, Korea: 2015. [Google Scholar]
- Soerensen AL, Jacob DJ, Streets DG, Witt ML, Ebinghaus R, Mason RP, Andersson M, Sunderland EM. Multi-decadal decline of mercury in the North Atlantic atmosphere explained by changing subsurface seawater concentrations. Geophys Res Lett. 2012;39(21):L21810. [Google Scholar]
- Sunderland EM. Mercury Exposure from Domestic and Imported Estuarine and Marine Fish in the U.S. Seafood Market. Environ Health Perspect. 2007;115(2):235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Dalziel J, Heyes A, Branfireun BA, Krabbenhoft DP, Gobas FA. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environ Sci Technol. 2010;44(5):1698–1704. doi: 10.1021/es9032524. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Driscoll CT, Hammitt JK, Grandjean P, Evans JS, Blum JD, Chen CY, Evers DC, Jaffe DA, Mason RP, Goho S, Jacobs W. Benefits of regulating hazardous air pollutants from coal and oil-fired utilities in the United States. Environ Sci Technol. 2016;50(5):2117–2120. doi: 10.1021/acs.est.6b00239. [DOI] [PubMed] [Google Scholar]
- Szczebak JT, Taylor DL. Ontogenetic patterns in bluefish (Pomatomus saltatrix) feeding ecology and the effect on mercury biomagnification. Environ Toxicol Chem. 2011;30(6):1447–1458. doi: 10.1002/etc.516. [DOI] [PubMed] [Google Scholar]
- Taylor VF, Carter A, Davies C, Jackson BP. Trace-level automated mercury speciation analysis. Anal Methods. 2011;3(5):1143–1148. doi: 10.1039/C0AY00528B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Jackson BP, Chen CY. Mercury speciation and total trace element determination of low-biomass biological samples. Anal Bioanal Chem. 2008;392(7–8):1283–1290. doi: 10.1007/s00216-008-2403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JM, Bergersen EP, Carlson CA, Kaeding LR. Role of size, condition, and lipid content in the overwinter survival of age– 0 Colorado Squawfish. T Am Fish Soc. 1991;120(3):346–353. [Google Scholar]
- Trudel M, Rasmussen JB. Bioenergetics and mercury dynamics in fish: a modelling perspective. Can J Fish Aquat Sci. 2006;63(8):1890–1902. [Google Scholar]
- Ward DM, Nislow KH, Chen CY, Folt CL. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. T Am Fish Soc. 2010;139(1):1–10. doi: 10.1577/T09-032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahira K, Conover DO. Intra- vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology. 2002;83:1252–1262. [Google Scholar]
- Yamahira K, Lankford TE, Conover DO. Intra- and interspecific latitudinal variation in vertebral number of Menidia spp. (Teleostei: Atherinopsidae) Copeia. 2006;2006(3):431–436. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.