Daniel et al. have previously published in JEM a study on the preventive effect of tolerogenic vaccination with a strong agonist insulin mimetope in type 1 diabetes. Bergman et al. now challenge these results.
Abstract
Daniel et al. (https://doi.org/10.1084/jem.20110574) have previously published in JEM a study on the preventive effect of tolerogenic vaccination with a strong agonist insulin mimetope in type 1 diabetes. Our study now challenges these results and shows that osmotic pump delivery of the modified insulin peptide R22E did not prevent hyperglycemia, accelerated disease onset, increased its incidence, and worsened insulitis.
The success of antigen-specific immunotherapies for allergy (Canonica et al., 2014) reinforces the notion that tolerogenic vaccination will be a therapeutic option for autoimmune diseases in a foreseeable future. However, and despite promising preclinical studies, results from clinical trials have been so far disappointing, and autoimmune diseases are still treated with nonspecific immunosuppressors. The difficulty in translating preclinical data may well relate to their lack of robustness, an issue that is amenable. Daniel et al. reported complete prevention of type 1 diabetes (T1D) in serum insulin autoantibody low (IAAlow) nonobese diabetic (NOD) mice treated with R22E (a.k.a. mimetope-3; B12:23-R22E), whether treatment was initiated at 4 or 12 wk of age. This spectacular outcome has prompted human studies testing this peptide (e.g., Nakayama et al., 2015). We sought to explore the mechanisms behind the preventive effect of R22E in NOD mice and specifically test the requirement for continuous thymic output for de novo tolerance induction in this system (Zelenay et al., 2010; Paiva et al., 2013), which lead us to probe its robustness.
We analyzed two independent NOD colonies, #1 and #2, derived from breeders imported 18 yr ago or during the course of this study, and presenting 53.2% and 83.3% T1D incidence in females, respectively (Fig. S1, A and B). In both colonies, IAA titers measured at 4 wk of age were low (4w-IAAlow) and provided no prediction power (Fig. S1, C, D, and G). In 6-wk-old mice (Fig. S1, E–H), titers spread over 3 logs, linear regression analysis established the optimal threshold for T1D predictability at 0.1, and selection for 6w-IAAlow titers (<0.1) delayed disease onset and reduced T1D incidence. These results are concordant with earlier experiments reporting that IAA titers are low at 4, and predictive of disease at 8, wk of age (Zhang et al., 2008). Daniel et al. (2011) state that 95% of the mice were 4w-IAAlow, although the representative data identify 25% of the mice as 4w-IAAhigh. Titer distribution in 12-wk-old animals is not indicated. Moreover, control animals, presumably selected as IAAlow (at 4 and 12 wk of age, in the respective figures), display high incidence and usual onset of disease, suggesting the measurements had little predictive power.
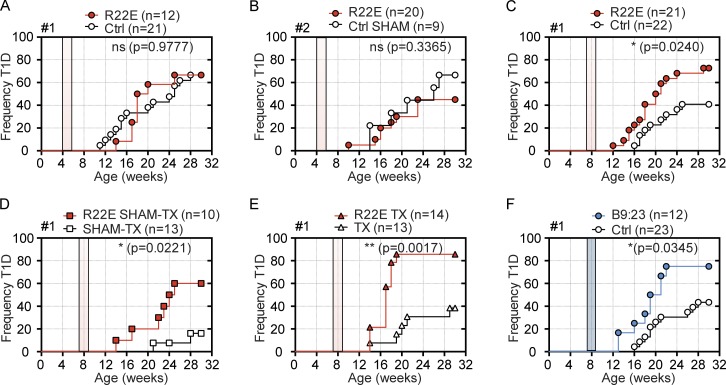
To test whether R22E treatment is protective in our NOD colonies, we followed the protocol described in Daniel et al. (2011). Females IAAlow (<0.1) received highly purified R22E for 14 d, through a subcutaneous osmotic pump delivering 5 µg/day. We chose this dose as it is shown to confer 100% protection in each of the several experiments presented in Daniel et al. (2011), and doses twofold lower or two- to fourfold higher are reported to also afford protection. We followed the manufacturer’s recommendation to remove the osmotic pump after the 2-wk treatment, both to ensure precision in the dose and regimen of peptide delivery and to avoid “swelling and leaking of a concentrated salt solution, resulting in local irritation around the pump” (Alzet mini-pump instructions). To ascertain that surgical intervention was not a confounding factor, control mice in colony #2 were sham operated. Moreover, mice from colonies #1 and #2 received peptide from independent batches, the latter from the same supplier as Daniel et al. (2011). Surprisingly, mice selected as 4w-IAAlow and immediately treated showed no reduction in hyperglycemia, irrespectively of whether they belonged to colony #1 (P = 0.9777) or #2 (P = 0.3365; Fig. 1, A and B). As we monitored a total of 21 treated mice, and Daniel et al. (2011) showed 100% protection in a group of 12 animals, a putative discrepancy in IAAlow preselection does not explain differences in the qualitative assessment of disease protection. We next monitored mice that were classified as IAAlow at 6 wk of age and were treated, or not, with R22E in the following days. We choose this age with confidence because Daniel et al. (2011) reported total prevention of disease when treating 12-wk-old animals. Mice were either maintained euthymic (Fig. 1 C), sham operated (Fig. 1 D), or thymectomized (Fig. 1 E) before treatment. Of note, sham surgery per se lowered the incidence of T1D, whereas effective thymus removal reverted this effect, emphasizing that our NOD colony is responsive to small effects promoting disease protection. Intriguingly, disease onset was precipitated, and T1D incidence was systematically higher in R22E-treated than in untreated mice (no surgery: 72.7% vs. 40.9%, P = 0.024; sham-thymectomy: 60% vs. 15.4%, P = 0.0221; thymectomy: 85.7% vs. 38.5%, P = 0.0017). The observed increased T1D incidence and earlier disease onset in animals treated at 7 but not at 4 wk of age is best explained by the high efficiency of IAA based selection in the former group of mice, with low and late T1D incidence allowing for worsening to be revealed.
Figure 1.
R22E treatment anticipates disease onset and increases T1D incidence. NOD females selected for serum IAA titers <0.1 were treated with the indicated peptides (filled symbols) for 14 d (filled area), and glycemia was monitored weekly from 10 to 30 wk of age. (A and B) Screen and osmotic pump insertion were performed at 4 wk of age in females from colonies #1 (A) and #2 (B). Control mice from colony #2 (B) underwent sham surgeries (Ctrl-Sham). (C–E) Females from colony #1, IAAlow at 6 wk of age, were implanted 1 wk later with osmotic pumps containing R22E (C) or were first submitted to sham-thymectomy (SHAM-TX; D) or full thymectomy (TX; E). (F) As in C, except B9:23-containing osmotic pumps. Treated mice are from two (A, C, and F), four (B), and three (D and E) independent experiments with n = 1 and 11 in A; n = 3, 5, 6, and 6 in B; n = 9 and 12 in C; n = 5, 1, and 4 in D; n = 7, 2, and 5 in E; and n = 5 and 7 in F. Log-rank tests statistical differences for T1D incidence in Kaplan-Meier survival plots: not significant in A (P = 0.977) and B (P = 0.3365); significant in C (P = 0.0240), D (P = 0.0221), E (P = 0.0017), and F (P = 0.0345). Controls are different animals between panel C and F, and from those presented in Fig. S1.
The finding that R22E treatment is not protective in our NOD cohorts while it fully prevents disease in Daniel et al. (2011) evokes previous discordant studies. Similar treatment with the native insulin peptide B9:23 prevented disease in 50% (Liu et al., 2002) or none (Daniel et al., 2011) of the NOD mice analyzed. In our setting, osmotic pump delivery of B9:23 in 6w-IAAlow females increased disease incidence when compared with control mice (75% vs. 40.9%, P = 0.0345; Fig. 1 F). The mice tested by Daniel et al. (2011) were imported from the Taconic facility, where segmented filamentous bacteria (SFB), reported to reduce T1D incidence in NOD females (Kriegel et al., 2011), is present. Specific 16S-PCR on fecal extract (Vaishnava et al., 2011) detected SFB in ≥80% of our experimental mice (colony #1) and in all breeders (colony #2) <3 mo after importation (not depicted), excluding this bacterium as a confounding factor.
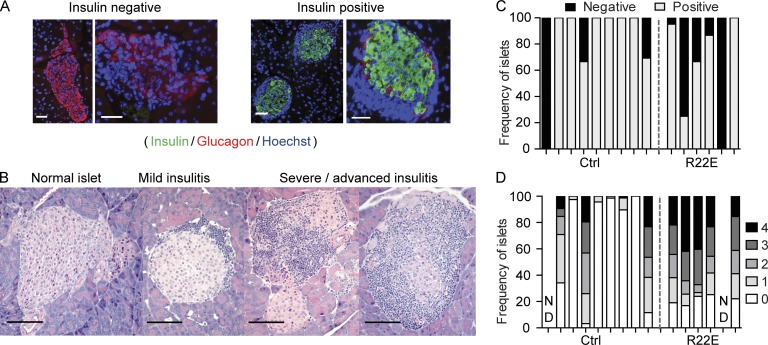
Finally, we assessed whether R22E treatment affected insulitis severity. Blinded examination of pancreatic sections from 30-wk-old normoglycemic mice revealed lower frequency of insulin-producing islets and higher frequency of severely infiltrated islets in treated compared with control animals (Fig. 2), indicating that R22E also worsens clinically silent insulitis.
Figure 2.
R22E treatment worsens insulitis in normoglycemic mice. Pancreas sections from nine control and six R22E-treated females normoglycemic at 30 wk of age (see Fig. 1 C) were scored blindly, all together at once, for insulitis severity. (A and B) Representative stainings used to identify insulin-producing islets (insulin/glucagon/Hoechst; A) and to score islets infiltration (hematoxylin and eosin; B). Bars, 100 µm. (C) Frequency of insulin-producing islets. (D) Frequency of infiltrated islets along a severity score (4 is most severe). ND, <50 islets detected.
By addressing the efficacy of R22E in NOD mice and several possible confounding physiological factors such as age, putative genetic drift, thymic output, or experimental factors such as peptide synthesis and purification, we found the protection reported previously not to be reproducible. Upon sharing these findings with C. Daniel, we could not ascertain repeats have been successful elsewhere or with other colonies (e.g., NOD-Foxp3GFP treated but not scored for disease in Daniel et al. [2011]) and came across a procedural discrepancy. Despite the specification that the treatment duration was of 14 d, and elsewhere in the text from 4 to 6 wk of age, the authors left the pump in place for the full length of the experiments, i.e., 40 wk. As referred above, the mishandling of osmotic pump can induce inflammation, and inflammation per se has been shown multiple times to lower T1D incidence, delay its onset, or even confer full protection in NOD mice. It is also expected that the pump would release another 22 µl of peptide solution after the 14 d, corresponding to an extension of the treatment for an estimated 3.5 d. Hence, it remains possible that pump-induced inflammation in combination or not with extended R22E administration generated a false-positive treatment outcome in Daniel et al. (2011).
Our work suggests that failure in translating preclinical studies may relate to their lack of robustness and, in turn, supports the notion that the NOD mouse remains a suitable model for screening candidate therapies for human T1D. As confounding factors in procedures are best revealed through independent repeats and because variability across inbred colonies pales compared with the heterogeneity of humans participating in clinical trials, we stress that translation must only be considered when peptide, or any other, therapy is robust across centers and animal facilities. This is particularly acute when results, such as ours, reveal cases of disease worsening.
Materials and methods
Mice
NOD/Lt breeders for colony #1 and #2 were purchased from The Jackson Laboratory in 1998 and 2014, respectively, and maintained at the Instituto Gulbenkian de Ciência (IGC) animal facility under specific pathogen–free conditions, according to the Federation for Laboratory Animal Science Association guidelines. Mice were sacrificed at 30 wk of age or when confirmed diabetic. Experimental protocols were approved by the Portuguese authority (Direção Geral de Alimentação e Veterinária) and the ethical committee of the IGC.
Diabetes monitoring
Blood glucose levels were monitored weekly from 10 wk of age with ACCU-CHEK AVIVA Glucose strips (Roche). Mice were considered diabetic after two consecutive measurements >200 mg/dl and one positive test of urine glycosuria (Diabur Test 5000 glucose strips, #10647659074; Roche).
IAA detection
Serum IAA levels were measured by Time-Resolved Fluorescence DELFIA immunoassay on a Victor3 Multilabel Counter fluorimeter (Wallac). ELISA Plates (#206072; Santa Cruz Biotechnology, Inc.) were coated with human recombinant insulin (100 U/ml, Humulin Regular; Lilly), blocked with 2% BSA (#A9647; Sigma Aldrich) and incubated sequentially with sera (1/10), biotinylated anti–mouse IgG1 Ab (1/5,000, #ab11587; Abcam), Europium-labeled Streptavidin (1/2,000, #1244-360; PerkinElmer) and DELFIA Enhancement solution (#1244, PerkinElmer). Each sera was measured in two independent assays. On each plate, a standard curve was generated from serial dilution of a pool of IAAhigh sera, and the first dilution was given a value 10. The best-fit trend line of the standard curve was determined using Excel. The equation of the trend line, the R2 value, and the concentration of each sample were generated using www.Elisaanalysis.com.
Histology and immunofluorescence
Pancreatic tissue from NOD mice was paraffin embedded, sectioned, and hematoxylin and eosin–stained. Islets were scored blindly for immune infiltration as follows: 0, no infiltration; 1, periinsulitis; 2, infiltration in <50% of the area; 3, >50% of the area; and 4, islets completely destroyed. Insulin and glucagon were stained with primary monoclonal mouse anti-insulin Ab (1/1000; SIGMA) and polyclonal rabbit anti-glucagon Ab (1/100; Dako) and secondary Abs Alexa Fluor 488 goat anti–mouse IgG (H+L) and Alexa Fluor 546 goat anti–rabbit IgG (H+L) (1/500; Molecular Probes, Invitrogen), respectively. After Hoechst 33342 nuclear staining, immunofluorescence was visualized on an Axio ImagerA1 microscope (ZEISS).
Peptides and osmotic pumps
R22E and B9:23 from Thermo Fisher Scientific (>95% purity) were used to treat colony #1 and R22E from New England Peptide (>90% purity) to treat colony #2. Peptides were administered subcutaneously at a rate of 0.25 µl/h, corresponding to 5 µg/day, over 14 d, through micro-osmotic pumps (Alzet model 1002, Charles River), inserted and explanted by aseptic surgery. Where indicated, control mice were sham operated, both at the insertion and the removal times.
Thymectomy and sham-thymectomy
Thymectomies were performed in aseptic conditions on 6-wk-old mice under ketamine/xylazine anesthesia. Sham-thymectomy was surgery without removal of thymic lobes.
Statistical analysis
T1D incidence was determined using Kaplan-Meier survival plots, statistically significant differences (P < 0.05) with Log-rank tests, and linear regression analysis were used to calculate the proportion of T1D onset explained by IAA titers. Statistical analysis was performed with Prism software (GraphPad Software).
Online supplemental material
Fig. S1 shows an age-dependent correlation between IAA titer and T1D onset in two NOD colonies.
Supplementary Material
Acknowledgments
We thank Manuel Rebelo for assistance with animal colony management, the Lymphocyte Physiology laboratory members for discussions and occasional help, and Mark Peakman and Antonio Coutinho for suggestions and critical reading.
This study was supported by the European Union’s (EU FP7) Large-Scale Focused Collaborative Research Project on Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes (NAIMIT, 241447).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- IAA
- insulin autoantibody
- NOD
- nonobese diabetic
- SFB
- segmented filamentous bacteria
- T1D
- type 1 diabetes
References
- Canonica G.W., Cox L., Pawankar R., Baena-Cagnani C.E., Blaiss M., Bonini S., Bousquet J., Calderón M., Compalati E., Durham S.R., et al. . 2014. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ. J. 7:6 10.1186/1939-4551-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Weigmann B., Bronson R., and von Boehmer H.. 2011. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 208:1501–1510. 10.1084/jem.20110574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel M.A., Sefik E., Hill J.A., Wu H.J., Benoist C., and Mathis D.. 2011. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA. 108:11548–11553. 10.1073/pnas.1108924108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E., Abiru N., Moriyama H., Miao D., and Eisenbarth G.S.. 2002. Induction of insulin autoantibodies and protection from diabetes with subcutaneous insulin B:9-23 peptide without adjuvant. Ann. N. Y. Acad. Sci. 958:224–227. 10.1111/j.1749-6632.2002.tb02974.x [DOI] [PubMed] [Google Scholar]
- Nakayama M., McDaniel K., Fitzgerald-Miller L., Kiekhaefer C., Snell-Bergeon J.K., Davidson H.W., Rewers M., Yu L., Gottlieb P., Kappler J.W., and Michels A.. 2015. Regulatory vs. inflammatory cytokine T-cell responses to mutated insulin peptides in healthy and type 1 diabetic subjects. Proc. Natl. Acad. Sci. USA. 112:4429–4434. 10.1073/pnas.1502967112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva R.S., Lino A.C., Bergman M.-L., Caramalho I., Sousa A.E., Zelenay S., and Demengeot J.. 2013. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc. Natl. Acad. Sci. USA. 110:6494–6499. 10.1073/pnas.1221955110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., and Hooper L.V.. 2011. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science. 334:255–258. 10.1126/science.1209791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S., Bergman M.-L., Paiva R.S., Lino A.C., Martins A.C., Duarte J.H., Moraes-Fontes M.F., Bilate A.M., Lafaille J.J., and Demengeot J.. 2010. Cutting edge: Intrathymic differentiation of adaptive Foxp3+ regulatory T cells upon peripheral proinflammatory immunization. J. Immunol. 185:3829–3833. 10.4049/jimmunol.1001281 [DOI] [PubMed] [Google Scholar]
- Zhang L., Nakayama M., and Eisenbarth G.S.. 2008. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 20:111–118. 10.1016/j.coi.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.