Abstract
Purpose of review
Pelvic inflammatory disease (PID) is a common and serious reproductive health disorder and disease rates remain unacceptably high among adolescent girls and young adult women in the United States. Despite data demonstrating that women experience major adverse health outcomes after PID, national recommendations for management of adolescents have become increasingly less cautious in an era of cost- containment. In this review, we take an alternative look at published data on adolescents with PID to frame the next steps for optimizing management for this vulnerable population.
Recent findings
Several findings emerge from review of the literature. First, there is limited evidence to guide the best practice strategies for adolescents with PID due to low enrolment of early and middle adolescents in national trials. Second, adolescents and adult women in the United States receive suboptimal treatment regimens per Centers for Disease Control and Prevention (CDC) standards. Third, available evidence suggests that adolescents are at an increased risk for poor adherence to CDC recommendations for self- care, reacquisition of sexually transmitted infections (STIs) and PID, and subsequent adverse reproductive health outcomes.
Summary
Efforts to develop and integrate adolescent-focused, evidence-based strategies for PID management and prevention of subsequent STIs and recurrent PID are warranted.
Keywords: adolescents, pelvic inflammatory disease, treatment
INTRODUCTION
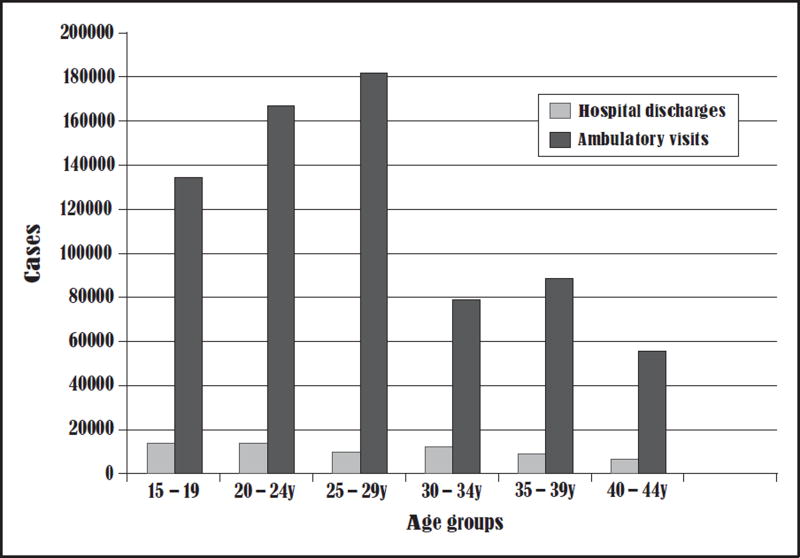
Pelvic inflammatory disease (PID) remains a serious reproductive health disorder and disease rates remain unacceptably high among adolescent girls and young adult women. Although asymptomatic screening and early treatment have resulted in a reduction in PID diagnoses in the United States [1], approximately 800 000 women will still experience a PID diagnosis [2] (Fig. 1). Each episode of this upper reproductive tract infection, usually caused by a sexually transmitted infection (STI), increases the risk for multiple sequelae, including tubal infertility, ectopic pregnancy and chronic pelvic pain (CPP). Care for PID has shifted from the inpatient to the outpatient setting in an effort to contain costs and in response to preliminary efficacy and cost-effectiveness data from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) trial, which demonstrated that hospitalization for mild – moderate disease did not improve longitudinal outcomes for affected women [3,4]. The Centers for Disease Control and Prevention (CDC) guide- lines actually changed prior to the first publication of PEACH trial data and no subsequent translational work demonstrating effectiveness among adolescents in the general population of women with PID who may be cared for outside of a randomized trial is available [5]. Unfortunately, many experts focused on the differences in longitudinal outcomes and failed to acknowledge that there were few early and middle adolescents enrolled in the trial and that both adolescent and adult women in the trial generally had unacceptably high rates of recurrent PID, infertility and CPP as longitudinal outcomes. Further, age-stratified analyses of the PEACH trial data reveal that adolescent and young adult women continue to have high rates of repeat STIs and CPP, regardless of treatment strategy [6,7]. In this review, we take an alternative look at the available data specific to adolescents with PID to better understand the shift to outpatient treatment and the potential next steps towards improving both quality of care and outcomes for young women so early in their reproductive trajectories.
FIGURE 1.
Average annual hospital discharge and ambulatory visit for women with pelvic inflammatory disease in the United States by age [2].
BACKGROUND
PID is most commonly characterized by the acute presentation of abdominal pain, but may often be subclinical with minimal symptoms among affected patients [8]. PID is associated with significant short-term and long-term sequelae. Short-term sequelae include the development of tubo-ovarian abscesses requiring triple antibiotic therapy, radiological procedures and protracted hospitalizations [9 – 12]. PID is also associated with significant long-term sequelae such as ectopic pregnancy, CPP and tubal infertility [9 – 15]. Further, each episode of PID increases the risk of complications such as infertility [16].
More recent studies examining data from women with microscopically and laparoscopically confirmed PID have demonstrated that oral regimens have good efficacy for treating etiologic organisms causing PID [17,18], resolve symptomatology, that patients with mild – moderate disease have a high conception rate longitudinally if properly treated with these regimens [19] and that high rates of infertility secondary to tubal occlusion are likely the result of recurrent disease [20]. This suggests that although treatment of a single episode of PID is important, prevention of recurrent disease among affected adolescents is critical for fertility preservation.
ADOLESCENTS AND PELVIC INFLAMMATORY DISEASE
The most recent reports from the National Survey of Family Growth indicate that the highest prevalence of PID was among women who initiated vaginal intercourse at less than 15 years of age and had greater numbers of sexual partners in the 12 months prior to data collection [21]. Data from a PID treatment adherence trial in a high STI prevalence, urban, minority community demonstrated that adolescents with PID in this context were often poor and had multiple sexual risk factors for PID (early age of sexual intercourse, multiple lifetime partners, pregnancy history, lack of condom and hormonal contraceptive use) [22]. These local data are also consistent with demographic findings from the PEACH trial [23]. National data demonstrate that minority adolescents are disproportionately more likely to be diagnosed with PID than nonminority adolescents [24&&]; however, adult fertility data suggest that there may be underdiagnosis amongst other populations of women who may present without this demographic profile or have mild disease [25].
Adolescents with a history of STIs have traditionally been considered a ‘special population’ by the CDC [26]. They are at a higher risk for STIs due to inaccurate perception of risk [27], biological susceptibility [28,29], frequent unprotected intercourse [30 – 32] and engagement in sexual partnership patterns such as serial monogamy and partnership concurrency [33 – 35]. They also face difficulties easily accessing comprehensive adolescent-friendly reproductive health services [36 – 38]. As such, the CDC treatment guidelines continue to assert that counselling for STDs should be non-judgemental, aimed at addressing risky sexual behaviours, and appropriate for the patient’s developmental level [26]. Past guidelines for PID treatment in adolescents also included universal hospitalization for 48 – 72 h for aggressive management with intravenous (i.v.) antibiotics followed by discharge and close follow-up during the remaining days of the 2-week course of oral antibiotic therapy. During hospitalization, patients not only received medical care but were also often provided intensive education programmes and social support to assist with self-care at discharge and prevention of future episodes [39 – 41]. Unfortunately, the effectiveness of these comprehensive approaches was not evaluated and so the current designation by the CDC focuses on the ‘lack of evidence’, which appears related to the small sample size and inability to reach a conclusion rather than a study powered to answer the question about whether hospitalization improves the outcomes for young adolescents with PID. Although the CDC states that younger adolescents have similar outcomes to adult women with PID [26], only 25.1% (N ¼ 209) of the PEACH cohort were adolescents aged 19 years or less and the mean age of this subgroup was 17.9 ± 1.1 years, limiting one’s ability to generalize to early and/or middle adolescents [6].
UNDERSTANDING THE SHIFT TO OUTPATIENT THERAPY
In 1998, a seminal research design from the PEACH trial called into question the role of inpatient stays for PID treatment. The PEACH trial was a multicentre randomized controlled clinical trial involving emergency departments, obstetrics and gynaecology clinics, sexually transmitted disease clinics and private practices in 13 cities in the United States. The trial was designed to recruit patients aged 14 – 38 years and the primary outcomes of interest were recurrent STI at 30 days, recurrent PID, pregnancy, chronic abdominal pain and infertility [42]. The 35-month and 84-month data demonstrated that patients in the inpatient and outpatient treatment groups had similar outcomes for ectopic pregnancy, live births, tubal infertility and CPP [42,43].
Although the focus of the PEACH trial was to look at differences between women treated using the two key therapeutic options available, the study did not identify a strategy to solve the problem of adverse sequelae in affected patients. Close examination of the combined data demonstrates that participants with mild to moderate PID are still at a risk for significant sequelae. At 84 months, 43% of this predominately minority sample of women reported CPP, 19% were categorized as infertile and 21% had recurrent PID [3,43,44]. Further analyses from the trial have also demonstrated that women with PID-associated CPP had significant reductions in health-related quality of life [45]. Age-stratified analyses also demonstrated that 20% of women aged less than 19 years experienced a repeat or persistent STI at 30 days and repeat PID over the 7-year follow-up period was associated with a five-fold risk of CPP [46]. An adolescent- focused trial in an urban community demonstrated that adolescents have poor adherence to the CDC-recommended 72-h clinical follow-up visit, are at a high risk for STI at the 90-day STI rescreening visit and that brief theory-based interventions have positive, but modest effects on adherence to self-management behaviours. Four-year follow-up of patients with PID in this setting demonstrates that recurrent STIs and PID are common and that on average the time to subsequent STI or PID is about 1 year [47].
The other major factor driving the push to design cost-effective strategies for management of PID is the high cost of inpatient therapy. In 2000, the CDC published a study estimating the 1998 direct medical expenditures for PID and its sequelae at $1.88 billion. Of these expenses, $1.06 billion was for acute PID, $166 million was for evaluation and treatment of CPP, $295 million was for evaluation and treatment of ectopic pregnancy and $360 million was for evaluation and treatment of PID-associated infertility. Using a probability model, 73% of the costs were associated with acute management of PID rather than the long-term sequelae [48]. The mean productivity losses for a single acute episode of PID have been estimated at $649 [49] and the average lifetime costs of PID range between $1060 and $3180. As a result, there was a call for prevention efforts to reduce the incidence of PID and to provide alternative management strategies for affected women [50]. Smith et al. [4] have recently published cost-effectiveness analyses utilizing data from the PEACH trial and concluded that hospitalization to preserve fertility in nulliparous young women and adolescents with PID would be economically unfeasible even if substantial improvements in complication rates were assumed. This is not surprising given that outpatient treatment regimens are so inexpensive compared with the costs of inpatient treatment. In a recent study [51] designed to examine the direct costs of PID for adolescents in a major academic centre, inpatient hospitalization was on average $7440 more than treatment in ambulatory sites, including the emergency department. This wide difference in costs suggests that there may be unexplored cost-effective, nonhospital-based management approaches that are worth evaluating for vulnerable adolescents.
BARRIERS TO TREATMENT ADHERENCE IN THE OUTPATIENT SETTING
The CDC provides evidence-based, expert-guided recommendations for treatment of PID and other STIs and is designed to serve as the guide for clinical practice in the United States. In 2006, the CDC expanded the diagnostic criteria for PID by adding an ‘OR’ after each of the minimum diagnostic criteria, but the rates of PID diagnoses have continued to decrease, as infections with Chlamydia trachomatis soar amongst adolescents and young adult women [52&]. Although PID is a nonreportable STI subject to mandated reporting to public health departments, much of this general decline is likely due to early diagnosis and treatment as noted above [1]. Researchers in health settings with universal healthcare systems and tracking of diagnoses have postulated that this decline in PID may be due to an arrested immune response to C. trachomatis [53&&]; however, the rise in ectopic pregnancy also observed may also suggest that there may be issues in making initial PID diagnoses. One factor that has not been considered in the literature is that increased use of (noninvasive) STI testing methods (urine and vaginal swabs) and avoidance of the routine bimanual examination in patients seeking STI testing may have occurred, resulting in fewer PID diagnoses. Although there is poor adherence to asymptomatic screening guidelines for women aged under 25 years [54 – 56], widespread use of a non- invasive-only approach in public health and emergency medical systems for symptomatic patients may lead to missed diagnoses in patients with subclinical and/or mild – moderate disease without classical PID findings such as severe abdominal pain, ‘shuffling gait’ and/or the ‘chandelier sign’.
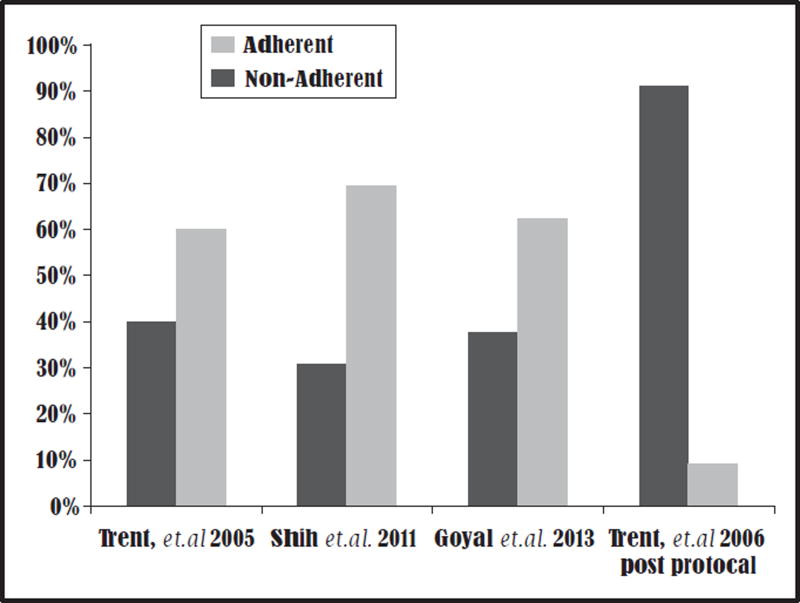
Several studies have demonstrated that the most significant barrier facing adolescents and young women nationally is poor adherence to national treatment guidelines (Table 1). A single-centre study demonstrated that 40% of providers caring for adolescents with PID in a large academic centre were nonadherent to CDC treatment regimens [57]. Shih et al. [58] conducted a multiyear cross-sectional analysis of emergency department data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) database, which spanned from 1999 to 2006 and demonstrated that out of 1.65 million emergency department visits in which patients were diagnosed with PID, only 30.5% of discharged patients received antibiotic treatment according to the CDC guidelines. Among those visits characterized by nonreceipt of a CDC regimen, 38.4% of patients did not receive any antibiotics or analgesia for pain management. Most noticeable was that in regions of the country where emergency medicine trained specialists do not staff emergency departments, patients were five times less likely to receive standard care therapies [58]. In a more recently analysis, Goyal et al. [24&&] examined data from adolescents aged 14 – 19 years in the NHAMC dataset from 2000 to 2009 representing an estimated 705 000 PID cases seen in the US emergency departments. These analyses demonstrated that only 37.1% of patients received care according to the CDC treatment guidelines (Fig. 2). Fortunately, clinical protocols with algorithms, treatment guidelines and medication resources for clinicians can result in improved adherence to the CDC treatment guidelines [40,59].
Table 1.
Antibiotic regimens recommended by the Centers for Disease Control and Prevention [26]
| Parenteral treatment | Regimen A | Cefoxitin 2 g i.v. q 6 or cefotetan 2 g i.v. q 12 þ doxycycline 100 mg p.o. b.i.d. × 14 days ± metronidazole 500 mg p.o. b.i.d. × 14 days |
| Regimen B | Clindamycin 900 mg i.v. þ gentamicin loading dose i.v. or i.m. (2 mg/kg), followed by a maintenance dose (1.5 mg/kg) every 8 h or gentamicin single-daily dosing (3 – 5 mg/kg/day) | |
| Alternate regimen | Ampicillin/sulbactam 3 g i.v. every 6 h þ doxycycline 100 mg p.o. b.i.d. × 14 days | |
| Oral treatment | Regimen A | Ceftriaxone 250 mg i.m. (single dose) þ doxycycline 100 mg p.o. b.i.d. × 14 days ± metronidazole 500 mg p.o. b.i.d. × 14 days |
| Regimen B | Cefoxitin 2 g i.m. þ probenecid 1 g p.o. (single dose) þ doxycycline 100 mg p.o. b.i.d. × 14 days ± metronidazole 500 mg p.o. b.i.d. × 14 days | |
| Alternate regimen | Other parenteral third-generation cephalosporin (e.g. ceftizoxime or cefotaxime) þ doxycycline 100 mg p.o. b.i.d. × 14 days ± metronidazole 500 mg p.o. b.i.d. × 14 days |
b.i.d., twice daily; i.m., intramuscular; i.v., intravenous.
Azithromycin is not currently considered standard therapy; however, if used (e.g. doxycycline allergy), concurrent use of twice-daily metronidazole for 14 days is recommended by the CDC.
FIGURE 2.
Summary of studied clinician adherence to Centers for Disease and Control Prevention STD Treatment Guidelines for pelvic inflammatory disease management [24&&, 57 – 59].
Patient adherence to PID treatment involves a complicated set of behaviours that integrate physician adherence to established treatment guidelines, patient-provider communication, agreement on a treatment regimen and adequate follow-up in the healthcare system [60]. Lack of adherence to a treatment plan is usually not solely due to the patient’s failure. The health provider’s role is to prescribe appropriate medication regimens on the basis of the individual patient’s needs, provide education on how the medications work and explain the importance of completing therapy [61]. Given the complexity of these roles and the low likelihood of being successful at all of them, health providers delivering (outpatient) PID care to adolescents assume some of the responsibility for adherence failure. Research from a large urban academic centre indicates that most patients diagnosed with PID are seen in urgent and emergency department settings [51]. Although high acuity care is delivered 24 h a day, there are also increased demands on provider time that often result in less time counselling patients on how treatment regimens can be integrated into adolescent patient lifestyles, thus resulting in poor adherence to prescribed treatment and follow-up [51,57,59]. This emphasizes the importance of the CDC-recommended short-term clinical follow-up visit for maximizing clinical adherence. Individuals who did not have a shor- term clinical follow-up visit reported that they did not understand the need for follow-up, had transportation issues and/or did not have a medical home or knowledge of a public health alternative for follow-up [59,62]. Although doxycycline as the treatment of choice poses some difficulties with adolescents’ ability to adhere to medications, other factors include access to medication and primary care provider ignorance regarding the polymicrobial nature of PID and need to continue medications for 14 days even if STI tests are negative [57]. Although there is hope that azithromycin will emerge as a potential panacea to antibiotic adherence issues, patients still need to take and/or return for observed delivery of the second dose. Further, the CDC still recommends concurrent use of twice-daily metronidazole with azithromycin if used as an alternative regimen [26].
Even though the CDC no longer recommends special management for adolescents with PID, a national survey has demonstrated that clinicians lack consensus for determining the disposition for adolescents in various contexts. In this study, clinicians used a visual analogue scale to assess how strongly they felt that a hypothetical 15-year-old adolescent girl with PID should be hospitalized versus discharged to outpatient care on the basis of 17 scenarios. Scenarios ranged from those with severe disease to demographic, social and/or clinical factors shown to interfere with outpatient adherence. The highest mean scores corresponded to the CDC recommendations, but there was significant variability and lower scores when the adolescent had other factors that could impair an adolescent’s ability to tolerate an outpatient regimen [63&&]. This may have resulted because clinicians also under- estimate the impact of a PID diagnosis on the adolescent girl. In a recent PID health utility study, a general survey of adolescents consistently rated health-related quality of life associated with PID health states lower than parents and clinicians. Further, in a time trade-off exercise, adolescents were willing to give up 1 – 2 years of their lives to prevent PID and associated sequelae [64].
Conclusion
Inpatient treatment for PID is expensive without incremental increases in efficacy when compared with outpatient treatment in affected women. The CDC’s assessment that there are no data to support hospitalization for adolescents with mild – moderate disease is technically true, but should not be misinterpreted. Available research suggests that the status quo for both inpatient and outpatient PID treatment is suboptimal and that additional cost-effective, evidence-based intervention is warranted to improve short and long-term reproductive health outcomes for all women.
KEY POINTS.
There is limited evidence available to guide the use of inpatient versus outpatient therapy for adolescents in the United States; therefore, clinicians will need to carefully assess an adolescent’s ability to tolerate an outpatient regimen per CDC guidelines.
Women in the United States receive suboptimal antibiotic regimens per Centers for Disease Control and Prevention standards; therefore, additional efforts to increase provider understanding of and adherence to the antibiotics regimens are warranted.
Adolescents are at increased risk for adherence failure, recurrent sexually transmitted infections and PID, and subsequent reproductive health outcomes; therefore, studies are needed to define the next steps for integrating cost-effective, developmentally appropriate, and evidence-based interventions into routine clinical practice.
Acknowledgments
Dr Trent is funded by the National Institute of Nursing Research 5 R01 NR13507-02 and National Institute of Minority Health and Health Disparities P20MD000198. The author is grateful for external review of the manuscript by Dr S. Jean Emans prior to submission.
Footnotes
Conflicts of interest
The author has no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
& of special interest
&& of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 419).
- 1.Moss NJ, Ahrens K, Kent CK, Klausner JD. The decline in clinical sequelae of genital Chlamydia trachomatis infection supports current control strategies. J Infect Dis. 2006;193:1336–1338. doi: 10.1086/503114. 1338 – 1339 author reply. [DOI] [PubMed] [Google Scholar]
- 2.Sutton MY, Sternberg M, Zaidi A, et al. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sex Transm Dis. 2005;32:778–784. doi: 10.1097/01.olq.0000175375.60973.cb. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002;186:929–937. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 4.Smith KJ, Ness RB, Roberts MS. Hospitalization for pelvic inflammatory disease: a cost-effectiveness analysis. Sex Transm Dis. 2007;34:108–112. doi: 10.1097/01.olq.0000225321.61049.13. [DOI] [PubMed] [Google Scholar]
- 5.Workowski K, Berman S. Centers for Disease Control and Prevention. 1998 guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep. 1998;47:80–86. [Google Scholar]
- 6.Trent M, Haggerty CM, Jennings JJ, et al. Adverse adolescent reproductive health outcomes after pelvic inflammatory disease. Arch Ped Adol Med. 2011;165:50–54. doi: 10.1001/archpediatrics.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38:879–881. doi: 10.1097/OLQ.0b013e31821f918c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32:400–405. doi: 10.1097/01.olq.0000154508.26532.6a. [DOI] [PubMed] [Google Scholar]
- 9.Mollen CJ, Pletcher JR, Bellah RD, Lavelle JM. Prevalence of tubo-ovarian abscess in adolescents diagnosed with pelvic inflammatory disease in a pediatric emergency department. Pediatr Emerg Care. 2006;22:621–625. doi: 10.1097/01.pec.0000227868.23568.9d. [DOI] [PubMed] [Google Scholar]
- 10.Banikarim C, Chacko MR. Pelvic inflammatory disease in adolescents. Semin Pediatr Infect Dis. 2005;16:175–180. doi: 10.1053/j.spid.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Slap GB, Forke CM, Cnaan A, et al. Recognition of tubo-ovarian abscess in adolescents with pelvic inflammatory disease. J Adolesc Health. 1996;18:397–403. doi: 10.1016/1054-139X(96)00020-1. [DOI] [PubMed] [Google Scholar]
- 12.Golden N, Cohen H, Gennari G, Neuhoff S. The use of pelvic ultrasonography in the evaluation of adolescents with pelvic inflammatory disease. Am J Dis Child. 1987;141:1235–1238. doi: 10.1001/archpedi.1987.04460110105035. [DOI] [PubMed] [Google Scholar]
- 13.Westrom L. Effect of pelvic inflammatory disease on fertility. Venereology. 1995;8:219–222. [PubMed] [Google Scholar]
- 14.Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168:1503–1509. doi: 10.1016/s0002-9378(11)90790-x. [DOI] [PubMed] [Google Scholar]
- 15.Shafer MA, Sweet RL. Pelvic inflammatory disease in adolescent females. Epidemiology, pathogenesis, diagnosis, treatment, and sequelae. Pediatr Clin North Am. 1989;36:513–532. doi: 10.1016/s0031-3955(16)36683-4. [DOI] [PubMed] [Google Scholar]
- 16.Westrom L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol. 1975;121:707–713. doi: 10.1016/0002-9378(75)90477-9. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55:1–94. [PubMed] [Google Scholar]
- 18.Haggerty CL, Ness RB. Newest approaches to treatment of pelvic inflammatory disease: a review of recent randomized clinical trials. Clin Infect Dis. 2007;44:953–960. doi: 10.1086/512191. [DOI] [PubMed] [Google Scholar]
- 19.Heinonen PK, Leinonen M. Fecundity and morbidity following acute pelvic inflammatory disease treated with doxycycline and metronidazole. Arch Gynecol Obstet. 2003;268:284–288. doi: 10.1007/s00404-002-0376-6. [DOI] [PubMed] [Google Scholar]
- 20.Pavletic AJ, Wolner-Hanssen P, Paavonen J, et al. Infertility following pelvic inflammatory disease. Infect Dis Obstet Gynecol. 1999;7:145–152. doi: 10.1002/(SICI)1098-0997(1999)7:3<145::AID-IDOG6>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23. 2005;25:1–160. [PubMed] [Google Scholar]
- 22.Trent M, Chung SE, Burke M, et al. Results of a randomized controlled trial of a brief behavioral intervention for pelvic inflammatory disease in adolescents. J Pediatr Adolesc Gynecol. 2010;23:96–101. doi: 10.1016/j.jpag.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162:585–590. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 24&&.Goyal M, Hersh A, Luan X, et al. Are emergency departments appropriately treating adolescent pelvic inflammatory disease? JAMA Pediatr. 2013;167:672–673. doi: 10.1001/jamapediatrics.2013.1042. This study demonstrates the lack of adherence to the STD treatment guidelines of management of PID in adolescents seen in US emergency departments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolner-Hanssen P. Silent pelvic inflammatory disease: is it overstated? Obstet Gynecol. 1995;86:321–325. doi: 10.1016/0029-7844(95)00177-S. [DOI] [PubMed] [Google Scholar]
- 26.Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC), Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 27.Ku L, St Louis M, Farshy C, et al. Risk behaviors, medical care, and chlamydial infection among young men in the United States. Am J Public Health. 2002;92:1140–1143. doi: 10.2105/ajph.92.7.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chacko MR, Lovchik JC. Chlamydia trachomatis infection in sexually active adolescents: prevalence and risk factors. Pediatrics. 1984;73:836–840. [PubMed] [Google Scholar]
- 29.Lee V, Tobin JM, Foley E. Relationship of cervical ectopy to chlamydia infection in young women. J Fam Plann Reprod Healthcare. 2006;32:104–106. doi: 10.1783/147118906776276440. [DOI] [PubMed] [Google Scholar]
- 30.Fortenberry JD, Tu W, Harezlak J, et al. Condom use as a function of time in new and established adolescent sexual relationships. Am J Public Health. 2002;92:211–213. doi: 10.2105/ajph.92.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrier LA, Goodman E, Emans SJ. Partner condom use among adolescent girls with sexually transmitted diseases. J Adolesc Health. 1999;24:357–361. doi: 10.1016/s1054-139x(98)00133-5. [DOI] [PubMed] [Google Scholar]
- 32.Shrier LA, Ancheta R, Goodman E, et al. Randomized controlled trial of a safer sex intervention for high-risk adolescent girls. Arch Pediatr Adolesc Med. 2001;155:73–79. doi: 10.1001/archpedi.155.1.73. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg MD, Gurvey JE, Adler N, et al. Concurrent sex partners and risk for sexually transmitted diseases among adolescents. Sex Transm Dis. 1999;26:208–212. doi: 10.1097/00007435-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kelly AM, Ireland M, Aughey D. Pelvic inflammatory disease in adolescents: high incidence and recurrence rates in an urban teen clinic. J Pediatr Adolesc Gynecol. 2004;17:383–388. doi: 10.1016/j.jpag.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Lenoir CD, Adler NE, Borzekowski DL, et al. What you don’t know can hurt you: perceptions of sex-partner concurrency and partner-reported behavior. J Adolesc Health. 2006;38:179–185. doi: 10.1016/j.jadohealth.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Yu SM, Bellamy HA, Schwalberg RH, Drum MA. Factors associated with use of preventive dental and health services among U.S. adolescents. J Adolesc Health. 2001;29:395–405. doi: 10.1016/s1054-139x(01)00252-x. [DOI] [PubMed] [Google Scholar]
- 37.Ford CA, Bearman PS, Moody J. Foregone healthcare among adolescents. JAMA. 1999;282:2227–2234. doi: 10.1001/jama.282.23.2227. [DOI] [PubMed] [Google Scholar]
- 38.Newacheck PW, Brindis CD, Cart CU, et al. Adolescent health insurance coverage: recent changes and access to care. Pediatrics. 1999;104:195–202. doi: 10.1542/peds.104.2.195. [DOI] [PubMed] [Google Scholar]
- 39.Rome ES, Moszczenski SA, Craighill M, et al. A clinical pathway for pelvic inflammatory disease for use on an inpatient service. Clin Perform Qual Healthcare. 1995;3:185–196. [PubMed] [Google Scholar]
- 40.Shrier LA, Moszczenski SA, Emans SJ, et al. Three years of a clinical practice guideline for uncomplicated pelvic inflammatory disease in adolescents. J Adolesc Health. 2000;27:57–62. doi: 10.1016/s1054-139x(00)00090-2. [DOI] [PubMed] [Google Scholar]
- 41.Pelvic inflammatory disease: guidelines for prevention and management. MMWR Recomm Rep. 1991;40:1–25. [PubMed] [Google Scholar]
- 42.Ness RB, Soper DE, Peipert J, et al. Design of the PID Evaluation and Clinical Health (PEACH) Study. Control Clin Trials. 1998;19:499–514. doi: 10.1016/s0197-2456(98)00022-1. [DOI] [PubMed] [Google Scholar]
- 43.Ness RB, Trautmann G, Richter HE, et al. Effectiveness of treatment strategies of some women with pelvic inflammatory disease: a randomized trial. Obstet Gynecol. 2005;106:573–580. doi: 10.1097/01.AOG.0000175193.37531.6e. [DOI] [PubMed] [Google Scholar]
- 44.Haggerty CL, Peipert JF, Weitzen S, et al. Predictors of chronic pelvic pain in an urban population of women with symptoms and signs of pelvic inflammatory disease. Sex Transm Dis. 2005;32:293–299. doi: 10.1097/01.olq.0000162361.69041.a5. [DOI] [PubMed] [Google Scholar]
- 45.Haggerty CL, Schulz R, Ness RB. PID Evaluation and Clinical Health Study Investigators. Lower quality of life among women with chronic pelvic pain after pelvic inflammatory disease. Obstet Gynecol. 2003;102:934–939. doi: 10.1016/s0029-7844(03)00695-1. [DOI] [PubMed] [Google Scholar]
- 46.Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38:879–881. doi: 10.1097/OLQ.0b013e31821f918c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trent M, Chung SE, Forrest L, Ellen JM. Subsequent sexually transmitted infection after outpatient treatment of pelvic inflammatory disease. Arch Pediatr Adolesc Med. 2008;162:1022–1025. doi: 10.1001/archpedi.162.11.1022. [DOI] [PubMed] [Google Scholar]
- 48.Rein DB, Kassler WJ, Irwin KL, Rabiee L. Direct medical cost of pelvic inflammatory disease and its sequelae: decreasing, but still substantial. Obstet Gynecol. 2000;95:397–402. doi: 10.1016/s0029-7844(99)00551-7. [DOI] [PubMed] [Google Scholar]
- 49.Blandford JM, Gift TL. Productivity losses attributable to untreated chlamydial infection and associated pelvic inflammatory disease in reproductive-aged women. Sex Transm Dis. 2006;33:S117–S121. doi: 10.1097/01.olq.0000235148.64274.2f. [DOI] [PubMed] [Google Scholar]
- 50.Yeh JM, Hook EW, 3rd, Goldie SJ. A refined estimate of the average lifetime cost of pelvic inflammatory disease. Sex Transm Dis. 2003;30:369–378. doi: 10.1097/00007435-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Trent M, Ellen JM, Frick KD. Estimating the direct costs of pelvic inflammatory disease in adolescents: a within-system analysis. Sex Transm Dis. 2011;38:326–328. doi: 10.1097/OLQ.0b013e3181fc6c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52&.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. This study updates prior data on the national prevalence and incidence estimates by age for women and men in the United States. [DOI] [PubMed] [Google Scholar]
- 53&&.Rekart ML, Gilbert M, Meza R, et al. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J Infect Dis. 2013;207:30–38. doi: 10.1093/infdis/jis644. An epidemiological trends study in British Columbia for which the author postulates potential immunological reasons for decline in PID rates despite increasing Chlamydia rates in this country. [DOI] [PubMed] [Google Scholar]
- 54.Hoover K, Tao G, Kent C. Low rates of both asymptomatic chlamydia screening and diagnostic testing of women in US outpatient clinics. Obstet Gynecol. 2008;112:891–898. doi: 10.1097/AOG.0b013e318185a057. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention (CDC) Chlamydia screening among sexually active young female enrollees of health plans – United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009;58:362–365. [PubMed] [Google Scholar]
- 56.Wiehe SE, Rosenman MB, Wang J, et al. Chlamydia screening among young women: individual- and provider-level differences in testing. Pediatrics. 2011;127:e336–344. doi: 10.1542/peds.2010-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trent M, Ellen JM, Walker A. Pelvic inflammatory disease in adolescents: care delivery in pediatric ambulatory settings. Pediatr Emerg Care. 2005;21:431–436. doi: 10.1097/01.pec.0000169432.14067.eb. [DOI] [PubMed] [Google Scholar]
- 58.Shih TY, Gaydos CA, Rothman RE, Hsieh YH. Poor provider adherence to the Centers for Disease Control and Prevention treatment guidelines in US emergency department visits with a diagnosis of pelvic inflammatory disease. Sex Transm Dis. 2011;38:299–305. doi: 10.1097/OLQ.0b013e31820b8bb4. [DOI] [PubMed] [Google Scholar]
- 59.Trent M, Judy SL, Ellen JM, Walker A. Use of an institutional intervention to improve quality of care for adolescents treated in pediatric ambulatory settings for pelvic inflammatory disease. J Adolesc Health. 2006;39:50–56. doi: 10.1016/j.jadohealth.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 60.DiClimente CC, Ferentz K, Velasquez MM. Handbook of primary care psychology. 1. New York: Oxford University Press; 2004. Health behavior change and the problem of ‘noncompliance’; pp. 157–158. [Google Scholar]
- 61.Eraker SA, Kirscht JP, Becker MH. Understanding and improving patient compliance. Ann Intern Med. 1984;100:258–268. doi: 10.7326/0003-4819-100-2-258. [DOI] [PubMed] [Google Scholar]
- 62.Baltimore City Health Department. School health services. [Accessed 22 March 2007]; http://www.baltimorehealth.org/schoolhealth.html.
- 63&&.Trent M, Lehmann HP, Butz A, et al. Clinician perspectives on management of adolescents with pelvic inflammatory disease using standardized patient scenarios. Sex Transm Dis. 2013;40:496–498. doi: 10.1097/OLQ.0b013e318284e3b5. This study describes the results of a national survey of adolescent-serving clinicians who were asked to consider 17 potential clinical presentations for a 15-year-old hypothetical adolescent and assess the need for hospitalization in these situations. The data show consistency with the CDC guidelines, but suggest that physicians struggle with the factors that impact a teen’s ability to tolerate an outpatient treatment regimen per the CDC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trent M, Lehmann HP, Qian Q, et al. Adolescent and parental utilities for the health states associated with pelvic inflammatory disease. Sex Transm Infect. 2011;87:583–587. doi: 10.1136/sextrans-2011-050187. [DOI] [PubMed] [Google Scholar]