Abstract
Background:
Orthognathic surgery can cause discomforts such as pain, inflammation, and edema. One of the challenges is bone regeneration at surgery area. The aim of this study is to evaluate the effect of low-intensity pulsed ultrasound (LIPUS) therapy on bone regeneration and pain relief after surgery.
Materials and Methods:
Following mandibular surgery of nine patients, LIPUS treatment was applied to the left or right side for 3 weeks and 20 min/day. The other side was treated with sham-LIPUS as the control group. Digital panoramic radiographies were obtained immediately after surgery and 3 weeks later. Bone density at surgery site was assessed using Digora version 2.8 software. The data were analyzed with one-sample Kolmogorov–Smirnov and t-paired test. Postoperation pain was evaluated by means of visual analog scale.
Results:
Increase in bone density at border and medulla was 23 and 28.33 for experimental group and 13 and 13.55 units in control group, respectively. The differences are statistically significant (P < 0.01). Variance analysis showed that decrease in experienced pain during 3 weeks after surgery was significantly different between groups (P < 0.01).
Conclusion:
LIPUS can be an effective way to increase bone modeling and decrease pain following orthognathic surgeries.
Keywords: Bone regeneration, low-intensity pulsed ultrasound, orthognathic surgery, pain
Introduction
Healing of bone fractures and surgical cuts is an important homeostatic process that depends on several factors including activation of specific cells and immobilization of the bones.[1,2] Pain after orthognathic surgery is an acute pain that develops because of surgical trauma and inflammatory processes. The inflammatory response is the main reason of pain and swelling after surgery. Leukotriene, bradykinin, and platelet-activating factors are examples of inflammatory chemicals that start cascades leading to vascular dilatation, modified permeability, and accumulation of interstitial fluid causing edema.[3]
One of the challenges following orthognathic surgery is bone healing at the surgery site. In past 50 years, investigators have studied different physical and biological methods to reduce healing time of bone fractures.[4] Physical interventions include mechanical stimuli,[5] electromagnetic fields,[6] capacitive paired electric field,[7,8] direct current,[9,10] microcurrent,[10] and low-level laser[11] are used as noninvasive and easy procedures. Drugs, laser therapy, cold pack, and ultrasound have been used to alleviate postsurgical pain.[11,12] It has been observed that applying low-level laser after orthognathic surgery decreases complications such as pain and swelling.[11] Cold packs have been used in several studies, and modern appliances such as water circulating devices have been introduced, but these methods have no effect on bone remodeling or neurologic problems. Furthermore, these methods depend on patient compliance.[12]
Application of ultrasound is one of the methods used to reduce bone healing time and complications of orthognathic surgery. In recent years, application of low-level therapeutic ultrasound has been introduced since higher levels may cause tissue damage.[13,14] Low-intensity pulsed ultrasound (LIPUS) with the intensity of 0.01–3 mW/cm2 and frequency of 1.5–4 MHz is a common physical intervention for diagnosis and treatment,[8,15] and it is the only physical intervention that has been approved by Food and Drug Administration for enhancement of fresh fractures.[13]
It seems that mechanism of ultrasound includes thermal and nonthermal (pulsed) effects which are the result of increased circulation of interstitial and vascular fluids.[8] Increased blood flow, reduced muscle spasm, increased capacity of collagen fiber elongation, and inflammatory reactions are the thermal effects of ultrasound which are mostly seen in continuous nonpulsed ultrasounds.[16,17] Nonthermal effects which are frequently seen in pulsed ultrasound divides into two groups: (1) effects resulted from cavitation that is the result of expansion and contraction of gas bubbles. This happens because of pressure alterations induced by ultrasound and causes increase in tissue fluids; (2) unidirectional flow of fluids along the cell membranes creates acoustic microcurrents that affect function and permeability of cell membranes which induce tissue repair. Eventually, cavitation and acoustic microcurrents induce fibroblast activation, protein synthesis, increased blood flow, tissue healing, and bone remodeling.[15,16]
The purpose of this study is to evaluate low-intensity pulsed ultrasound effects on bone healing and pain relief after orthognathic surgery.
Materials and Methods
Twelve patients (six males and six females) between 17 and 40 years of age were included in this study, which needed mandibular advancement or setback surgeries by means of the bilateral sagittal split osteotomy.
Informed consent of the participants was provided according to the Ethics Committee of Shahid Beheshti University of Medical Sciences.
Exclusion criteria were:
Patients with systemic or metabolic bone disorders
Patients consuming drugs affecting bone metabolism like bisphosphonates
Patients with significant facial asymmetry
Patients with history of medicine consumption
Patients with postsurgical complications.
After orthognathic surgery, LIPUS was performed with EXOGEN 4000+, Smith & Nephew Co., USA, at surgical sites [Figure 1]. LIPUS was used for 3 weeks, 20 min a day with the intensity of 30 mW/cm2, frequency of 1 MHz, and a pulse repetition frequency of 1 kHz 200-μs-wide (duty cycle 1:4). One side of the face was selected randomly to be treated with ultrasound, and the other side was treated with sham transducer as the control side. Digital panoramic radiographs were taken immediately (T1) and 3 weeks after surgery (T2). Bone density at surgery site was analyzed by Digora version 2.8 software (Soredex, Finland) [Figures 2 and 3]. Postsurgical pain was assessed using visual analog scale (VAS) method. All the dependent factors were measured and evaluated separately for both right and left sides.
Figure 1.
Low-level pulsed ultrasound appliance
Figure 2.
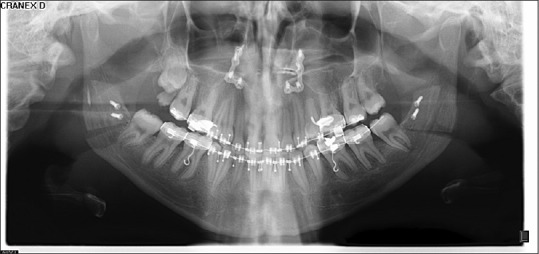
Orthopantomogram radiograph immediately after surgery (pins were applied in this patient for mandible stabilization)
Figure 3.
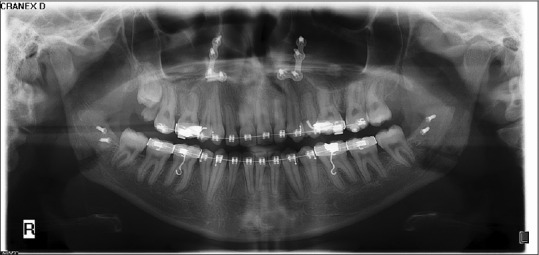
Orthopantomogram radiograph 3 weeks after surgery
Three patients were excluded from the study, one due to infection at the surgery site, and two because of noncompliance.
Initial data were assessed with ANOVA with repeated measures. One-sample Kolmogorov– Smirnov (K_S) test and t-paired test were used for statistical analysis.
Results
Bone density at mandibular border
Normal distribution of the data was confirmed by one-sample K_S test at the minimum probability of 0.624. ANOVA with repeated measures was used to compare density at mandibular borders of experimental and control groups between two times. The relation between time and exposure to LIPUS was significant [Chart 1]. Amount of increased density was assessed which is shown in Table 1.
Chart 1.
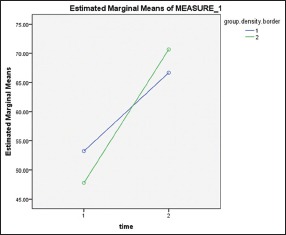
Density of border bone (1) control group, (2) experimental group. (Time 1) immediately after surgery, (Time 2) 3 weeks after treatment with low-intensity pulsed ultrasound
Table 1.
Measurements of bone density at border and medulla in experimental and control group at two stages: (T1) Immediately after surgery, (T2) 3 weeks after surgery
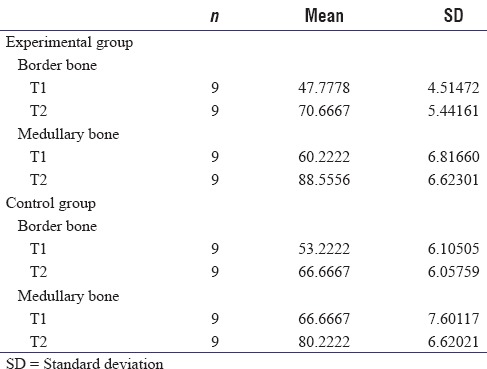
Bone density increase at the border of the mandible was 23 units for experimental group and 13 for the control group. The difference is statistically significant according to paired t-test (P = 0.01).
Density at medullary bone
One-sample K_S test was used to evaluate the normal distribution of data and was confirmed with minimum probability of 0.622.
Due to significant cross effect of time and group parameters [Chart 2], increased density was calculated in both groups at medullary bone [Table 1].
Chart 2.
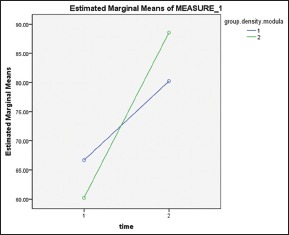
Density of medullary bone, (1) control group, (2) experimental group. (Time 1) immediately after surgery, (Time 2) 3 weeks after treatment with low-intensity pulsed ultrasound
Density was significantly increased in the experimental group (28.33) in comparison with control group (13.55) according to paired t-test (P < 0.001).
Postsurgical pain
For all the patients in the study, pain was zero at day 7 after surgery; so, day 7 was eliminated from analysis. Normal distribution of data was confirmed at the minimum probability of 0.01 with one-sample K_S test.
The results of two-way repeated measures ANOVA show that pain decrease was significant (P < 0.01). Furthermore, patients in experimental group experienced less pain than the control group at each interval (P = 0.048). As shown in Chart 3, pain decline pattern was different in two groups.
Chart 3.
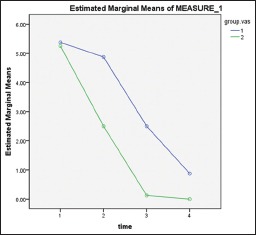
Average of VAS at three different times: (1) 1 day after surgery, (2) 2 days after surgery, (3) 4 days after surgery, (4) 7 days after surgery. Group 1: Control group, Group 2: Experimental group
Discussion
The results of this study showed that applying LIPUS after orthognathic surgery can enhance bone formation (border and medullary) at the surgery site.
LIPUS waves produces nanomotion that starts a process which involves integrins and focal adhesions. The most important molecule involved in the mechanism of LIPUS is cyclooxygenase 2 (COX2), which is produced as the result of a biologic cascade. Stimulation of COX2 enhances production of prostaglandin E2 and expression of osteogenic genes. LIPUS can affect all phases of fracture healing including inflammatory, intramembranous ossification, chondrogenesis, endochondral ossification, and remodeling phase in a positive way.[18,19,20,21,22]
Effects of LIPUS on mandibular fractures were investigated in a study by Patel et al.[23] After intermaxillary fixation, 28 healthy controls with fresh, undisplaced, or minimally displaced mandibular fracture were divided into two groups. Patients in the intervention group received LIPUS treatment with the frequency of 1 MHz and intensity of 1.5 W/cm for 24 days, 5 min each day. The control group did not receive any therapy after inframammary fold (IMF). The results showed that radiographic density was significantly higher in the experimental group than in control group at 3 and 5 weeks after IMF.
In another study, LIPUS with frequency of 3.0 MHz and intensity of 30 mW/cm2 was applied over the right alveolar bone of rats for 2 weeks and 10 min a day after extraction of the tooth. The left side did not receive LIPUS treatment. On histomorphometric analysis, it was observed that new bone formation in the sockets of LIPUS group was higher than in that of the control group.[24]
It also has been shown that initial stability of miniscrew implants and osseointegration is intensified after LIPUS application because of the increased bone formation around the miniscrews.[25,26]
The results of this study also demonstrated that VAS scores improved significantly at the LIPUS-treated side. In a similar study, LIPUS treatment in patients with mandibular fracture after intermaxillary fixation decreased pain perception significantly.[23]
Nonsteroidal anti-inflammatory drugs are commonly used after orthognathic surgery, but they may cause side effects such as allergic reactions, irritation of gastrointestine, cutaneous rash, neutropenia, and bleeding tendency.[27] Administration of corticosteroids decreases postoperative pain and edema, but it may cause serious side effects such as avascular osteonecrosis, diminished healing potential, and suppression of adrenal gland.[28]
As it is shown in Chart 3, pain in LIPUS-treated side declines rapidly 1 day after surgery; meanwhile, in control side, decrease in pain is slower 1 day after surgery. At day 4 after surgery, pain is almost zero at LIPUS-treated side, but at the control, side pain is present until day 7.
Application of low-level laser therapy has been shown to be an effective method to improve tissue remodeling and decrease pain and inflammation, and it has been claimed that it is almost side-effect free.[13,29,30]
Conclusion
It can be concluded from this study that application of low-intensity pulsed ultrasound is a noninvasive and effective way to enhance bone healing and patient comfort after orthognathic surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was supported by grant from Dentofacial Deformities Research Center, Research Institute of Dental Sciences, Dental School, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Childs SG. Stimulators of bone healing. Biologic and biomechanical. Orthop Nurs. 2003;22:421–8. doi: 10.1097/00006416-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Paterson D. Treatment of nonunion with a constant direct current: A totally implantable system. Orthop Clin North Am. 1984;15:47–59. [PubMed] [Google Scholar]
- 3.Ahiskalioglu A, Ince I, Aksoy M, Yalcin E, Ahiskalioglu EO, Kilinc A. Effects of a single-dose of pre-emptive pregabalin on postoperative pain and opioid consumption after double-jaw surgery: A randomized controlled trial. J Oral Maxillofac Surg. 2016;74:53.e1–7. doi: 10.1016/j.joms.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Trock DH. Electromagnetic fields and magnets. Investigational treatment for musculoskeletal disorders. Rheum Dis Clin North Am. 2000;26:51–62. doi: 10.1016/s0889-857x(05)70119-8. viii. [DOI] [PubMed] [Google Scholar]
- 5.Hannouche D, Petite H, Sedel L. Current trends in the enhancement of fracture healing. J Bone Joint Surg Br. 2001;83:157–64. doi: 10.1302/0301-620x.83b2.12106. [DOI] [PubMed] [Google Scholar]
- 6.Malizos KN, Hantes ME, Protopappas V, Papachristos A. Low-intensity pulsed ultrasound for bone healing: An overview. Injury. 2006;37(Suppl 1):S56–62. doi: 10.1016/j.injury.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–5. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 8.Watson T. Electrotherapy: Evidence-Based Practice. Chapter 10. Elsevier Health Sciences; 2008. p. 140. [Google Scholar]
- 9.Barden RM, Sinkora GL. Bone stimulators for fusions and fractures. Nurs Clin North Am. 1991;26:89–103. [PubMed] [Google Scholar]
- 10.Benazzo F, Mosconi M, Beccarisi G, Galli U. Use of capacitive coupled electric elds in stress fractures in athletes. Clin Orthop Relat Res. 1995;310:145–9. [PubMed] [Google Scholar]
- 11.Cundy PJ, Paterson DC. A ten-year review of treatment of delayed union and nonunion with an implanted bone growth stimulator. Clin Orthop Relat Res. 1990;259:216–22. [PubMed] [Google Scholar]
- 12.Gresh M. Microcurrent electrical stimulation: Putting it in perspective. Clin Manage. 1987;9:51–4. [Google Scholar]
- 13.Gasperini G, Rodrigues de Siqueira IC, Rezende Costa L. Does low-level laser therapy decrease swelling and pain resulting from orthognathic surgery? Int J Oral Maxillofac Surg. 2014;43:868–73. doi: 10.1016/j.ijom.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Rana M, Gellrich NC, Joos U, Piffkó J, Kater W. 3D evaluation of postoperative swelling using two different cooling methods following orthognathic surgery: A randomised observer blind prospective pilot study. Int J Oral Maxillofac Surg. 2011;40:690–6. doi: 10.1016/j.ijom.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–48. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 16.Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83-A:259–70. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Grieder A, Vinton PW, Cinotti WR, Kangur TT. An evaluation of ultrasonic therapy for temporomandibular joint dysfunction. Oral Surg Oral Med Oral Pathol. 1971;31:25–31. doi: 10.1016/0030-4220(71)90029-6. [DOI] [PubMed] [Google Scholar]
- 18.Naruse K, Sekiya H, Harada Y, Iwabuchi S, Kozai Y, Kawamata R, et al. Prolonged endochondral bone healing in senescence is shortened by low-intensity pulsed ultrasound in a manner dependent on COX-2. Ultrasound Med Biol. 2010;36:1098–108. doi: 10.1016/j.ultrasmedbio.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Harrison A, Lin S, Pounder N, Mikuni-Takagaki Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics. 2016;70:45–52. doi: 10.1016/j.ultras.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16:671–80. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa T, Miwa M, Sakai Y, Niikura T, Kurosaka M, Komori T. Osteogenic activity of human fracture haematoma-derived progenitor cells is stimulated by low-intensity pulsed ultrasound in vitro. J Bone Joint Surg Br. 2009;91:264–70. doi: 10.1302/0301-620X.91B2.20827. [DOI] [PubMed] [Google Scholar]
- 22.Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF, et al. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol. 2006;69:2047–57. doi: 10.1124/mol.105.022160. [DOI] [PubMed] [Google Scholar]
- 23.Patel K, Kumar S, Kathiriya N, Madan S, Shah A, Venkataraghavan K, et al. An Evaluation of the Effect of Therapeutic Ultrasound on Healing of Mandibular Fracture. Craniomaxillofac Trauma Reconstr. 2015;8:299–306. doi: 10.1055/s-0034-1544104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang KL, Kim EC, Park JB, Heo JS, Choi Y. High-frequency, low-intensity pulsed ultrasound enhances alveolar bone healing of extraction sockets in rats: A pilot study. Ultrasound Med Biol. 2016;42:493–502. doi: 10.1016/j.ultrasmedbio.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Ganzorig K, Kuroda S, Maeda Y, Mansjur K, Sato M, Nagata K, et al. Low-intensity pulsed ultrasound enhances bone formation around miniscrew implants. Arch Oral Biol. 2015;60:902–10. doi: 10.1016/j.archoralbio.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhou H, Hou Y, Zhu Z, Xiao W, Xu Q, Li L, et al. Effects of low-intensity pulsed ultrasound on implant osseointegration in ovariectomized rats. J Ultrasound Med. 2016;35:747–54. doi: 10.7863/ultra.15.01083. [DOI] [PubMed] [Google Scholar]
- 27.Merry AF, Gibbs RD, Edwards J, Ting GS, Frampton C, Davies E, et al. Combined acetaminophen and ibuprofen for pain relief after oral surgery in adults: A randomized controlled trial. Br J Anaesth. 2010;104:80–8. doi: 10.1093/bja/aep338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dan AE, Thygesen TH, Pinholt EM. Corticosteroid administration in oral and orthognathic surgery: A systematic review of the literature and meta-analysis. J Oral Maxillofac Surg. 2010;68:2207–20. doi: 10.1016/j.joms.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Carroll JD, Milward MR, Cooper PR, Hadis M, Palin WM. Developments in low level light therapy (LLLT) for dentistry. Dent Mater. 2014;30:465–75. doi: 10.1016/j.dental.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Pol R, Ruggiero T, Gallesio G, Riso M, Bergamasco L, Mortellaro C, et al. Efficacy of anti-inflammatory and analgesic of superpulsed low level laser therapy after impacted mandibular third molars extractions. J Craniofac Surg. 2016;27:685–90. doi: 10.1097/SCS.0000000000002523. [DOI] [PubMed] [Google Scholar]


