Abstract
Background
Post-traumatic joint contracture (PTJC) in the elbow is a challenging clinical problem due to the anatomical and biomechanical complexity of the elbow joint.
Methods
Previously, we established an animal model to study elbow PTJC, wherein surgically induced soft tissue damage followed by six-weeks of unilateral immobilization in Long-Evans rats led to stiffened and contracted joints that exhibited features similar to the human condition. In this study following the six-weeks of immobilization, we remobilized the animal (i.e. external bandage removed and free cage activity) for an additional six-weeks; after which the limbs were evaluated mechanically and histologically.
Hypothesis
The objective of this study was to evaluate whether this decreased joint motion would persist following six-weeks of free mobilization.
Results
After free mobilization (FM), flexion-extension demonstrated decreased total range of motion (ROM) and neutral zone length, and increased ROM midpoint for injured limbs compared to control and contralateral limbs. Specifically, following FM total ROM demonstrated a significant decrease of approximately 22% and 26% compared to control and contralateral limbs for Injury I (anterior capsulotomy) and Injury II (anterior capsulotomy with lateral collateral ligament transection), respectively. Histological evaluation showed increased adhesion, fibrosis and thickness of the capsule tissue in the injured limbs after FM compared to control and contralateral limbs, which is consistent with patterns previously reported in human tissue.
Conclusion
Therefore, even with free mobilization, injured limbs in this model demonstrate persistent joint motion loss and histological results similar to the human condition. Future work will use this animal model to investigate the mechanisms responsible for PTJC and responses to therapeutic intervention.
KEY TERMS: elbow joint, post-traumatic joint contracture, free mobilization, joint mechanics, range of motion, flexion-extension
INTRODUCTION
Post-traumatic joint contracture (PTJC) as a result of injury to the elbow is a common and challenging clinical problem because the elbow is anatomically and biomechanically one of the most complex joints in the body9,18. The highly congruent joint surfaces of the three bones that comprise the elbow create a complex articulation which allows precise positioning of the forearm and hand in space2. Joint articulation is stabilized by several surrounding periarticular soft tissues (i.e., capsule, ligaments, tendons, muscles). Injury to the elbow often disrupts the periarticular structures potentially causing changes in ligament tension, bone anatomy or cartilage congruity and leads to an onset of PTJC. Injury is poorly tolerated in the elbow such that even a relatively minor injury can result in significant functional impairment affecting routine daily and vocational activities7. Given that injury severity does not always correlate with the degree of functional deficit, it is difficult to predict who is at risk for developing PTJC. This presents a significant clinical challenge in managing elbow injuries with contracture. Thus, there is a critical need to study the development of elbow PTJC in a relevant model.
Previously, our group developed an animal model of elbow PTJC10. We demonstrated that elbow contracture could be induced in Long-Evans rats by surgically creating a soft tissue injury followed by six-weeks of immobilization. Our animal model was evaluated biomechanically in flexion-extension (F-E) and histologically and was found to replicate characteristics similar to the human condition, including decreased total range of motion (ROM) and neutral zone (NZ) length as well as increased cellularity, adhesion and capsule thickness. However, more research is needed to determine if symptoms of PTJC persist long-term in this animal model. Long-standing contracture would indicate that periarticular joint tissues are permanently altered, which would further validate the use of our rat elbow model for studying PTJC pathophysiology and treatment methodologies. Therefore, the objective of this study was to evaluate whether decreased joint mechanics induced by injury and immobilization resolves after joint free mobilization in our recently developed rat model of elbow PTJC.
MATERIALS AND METHODS
Animal Model
Long-Evans rats (Charles River Laboratories International, Wilmington, MA) were selected and used based on criteria previously described10. Briefly, these animals were evaluated based on their (1) anatomical similarities, (2) functional ROM of the joint and (3) use of their upper extremities. Anatomically, Long-Evans rats exhibit many features that are analogous to the human elbow, in which three bones (humerus, radius and ulna) form a complex articulation. The periarticular structures surrounding the elbow are also similar to human anatomy.
Injury Model
This IACUC approved study used male Long-Evans rats (250–300g) that were randomized into three surgical groups (Sham, Injury I, Injury II) and a group of age-matched control animals. The study utilized forty rats initially; after excluding four samples because of dissection and testing abnormalities, a total of thirty-six rats were included (n = 7–10/group). Clinically relevant elbow injuries were surgically created to replicate varying degrees of soft-tissue injury seen in elbow subluxation/dislocation, as described previously10. Briefly, the animals in each surgical group were anesthetized and surgery was performed under sterile conditions on the left limb: Sham (superficial lateral incision without violation of joint structures), Injury I (anterior capsulotomy) and Injury II (anterior capsulotomy combined with lateral collateral ligament transection). Sham animals were used to evaluate the effect of joint immobilization combined with a minor surgical procedure (superficial lateral incision) but no periarticular joint tissue injury. Thus, Sham represents the least severe injury (i.e., no joint injury with immobilization) while Injury II represents the most severe injury (i.e., anterior capsulotomy and lateral collateral ligament transection with immobilization). Animals received single doses of antibiotic (7.5 mg/kg enrofloxacin, Bayer Health LLC, Shawnee Mission, KS) and non-steroidal anti-inflammatory drug (NSAID) (5 mg/kg, carprofen, Pfizer Animal Health, New York, NY) pre-operatively via subcutaneous injection and one dose of analgesic (0.5 cc of 5 mg/mL bupivacaine, Hospira, Lake Forest, IL) post-operatively via subcutaneous injection under the closed incision. Contralateral (CL) and control limbs were not injured and served as comparisons.
Following surgery, operated limbs were immobilized in flexion (151° ± 2°) for six weeks, while CL limbs and control animals were not immobilized and allowed unrestricted motion. As described previously10, the injured limbs were immobilized using tubular elastic netting (Nich Marketers Inc., Gulf Breeze, FL) and self-adhering Vetrap bandaging (3M™, St. Paul, MN). An access hole was cut to leave the CL limb unconstrained. During the six-week immobilization period, animals were evaluated 5X/week to ensure the injured limb was constrained and to identify any pain or distress. Clean wraps were applied weekly and additional details regarding animal care and observation during the immobilization period were provided previously10. Any time an animal was rewrapped, any sores or cuts caused by scratching or rubbing of the wrap were treated topically with antibiotic powder/cream (nitrofurazone (Neogen Corporation, Lexington, KY), silver sulfadiazine (Dr. Reddy’s Laboratories Louisiana, Shreveport, LA)) and/or chafing cream (Prestige Brands, Tarrytown, NY). After six weeks the wrapping restraints were removed and animals were allowed unrestricted cage activity for the remaining six weeks to remobilize their left limb (free mobilization or FM). At the conclusion of the FM period, animals were sacrificed via CO2 inhalation and immediately stored in a −20°C freezer.
Mechanical Testing
Mechanical testing was performed on both the injured and CL limbs for each animal so paired comparisons could be made, in addition to comparisons with controls. Forelimbs were prepared for mechanical testing as described previously10. To summarize, forelimbs were skinned, the glenohumeral joint was disarticulated and the paw was removed. The humeral head and distal ulna/radius were then secured in test fixtures. In the test setup, the limb was aligned to allow smooth articulation of the joint and had a starting position of ~90° flexion.
A custom mechanical test system was used to evaluate rat elbow stiffness and joint-contracture in F-E. The design of this system and post-test analysis were published previously10 and is similar to other setups used to test animal limbs in F-E6.
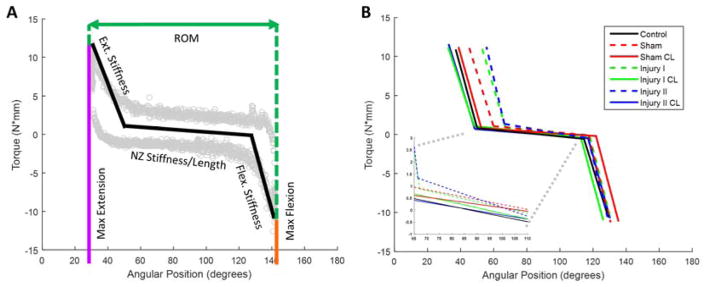
Force and displacement data from the fifth cycle was converted to torque and angular position for F-E. A custom written Matlab program (Mathworks, Natick, MA) analyzed the torque-angle curves to quantify joint motion in F-E (Fig. 1A). F-E measurements include: total ROM, ROM midpoint, maximum flexion, maximum extension, NZ length, flexion stiffness, extension stiffness and NZ stiffness. ROM data (i.e., total ROM, ROM midpoint, NZ length) represent a measure of joint contracture, while stiffness values (i.e., NZ, flexion, extension) represent various aspects of overall joint stiffness. Total ROM is the summation of the angular positions of the limits of motion in either direction. The ROM midpoint shows the relative shift of the overall curve, which can demonstrate decreased joint motion. The NZ region falls between the linear fits of flexion/extension stiffness. As defined previously, NZ stiffness and NZ length are averages of the loading and unloading curves in that region10. Post-test analysis also calculated the average curves for each group by averaging the maximum extension, maximum flexion and both end points of the NZ stiffness/length curve within each group to present a graphical visualization of qualitative differences in joint motion between groups (Fig. 1B).
Figure 1.
(A) Schematic of torque-angle loading curve with parameters quantified for flexion-extension biomechanical joint testing. The light gray circles are data from a representative sample. The black line for neutral zone stiffness/length is the average of the loading and unloading curves. (B) Average curves for each group (injury, contralateral, control) demonstrate decreased joint motion in flexion-extension for injured limbs following free mobilization (dashed line = injured limb, solid line = contralateral).
Histological Analysis
After mechanical testing, a subset of samples (n = 3/group) that exhibited average joint motion in mechanical testing were prepared for histological assessment using standard protocols as described previously10. Three sagittal sections (5 μm) were cut for each limb and stained with hematoxylin and eosin (H&E). A musculoskeletal pathologist completed a blind analysis on each anterior capsule and semi-quantitative scores were assigned for biological characteristics of interest (adhesion, fibrosis, cellularity, inflammation, synovial proliferation and vascularity) using the same evaluation criteria used previously10. Briefly, adhesion, fibrosis and synovial proliferation were scored as either present or absent, cellularity was scored as minimal, mild, moderate or marked and inflammation was scored as none, mild, moderate or marked. Vascularity was scored as < 6 vessels, 6–10 vessels and > 10 vessels per field at 40× magnification. Capsular thickness was also measured on each section and reported semi-quantitatively to account for variation in absolute capsule thickness values for sections cut at different depths and varying angular orientations. Specifically, numerical scores were averaged across each group, normalized by the thickness of control capsules, and converted to a symbolic grading scheme for comparison between groups: < 0 μm (− −), 0–150 μm (−), 151–300 μm (+), 301–450 μm (+ +), 451–600 μm (+ + +) and > 601 μm (+ + + +).
Statistical Analysis
One-way ANOVA tests were used to compare mechanical test parameters between (1) control and injured limbs for each group and (2) control and CL limbs for each group. ANOVAs were run separately since data for injured and CL limbs are not independent and because this enabled comparison of injured only and CL only groups. When there was significance, post-hoc Bonferroni analyses compared each experimental group to control. We also evaluated side-to-side limb differences with paired t-tests to compare injured limbs to CL limbs for each group. Correlations between mechanical testing measurements including data from all groups (i.e., Sham, Injury I, Injury II, control and CL limbs) were completed and r values reported. All statistical analysis was performed in GraphPad Prism (GraphPad Software Inc., La Jolla, CA) and significance was defined as p < 0.05.
RESULTS
Mechanical Testing
Qualitatively, group-averaged mechanical test data show differences in the overall loading profiles for F-E after free mobilization (FM) when comparing each injured group to its respective uninjured CL group and control (Fig. 1B). Average curves for injured limbs (i.e., dashed lines in Fig. 1B) have decreased total ROM, NZ length and maximum extension as well as an increased NZ stiffness (Fig. 1B inset) and ROM midpoint in comparison to uninjured CL and control (i.e., solid lines), thereby illustrating qualitatively that joint mechanics remain altered even following FM. Among the different injury groups, Injury II exhibited the most apparent qualitative differences compared to CL and control.
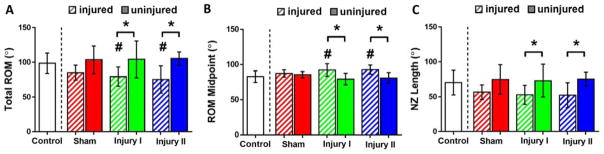
Quantitatively, Injury I and Injury II demonstrated significantly smaller total ROM values than control (p = 0.032, p = 0.007, respectively) and their CL limb (p = 0.016, p = 0.011, respectively) (Table 1, Fig. 2A). Injury I and Injury II had a significantly greater ROM midpoint values than control (p = 0.031, p = 0.020, respectively) and their CL limb (p = 0.020, p = 0.001, respectively) (Table 1, Fig. 2B). Injury I and Injury II had a significantly smaller NZ length than their CL limb (p = 0.024, p = 0.006, respectively) (Table 1, Fig. 2C). Importantly, total ROM, ROM midpoint and NZ length exhibited the most drastic impairment for the most severe injury (Injury II) while the least severe injury (Sham) did not exhibit any statistically significant differences compared to control and their CL limb.
Table 1.
Quantitative results for flexion-extension joint mechanics: total range of motion, range of motion midpoint and neutral zone length values (average ± standard deviation; *different from control; bolded values = different from contralateral limb; p < 0.05).
| Total ROM1 (°) | ROM Midpoint (°) | NZ2 Length (°) | |
|---|---|---|---|
| Control (n = 9) | 98.7 ± 14.6 | 82.9 ± 8.2 | 70.3 ± 17.6 |
| Sham (n = 7) | 85.2 ± 10.7 | 87.4 ± 5.2 | 56.7 ± 10.4 |
| Sham CL (n = 7) | 103.6 ± 19.9 | 85.6 ± 4.4 | 74.9 ± 21.0 |
| Injury I (n = 10) | 79.4 ± 13.9* | 92.3 ± 8.9* | 52.9 ± 13.6 |
| Injury I CL (n = 9) | 104.4 ± 26.5 | 79.4 ± 8.3 | 73.2 ± 23.4 |
| Injury II (n = 10) | 75.3 ± 19.7* | 92.9 ± 6.4* | 52.3 ± 17.8 |
| Injury II CL(n = 9) | 105.4 ± 9.5 | 80.9 ± 7.9 | 75.3 ± 10.2 |
ROM = Range of Motion
NZ = Neutral Zone
Figure 2.
Quantitative results from flexion-extension joint mechanics: (A) total range of motion, (B) range of motion midpoint and (C) neutral zone length were significantly different for Injury I and Injury II. (average ± standard deviation; diagonally shaded bars = injured; solid bars = contralateral; # = different from control, * = different from contralateral limb; p < 0.05).
Maximum extension values for Injury I and Injury II were significantly different from control (p < 0.0002, p < 0.0001, respectively) and all three experimental groups (Sham, Injury I and Injury II) were significantly different from their CL limb (p = 0.046, p < 0.0001, p = 0.0002, respectively) (Table 2). There were no significant differences for any group in maximum flexion and extension stiffness. For flexion stiffness, only Sham was significantly different from its CL limb (p = 0.027).
Table 2.
Quantitative results for flexion-extension joint mechanics: range of motion limits and stiffness values (average ± standard deviation; *different from control; bolded values = different from contralateral limb; p < 0.05).
| Maximum Limits (°) | Stiffness (N-mm/°) | |||
|---|---|---|---|---|
|
|
|
|||
| Extension | Flexion | Extension | Flexion | |
|
|
|
|||
| Control (n = 9) | 33.6 ± 7.4 | 132.2 ± 13.6 | 0.70 ± 0.47 | 0.91 ± 0.38 |
| Sham (n = 7) | 44.8 ± 4.1 | 130.0 ± 9.7 | 0.70 ± 0.30 | 0.83 ± 0.30 |
| Sham CL (n = 7) | 33.8 ± 9.3 | 137.4 ± 12.2 | 0.85 ± 0.45 | 0.78 ± 0.19 |
| Injury I (n = 10) | 52.5 ± 10.2* | 132.0 ± 12.4 | 0.82 ± 0.41 | 0.77 ± 0.17 |
| Injury I CL (n = 9) | 27.2 ± 7.0 | 131.6 ± 20.9 | 0.80 ± 0.21 | 0.72 ± 0.27 |
| Injury II (n = 10) | 55.2 ± 11.6* | 130.5 ± 11.9 | 0.68 ± 0.24 | 0.76 ± 0.13 |
| Injury II CL (n = 9) | 28.2 ± 5.31 | 133.6 ± 11.9 | 0.71 ± 0.24 | 0.71 ± 0.26 |
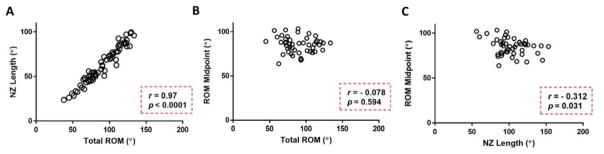
Total ROM and NZ length values exhibited a statistically significant and strong correlation (p < 0.0001, r = 0.970) (Fig. 3A). Total ROM and ROM midpoint values were not significantly correlated (p = 0.594, r = −0.078) (Fig. 3B). NZ Length and ROM midpoint values showed a significant yet moderate correlation (p = 0.031, r = −0.312) (Fig. 3C).
Figure 3.
Correlations included data from all groups (i.e., Sham, Injury I, Injury II, control and CL limbs) and were evaluated for relationships between (A) total range of motion and neutral zone length, (B) total range of motion and range of motion midpoint and (C) neutral zone length and range of motion midpoint for flexion-extension free mobilization (p < 0.05).
Histological Analysis
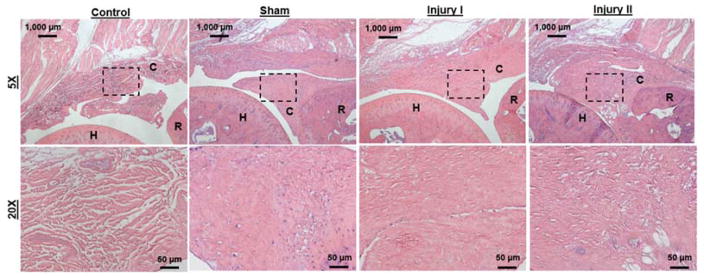
Altered biological properties in the anterior capsule persisted in injured limbs following FM in comparison to control and CL (Table 3, Fig. 4). Specifically, injured limbs showed increased adhesion to osseous surfaces, evidence of fibrosis and thicker capsule/scar tissue compared to CL and control limbs. No groups showed evidence of inflammation, synovial proliferation or increased vascularity. The only difference between control and CL limbs in all of the metrics assessed occurred in Sham CL thickness, which was smaller than control. The largest difference between injured and uninjured limbs occurred in capsule thickness.
Table 3.
Histological evaluation of the anterior capsule showed altered tissue properties for free mobilization limbs compared to control and contralateral joints (n = 3/group).
| Adhesion | Fibrosis | Cellularity | Inflammation | Synovial Proliferation | Thickness | Vascularity | |
|---|---|---|---|---|---|---|---|
| Control | − | − | + | − | − | − | + |
| Sham | + | + | + | − | − | + + | + |
| Sham CL | − | − | + | − | − | − − | + |
| Injury I | + | + | + | − | − | + + + + | + |
| Injury I CL | − | − | + | − | − | − | + |
| Injury II | + | + | + | − | − | + + + | + |
| Injury II CL | − | − | + | − | − | − | + |
Figure 4.
Representative sagittal histological sections (H&E stain) of control and injured elbow joints. Low magnification images (5x) demonstrate joint anatomy and general morphological characteristics of sections from (A) control, (B) sham, (C) Injury I and (D) Injury II joints for free mobilization (C = capsule; H = humerus; R = radius; scale bar = 1000 μm). Higher magnification images (20x, correspond to dashed boxes in A, B, C and D) show anterior capsule in (E) control, (F) sham, (G) Injury I and (H) Injury II joints (scale bar = 50 μm).
DISCUSSION
This study shows that our rat elbow model of PTJC exhibits impaired joint function that persists even after joint free mobilization (FM). Flexion-extension biomechanical joint testing demonstrated a decreased total ROM and NZ length and an increased ROM midpoint for injured limbs compared to uninjured control and CL limbs after FM. Compared to our previous data for limbs at the end of the immobilization period10, the immobilized-remobilized limbs exhibited ~11%, ~8% and ~20% increases in total ROM for Sham, Injury I and Injury II, respectively. However, after FM total ROM values were still ~16%, ~22% and ~26% decreased compared to control and CL limbs for Sham, Injury I and Injury II, respectively. Thus, while injured limbs did regain some motion after FM, the ROM lost following immobilization was never fully regained for any group. Biomechanical parameters of contracture (total ROM, ROM midpoint and NZ length) were slightly altered for sham limbs (Fig. 1B and Fig. 2), demonstrating some impact due to immobilization without joint injury. However, these parameter values for the sham group were not significantly different from either control or CL limbs (Fig. 2) demonstrating that soft-tissue injury combined with immobilization is necessary to develop more consistent and persistent joint contracture long-term (i.e., Injury I and Injury II). The severity of the induced injury correlated with the amount of total motion loss, with injury II animals exhibiting the most dramatic differences compared to control and CL limbs. Therefore, the injury and immobilization protocol developed in our previous study not only altered joint mechanics immediately following an immobilization period but induced changes that remained long-term, which is similar to persistent symptoms common to human patients with elbow PTJC1,8,10,12,16.
To our knowledge, there are no previous animal models of elbow injury. Previous work has focused on understanding PTJC in rabbit or rat knees and then extrapolated results to the elbow joint6,11,17. Hildebrand and colleagues developed a rabbit knee model of PTJC by surgically removing 5mm2 cortical windows from non-articulating surfaces of the medial and lateral femoral condyles and then immobilizing the joint in a flexed position for eight weeks using internal fixation6. These rabbit knees lost ~25–30° range of motion when loaded in extension5,6,14. Nesterenko and colleagues reported a similar change in rabbits using a related injury and immobilization protocol, with ~20° loss of extension17. These studies developed contracture using a more severe injury in order to initiate joint contractures that remained after FM. However, we have shown that joint motion loss can persist using a less severe injury that mimics soft tissue damage that occurs in elbow dislocation and a clinically relevant immobilization protocol in our rat model, which is specific to the complex elbow joint.
Compared to these previous studies, our current work found similar biomechanical results following FM of the injured joint. After FM, there was an average of 20° decrease in total ROM compared to control and CL limbs (Fig. 2A). While there was improvement with FM compared to injury-immobilization only, no group regained all motion demonstrating that contracture persists long-term in F-E. A similar trend was found when measuring NZ length (Fig. 2C). A deficiency in NZ length is clinically relevant because it represents functional range of motion and corresponds to the amount of motion possible before a larger force is required for further joint movement. Total ROM and NZ length also showed a significant and strong, positive correlation (Fig. 3A) demonstrating a direct relationship between these two parameters.
ROM midpoint values also showed altered joint function by the increase in the relative shift of the overall curve for injured groups in comparison to control and CL limbs (Fig. 2B). The increase in ROM midpoint demonstrates a shift of joint motion towards more flexion; however, with no significant changes in maximum flexion, the shift in ROM midpoint most strongly corresponds to a change in maximum extension (Table 2, Fig. 1B). In our injury model, the clinically motivated surgical injury includes an anterior capsulotomy and/or a lateral collateral ligament transection. Since the posterior capsule is not injured during surgical intervention, only maximum extension (and not maximum flexion) would be expected to be altered as shown in Figure 1B and Table 2.
The correlation of total ROM and NZ length with ROM midpoint both have a weak to moderate r value thus exhibiting the lack of a strong relationship between these parameters (Fig. 3B, 3C). This demonstrates that a shift in ROM midpoint does not reflect a similar change in either total ROM or NZ length. Ultimately, this shows that the overall shift in joint motion can correspond to a change in the maximum limits without causing a similar change in total ROM or NZ length, yet still be indicative of joint motion loss.
Histologically, the injury and immobilization protocol developed previously10 induces changes in the capsule tissue that remain following FM. The persistent alterations in the capsule are similar to what has been reported for human patients. Specifically, previous studies of human tissues have reported thickened capsular tissue1,2,15 and evidence of capsular fibrosis13, which is consistent with our findings (Table 3, Fig. 4). In addition, human data also exhibits limited neovascularization and synovial proliferation, similar to our observations3. Surprisingly, similar histological results were observed for all three injury groups (Sham, Injury I and Injury II), which was unexpected since Injury I and Injury II involve direct disruption of the anterior capsule during surgery. It was also surprising that increased capsule thickness and decreased ROM did not correspond to increased stiffness in the injured limbs. However, there may be other features of the capsule we did not evaluate histologically (e.g., different collagen type and collagen organization) that could result in the decreased joint mechanics in Injury I and Injury II but not Sham as well as explain why the stiffness was not altered. We also focused our histological analysis on the anterior capsule, but other periarticular joint tissues could also be affected and potentially contribute to the altered joint mechanics of Injury I and Injury II. A more in depth biological evaluation is needed to identify distinctions between these three different groups. However, the persistent histological changes following FM demonstrate the clinical relevance of this model to develop contracture long-term within the elbow.
This study is not without limitations. First, rats are quadruped animals and thus they use their upper extremities in different ways than humans (i.e., locomotion) and bear different loads than humans. However, this species/breed of rat was carefully selected to match key similarities to humans (i.e., range/types of motion, similarity in joint articulations) to maximize clinical relevance of this animal model10. Second, all mechanical testing was completed post-mortem. Post-mortem testing only divulges information about passive mechanical properties of tissue. Ongoing work is investigating the properties of active tissue testing to evaluate physiological and mechanical changes in muscle strength. Third, only one FM time point (i.e., six-weeks) was evaluated following the injury and immobilization protocol. However, Evans and colleagues showed that after 45 days (6.3 weeks) of immobilization in the rat knee, there were no additional differences in the range of motion that was regained in the joint after 35 days (5 weeks) of FM4. Since no additional improvements occurred in the rat knee after five-weeks of FM, our six-weeks of FM is a reasonable time point for evaluation. Future research could examine other time points of FM to evaluate the persistence of PTJC symptoms in the rat elbow.
CONCLUSION
In conclusion, while no active therapy was used in this study to mobilize the joint following injury and immobilization, free cage activity alone was not enough to restore joint motion demonstrating that our animal model of post-traumatic elbow contracture exhibits significant contracture that persists after free mobilization. Biomechanical quantification of F-E motion exhibited a decreased total ROM and NZ length and increased ROM midpoint, which are all clinically relevant measurements of joint function. Future investigations will use this animal model to evaluate pronation-supination joint motion and utilize genetic/biochemical assays to identify biologic changes within the elbow that contribute to contracture.
Acknowledgments
Funding: American Shoulder and Elbow Surgeons for research funding and the NIH for a training fellowship (NIBIB T32 EB018266) to C.L.D. and research funding (NIAMS R03 AR067504).
The authors thank the American Shoulder and Elbow Surgeons for research funding, the NIH for a training fellowship (NIBIB T32 EB018266) to C.L.D. and research funding (NIAMS R03 AR067504) and the Washington University Musculoskeletal Research Center (NIH P30 AR057235) for histology processing.
ABBREVIATIONS
- PTJC
Post-traumatic joint contracture
- F-E
Flexion-Extension
- FM
Free mobilization
- ROM
Range of Motion
- NZ
Neutral Zone
Footnotes
Illustrations: Figures should be published in color online only.
Disclaimer: Chelsey L. Dunham, None; Ryan M. Castile, None; Necat Havlioglu, None; Aaron M. Chamberlain, None; Leesa M. Galatz, None; Spencer P. Lake, None.
LEVEL OF EVIDENCE: Basic science study
References
- 1.Charalambous CP, Morrey BF. Posttraumatic elbow stiffness. J Bone Joint Surg Am. 2012;94:1428–1437. doi: 10.2106/jbjs.k.00711. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Schimmel DR, Masuda K, Hasting H, Muehleman C. Structural and biochemical evaluation of the elbow capsule after trauma. J Shoulder Elbow Surg. 2007;16:484–490. doi: 10.1016/j.jse.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doornberg JN, Bosse T, Cohen MS, Jupiter JB, Ring D, Kloen P. Temporary presence of myofibroblasts in human elbow capsule after trauma. J Bone Joint Surg Am. 2014;96:e36. doi: 10.2106/jbjs.m.00388. [DOI] [PubMed] [Google Scholar]
- 4.Evans EB, Eggers GWN, Butler JK, Blumel J. Experimental immobilization and remobilization of rat knee joints. J Bone Joint Surg Am. 1960;42:737–758. [Google Scholar]
- 5.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Hildebrand KA, Holmberg M, Shrive N. A new method to measure post-traumatic joint contractures in the rabbit knee. J Biomech Eng. 2003;125:887–892. doi: 10.1115/1.1634285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawa A, Jupiter J, Ring D. Operative Elbow Surgery. New York: Churchill Livingston; 2012. Pathogenesis and classification of elbow stiffness Elsevier; pp. 409–16. [Google Scholar]
- 8.Keener JD, Galatz LM. Arthroscopic management of the stiff elbow. J Am Acad Orthop Surg. 2011;19:265–274. doi: 10.5435/00124635-201105000-0004. [DOI] [PubMed] [Google Scholar]
- 9.King GJ, Morrey BF, An KN. Stabilizers of the elbow. J Shoulder Elbow Surg. 1993;2:165–174. doi: 10.1016/S1058-2746(09)80053-0. [DOI] [PubMed] [Google Scholar]
- 10.Lake SP, Castile RM, Borinsky S, Dunham CL, Havlioglu N, Galatz LM. Development and use of an animal model to study post-traumatic stiffness and contracture of the elbow. J Orthop Res. 2016;34:354–364. doi: 10.1002/jor.22981. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Liu S, Fan C. Lentivirus-mediated ERK2 siRNA reduces joint capsule fibrosis in a rat model of post-traumatic joint contracture. Int J Mol Sci. 2013;14:20833–20844. doi: 10.3390/ijms141020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindenhovius ALC, Jupiter JB. The posttraumatic stiff elbow: a review of the literature. J Hand Surg Am. 2007;32:1605–1623. doi: 10.1016/j.jhsa.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Ling SK, Lui TH, Faan YS, Lui PW, Ngai WK. Post-traumatic elbow rotational stiffness. Shoulder Elbow. 2014;6:119–123. doi: 10.1177/1758573214524935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monument MJ, Hart DA, Befus AD, Salo PT, Zhang M, Hildebrand KA. The mast cell stabilizer ketotifen fumarate lessens contracture severity and myofibroblast hyperplasia: a study of a rabbit model of posttraumatic joint contractures. J Bone Joint Surg Am. 2010;92:1468–1477. doi: 10.2106/jbjs.1.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monument MJ, Hart DA, Salo PT, Befus D, Hildebrand KA. Posttraumatic elbow contractures: targeting neuroinflammatory fibrogenic mechanisms. J Orthop Sci. 2013;18:869–877. doi: 10.1007/s00776-013-0447-5. [DOI] [PubMed] [Google Scholar]
- 16.Myden C, Hildebrand KA. Elbow joint contracture after traumatic injury. J Shoulder Elbow Surg. 2011;20:39–44. doi: 10.1016/j.jse.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Nesterenko S, Morrey ME, Abdel MP, An KN, Steinmann SP, Morrey BF, et al. New rabbit knee model of posttraumatic joint contracture: indirect capsular damage induces a severe contracture. J Orthop Res. 2009;27:1028–1032. doi: 10.1002/jor.20845. [DOI] [PubMed] [Google Scholar]
- 18.Safran MR, Baillargeon D. Soft-tissue stabilizers of the elbow. J Shoulder Elbow Surg. 2005;14:S179–S185. doi: 10.1016/j/jse.2004.09.032. [DOI] [PubMed] [Google Scholar]