Abstract
Anti–PD-1 therapy has improved clinical outcomes in advanced melanoma, but most patients experience intrinsic resistance. Responding patients can develop acquired resistance to anti–PD-1. We retrospectively reviewed 488 patients treated with anti–PD-1 from three academic centers and identified 36 patients with acquired resistance, defined as disease progression following objective response. The incidence, timing, disease sites, post-progression survival (PPS), and outcomes were evaluated descriptively. The acquired-resistance cohort consisted of 67% with more than 1 feature of poor prognosis (stage M1c, elevated LDH, or brain metastasis), and 67% had previously received ipilimumab. Partial and complete responses were achieved in 89% (n = 32) and 11% (n = 4) of patients, respectively, and median time to resistance (progression-free survival; PFS) was 11.1 months (range 4.3–32.8 months). Most progression was isolated (78% of patients, n = 28) and occurred while receiving therapy (78%, n = 28). The median PPS was 12.8 months (range 0.1–51.8 months), and the median overall survival was 33.7 months. Among isolated progressors, 15 received localized therapy (12 with surgery, 3 with radiation). Patients with isolated vs. systemic progression exhibited a trend for improved PPS (P = 0.081), and patients with an initial PFS ≥ 15 months showed significant PPS improvement (P = 0.036). Two patients experienced subsequent responses to anti–PD-1 resumption. In conclusion, acquired resistance to anti–PD-1 was frequently associated with excellent clinical outcomes and often presented as isolated progression amenable to localized therapy (surgery or radiation) or systemic progression sensitive to therapy resumption.
Keywords: PD-1, nivolumab, pembrolizumab, melanoma, acquired resistance
Introduction
Agents blocking the interaction between the checkpoint inhibitor programmed cell death-1 (PD-1) and its ligand (PD-L1) have dramatically improved clinical outcomes for patients with advanced melanoma compared to conventional therapies. Up to 45% of patients receiving anti–PD-1 therapy experience an objective response, and a 34% 5-year survival rate with single-agent anti–PD-1 was reported in a small set of patients (1). Despite these advances, most patients ultimately experience intrinsic (lack of initial response) or acquired (response followed by progression) resistance (2–5). Reliable predictors of resistance have yet to be characterized. However, a number of studies have identified clinical features (elevated lactate dehydrogenase, bulky disease, presence of liver metastases) and molecular correlates (low nonsynonmous mutation load, angiogenesis/wound healing signatures, lack of CD8+ T cell infiltrate, lack of PD-L1 expression by tumor or inflammatory cells, particular oncogenic pathway activation) of intrinsic resistance (6–16).
Although intrinsic resistance has received the most focus, a modest number of patients clearly develop acquired resistance to anti–PD-1. For example, long-term analysis of phase I trial participants with advanced melanoma treated with nivolumab revealed that nearly half (42%) of responders ultimately experienced disease progression at a median of 24 months (17). Overall, this represented approximately 13% of patients in this clinical trial cohort. Likewise, the phase I study of pembrolizumab revealed a similar percentage (8.1% of total cohort, 24% of responders) of patients who progressed after response.(18) Acquired resistance is a familiar limitation of targeted therapy (e.g., inhibitors of BRAF or EGFR) for various cancers, but this concept is less well explored for immune therapies, including anti–PD-1 antibodies. Molecular characterization of acquired anti–PD-1 resistance has been performed on a limited number of samples, and the clinical features of resistance are unexplored.(6, 19)
We sought to characterize the incidence, timing, and clinical features of acquired resistance to anti–PD-1 therapy in advanced melanoma. We also aimed to assess the post-progression outcomes in this population. To accomplish this, we reviewed the clinical courses of patients at five large academic institutions who received single-agent anti–PD-1 and achieved an objective response with subsequent progression of disease.
Methods
Patients
Following approval from each site’s institutional review board (IRB), we screened all patients who received single-agent anti–PD-1 (nivolumab or pembrolizumab) at each participating center (n = 488). From these, we identified patients who experienced response followed by progression (n = 36). These centers included Dana Farber Cancer Institute (n = 9), Moffitt Cancer Center (n = 12), and Vanderbilt University Medical Center (n = 15). As the data was retrospective, waiver of consent was obtained at all sites. We included patients with unresectable or metastatic melanoma who had received at least one dose of anti–PD-1 therapy and achieved either partial or complete response as measured by Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 criteria.(20) Patients treated with combination nivolumab and ipilimumab, or other anti–PD-1-based combinations were not included. Patients were included only if they experienced RECIST-defined disease progression of disease following their initial response.
Study Design
We obtained baseline demographic data for each patient including age, gender, American Joint Committee on Cancer (AJCC, 7th ed. 2010) pathologic stage, performance status defined by the Eastern Cooperative Oncology Group (ECOG), and serum lactate dehydrogenase level (LDH). Additional information regarding prior treatments and responses were also recorded. To assess the efficacy of initial anti–PD-1 therapy, we evaluated the objective response based on RECIST v1.1 criteria, progression free survival (PFS), and overall survival (OS) of patients with acquired resistance. To explore whether increasing PFS would correlate with improved survival after progression, we assessed a range of PFS cut-off points (6, 9, 12, and 15 months) (Supplementary Fig. S1). Finally, to characterize post-progression clinical outcomes, we collected data following disease progression including sites of progression, subsequent treatments, treatment responses, and survival. Isolated disease progression was defined as progression in one organ, whereas systemic progression involved >1 organ sytem. We considered “new” lesions at progression as tumors not present at treatment initiation (even if in the same organ as a pre-existing lesion), whereas “existing” lesions were those that responded then progressed.
Statistical Analysis
OS and PFS were calculated based on the Kaplan-Meier method. PFS was defined as time from the start of treatment until disease progression. Post-progression survival was defined as time from disease progression until death for any reason. OS was defined as time from the start of treatment until death for any reason. Patients were censored at their last follow-up. Survival was compared between groups using the logrank test. Continuous and categorical variables were described using means and percentages, respectively. Analysis were performed using the statistical software R version 3.3.0 and GraphPad Prism 7.
Results
Baseline patient characteristics
From a total of 488 patients screened from 3 centers, 166 (34%) responded initially to anti–PD-1 therapy and 36 (7.4%) developed acquired resistance. There was a 21.7% incidence of acquired resistance among responders to anti–PD-1 therapy (21 received pembrolizumab alone, 14 received nivolumab alone, and 1 received nivolumab and vaccine). Patient baseline characteristics are described in Table 1. All subsequent percentages refer to the 36 patients in the acquired resistance cohort unless specified. Of these, 67% were male (n = 24) and ages ranged from 31 to 88 with a median of 62. Most patients had melanoma with a cutaneous primary (89%, n = 32). Most patients who acquired resistance had received previous treatment (89%, n = 32), including 24 patients with prior ipilimumab therapy. Twenty-six patients (67%) had at least one poor prognostic feature including brain metastasis, elevated LDH or stage IV M1c disease. Of the patients with evaluable mutational status, 26% had a BRAF mutation and 35% had an NRAS mutation. Four (11%) patients with acquired resistance achieved a CR, while the remainder experienced PR (Fig. 1). The median follow-up for the cohort was 33.1 months [range 6.1–64.6 months]. The Supplementary Fig. S2 provides a radiographic and clinical example of one individual patient who had an initial response with an isolated site of disease progression.
Table 1.
Patient Baseline Demographics
| Characteristic | No. (%) (n = 36) |
|---|---|
| Age, Median (range) | 62 (31 – 88) |
| Sex | |
| Male | 24 (66.7) |
| Female | 12 (33.3) |
| Primary Site | |
| Skin | 33 (88.9) |
| Acral | 2 (5.6) |
| Mucosal | 1 (2.8) |
| Unknown | 1 (2.8) |
| Stage | |
| IV M1a/b | 12 (33.3) |
| IV M1c | 24 (66.7) |
| Brain metastases | 9 (25.0) |
| Elevated LDH | 9 (25.0) |
| Mutational status* | |
| BRAF | 8 (25.8) |
| NRAS | 7 (35) |
| Prior treatment | |
| BRAF and/or MEK inhibitor | 4 (11.1) |
| CTLA-4 inhibitor | 24 (66.7) |
| None | 4 (11.4) |
| Type of response | |
| PR | 32 (88.9) |
| CR | 4 (11.1) |
Percentage is of evaluable patients
Figure 1.
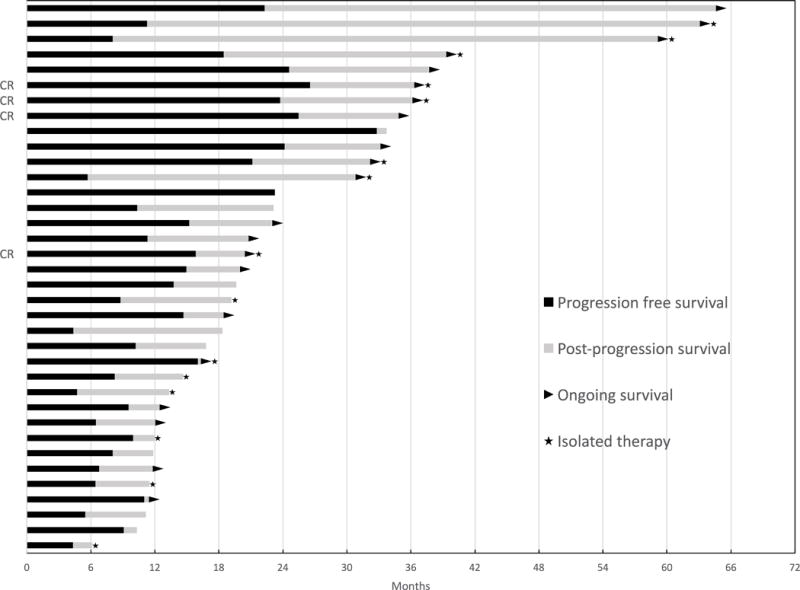
Swimmer’s plot for progression free and post-progression survival of the patients with acquired resistance. CR indicates complete response.
Characteristics at progression
The median PFS (i.e. time to acquired resistance) was 11.1 months (range 4.3 – 32.8 months) (Fig. 2A). Patient progression characteristics are described in Table 2. The majority of patients had isolated (single organ) sites of progression (78%, n = 28), usually with visceral involvement (n = 18, 50%), including 4 patients with brain metastases. Other sites of progression were either in lymph nodes (n = 8, 22%) or as soft tissue sites (n = 2, 6%). Overall, there was a slight preponderance of progression at new site(s) of disease only (n = 19, 53%); 14 patients in the acquired resistance cohort (39%) experienced progression at existing site(s) only and 3 patients (8%) had disease progression at both new and existing sites. Most patients were receiving therapy at the time of progression, although 8 (21%) had discontinued therapy for at least 3 months prior to progression.
Figure 2.
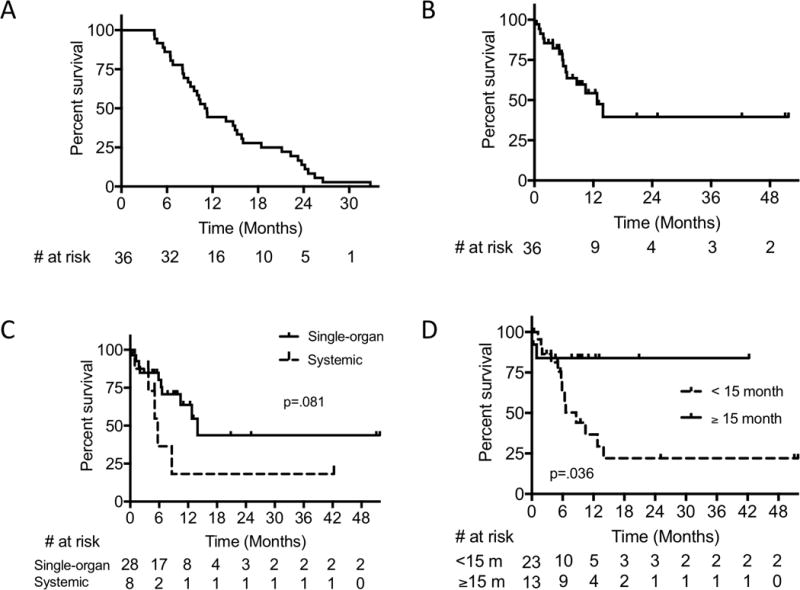
(A) Progression-free survival, (B) Post-progression survival (PPS) in the acquired resistance cohort, (C) PPS in single-organ versus systemic progression, and (D) PPS cut-off at 15 months.
Table 2.
Progression Characteristics
| Characteristic | No. (%) (n = 36) |
|---|---|
| Type of Progression | |
| Systemic | 8 (22.2) |
| Localized | 28 (77.8) |
| Site of Progression | |
| New only | 19 (52.8) |
| Existing only | 14 (38.9) |
| Both | 3 (8.3) |
| Initial Treatment at Progression | |
| Surgery | 12 (33.3) |
| Radiation | 3 (8.3) |
| Systemic | 15 (41.7) |
| None/Hospice | 5 (13.9) |
| Undecided | 1 (2.8) |
| Type of Systemic Therapy after Progression | |
| PD-1 | 11 (30.6) |
| Continued | 8 (22.2) |
| Switched | 3 (8.3) |
| CTLA-4 | 3 (8.3) |
| Combination PD-1/CTLA-4 | 1 (2.8) |
| BRAF and/or MEK inhibitor | 5 (13.9) |
| Clinical Trial* | 4 (11.1) |
| Chemotherapy | 1 (2.8) |
| Alive | |
| Yes | 21 (58.3) |
| No | 15 (41.7) |
Included trials (2) with ERK inhibitor and antibody-drug conjugate
Post-progression treatments
Following progression in the acquired resistance cohort, 15 patients (42%) received systemic treatment initially, and another 15 (42%) received localized treatments for isolated sites of disease progression (surgery in 12, radiation in 3). Of those that received post-progression systemic therapy (n = 22) either immediately following progression or following initial surgery or radiation, 11 patients received anti–PD-1 therapy with 8 patients continuing the same anti–PD-1 agent and 3 patients switching to another anti–PD-1 drug (e.g., pembrolizumab to nivolumab or vice versa). Other types of systemic therapy including BRAF and/or MEK inhibitors (n = 5), ipilimumab (n = 4), clinical trials (n = 4), or chemotherapy (n = 1).
Patient outcomes
The median overall survival in the acquired resistance cohort was 33.7 months (Supplementary Fig. S3) with a median post-progression survival (PPS) after disease progression of 12.8 months (Fig. 2B). When comparing the median PPS of patients with isolated (single-organ) vs. systemic progression, patients with isolated progression trended toward improved overall survival (median PPS 14.0 months vs 5.7 months, P = 0.081) (Fig. 2C). Patients with initial PFSs of ≥ 15 months had significantly improved PPS as compared to those with a PFS < 15 months (not reached vs 8.6 months, P = 0.036) (Fig. 2D). Similar trends existed for other PFS cutoffs (Supplementary Fig. S1). The PPSs of patients with BRAF or NRAS mutation were not different from respective wild-type cohorts.
Eight patients underwent surgery for an isolated site of disease progression with curative intent (goal of “surgical CR”). Of these, 7 have not had any further progression, and only one patient has died, with a median follow up of 36.2 months. Four other patients had surgery for symptomatic disease progression in the presence of systemic or other disseminated disease progression, and all of these patients have died. Regarding systemic therapy, 3 patients received ipilimumab with evaluable responses, including 1 with a subsequent partial response (below). One patient experienced an excellent partial response to dabrafenib and trametinib, one had primary disease progression on dabrafenib, and 3 patients progressed on trametinib (administered in the setting of NRAS or non-V600E/K BRAF mutations).
A major clinical question currently is the timing of anti–PD-1 cessation, and whether progression following cessation would remain sensitive to therapy. Although most patients progressed while still receiving anti–PD-1, 8 experienced disease progression following cessation of therapy (because of patient preference, completion of clinical trials, or toxicity) for at least 3 months. Of these, two patients experienced a subsequent response after resumption of anti–PD-1 therapy, two experienced further progressive disease following resumption of anti–PD-1 therapy, and three had surgery or radiation to isolated sites of progression without further recurrence or treatment (4.6 – 12.4 months later). The final patient had an excellent response to ipilimumab that has persisted > 3 years.
Four patients (11%) also experienced isolated disease progression in the brain. Of these, one underwent radiation followed by resumption of pembrolizumab (no evaluable response yet), two had surgery for a symptomatic brain metastasis (both ultimately died within 6 months later), and one was transitioned to hospice care.
Discussion
Anti–PD-1 agents have clearly transformed treatment paradigms for numerous malignancies, producing durable responses in a sizable fraction of patients. These agents, however, are encumbered both by intrinsic and acquired resistance. With the increasing use of anti–PD-1 agents, characterizing resistance at a clinical and molecular level is particularly critical. In this study, we observed that acquired resistance in advanced melanoma occured in approximately 22% of patients who respond to anti–PD-1 therapy with a median onset of 11 months after initiation of anti–PD-1 therapy. Therefore, clinical characterization of this defined population provides important prognostic information for patients who progress after initial anti–PD-1 response. Additionally, further understanding of post-progression outcomes improves our ability to make appropriate therapeutic choices.
Importantly, we observed that many patients experienced excellent post-progression outcomes. Patients who had isolated disease progression (78%) had improved survival compared to those with systemic progression. Many of these patients received localized therapies (42%) with either surgery or radiation. Thus, there appears to be a frequent clinical pattern of patients with isolated progression that may still experience durable benefit with localized therapy or anti–PD-1 resumption following progression.
At a molecular level, acquired anti–PD-1 resistance could reflect changes in the tumor (loss of responsive tumor antigens or loss of IFNγ/MHC signaling), tumor microenvironment (myeloid-derived suppressor cells, tumor associated macrophages, Tregs), or PD-1 independent mechanisms of immunosuppression (upregulation of other immune checkpoints).(6, 7, 19, 21–25) Molecular studies have begun to shed light on these mechanisms. In melanoma patients with acquired resistance to pembrolizumab, loss of function mutations in JAK1 and JAK2 mediated resistance through disrupted IFNγ receptor signaling. Another resistant sample in this study demonstrated a defect in the antigen-presenting protein β2-microglobulin (β2M), causing loss of MHC class I heavy chain outer-membrane localization, despite constitutive production, suggesting a defect in antigen presentation (19). Additionally, in preclinical mouse models of non-small cell lung cancer with acquired anti–PD-1 resistance, other immune checkpoints, notably TIM-3, can be upregulated in tumor infiltrating lymphocytes (25). These studies highlight the heterogeneous nature of adaptive resistance, which could potentially reflect the diverse clinical patterns in our study.
Clinical definitions of anti–PD-1 resistance have not been established, and remain an unmet need. For example, resistance to platinum-based chemotherapy has been classified in many tumor types depending on the time of progression (e.g. platinum-refractory vs. platinum-sensitive ovarian cancer), and remain a useful tool for clinical decision-making and trial accrual. Similarly, we speculate that there may be clinical phenotypes of disease progression on anti–PD-1 therapy. Based on our data, one could conjecture that “anti–PD-1 sensitive” could be used to define patients who relapse after treatment discontinuation (either in the metastatic or perhaps in the adjuvant setting), or have isolated relapse amenable to local therapy (with continued systemic disease control). In contrast, patients with systemic disease progression while still receiving therapy may have a more aggressive course and could be termed “anti–PD-1 resistant.” At this time, these concepts are speculative and large prospective studies are needed to establish more refined and biologically relevant classifications of anti–PD-1 resistance.
This study has several limitations. First, the sample size is relatively small but represents the largest retrospective study of acquired resistance in anti–PD-1 therapy. Further, this represents a concerted effort to evaluate patients from multiple large centers who received anti–PD-1 (488 patients). Second, due to the retrospective nature of this study, the protocols for treatment of localized or systemic progression were not standardized, and were largely driven by physician/patient preference. Third, we could not compare our data to detailed demographic information of responders, who did not experience acquired resistance, as it was not feasible to collect all these data. Finally, some patients had relatively short follow-up times. Despite these limitations, our study begins to characterize acquired resistance to these transformative agents and provides a foundation for future clinical investigations with combination immunotherapy and in other tumor types.
As the use of anti–PD-1 therapy becomes more prevalent, acquired resistance will become a challenging and more common clinical dilemma for oncologists. We observed a predominant clinical pattern of resistance with patients developing isolated disease that is amenable to local therapy or anti–PD-1 resumption with subsequent durable benefit. In the setting of isolated progression, therefore, multidisciplinary management should occur, with strong consideration for surgery or radiation to the single lesion. Further, anti–PD-1 resumption should be considered in patients progressing after cessation of therapy. Ultimately, further studies on larger cohorts will be needed to fully characterize the mechanisms that drive these relapses and their link to clinical outcomes.
Supplementary Material
Acknowledgments
Financial support: This study was supported by National Institutes of Health (NIH) grant K23 CA204726 (Johnson) and Moffitt Cancer Center Skin SPORE 5P50CA168536 (Eroglu).
Financial Disclosures:
Dr. Luke has consulted for Merck, and has received research funding from Bristol-Myers Squibb (BMS) and Merck. Dr. Joseph has consulted for BMS, Castle Biosciences, Cerulean, Eisai, Merck, Nektar, and Novartis, and received research funding from Amgen, BMS, Merck, and Roche. Dr. Ott has consulted for Alexion, Amgen, BMS, Celldex, CytomX, and Genentech, and has received research funding from Armo Biosciences, AstraZeneca/MedImmune, BMS, Celldex, and Merck. Dr. Hodi has consulted for EMF Serono, Genentech, Merck, and Novartis, and received research funding from BMS. Dr. Sosman has consulted for Array, Genentech, Merck, and Novartis. Dr. Johnson has consulted for BMS and Genoptix, and has received research funding from Incyte. Dr. Buchbinder has received research funding from Merck and BMS.
Footnotes
Conflict of Interest:
No other disclosures are reported.
References
- 1.Hodi FS, Kluger H, Sznol M, Carvajal RD, Lawrence D, Atkins MB, et al. Proceedings of the 107th Annual Meeting of the American Association for Cancer Research. Vol. 2016 New Orleans, LA: Philadelphia (PA): AACR; 2016. Apr 16–20, Durable, long-term survival in previously treated patients with advanced melanoma (MEL) who received nivolumab (NIVO) monotherapy in a phase I trial. 2016. [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. The New England journal of medicine. 2012 Jun 28;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013 Jul 11;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015 Jan 22;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015 Jun 25;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 6.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016 Mar 24;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014 Dec 4;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015 Oct 9;350(6257):207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014 Nov 27;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016 Feb;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger S, Gajewski TF. A new paradigm for tumor immune escape: beta-catenin-driven immune exclusion. Journal for immunotherapy of cancer. 2015;3:43. doi: 10.1186/s40425-015-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin D, Garcia-Diaz A, Zaretsky J, Escuin-Ordinas H, Hu-Lieskovan S, Palaskas NJ, et al. Innate resistance of PD-1 blockade through loss of function mutations in JAK resulting in inability to express PD-L1 upon interferon exposure. Journal for immunotherapy of cancer. 2015;3(Suppl 2):311. [Google Scholar]
- 14.Tumeh PC, Rosenblum M, Handley N, Tsai K, Rodriguez RRS, Khurana N, et al. Abstract 2857: Metastatic site and response to pembrolizumab (anti-PD1 antibody) in melanoma. Cancer Research. 2015 Aug 02;75(15 Supplement):2857. 2015. 21:20:47. [Google Scholar]
- 15.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114(3):256–61. doi: 10.1038/bjc.2015.467. 02/02/print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 May 16; doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014 Apr 1;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016 Apr 19;315(15):1600–9. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 19.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996 Jan 17;88(2):100–8. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 Sep 1;21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015 Sep;125(9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015 Jul 9;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 25.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


