Significance
Organosiloxanes are components in a huge variety of consumer products and play a major role in the synthesis of fine chemicals. However, their synthetic manipulation primarily relies on the use of chlorosilanes, which are energy-intensive to produce and environmentally undesirable. Synthetic routes that operate under ambient conditions and circumvent the need for chlorinated feedstocks would therefore offer a more sustainable route for producing this class of compounds. Here, a systematic survey is reported for the silicatein enzyme, which is able to catalyze the hydrolysis, condensation, and exchange of the silicon–oxygen bond in a variety of organosiloxanes under environmentally benign conditions. These results suggest that silicatein is a promising candidate for development of selective and efficient biocatalysts for organosiloxane chemistry.
Keywords: silicatein, biocatalysis, organosilicon, organosiloxane, silyl ether
Abstract
The family of silicatein enzymes from marine sponges (phylum Porifera) is unique in nature for catalyzing the formation of inorganic silica structures, which the organisms incorporate into their skeleton. However, the synthesis of organosiloxanes catalyzed by these enzymes has thus far remained largely unexplored. To investigate the reactivity of these enzymes in relation to this important class of compounds, their catalysis of Si–O bond hydrolysis and condensation was investigated with a range of model organosilanols and silyl ethers. The enzymes’ kinetic parameters were obtained by a high-throughput colorimetric assay based on the hydrolysis of 4-nitrophenyl silyl ethers. These assays showed unambiguous catalysis with kcat/Km values on the order of 2–50 min−1 μM−1. Condensation reactions were also demonstrated by the generation of silyl ethers from their corresponding silanols and alcohols. Notably, when presented with a substrate bearing both aliphatic and aromatic hydroxy groups the enzyme preferentially silylates the latter group, in clear contrast to nonenzymatic silylations. Furthermore, the silicateins are able to catalyze transetherifications, where the silyl group from one silyl ether may be transferred to a recipient alcohol. Despite close sequence homology to the protease cathepsin L, the silicateins seem to exhibit no significant protease or esterase activity when tested against analogous substrates. Overall, these results suggest the silicateins are promising candidates for future elaboration into efficient and selective biocatalysts for organosiloxane chemistry.
The organosiloxanes, compounds containing C–Si–O moieties, represent a class of compounds with a truly diverse range of applications. They are commonly used in the form of polysiloxane “silicone” polymers as components of industrial and consumer products for a variety of purposes such as bulking agents, separation media, protective coatings, lubricants, emulsifiers, and adhesives (1–3). Their use as auxiliaries in the chemical synthesis of complex molecules is also long-established (4–6). However, the production and synthetic manipulation of these compounds are almost entirely dependent on chlorosilane feedstocks, which are environmentally undesirable and energy-intensive to produce (7, 8). Furthermore, organosiloxanes, which are entirely anthropogenic in origin, are now known to be persistent environmental contaminants because little attempt is made to recover and recycle them (3). Synthetic routes that use, and ultimately recycle, siloxanes and silanols as alternatives would in principle be more environmentally sound.
One possible strategy toward improved sustainability is to harness enzymes for chemical processing. Such biocatalysts are attractive because they offer highly efficient synthesis in terms of yields and regio- and stereospecificity, together with an ability to promote reactions under mild conditions and a minimal reliance on halogenated or metallic feedstocks (9, 10). The use of enzymes to manipulate the Si–O bond would therefore potentially offer more sustainable routes to the synthesis of many compounds, as well as for the eventual recycling and reuse of organosiloxanes. Several attempts have been made to use hydrolytic enzymes such as lipases and proteases for the hydrolysis and condensation of the Si–O bond (11–13). Although they clearly demonstrate the feasibility of the general concept, the range of enzymes tested have so far met with only limited success in regard to synthetic yield and substrate scope.
In contrast, poriferans (marine sponges) that use silica as part of their inorganic skeleton use a family of enzymes termed the silicateins to catalyze the polymerization of soluble silicates into silica (14–16). The primary sequences of these enzymes have been reported and they bear a remarkable homology with proteases of the cathepsin family, with ∼65% sequence similarities and ∼50% sequence identities relative to cathepsin L. Both enzymes share a similar Xaa–His–Asn catalytic triad at their active site, although in the silicateins a Ser residue occupies the Xaa position rather than Cys in cathepsin L. Previous reports have shown that silicatein-α (Silα), the prototypical member of this family, can catalyze the hydrolysis of ethoxysilanes such as tetraethoxysilane (TEOS) and triethoxyphenylsilane (17).
Because the silicateins have evolved specifically to manipulate the Si–O bond, these enzymes may offer a better starting point for further elaboration into practical biocatalysts in organosiloxane chemistry. This paper outlines the performance of heterologously produced Silα for both the hydrolysis and condensation of a range of model organosiloxanes. In the process, the development of a colorimetric high-throughput screening method for silyl ether bond hydrolysis based on the 4-nitrophenoxylate chromophore is also reported. Additionally, the protease and esterase activity of Silα against analogous substrates is described.
Results and Discussion
Production and Characterization of Silα.
To acquire this enzyme, a synthetic vector containing cDNA encoding for the mature wild-type Silα from Suberites domencula, fused to an N-terminal hexahistidine tag and codon optimized for expression in Escherichia coli, was used. It is known that the mature form of the protein is highly hydrophobic and difficult to produce in soluble form (16, 18). Thus, in attempts to improve its solubility the gene was also subcloned with the sequences for a number of proteins known to enhance solubility and folding. Sequences encoding for GST, thioredoxin, small ubiquitin-like modifier, maltose binding protein, or trigger factor (TF) were inserted between the hexahistidine tag and Silα and the genes transformed into a variety of E. coli BL21(DE3) strains including Arctic-Express and Origami. Expression trials were then carried out by varying the induction conditions, including the concentration of the induction agent (isopropyl β-d-1-thiogalactopyranoside), incubation temperature, and incubation time.
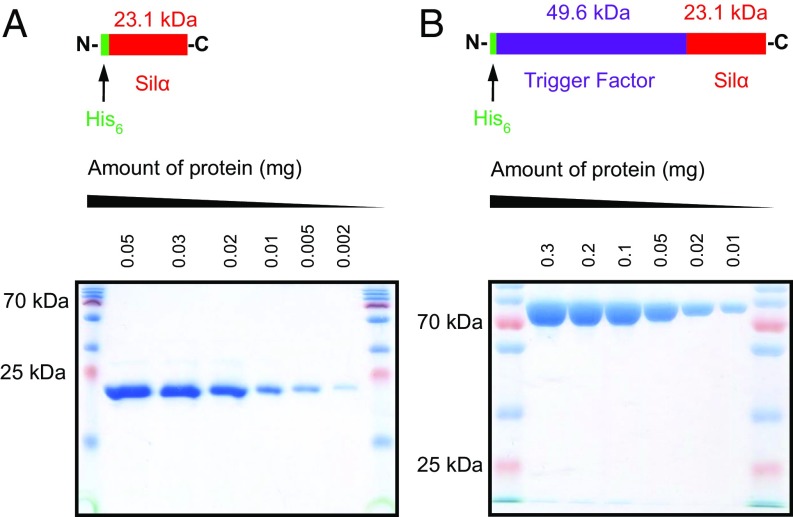
Overall, these optimization experiments showed that all of the candidate proteins expressed well in E. coli but only the TF-Silα fusion gave the protein in soluble form. As expected, the Silα (without any fusion tag) was almost entirely insoluble. However, it was found that addition of the nondenaturing detergents Triton X-100 and CHAPS into the lysis buffer enabled the recovery of sufficient levels of the soluble protein for some further studies. Both the TF-Silα fusion and the solubilized wild-type Silα were then purified to homogeneity (Fig. 1).
Fig. 1.
Images of SDS/PAGE gels for Silα (A) and TF-Silα (B) after purification, over a range of dilutions, demonstrating the homogeneity of the isolated proteins. Also included above each gel image are the schematic representations of the protein constructs.
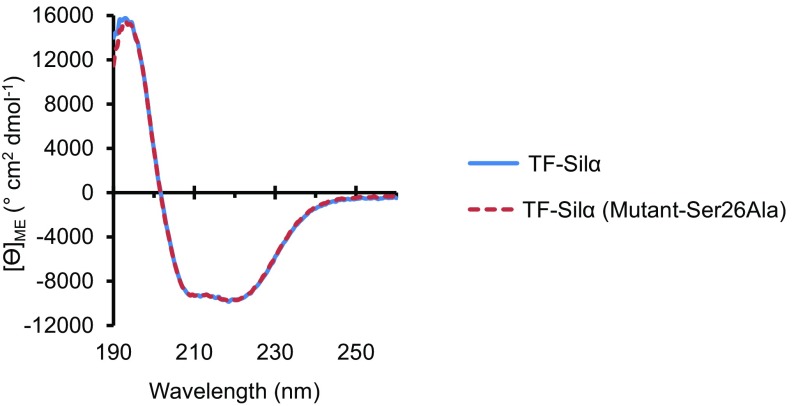
The isolated TF-Silα, and a Ser→Ala mutant at position 26 (Ser26Ala) of this protein produced using the same procedures, were fully soluble and could be handled without any special precautions. Size-exclusion chromatography of both these proteins in isolated form showed a single well-defined species (SI Appendix, Fig. S1A). The CD spectrum of each protein showed clear secondary structural features, indicating the proteins were not denatured or disordered (Fig. 2 and SI Appendix, Fig. S2). The spectra of the mutant and unmodified protein essentially overlap, showing that the mutation did not affect its overall folding. The relative proportions of secondary structures were also calculated from these spectra and were found to be within 4% of the values calculated from combining the crystallographically derived data of a cathepsin-silicatein chimera (18) and TF (SI Appendix, Tables S1–S3).
Fig. 2.
CD spectra plots of molar ellipticity against wavelength for TF-Silα and TF-Silα(Ser26Ala).
In contrast, Silα could be maintained in a homogeneous state only at dilute concentrations (micromolar range), which were insufficient for CD measurements. However, analysis by nondenaturing gel electrophoresis for Silα clearly showed a single band, indicating nonaggregation (SI Appendix, Fig. S1B). The protein advanced through the gel at a similar rate as the 25 kDa reference protein, suggesting that it was of approximately similar size, globular, and monomeric.
Determination of Enzymatic Activity.
To confirm that the two candidate proteins (Silα and TF-Silα) were catalytically competent, their hydrolytic activity against TEOS was tested and the amount of precipitated silica quantified by the previously reported silicomolybdic acid assay (17). The amount of silica produced upon exposure of silicic acid (derived from acid hydrolysis of TEOS) to these enzymes was also measured. Both assays demonstrated that the enzymes were active for both the hydrolysis and subsequent condensation of silicate Si–O bonds (SI Appendix, Fig. S3). In comparison, the heat-denatured enzyme and chymotrypsin (as a representative serine protease) displayed only a small amount of nonspecific activity, likely due to general hydrophobic interactions and nonspecific basic catalysis (19). These results were in agreement with previous reports using Silα derived from the native organisms (15, 17) and tests with trypsin and papain that are known to reject TEOS as a substrate (11, 17).
High-Throughput Colorimetric Assays for Silyl Ether Hydrolysis.
To investigate the scope of these recombinant silicateins in the manipulation of a wider range of siloxanes, it was necessary to develop a new screening methodology for this class of compounds, because the silicomolybdic acid assay is incompatible with organosilanes (compounds with C–Si bonds). For this purpose, a series of 4-nitrophenoxy silyl ethers 1–3 was synthesized for use as model substrates (Scheme 1). Here, it was envisaged that hydrolysis of the Si–O bond would result in the release of the corresponding silanols 4–6 and the strongly absorbing nitrophenoxylate ion, which can be quantified by UV-visible (UV-Vis) spectrophotometry.
Scheme 1.
Hydrolysis of model 4-nitrophenoxy silyl ethers. aNp, 4-nitrophenyl; TBDMS, tert-butyldimethylsilyl; TDS, thexyldimethylsilyl; Thx, thexyl TIPS, tri(iso-propyl)silyl.
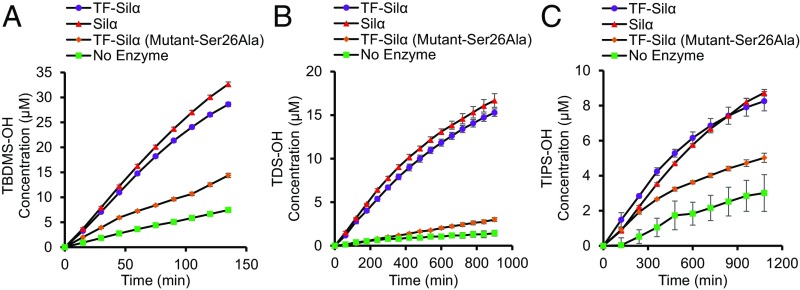
These substrates were used to perform time-course experiments with the two enzyme candidates (Fig. 3 and SI Appendix, Figs. S4 and S5) and the data were further verified by GC-MS analysis. It was further observed that the assay in fact generated a yellow color that is visually observable (SI Appendix, Fig. S6), thus making it fully colorimetric rather than only spectrometric. These assays showed the 4-nitrophenoxy substrates are somewhat susceptible to hydrolysis, and even in the absence of any catalyst an appreciable rate of product formation is detectable. Nevertheless, assays that used the fully competent enzyme always gave enhanced rates of hydrolysis compared with the control experiments or the uncatalyzed reaction.
Fig. 3.
Graphs of the extent of hydrolysis of the 4-nitrophenoxy silyl ether substrates 1 (A), 2 (B), and 3 (C) against time, as measured by UV-Vis absorbance at 405 nm corresponding to the 4-nitrophenoxylate anion (calibrated using data from SI Appendix, Fig. S4). Assays are conducted with 0.00067 mol eq of enzyme relative to 0.1 mM substrate in 5% 1,4-dioxane, 50 mM Tris, and 100 mM NaCl, pH 8.5 at 22 °C.
Taken together, these results demonstrate the feasibility of using 4-nitrophenoxy silyl ethers as a high-throughput assay and show that both enzymes accelerated silyl ether bond hydrolysis significantly above that of background uncatalyzed hydrolysis.
pH Dependence of TF-Silα Hydrolytic Activity.
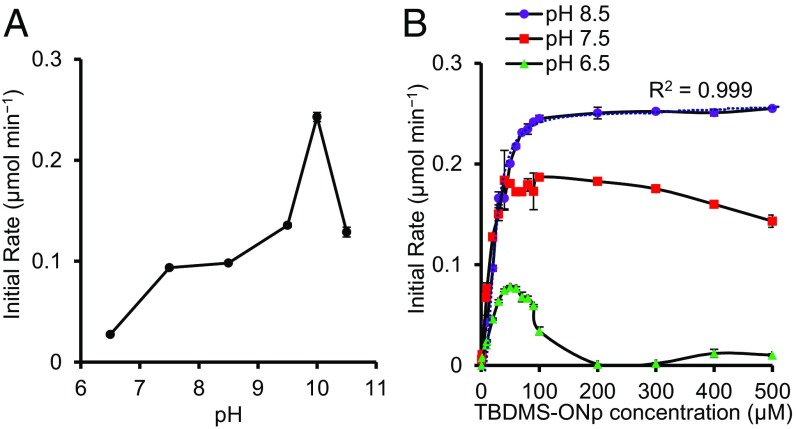
To investigate the effect of pH on enzymatic silyl ether hydrolysis the initial rates of hydrolysis of 1 catalyzed by TF-Silα over pH 6.5–10.5 were determined. Here, the initial rates of reaction were used as a measure of activity and the net enzyme-catalyzed rate was calculated after accounting for the background nonenzymatic hydrolysis. It was found that at low substrate concentrations (≤0.05 mM) higher activities were recorded in the basic pH range with a maximum at pH ∼10 (Fig. 4A), consistent to the optimum pH range of “alkaline” serine proteases (20–22). This optimum is related to the ionization state of catalytic His residue, which must be unprotonated for activity, and the concentration of hydroxide ions that participate in the hydrolysis of the acyl-enzyme intermediate (22).
Fig. 4.
(A) Graph of the rate of hydrolysis of 1 catalyzed by TF-Silα against pH with an initial 1 concentration of 0.05 mM. (B) Graph of initial reaction velocity for the hydrolysis of 1 catalyzed by TF-Silα against a range of initial substrate concentrations. The plotted data are obtained after subtraction of the rate of background hydrolysis and calibrated using data from SI Appendix, Figs. S4 and S5. The best fit Michaelis–Menten curve (dotted line) and associated R2 value are also shown for the pH 8.5 data.
Substrate inhibition was observed at lower pH values with this effect evident at substrate concentrations above ∼0.06 mM and ∼0.10 mM at pH 6.5 and 7.5, respectively (Fig. 4B). This phenomenon of substrate inhibition, though not frequently discussed, is known in many enzyme systems through a variety of mechanisms (23, 24). Of the proposed mechanisms, the classical substrate inhibition model where the substrate acts as an allosteric regulator is unlikely in this case because 1 bears little resemblance to silicic acid, the presumed natural substrate, and there are no known compounds bearing C–Si bonds of biological origin (3). Models where the increasing amounts of substrate result in the formation of noncatalytic conformations of the enzyme could be invoked, although how this effect is related to the pH is unclear at present.
Kinetic Analysis of TF-Silα and Silα-Catalyzed Silyl Ether Hydrolysis.
To further characterize this enzyme-catalyzed hydrolysis, a series of assays were conducted to extract the Michaelis–Menten kcat and Km values (Table 1 and SI Appendix, Figs. S7–S9). Based on the pH study above, these assays were performed at pH 8.5 as an acceptable compromise between relatively good enzyme activity, low levels of background (uncatalyzed) hydrolysis, and avoidance of substrate inhibition.
Table 1.
Table of Michaelis–Menten constants determined for Silα and TF-Silα against the model substrates 1–3 (the hydrolysis of model peptide 7 by cathepsin L is also listed for comparison)
| Enzyme* | Substrate | Km, µM | kcat, min−1 | kcat/Km, min−1⋅µM−1 |
| TF-Silα | 1 | 22.4 ± 2.2 | 988.3 ± 416.4 | 55.7 ± 21.2 |
| TF-Silα | 2 | 8.7 ± 4.5 | 79.1 ± 19.9 | 12.3 ± 7.5 |
| TF-Silα | 3 | 49.8 ± 17.2 | 92.1 ± 29.4 | 2.3 ± 1.4 |
| Silα | 1 | 12.9 ± 2.1 | 611.1 ± 32.8 | 48.3 ± 6.0 |
| Silα | 2 | 7.5 ± 2.6 | 61.5 ± 5.4 | 4.6 ± 1.9 |
| Silα | 3 | 76.4 ± 47.2 | 166.5 ± 89.7 | 4.7 ± 4.0 |
| Cathepsin L† | 7 | 36 | 1,200 | 33.3 |
Assays for silicateins performed in 5% vol/vol 1,4-dioxane, 50 mM Tris, and 100 mM NaCl, pH 8.5.
Assay performed in 5% vol/vol N,N-dimethylformamide and 100 mM phosphate buffer, pH 6.0 (data taken from ref. 25).
In general, the binding of both candidate proteins to all of the substrates was relatively weak, with Km values in the micromolar range. However, it did not follow in the expected trend of decreasing Km with increasing substrate steric bulk. The TDS silyl ether 2 gave a lower Km than the smaller tert-butyldimethylsilyl (TBDMS) analog across both TF-Silα and Silα. This observation suggests that the enzymes display some selectivity in regard to substrate shape that is not simply a function of overall steric bulk of the substrate. The kcat values also followed this general trend, with substrate 2 giving the lowest turnover.
The overall catalytic efficiency (kcat/Km) did follow a trend of falling with increasing bulk of the substrates. These values were comparable with the data from cathepsin L against its standard Cbz-Phe-Arg-NHNp dipeptide nitrophenylanilide substrate 7 (25), suggesting the kinetics data obtained for the silicateins were plausible.
Ser26Ala Mutation at the TF-Silα Active Site.
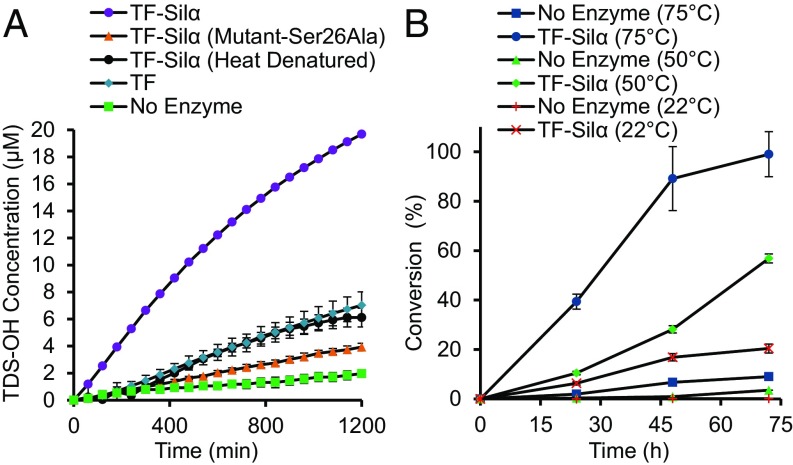
To confirm that the catalysis did indeed involve the serine residue at the active site, the Ser26Ala mutant of TF-Silα was then tested. Using TEOS as a substrate, only a very small amount of silica was formed, comparable to that of the heat-denatured enzyme and chymotrypsin (SI Appendix, Fig. S3A). These data are in agreement with a previous report where the equivalent mutation in wild-type Silα from Tethya aurantia resulted in the abolition of specific catalysis (26). When tested against the chromogenic substrate 2 a similar result was obtained, with the unmodified TF-Silα resulting in a clear positive result whereas only low activity was found in the mutant, comparable to the other control experiments (Fig. 5A). Although greatly reduced in all cases, total loss of catalysis is never observed due to residual nonspecific catalysis, as previously noted.
Fig. 5.
(A) Graph of the extent of hydrolysis of 2 over time catalyzed by TF-Silα, its Ser26Ala mutant, heat-denatured TF-Silα, and the TF protein alone. Assays are conducted under the same conditions as noted for Fig. 3. (B) Graph of percentage conversion to silyl ether 15 against reaction time at three different temperatures.
Molecular Dynamics Modeling of Silα Binding with TBDMS-ONp.
It was notable that the enzymes were able to process large nonpolar organosiloxanes, albeit at a low rate. There is currently no experimentally derived structure for any of the silicateins, but previous models (27) have suggested that the active site pocket of the enzyme is relatively wide, which may allow it accommodate these larger molecules.
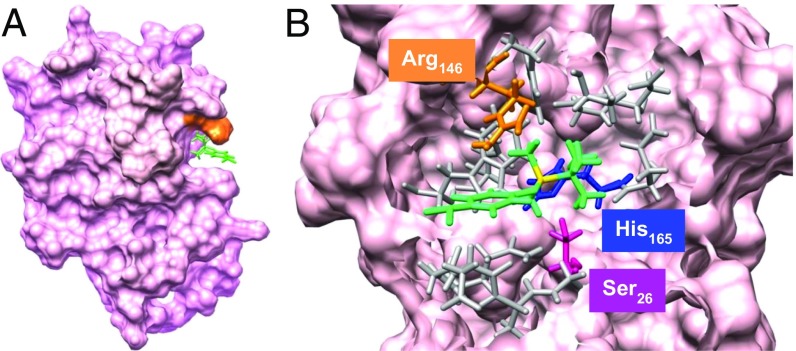
To rationalize the experimental observations, molecular dynamics (MD) simulations with CHARMM were carried out using a homology model constructed from the known crystallographic structure of cathepsin L (28). The silicatein model structure bound to substrate 1 was created and two sets of MD calculations were performed for a period of 1 ns, one with constrained substrate geometry and one fully relaxed structure. After completion of the calculations, the final snapshot of the latter model showed that the substrate is bound to the enzyme in close proximity to the active site catalytic triad (Fig. 6 and SI Appendix, Figs. S10–S14). Here, the binding cavity appears as a wide cleft that is able to accommodate large molecules. The model shows that 1 is oriented such that the Si atom and its substituent alkyl groups point toward the catalytic residues His165 and Ser26. The 4-nitrophenyl group points outward to the solvent and fits between the protein backbone and the Arg146 residue, which projects over the substrate (Fig. 6A). The proximity of the phenyl ring face of 1 with the guanidinium terminal of the Arg residue (∼3 Å) suggests the intriguing possibility of a cation–π interaction (29), which may contribute toward the binding of a hydrophobic substrate to the relatively polar binding site.
Fig. 6.
Images of substrate 1 bound to Silα at the end of the MD simulation without substrate constraints. (A) Image of the overall protein structure showing the substrate (green) bound at the large active site cavity and the overhanging Arg146 (orange). (B) Magnification of the area around the bound substrate, with the substrate (green with the silicon atom in yellow), Ser26 (magenta), His165 (blue), and Arg146 (orange). Other residues within 5 Å of the substrate are shown in gray. Asn185 is located behind His165 and is not visible from this perspective.
At the end of the simulation the interatomic distance between the Si atom and the O atom of Ser26 remains large (∼6 Å) and suggests further molecular motions would be required to enable the attack of the catalytic hydroxy group at the Si atom. The MD simulation where the geometry of 1 is constrained shows that the substrate does not fit into this cleft cavity and is, therefore, displaced from the protein surface (SI Appendix, Fig. S13). Nevertheless, in all cases large structural changes to the protein are recorded, indicating that both substrate and protein must undergo significant adjustments to match their shape upon binding.
Silyl Ether Condensation Catalyzed by TF-Silα.
It is well-established that hydrolytic enzymes such as proteases and esterases can be driven in the reverse direction (i.e., condensation) by alteration of the reaction equilibrium (30, 31). To explore the applicability of this general concept to the silicateins, the condensation of silanols and alcohols to give the corresponding silyl ethers was investigated. For this purpose, the enzymes were lyophilized and the reactions performed in octane. In the first instance, condensation of 1-octanol (OcOH, 8) with trimethylsilanol (TMS-OH, 9) to give the corresponding silyl ether (TMS-OOc, 15; Scheme 2) was studied. Analysis of the reaction mixtures at various time intervals by GC-MS showed that the desired product was generated (SI Appendix, Figs. S15 and S16) with reaction conversions of 20% achieved after 72 h. Control experiments using equimolar amounts of heat-denatured enzyme or when the enzyme was omitted gave only small amounts of product (3.6% and 1.1%, respectively), via nonspecific catalysis or the uncatalyzed reaction.
Scheme 2.
Silyl ether synthesis by silanol condensation or ethoxysilane transetherification. aFive mole equivalents of substrates used relative to octanol. bDMPS, dimethylphenylsilyl; TES, triethylsilyl; TMS, trimethylsilyl. cAs determined by GC-MS quantification. Reaction using TF-Silα lyophilized from the buffered lyoprotectant mixture for 72 h at 75 °C. dReaction using TF-Silα lyophilized from NH3HCO3 buffer only at 75 °C, shown for comparison. eMaximum conversion achieved after 48 h.
To optimize the condensation reaction, a number of parameters were investigated. It has previously been reported that when used in organic solvents, enzymes lyophilized from buffers at different pH values gave different activities due to the retention of the protonation state related to that pH before lyophilization (32). This effect was investigated for the formation of 15, using TF-Silα lyophilized from buffered ammonium bicarbonate at pH 7, 8, and 9 (SI Appendix, Fig. S18). It was found that the highest conversion was observed with the enzyme sample lyophilized from pH 7. The effect of reactant stoichiometry was then investigated and a 5 mol. eq. excess of silanol 9 was found to give the best conversion (SI Appendix, Fig. S19). Much lower conversions were observed with larger excesses of silanol, possibly due to the apparent substrate inhibition noted above. Using neat octanol as the solvent resulted in negligible conversions, thought to be due to the denaturation of the enzyme and displacement of essential structural water molecules by the alcohol (32).
To maximize the rate of reaction and to test the thermal stability of the system, the condensations were also carried out at 50 °C and 75 °C. These elevated temperatures gave major improvements to conversions and were essentially quantitative after 72 h at 75 °C (Fig. 5B). In comparison, control experiments where the enzyme was omitted gave <9% conversion. The ability of the protein to function at such elevated temperatures is notable but conforms with previous reports that nonpolar organic solvents improve the stability of enzymes through hydrophobic confinement and thus suppression of denaturation (33, 34).
Another factor that was investigated was the addition of lyoprotectants such as potassium salts and the crown ether 18-crown-6 (18C6) before enzyme lyophilization (33, 35, 36). When TF-Silα was colyophilized with KCl and 18C6, then used for the condensation of 15, conversions could be further improved compared with when these additives were omitted (SI Appendix, Figs. S20 and S21). Using these optimized conditions, the condensation with octanol 8 with further silanol examples, dimethylphenylsilanol (DMPS-OH, 10) and triethylsilanol (TES-OH, 11), were then performed to give the corresponding ethers 16 and 17 (Scheme 2 and SI Appendix, Figs. S22–S25). In all cases >80% conversions were achieved, demonstrating the use of this enzyme as a silyl ether condensation catalyst.
Silyl Transfer by Transetherification.
A major limitation of using silanols for condensation is their propensity to form disiloxanes. As an alternative, the transfer of the silyl group from their corresponding ethoxysilanes was investigated. Such intermolecular transetherifications would be a useful route to the silylation of more valuable substrates using readily available silyl donors while avoiding the use of chlorosilanes. To demonstrate this approach, TMS-OEt, DMPS-OEt, and TES-OEt (12–14, Scheme 2) were used as silyl donors for the silylation of octanol 8 with TF-Silα catalysis under the optimized conditions previously used for the condensation reactions (lyoprotectants added, n-octane, 75 °C). Analysis of the reaction products showed that the silyl groups could be successfully transferred (SI Appendix, Figs. S26–S31). However, the reactions were slower compared with the analogous condensation reactions and generally gave poorer conversions (Scheme 2).
Regioselective Silylation.
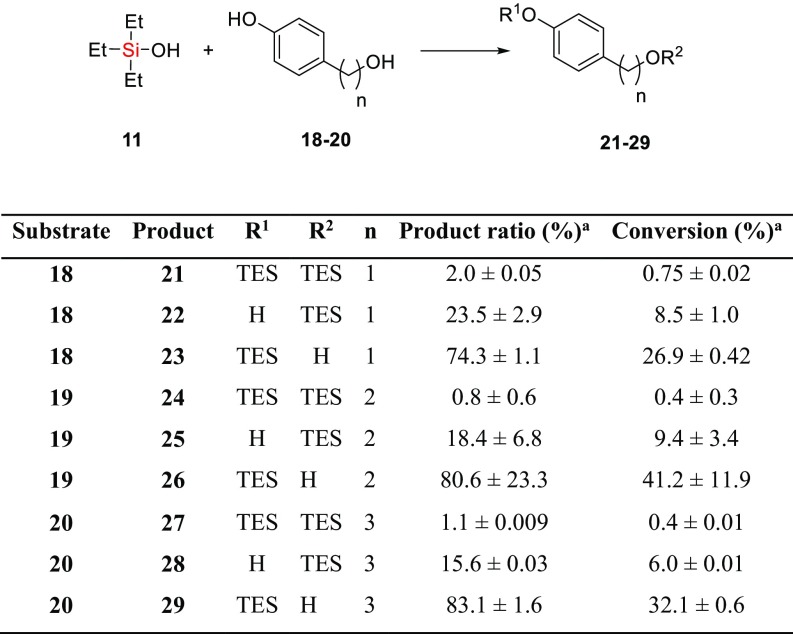
The differentiation of hydroxy groups remains an important step toward the chemical synthesis of complex molecules. Such regioselectivity is typically achieved through the selective deprotection of persilylated substrates (6, 37) but necessitates wasteful global protection beforehand. Direct, selective silylation would circumvent the deprotection step and would therefore be more efficient. As an initial investigation into the regioselectivity of biocatalytic silylation, model 4-(ω-hydroxyalkyl)phenol substrates 18–20 that each possess a phenolic and aliphatic alcohol (Scheme 3) were subjected to TF-Silα catalyzed silylation with TES-OH (11). For each of these dihydroxy substrates, analysis of the reaction mixtures indicated the formation of all three possible products (21–29, Scheme 3 and SI Appendix, Figs. S32–S34). Notably, although the enzyme eventually catalyzed the silylation of both hydroxy groups, in all cases there is an initial preference for the phenolic group. For example, the silylation of 19 gave a 52% substrate conversion after 24 h, of which over 80% was the aryl siloxane 26. The aliphatic siloxane 25 comprised most of the remainder of the product, with only trace amounts (∼1%) of the disilylated material 24. In contrast, negative control experiments where the enzyme was omitted gave very low total conversions and silylation of the aliphatic alcohol was preferred (∼0.5% of 25 was produced after 24 h), as would be expected due to the higher nucleophilicity of the aliphatic alcohol under these conditions. This general trend was repeated for the other substrates that were tested.
Scheme 3.
Regioselective silyl ether synthesis by silanol condensation. aAfter 24 h using TF-Silα lyophilized with lyoprotectant mixture, 5 mol eq 11 relative to diol, 75 °C.
Comparisons with Small-Molecule Catalysts.
Using the condensation reaction of octanol 8 and TMS-OH (9) to form 15 as a model reaction, the effectiveness of common catalysts such as imidazole, triethylamine, and histidine were compared. At similar catalyst loadings, these bases gave negligible conversions (<0.5% after 24 h) compared with the enzyme-catalyzed reaction (17%, SI Appendix, Fig. S35). These small-molecule catalysts also gave low regioselectivity with the dihydroxy compound 19. Taking imidazole as an example, if a large amount of catalyst was used (3 mol eq relative to alcohol) and the reaction was halted at an early time point (after 5 h), the aliphatic silyl ether 24 and disilylated 25 were clearly the dominant products (SI Appendix, Fig. S36).
These results are significant because they show that the enzymatic catalysis is not simply due to the presence of basic functional groups, and that the enzymatic reaction results in contrasting regioselectivity compared with small-molecule catalysis.
Esterase and Protease Activity.
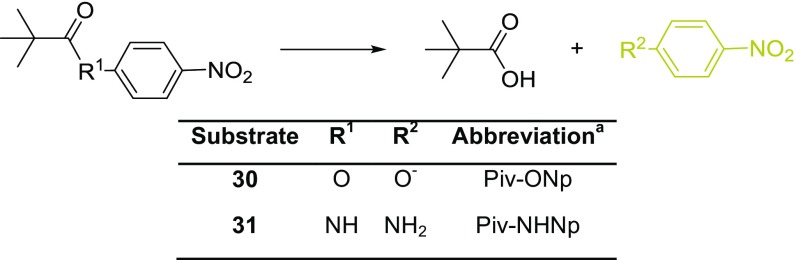
Since Silα has a high degree of homology with the protease cathepsin L and employs a catalytic triad common to many other hydrolytic enzymes, its esterase and protease activity was surveyed. Here, analogs of 1 where the dimethylsiloxy moiety was replaced with an ester or amide (30 and 31 respectively, Scheme 4) were tested. Time-course experiments for the hydrolysis of these substrates catalyzed by TF-Silα and Silα showed no specific hydrolysis, comparable with control experiments where the enzyme was omitted (Fig. 7A and Table 2). Both the candidate enzymes therefore seem to have negligible esterase or amidase activity against such analogous substrates.
Scheme 4.
Enzyme catalyzed hydrolysis of 4-nitrophenyl pivaloate and pivalamide. aNp, 4-nitrophenyl; Piv, pivaloyl.
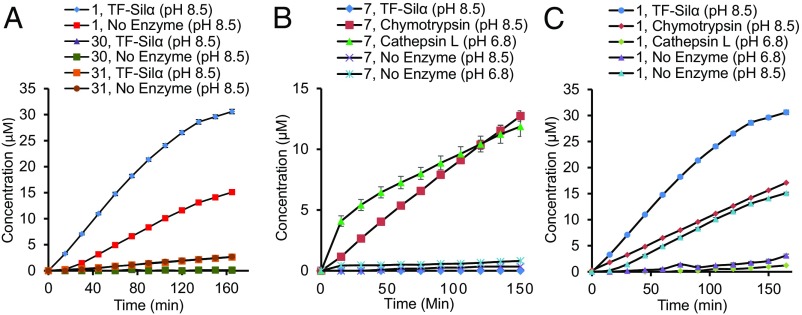
Fig. 7.
Graphs of the concentration of chromophores (4-nitroaniline and 4-nitrophenoxylate ion) generated against time for the hydrolysis of: (A) TBDMS-ONp (1), Piv-ONp (30), or Piv-NHNp (31) catalyzed by TF-Silα; (B) Cbz-Phe-Arg-NHNp (7) catalyzed by TF-Silα, chymotrypsin, or cathepsin L; and (C) 1 catalyzed by TF-Silα, chymotrypsin, or cathepsin L.
Table 2.
Table of percentage conversions for the enzyme catalyzed hydrolysis of the various substrates
| Enzyme | Net % conversion | |||
| 1 | 30 | 31 | 7 | |
| TF-Silα | 19.2 ± 1.2 | <0.01 ± 0.0 | <0.01 ± 0.0 | <0.01 ± 0.0 |
| Silα | 21.4 ± 0.3 | 1.3 ± 0.8 | <0.01 ± 0.0 | <0.01 ± 0.0 |
| Chymotrypsin | 6.9 ± 0.05 | 17.5 ± 7.1 | <0.01 ± 0.0 | 21.4 ± 0.5 |
| Cathepsin L* | 2.5 ± 1.3 | 1.6 ± 1.7 | <0.2 ± 0.01 | 26.7 ± 1.8 |
Net conversions are calculated after subtraction of background hydrolysis. All reactions were performed for 4 h with 0.00067 mol eq enzyme with 0.2 mM substrate in 5% vol/vol dioxane, 50 mM Tris, and 100 mM NaCl.
Assay was conducted at pH 6.8, the optimum for this enzyme; all other assays were performed at pH 8.5.
When tested against dipeptide 7 both silicateins also displayed negligible activity in comparison with both chymotrypsin and cathepsin L, which readily hydrolyzed this dipeptide (Fig. 7B and Table 2). These results are consistent with a previous report where a Cys→Ser mutation in the cathepsin L active site largely abolishes protease activity (38). However, it does not exclude the possibility that Silα may have protease activity against very specific sequences such as in the autolysis of its own propeptide during maturation of the enzyme. Indeed, previous work with the silicatein proenzyme has shown that self-cleavage is possible (15).
In contrast, chymotrypsin and cathepsin L only catalyzed the hydrolysis of siloxane 1 at low levels, which is attributable to nonspecific catalysis (Fig. 7C and Table 2). In all cases, no hydrolysis of the amide 31 was detectable, although chymotrypsin demonstrated a good level of hydrolytic activity against the ester 30 (Table 2), in agreement with the known promiscuous esterase activity of this enzyme even against bulky substrates (39).
Conclusions
In summary, the production of two recombinant silicateins and a systematic survey into their reactivity against various organosiloxanes has been conducted. In the process, a high-throughput colorimetric assay for silyl ether hydrolysis was developed. This assay showed that the enzymes are able to catalyze the hydrolysis of a range of silyl ethers, including those with very bulky substituents, albeit at a relatively slow rate. The silicateins display good activity from pH 7.5–10.0, with substrate inhibition observed below this pH range. Supporting MD modeling of the enzyme with a model substrate showed that substrate fits into the binding cavity, with the silicon center oriented toward the presumed catalytic residues. The binding of these large substrates is possible as a result of large conformational changes in the protein and substrate distortion. These results are consistent with the proposed hydrolytic mechanism that is used by enzymes possessing a catalytic triad motif.
The silicateins were also shown to catalyze the condensation of organosilanols and alcohols to give the corresponding silyl ethers when used in organic solvents. Furthermore, they can catalyze transetherifications, where the silyl group from one silyl ether may be transferred to a recipient alcohol. Notably, when presented with a substrate bearing both aliphatic and aromatic hydroxy groups the enzyme preferentially catalyzes the silylation of the latter group, in clear contrast to nonenzymatic silylations.
Despite sequence similarities with the cathepsins, the silicateins seem to exhibit no significant protease or esterase activity when tested against analogous substrates. The silicateins thus represent an example of divergent evolution where an existing ancestral enzyme has evolved to catalyze reactions in a new “niche” of chemical space.
The results reported herein therefore suggest that the silicateins are promising candidates for future development into efficient and selective biocatalysts for organosiloxane chemistry. By providing a new chemical context (e.g., the condensation of organic silyl ethers in organic solvents), they may be considered prototype “silyl etherase” enzymes that can be subjected to further evolutionary optimization (40). Indeed, powerful directed evolution strategies are now available to generate highly specific and robust biocatalysts for applications in the production of fine chemicals and functional materials (10, 41). It is envisaged that such biocatalysts could be used in the chemical synthesis of complex molecules, where they could be used to selectively introduce or cleave silyl protecting groups (6) or to recycle these relatively expensive silyl groups by transetherification (13). In the area of materials chemistry, they could be applied for the synthesis of silicone polymers from nonhalogenated feedstocks (42).
Further work will be needed to develop an in-depth understanding of the structure and mechanism of this family of enzymes. Such structural elucidation of the silicateins remains a significant challenge (18) due to the low protein production yield and their hydrophobicity, but it is anticipated that the work reported here will provide a solid foundation toward this end and to the wider goal of using enzymes for applied biocatalysis.
Materials and Methods
The proteins were heterologously produced from synthetic genes in E. coli and isolated using standard procedures. The silyl ether substrates were synthesized from the corresponding chlorosilanes, silyl triflates, or silazanes. The GC-MS analyses of the reactions were performed by comparison and calibration with chemically synthesized samples. Full details for the materials and methods are given in SI Appendix. The tabulated CD data are given in Dataset S1.
Supplementary Material
Acknowledgments
We thank Emily I. Sparkes for technical assistance and Prof. Peter G. Taylor (Open University, Milton Keynes, United Kingdom) for assistance with the chemical data analysis. This work was supported by Engineering and Physical Sciences Research Council Grants EP/K011685/1 and EP/K031465/1 (to L.S.W.), Biotechnology and Biological Sciences Research Council Doctoral Training Partnership Graduate Studentship BB/J014478/1 (to S.A.C.), and a graduate studentship from the Tertiary Education Trust Fund of Nigeria (to A.S.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613320114/-/DCSupplemental.
References
- 1.Andriot M, et al. Silicones in industrial applications. In: De Jaeger R, Gleria M, editors. Inorganic Polymers. Nova Science Publishers; Hauppauge, NY: 2007. pp. 61–161. [Google Scholar]
- 2.Kung MC, Riofski MV, Missaghi MN, Kung HH. Organosilicon platforms: Bridging homogeneous, heterogeneous, and bioinspired catalysis. Chem Commun (Camb) 2014;50:3262–3276. doi: 10.1039/c3cc48766k. [DOI] [PubMed] [Google Scholar]
- 3.Rücker C, Kümmerer K. Environmental chemistry of organosiloxanes. Chem Rev. 2015;115:466–524. doi: 10.1021/cr500319v. [DOI] [PubMed] [Google Scholar]
- 4.Mewald M, Schiffner JA, Oestreich M. A new direction in C-H alkenylation: Silanol as a helping hand. Angew Chem Int Ed Engl. 2012;51:1763–1765. doi: 10.1002/anie.201107859. [DOI] [PubMed] [Google Scholar]
- 5.Xu L-W, Li L, Lai G-Q, Jiang J-X. The recent synthesis and application of silicon-stereogenic silanes: A renewed and significant challenge in asymmetric synthesis. Chem Soc Rev. 2011;40:1777–1790. doi: 10.1039/c0cs00037j. [DOI] [PubMed] [Google Scholar]
- 6.Crouch RD. Recent advances in silyl protection of alcohols. Synth Commun. 2013;43:2265–2279. [Google Scholar]
- 7.Rösch L, John P, Reitmeier R. Ullmann’s Encyclopedia of Industrial Chemistry. Vol 32. Wiley-VCH; Weinheim, Germany: 2002. Silicon compounds, organic; p. 637. [Google Scholar]
- 8.Ikeda Y, Huang W, Oku A. Recycling of monomers and fillers from high-temperature-vulcanized silicone rubber using tetramethylammonium hydroxide. Green Chem. 2003;5:508–511. [Google Scholar]
- 9.Turner NJ. Directed evolution drives the next generation of biocatalysts. Nat Chem Biol. 2009;5:567–573. doi: 10.1038/nchembio.203. [DOI] [PubMed] [Google Scholar]
- 10.Bornscheuer UT, et al. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 11.Maraite A, Ansorge-Schumacher MB, Ganchegui B, Leitner W, Grogan G. On the biocatalytic cleavage of silicon–oxygen bonds: A substrate structural approach to investigating the cleavage of protecting group silyl ethers by serine-triad hydrolases. J Mol Catal B Enzym. 2009;56:24–28. [Google Scholar]
- 12.Abbate V, Bassindale AR, Brandstadt KF, Taylor PG. A large scale enzyme screen in the search for new methods of silicon-oxygen bond formation. J Inorg Biochem. 2011;105:268–275. doi: 10.1016/j.jinorgbio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Abbate V, Brandstadt KF, Taylor PG, Bassindale AR. Enzyme-catalyzed transetherification of alkoxysilanes. Catalysts. 2013;3:27–35. [Google Scholar]
- 14.Shimizu K, Cha J, Stucky GD, Morse DE. Silicatein α: Cathepsin L-like protein in sponge biosilica. Proc Natl Acad Sci USA. 1998;95:6234–6238. doi: 10.1073/pnas.95.11.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller WEG, et al. Molecular mechanism of spicule formation in the demosponge Suberites domuncula: Silicatein–collagen–myotrophin. Prog Mol Subcell Biol. 2003;33:195–221. doi: 10.1007/978-3-642-55486-5_8. [DOI] [PubMed] [Google Scholar]
- 16.Schröder HC, et al. Acquisition of structure-guiding and structure-forming properties during maturation from the pro-silicatein to the silicatein form. J Biol Chem. 2012;287:22196–22205. doi: 10.1074/jbc.M112.351486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha JN, et al. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc Natl Acad Sci USA. 1999;96:361–365. doi: 10.1073/pnas.96.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairhead M, et al. Crystal structure and silica condensing activities of silicatein α-cathepsin L chimeras. Chem Commun (Camb) 2008:1765–1767. doi: 10.1039/b718264c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedstrom L. Serine protease mechanism and specificity. Chem Rev. 2002;102:4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 20.Sipos T, Merkel JR. An effect of calcium ions on the activity, heat stability, and structure of trypsin. Biochemistry. 1970;9:2766–2775. doi: 10.1021/bi00816a003. [DOI] [PubMed] [Google Scholar]
- 21.Kirschke H, Langner J, Wiederanders B, Ansorge S, Bohley P. Cathepsin L. A new proteinase from rat-liver lysosomes. Eur J Biochem. 1977;74:293–301. doi: 10.1111/j.1432-1033.1977.tb11393.x. [DOI] [PubMed] [Google Scholar]
- 22.Craik CS, Roczniak S, Largman C, Rutter WJ. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987;237:909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- 23.Reed MC, Lieb A, Nijhout HF. The biological significance of substrate inhibition: A mechanism with diverse functions. Bioessays. 2010;32:422–429. doi: 10.1002/bies.200900167. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser PM. Substrate inhibition as a problem of non-linear steady state kinetics with monomeric enzymes. J Mol Catal. 1980;8:431–442. [Google Scholar]
- 25.Tchoupé JR, Moreau T, Gauthier F, Bieth JG. Photometric or fluorometric assay of cathepsin B, L and H and papain using substrates with an aminotrifluoromethylcoumarin leaving group. Biochim Biophys Acta. 1991;1076:149–151. doi: 10.1016/0167-4838(91)90232-o. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Shimizu K, Cha JN, Stucky GD, Morse DE. Efficient catalysis of polysiloxane synthesis by silicatein α requires specific hydroxy and imidazole functionalities. Angew Chem Int Ed. 1999;38:779–782. doi: 10.1002/(SICI)1521-3773(19990315)38:6<779::AID-ANIE779>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Schröder HC, et al. Enzymatic production of biosilica glass using enzymes from sponges: Basic aspects and application in nanobiotechnology (material sciences and medicine) Naturwissenschaften. 2007;94:339–359. doi: 10.1007/s00114-006-0192-0. [DOI] [PubMed] [Google Scholar]
- 28.Quesne MG, Borowski T, de Visser SP. Quantum mechanics/molecular mechanics modeling of enzymatic processes: Caveats and breakthroughs. Chemistry. 2016;22:2562–2581. doi: 10.1002/chem.201503802. [DOI] [PubMed] [Google Scholar]
- 29.Mahadevi AS, Sastry GN. Cation-π interaction: Its role and relevance in chemistry, biology, and material science. Chem Rev. 2013;113:2100–2138. doi: 10.1021/cr300222d. [DOI] [PubMed] [Google Scholar]
- 30.Gais H-J, Jungen M, Jadhav V. Activation of pig liver esterase in organic media with organic polymers: Application to the enantioselective acylation of racemic functionalized secondary alcohols. J Org Chem. 2001;66:3384–3396. doi: 10.1021/jo0016881. [DOI] [PubMed] [Google Scholar]
- 31.Riva S, Chopineau J, Kieboom APG, Klibanov AM. Protease-catalyzed regioselective esterification of sugars and related compounds in anhydrous dimethylformamide. J Am Chem Soc. 1988;110:584–589. [Google Scholar]
- 32.Zaks A, Klibanov AM. Enzyme-catalyzed processes in organic solvents. Proc Natl Acad Sci USA. 1985;82:3192–3196. doi: 10.1073/pnas.82.10.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stepankova V, et al. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013;3:2823–2836. [Google Scholar]
- 34.Griebenow K, Klibanov AM. On protein denaturation in aqueous−organic mixtures but not in pure organic solvents. J Am Chem Soc. 1996;118:11695–11700. [Google Scholar]
- 35.Khmelnitsky YL, Welch SH, Clark DS, Dordick JS. Salts dramatically enhance activity of enzymes suspended in organic solvents. J Am Chem Soc. 1994;116:2647–2648. [Google Scholar]
- 36.Broos J, Sakodinskaya IK, Engbersen JFJ, Verboom W, Reinhoudt DN. Large activation of serine proteases by pretreatment with crown ethers. J Chem Soc Chem Commun. 1995;68:255–256. [Google Scholar]
- 37.Crouch RD. Selective deprotection of silyl ethers. Tetrahedron. 2013;69:2383–2417. [Google Scholar]
- 38.Coulombe R, et al. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 1996;15:5492–5503. [PMC free article] [PubMed] [Google Scholar]
- 39.Tanizawa K, Yamada H, Itoh K, Kanaoka Y. Comparison of deacylation rates of chymotryptic catalysis within an enantiomeric pair of p-nitrophenyl esters. Chem Pharm Bull (Tokyo) 1991;39:2748–2749. [Google Scholar]
- 40.Arnold FH. The nature of chemical innovation: New enzymes by evolution. Q Rev Biophys. 2015;48:404–410. doi: 10.1017/S003358351500013X. [DOI] [PubMed] [Google Scholar]
- 41.Reetz MT. Laboratory evolution of stereoselective enzymes: A prolific source of catalysts for asymmetric reactions. Angew Chem Int Ed Engl. 2011;50:138–174. doi: 10.1002/anie.201000826. [DOI] [PubMed] [Google Scholar]
- 42.Wolf SE, et al. Formation of silicones mediated by the sponge enzyme silicatein-α. Dalton Trans. 2010;39:9245–9249. doi: 10.1039/b921640e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.