Significance
Hypertension affects one in four adults in the United States and is a major risk factor for heart failure. A plasmonic nano-gold platform with excellent reproducibility and sensitivity is developed to address the currently unmet need of profiling circulating cardiovascular autoantibodies in hypertensive patients. We observed that patients with early-stage hypertensive heart diseases displayed a higher level of cardiovascular autoantibodies than hypertensive patients with normal cardiac function. Autoantibodies to troponin I, adrenergic beta-1 receptor, and annexin-A5 could best discriminate hypertensive heart disease. Ultimately, this nanoscience-based platform could be deployed to facilitate screening autoantibodies and identify at-risk patients for early stages of heart failure. Furthermore, this platform could have broad applications in myocarditis, heart transplantation, and other autoimmune diseases.
Keywords: hypertensive heart disease, heart failure, autoantibodies, early detection, high-sensitivity multiplexed assay
Abstract
The role of autoimmunity in cardiovascular (CV) diseases has been increasingly recognized. Autoimmunity is most commonly examined by the levels of circulating autoantibodies in clinical practices. Measurement of autoantibodies remains, however, challenging because of the deficiency of reproducible, sensitive, and standardized assays. The lack of multiplexed assays also limits the potential to identify a CV-specific autoantibody profile. To overcome these challenges, we developed a nanotechnology-based plasmonic gold chip for autoantibody profiling. This approach allowed simultaneous detection of 10 CV autoantibodies targeting the structural myocardial proteins, the neurohormonal regulatory proteins, the vascular proteins, and the proteins associated with apoptosis and coagulation. Autoantibodies were measured in four groups of participants across the continuum of hypertensive heart diseases. We observed higher levels of all 10 CV autoantibodies in hypertensive subjects (n = 77) compared with healthy participants (n = 30), and the autoantibodies investigated were related to each other, forming a highly linked network. In addition, we established that autoantibodies to troponin I, annexin-A5, and beta 1-adrenegic receptor best discriminated hypertensive subjects with adverse left ventricular (LV) remodeling or dysfunction (n = 49) from hypertensive subjects with normal LV structure and function (n = 28). By further linking these three significant CV autoantibodies to the innate and growth factors, we revealed a positive but weak association between autoantibodies to troponin I and proinflammatory cytokine IL-18. Overall, we demonstrated that this platform can be used to evaluate autoantibody profiles in hypertensive subjects at risk for heart failure.
Both the innate and adaptive immune systems play an important role in the development and progression of heart failure (HF). The association between HF and inflammation was first recognized in 1990 by Levine et al. (1) who reported elevated levels of TNF in patients with HF with a reduced ejection fraction (EF). A recent study established that patients with hypertensive heart diseases demonstrated higher levels of IL-18, a marker of inflammasome activation (2).
Whereas much research effort focused on the innate immune system, an increasing number of studies have shown that autoimmunity was also involved in HF. In the study from Latif et al. (3), IgG of antiheart antibodies was detected significantly more in patients with dilated cardiomyopathy (DCM) than in patients with ischemic heart disease. More recently, there is also evidence that autoantibodies are involved in systemic hypertension (HTN); for example, autoantibodies directed against angiotensin II type I receptors have been associated with HTN, especially in patients with pre-eclampsia (4, 5). Experimental studies support a potential role of autoantibodies in ventricular hypertrophy. Matsui et al. (6) showed that immunization of combined β1-adrenoreceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. In another study, Wallukat et al. (7) found that aging spontaneous hypertensive rats develop antibodies against β1-adrenoreceptor. To date, however, the clinical data supporting a role for autoantibodies in hypertensive heart disease have been sparse.
Measuring cardiovascular (CV) autoantibodies remains challenging due to the lack of a standardized and sensitive assay. Moreover, because autoantibodies are often associated with different structural components or pathways, a multiplexed assay would be useful to establish the autoantibody profile. Whereas the available ELISA lacks multiplexing ability, the multiplexed Luminex platform requires relatively large amount of often precious and expensive antigens. To overcome these limitations, we have recently developed a nanotechnology-based plasmonic gold chip that affords multiplexed profiling of autoantibodies in large numbers with only ∼2-μL serum samples using down to ∼15 μL of antigens. This platform also offers high detection specificity and sensitivity, equivalent to the gold standards of RIA, owing to its ∼100-fold fluorescence enhancement ability in the near-infrared (NIR) region (8–12).
Here, we describe the development of a multiplexed specific CV autoantibody detection panel on a plasmonic gold chip for measuring key components involved in the pathophysiology of HF. Our study tests the hypothesis that CV autoantibodies are associated with hypertensive heart diseases. The discovery of new biomarkers of hypertensive heart disease is of great importance because routine markers such as B-type natriuretic peptide lack sensitivity for the detection of early-stage hypertensive heart diseases (13). We compared the prevalence of CV autoantibodies in four groups of subjects using a nested case control study design building on a large, well-phenotyped population-based study (14, 15). The selected groups of subjects represented a continuum of hypertensive heart diseases including healthy participants, participants with HTN but without left ventricular (LV) hypertrophy or diastolic dysfunction, and participants with systemic HTN and evidence of LV involvement with or without clinical HF or ischemic events (CV events).
Results
Development of CV Autoantibody Detection Panel on Plasmonic Gold Chips.
We recently developed a plasmonic gold chip composed of a layer of nanostructured gold islands deposited on a glass surface through a solution phase growth method. The gold nano-islands were separated by nano-gaps (∼10 nm), affording strong local electric-field and surface plasmon resonance enhancements to fluorescent signals in the NIR region of 650–800 nm by up to 100-fold (8–12).
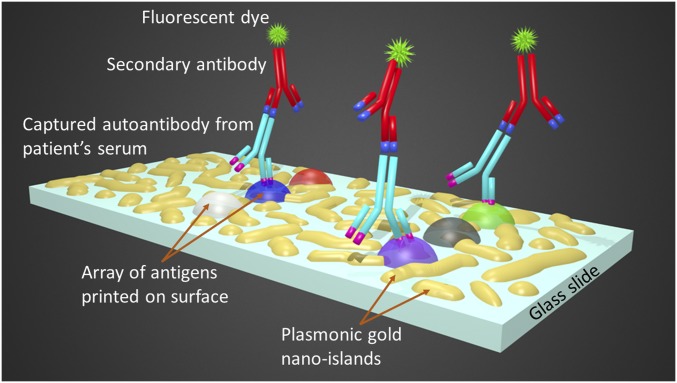
Here, we constructed a sandwich immunoassay on this platform for specific CV autoantibody quantification in a microarray format by first immobilizing on the plasmonic gold chip 10 CV antigen targets that were associated with different pathways related to HF (Fig. 1). The antigens were categorized into four groups: (i) the structural myocardial cellular proteins [i.e., troponin I, titin, β-chain myosin heavy chain 7 (MYH7), and α-chain myosin heavy chain 6 (MYH6)]; (ii) the neurohormonal regulatory proteins [i.e., beta 1-adrenegic receptor (ADRBK1), angiotensin II receptor 1 (AGTR1), and cholinergic receptor muscarinic 2 (CHRM2)]; (iii) the vascular proteins endothelin receptor A and B (EDNR-A and -B); and (iv) coagulation and apoptosis protein annexin-A5. An ultralow volume of 2 μL of each human serum was diluted and incubated over the plasmonic gold chip with printed antigens, during which the corresponding human IgG autoantibodies from a patient’s serum sample were captured on the target antigen spots. The abundance of each autoantibody in serum was quantified by measuring the signal of IRDye 800 fluorescence on the spots after incubating the spots with antihuman IgG labeled with IRDye 800 fluorophores (Fig. 1 and SI Construction of CV Autoantibody Detection on Plasmonic Gold Platform).
Fig. 1.
The multiplexed detection of CV-specific IgG autoantibodies on plasmonic gold chips using an ultralow volume of serum samples. Schematic depicting the plasmonic nano-gold islands deposited on glass slide surface, the sandwich microarray structure, and the actual assay steps including (i) the immobilization of an array of CV antigens on the chip, (ii) the capture of the targeted autoantibodies from diluted patients’ serum, and (iii) the binding of secondary detection antibodies conjugated with IRDye 800 fluorescent dye for signal reporting.
Detection of Antibodies on Plasmonic Gold Chips.
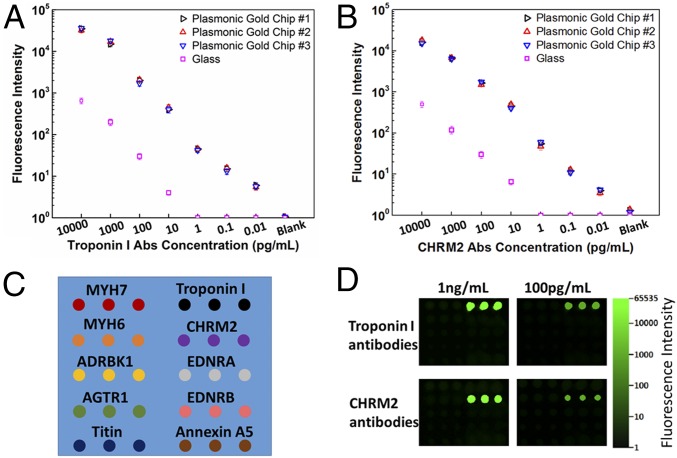
To establish the dynamic range and sensitivity of antibody detection on plasmonic gold chips we generated calibration curves of antibodies to troponin I and CHRM2. By using commercially available samples with known concentrations of antibodies to troponin I and CHRM2 we obtained and repeated the calibration of both antibodies on three different plasmonic chips and on glass slides. We observed ∼100-fold NIR fluorescence enhancement on plasmonic gold chips compared with glass, accompanied by a three-orders-of-magnitude increase in detection dynamic range and sensitivity with signal linearity down to ∼10 fg/mL (Fig. 2 A and B). The coefficient variation of calibration curves obtained from different plasmonic chips in independent experiments was less than 14%. Each of the two commercial troponin I and CHRM2 antibodies was also tested individually over the 10 printed target antigens for specificity checking (Fig. 2 C and D). For the microarray probed with troponin I antibody, positive fluorescence signals were only detected on the troponin I antigen spots and there was no signal on any other antigen spots in the microarray even at high concentrations of troponin I antibody (Fig. 2D). We observed the same trend in the microarray probed only with CHRM2 antibody (Fig. 2D).
Fig. 2.
The dynamic range, sensitivity, and specificity of multiplexed antibody detection on plasmonic gold chips. Calibration curves for antibody quantification and for comparing dynamic range and sensitivity of the detection of (A) antibodies to troponin I and (B) antibodies to CHRM2 on plasmonic gold chips and on glass. Samples used for generating the calibration curves were commercial standards with known concentrations of antibodies. Each experiment was performed three times, and the error bars represent the SD. The calibration curve of each antibody was repeated on plasmonic gold chips produced from various batches and the coefficient variation was <14%. (C) Schematic of the 10-plexed antibody detection microarray containing the 10 target antigens immobilized on a gold plasmonic chip for interacting with each of the two commercial troponin I and CHRM2 antibodies. (D) Fluorescence images of the two microarrays on a plasmonic gold chip probed with 1 ng/mL and 100 pg/mL troponin I and CHRM2 antibodies separately. Each array was subsequently detected with 2 nM IRDye 800-labeled secondary antibodies. Fluorescent signals were only observed on the troponin I antigen spots in the microarray probed with troponin I antibodies (Top), whereas only CHRM2 spots showed signals in the microarray probed with CHRM2 antibodies (Bottom).
For signal normalization purposes and assay quality control the serum sample of a patient with sustained HTN and LV remodeling was included on every plasmonic gold chip used. Thus, we obtained the autoantibodies signals from the same sample 30 times on different chips from independent experiments. The calculated coefficient of variations for the detection of each of the 10 specific autoantibodies in this control sample were 8–13% (Table S1).
Table S1.
Protein abbreviations and coefficient of variation for autoantibodies to the proteins
| Protein | Abbreviation | CoV for autoantibodies, % |
| Troponin I | Troponin I | 12.9 |
| Annexin-A5 | Annexin-A5 | 11.5 |
| Adrenergic beta-1 receptor | ADRBK1 | 11.9 |
| Cholinergic receptor muscarinic 2 | CHRM2 | 11.8 |
| Myosin heavy chain 7, β-isoform | MYH7 | 11.0 |
| Myosin heavy chain 6, α-isoform | MYH6 | 12.7 |
| Titin | Titin | 10.8 |
| Endothelin receptor, type A | EDNR-A | 8.3 |
| Endothelin receptor, type B | EDNR-B | 12.6 |
| Angiotensin II receptor, type 1 | AGTR1 | 12.6 |
The same positive control serum sample was included on each plasmonic gold chip and was tested for 30 times in total on different chips on various days independently. The coefficient of variation (CoV) of each autoantibody in this positive control serum sample was calculated based on the MFI of the 30 test results of each autoantibody.
Clinical Cohort.
From a well-characterized population-based study with available baseline echocardiography and circulating CV biomarker measurements of subjects (Tables S2 and S3) we identified 49 patients with sustained HTN and LV concentric remodeling, hypertrophy, and/or diastolic dysfunction (LV+), of which 20 patients had a prior history of a CV event (LV+/CV+) (14, 15). In the LV+/CV+ group, 9 out of 20 patients reported a previous history of cardiac diseases before the baseline examination. During the follow-up period, nonfatal CV events occurred in 18 patients from this group. Overall, five patients experienced a coronary (myocardial infarction and/or PTCA) event, six patients had symptomatic HF, and seven patients experienced vascular (stroke or peripheral arteries revascularization) events. Then, we selected 29 age-matched (within 10 y) patients with sustained HTN but without LV involvement (LV−). In addition, we selected 30 age-matched (within 10 y to HTN LV+ patients) healthy, normotensive subjects (NTN).
Table S2.
Clinical characteristics of participants
| Groups | ||||
| Characteristic | NTN controls (n = 30) | HTN (LV−) (n = 28) | HTN (LV+/CV−) (n = 29) | HTN (LV+/CV+) (n = 20) |
| Age, y | 57.7 ± 6.12 | 57.5 ± 5.51 | 62.1 ± 8.29*† | 65.7 ± 7.03*† |
| Women, n (%) | 20 (66.7) | 19 (67.9) | 13 (44.8) | 5 (25.0) *† |
| Body mass index, kg/m2 | 24.0 ± 2.53 | 25.6 ± 3.32 | 29.1 ± 4.88*† | 28.8 ± 3.50*† |
| Systolic pressure, mmHg | 120.1 ± 8.44 | 142.8 ± 16.4* | 144.6 ± 16.0* | 149.0 ± 16.6* |
| Diastolic pressure, mmHg | 77.7 ± 4.85 | 85.4 ± 9.76* | 86.0 ± 10.6* | 84.2 ± 10.2* |
| Heart rate, beats per min | 58.9 ± 7.58 | 58.5 ± 9.39 | 63.0 ± 10.4 | 61.4 ± 11.3 |
| Questionnaire data | ||||
| Current smoking, n (%) | 11 (36.7) | 6 (21.4) | 4 (13.8) | 5 (25.0) |
| Drinking alcohol, n (%) | 10 (33.3) | 12 (42.9) | 11 (37.9) | 13 (65.0)* |
| Treated for HTN, n (%) | 0 | 20 (71.4) | 20 (69.0) | 16 (80.0) |
| Diabetes mellitus, n (%) | 0 | 1 (3.6) | 7 (24.1) | 3 (15.0) |
| Coronary heart disease, n (%) | 0 | 0 | 0 | 14 (70.0) |
| NYHA class II–III, n (%) | 0 | 0 | 0 | 8 (40.0) |
| Biochemical data | ||||
| White blood cells, x 109 per L | 6.43 ± 1.67 | 6.58 ± 2.19 | 6.11 ± 1.58 | 6.37 ± 1.40 |
| eGFR, mL⋅min−1⋅1.73 m−2 | 80.0 ± 12.9 | 76.4 ± 14.8 | 77.1 ± 17.4 | 72.6 ± 14.4 |
| Serum insulin, μmol/L | 3.63 (2.00–9.33) | 4.68 (2.00–13.0) | 6.24* (2.00–31.0) | 6.32* (2.45–13.8) |
| hs-cTnT, µg/L | 0.005 (0.004–0.007) | 0.007* (0.005–0.010) | 0.009*† (0.006–0.015) | 0.007* (0.004–0.012) |
| NT-proBNP, pmol/L | 200 (98–508) | 195 (78–363) | 242 (96–528) | 191 (78–457) |
| Annexin-A5 | 1.43 (0.81–2.91) | 1.27 (0.79–2.10) | 1.74† (1.00–3.97) | 1.84† (0.97–3.24) |
Values are mean (±SD), number of subjects (percent), or geometric mean (10–90% interval). hs-cTnT, high-sensitive cardiac troponin T; NT-proBNP, amino terminal-pro-BNP; and eGFR, estimated glomerular filtration rate. Significance for between-groups differences: *P < 0.05 vs. NTN controls; †P < 0.05 vs. HTN (LV−).
Table S3.
Echocardiographic characteristics of participants
| Groups | ||||
| Characteristic | NTN (n = 30) | HTN (LV−) (n = 28) | HTN (LV+/CV−) (n = 29) | HTN (LV+/CV+) (n = 20) |
| LV mass index, g/m2 | 80.7 ± 13.6 | 86.1 ± 12.4 | 114.2 ± 23.0*† | 120.0 ± 23.7*† |
| Relative wall thickness | 0.34 ± 0.04 | 0.35 ± 0.04 | 0.45 ± 0.06*† | 0.43 ± 0.07*† |
| Ejection fraction, % | 62.6 ± 5.8 | 62.3 ± 5.7 | 60.1 ± 7.3 | 60.0 ± 6.7 |
| GLS, % | 20.4 ± 2.50 | 19.8 ± 2.14 | 18.8 ± 3.10* | 17.8 ± 2.79*† |
| TDI e′ peak, cm/s | 10.8 ± 1.90 | 10.2 ± 1.56 | 7.45 ± 1.83*† | 7.09 ± 1.66*† |
| E/e′ ratio | 6.65 ± 1.53 | 7.29 ± 1.04 | 9.30 ± 2.44*† | 8.51 ± 1.94*† |
| LV concentric remodeling, n (%) | 0 | 0 | 17 (58.6) | 8 (40.0) |
| LV hypertrophy, n (%) | 0 | 0 | 9 (31.0) | 7 (35.0) |
| Diastolic dysfunction, n (%) | 0 | 0 | 14 (48.3) | 9 (45.0) |
Values are mean (±SD), number of subjects (percent), or geometric mean (10–90% interval). LV concentric remodeling was relative wall thickness exceeding 0.42. LV hypertrophy was an LV mass index exceeding 110 g/m2 in women and 125 g/m2 in men. Moderate LV diastolic dysfunction was defined as having mildly to moderately elevated LV filling pressure (E/e′ >8.5) and transmitral E/A ratio within the normal age-specific range. We also used the differences in durations between the mitral A flow and the reverse pulmonary vein flow during atrial systole (Ad < ARd + 10) and/or left atrial volume index (≥29 mL/m2) to confirm possible elevation of the LV filling pressures in this group. Moreover, this group also included subjects with an elevated E/e′ ratio and an abnormally low age-specific E/A ratio (combined dysfunction). GLS, global longitudinal strain. Significance for between-groups differences: *P < 0.05 vs. NTN controls; †P < 0.05 vs. HTN (LV−).
CV Autoantibody Distribution in HTN Cases and Controls and Network Analysis.
Patients with HTN and LV remodeling or dysfunction (LV+/CV− and LV+/CV+) displayed higher levels of high-sensitive cardiac troponin T and annexin-A5 compared with NTN controls and HTN patients without LV involvement (LV−). In addition, insulin levels were also higher in patients with hypertensive heart disease (Table S2).
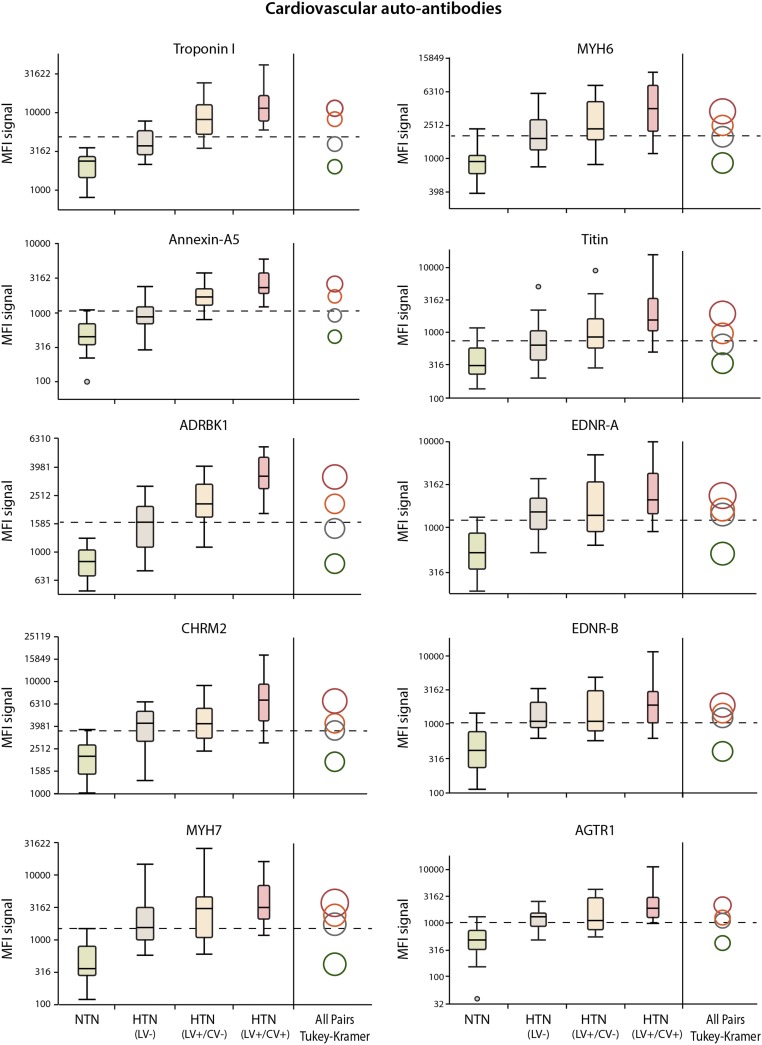
Fig. 3 shows the geometric means and distributions of the mean fluorescence intensity (MFI) signals of the 10 autoantibodies measured in serum samples of three groups of HTN subjects and one group of healthy participants. We identified that all autoantibodies measured could distinguish the healthy group from the HTN subjects. Autoantibodies to troponin I, annexin-A5, and ADRBK1 were the most significant markers that best discriminated these four groups of subjects.
Fig. 3.
Box plots of distributions of the MFI signals of the 10 measured autoantibodies in three groups of hypertensive subjects and healthy participants. Each pair of group means could be compared visually by examining the intersection of the comparison circles. Circles for means that are significantly different between groups either do not intersect or intersect slightly. If the circles intersect by an angle of more than 90°, the means are not significantly different between groups. The radii of the circles depend on the biomarker distribution, the number of tests, and number of subjects per group. The autoantibodies to troponin I, annexin-A5, and ADRBK1 were the most significant markers that best discriminated these four groups of subjects.
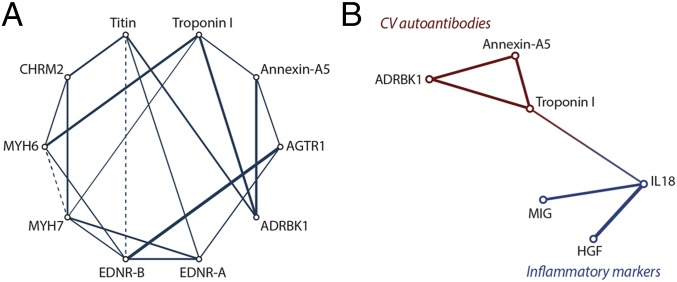
We also observed significant intracorrelations among the 10 measured autoantibodies (Fig. 4A). We explored this by constructing partial correlation diagram in which partial correlations were estimated and each variable was plotted as a node (SI Statistical Methods). The strongest correlation (r = 0.54; P < 0.0001) was observed between autoantibodies to AGTR1 and EDNR-B. Autoantibodies to ADRBK1 were significantly and directly associated with autoantibodies to troponin I, annexin-A5, and titin (r ≥ 0.33; P < 0.0001). Autoantibodies to troponin I also displayed strong and direct correlation to autoantibodies to MYH6 (r = 0.40; P < 0.0001).
Fig. 4.
Partial correlation diagrams illustrating (A) the intracorrelations among the 10 measured autoantibodies and (B) the correlation between selected CV autoantibodies and proinflammatory cytokines in all participants. Solid lines represent significant (P < 0.01 for all) direct correlations, and dashed lines show significant inverse correlation. Thicker lines correspond to stronger relationships. HGF, hepatocyte growth factor; MIG, monokine induced by gamma IFN.
Discrimination Analyses.
We applied the partial least squares-discriminant analysis (PLS-DA) model for identifying CV autoantibodies responsible for group separation (16). First, we compared HTN patients with adverse LV remodeling or dysfunction (LV+) vs. HTN patients without LV involvement (LV−). Then, we compared healthy controls vs. HTN patients with LV involvement. For each group comparison we identified the minimum number of uncorrelated latent factors derived from the measured autoantibodies, which explained from 57.4 to 89.3% of the variations between groups (P ≤ 0.006 for all; Table 1).
Table 1.
Summary data for each of the models generated by PLS-DA
| Group comparison | ||||
| Characteristics | HTN (LV+/CV-) vs. HTN (LV−) | HTN (LV+/CV+) vs. HTN (LV−) | HTN (LV+/CV−) vs. NTN | HTN (LV+/CV+) vs. NTN |
| No. of used latent factors | 3 | 2 | 3 | 3 |
| % of variation explained by latent factors | ||||
| For predictor variables (CV autoantibodies) | 69.0 | 72.4 | 85.8 | 90.1 |
| For outcome variables (LV remodeling) | 57.4 | 68.0 | 82.0 | 89.3 |
| AUC | 0.88 | 0.76 | 0.94 | 0.96 |
| No. of correctly classified | 0.83 | 0.71 | 0.86 | 0.90 |
| PAUC value | <0.0001 | 0.0062 | <0.0001 | <0.0001 |
| Top CV autoantibodies responsible for class discrimination | Troponin I (VIP = 1.50; CC = 0.59) | Troponin I (VIP = 1.34; CC = 0.26) | Troponin I (VIP = 1.11; CC = 0.31) | Troponin I (VIP = 1.08; CC = 0.27) |
| Annexin-A5 (VIP = 1.38; CC = 0.49) | Annexin-A5 (VIP = 1.25; CC = 0.22) | Annexin-A5 (VIP = 1.13; CC = 0.39) | ADRBK1 (VIP = 1.18; CC = 0.48) | |
| ADRBK1 (VIP = 1.25; CC = 0.23) | ADRBK1 (VIP = 1.18; CC = 0.40) | |||
CC, correlation coefficient for centered and scaled data.
The accuracy of the discrimination model was assessed using the number of correctly classified samples and the receiver operating characteristic (ROC) plots (SI Statistical Methods). We established that the area under the ROC curve (AUC) for the discrimination between HTN (LV−) and the two groups of HTN (LV+) patients with or without CV events was 0.76 and 0.88, respectively (P ≤ 0.006) and the AUC for the discrimination between healthy controls and the two groups of HTN (LV+) patients was as high as 0.96 (P < 0.0001) (Table 1).
Next, the importance of each autoantibody in the construction of the latent variables was assessed using the variance importance in projection (VIP) scores (SI Statistical Methods) and we identified the most significant autoantibodies responsible for group discrimination. Autoantibodies to cardiac-specific troponin I and annexin-A5 showed higher MFI signal in HTN patients with LV remodeling or dysfunction (LV+) compared with the HTN patients without LV involvement (LV−) (Table 1). For comparisons between HTN (LV+) patients with CV events (LV+/CV+) and HTN (LV−) patients, in addition to autoantibodies to troponin I and annexin-A5 we identified autoantibodies to ADRBK1 as another important biomarker for group discrimination (Table 1). These autoantibodies remained significant when we compared HTN (LV+) patients and healthy subjects.
With adjustment for sex, age, and body mass index (Table S2), level of CV autoantibodies to troponin I (P = 0.0009), annexin-A5 (P = 0.0004), and ADRBK1 (P = 0.002) remained considerably different between HTN (LV+) patients and HTN (LV−) patients. With additional adjustments of the model for the serum level of two cardiac-specific biomarkers, NT-proBNP and troponin T (Table S2), the significance of the above-mentioned CV autoantibodies still persisted.
In analyzing the relationship between autoantibodies and innate system activation we observed that autoantibodies to troponin I, annexin-A5, and ADRBK1 were linked to the innate and growth factors determined by other platforms (17). In particular, there was a significant but weak positive correlation between autoantibodies to troponin I and proinflammatory cytokine IL-18 (r = 0.09; P = 0.012) (Fig. 4B).
Temporal Stability of the Measured Autoantibodies.
Two echocardiographic examinations were available for each of the 65 participants from the LV diastolic dysfunction group, the normal diastolic function group, and the NTN group. We tested the temporal stability of all 10 CV autoantibodies in baseline and follow-up serum samples in these 65 subjects. The median time interval between two blood samplings was 4.7 y (5th to 95th percentiles are 3.7–5.4 y). The analysis of the repeated biomarkers measurements from two visits showed that the intraclass correlation coefficient (ICC) was over 93.7% for the measured autoantibodies (P < 0.0001 for all; Table S4) (18). This implies that the selected autoantibodies in this study were relatively stable between the two time points in comparison to the variability observed across the different individuals in the study.
Table S4.
MFI signal and ICC for CV autoantibodies in 65 participants
| MFI signal | ICC, % | PICC | ||
| Autoantibodies | Baseline | Follow-up | ||
| Troponin I | 5,689 (2,018–16,482) | 5,702 (1,901–15,849) | 99.7 | <0.0001 |
| Annexin-A5 | 1,161 (376–3,206) | 1,145 (383–3,148) | 99.5 | <0.0001 |
| ADRBK1 | 1,770 (798–3,990) | 1,738 (793–3,767) | 99.4 | <0.0001 |
| CHRM2 | 3,873 (1,435–9,078) | 3,855 (1,445–8,954) | 99.6 | <0.0001 |
| MYH7 | 1,710 (302–7,112) | 1,633 (282–6,855) | 98.7 | <0.0001 |
| MYH6 | 2,213 (794–6,252) | 2,178 (794–6,457) | 99.6 | <0.0001 |
| Titin | 824 (274–2,576) | 859 (267–2,455) | 93.7 | <0.0001 |
| EDNR-A | 1,330 (357–3,837) | 1,312 (331–3,776) | 99.4 | <0.0001 |
| EDNR-B | 1,330 (357–3,837) | 1,312 (331–3,776) | 99.4 | <0.0001 |
| AGTR1 | 1,178 (339–3,214) | 1,180 (325–3,388) | 99.6 | <0.0001 |
MFI values are geometric mean (10–90% interval). Each pair of baseline and follow-up samples was tested side-by-side on the same chip simultaneously; therefore, the interchip variation was not reflected in the analysis of temporal variability results.
CV Autoantibody Distribution in Subjects with DCM and Healthy Controls.
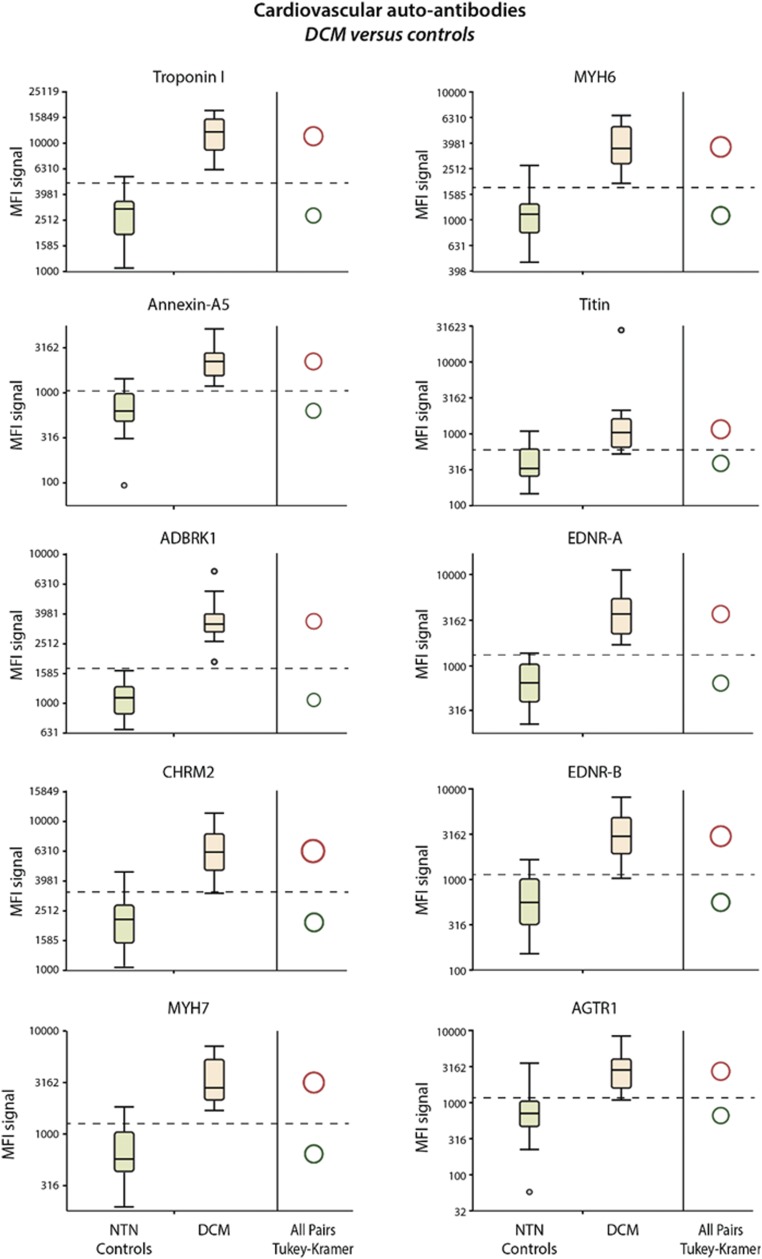
We measured CV autoantibodies in 23 subjects with DCM as well as 30 healthy control subjects (SI CV Autoantibody Distribution in Subjects with DCM and Healthy Controls). The distribution of the MFI signals of the 10 measured autoantibodies in the sera of DCM subjects and in the healthy subjects is presented in Fig. S1. We observed that all autoantibodies measured were able to discriminate the two groups of subjects.
Fig. S1.
Box plots of distributions of the MFI signals of the 10 measured autoantibodies in DCM subjects and healthy participants. All autoantibodies measured could discriminate DCM subjects and the healthy participants. The autoantibody profile established in DCM could help guide immune modulation in the future.
Discussion
We developed a high-sensitivity platform for the detection of CV autoantibodies; this multiplexed assay targeted key structural cardiac proteins as well as several key pathways involved in the pathophysiology of HF. We demonstrated that CV autoantibodies could discriminate patients with hypertensive heart disease and that autoantibodies to troponin I, ADRBK1, and annexin-A5 were the most discriminatory. In addition, we have shown that our CV autoantibody detection platform can be used to detect autoantibodies in patients with DCM.
The plasmonic gold chips had high reproducibility and consistency for measurements of CV autoantibodies. The coefficient of variation for the calibration curves of antibody detection in independent experiments was less than 14% even at a low concentration of antibodies (Fig. 2). Although ELISA is the standard for protein quantification and has been widely used for research and clinical practice, it requires a relatively large amount (submilliliters) of serum from an individual subject. In addition, both ELISA and Luminex assays require hundreds of microliters to milliliters of CV antigen (50 μL for up to $1,000) for single-autoantibody detection, making these techniques much more expensive for the detection of large numbers of autoantibodies. In contrast, the plasmonic gold chip offers the advantage of multiplexed profiling and quantification of autoantibodies using a very low serum sample volume of ∼2 μL for hundreds to thousands of samples with down to ∼15 μL of antigens.
The three autoantibodies that best differentiated hypertensive heart diseases emerged from our study were autoantibodies to troponin I, annexin-A5, and ADRBK1. Autoantibodies to titin also provided some discrimination for hypertensive heart diseases. Autoantibodies specific to the vasculature such as autoantibodies to EDNR-A and EDNR-B as well as CHRM2 or AGTR1 were more closely associated with hypertensive status than with hypertensive heart diseases (Fig. 3 and Table 1). Our study established that the discriminatory ability of CV autoantibody profiling was highly promising and autoantibody profiling could complement B-type natriuretic peptide or high sensitivity troponin for the diagnosis of early stages of hypertensive heart disease (13).
Because cardiac troponins are the biomarkers of choice for the assessment of myocardial injury, several previous studies analyzed the autoantibodies against cardiac-specific troponin I and T using the classical ELISA method or affinity chromatography with surface plasmon resonance analysis (19). The reported prevalence of autoantibodies to cardiac-specific troponin I varies from 7.3% in patients with acute coronary syndrome up to 27.9% in patients with cardiomyopathies (20–22). Of note, the experimental study showed that autoimmune response to recombinant murine cardiac troponin I induced inflammation in the myocardium followed by fibrosis and HF in mice (23). Therefore, cardiac-specific autoantibodies against troponin I might also be considered as a mediator of cardiotoxicity, with further validation needed in hypertensive heart diseases.
Circulating annexin-A5 as well as antibodies directed against annexin-A5 were associated with evidence of hypertensive heart diseases. Annexin-A5 is a cellular protein in the annexin group that is commonly used as a biomarker to detect apoptotic cells and may also play a role in the inhibition of blood coagulation. Using annexin-A5 knockout mice, Schurgers et al. (24) demonstrated that annexin-A5 is highly expressed in organs that are often affected by HF including lung, kidney, liver, and spleen. The authors suggested that cell lysis in these organs due to hypoperfusion resulted in a marked and significant increase in annexin-A5 concentrations. Along similar lines, in our study we observed that a higher level of circulating annexin-A5 in hypertensive patients with LV remodeling was accompanied by an increased level of annexin-A5 autoantibodies (Fig. 3, Table 1, and Table S2). Interestingly, antibodies against annexin-A5 were found in patients with the antiphospholipid syndrome (25). In this disease, antibodies to annexin-A5 can cause enhanced coagulation and thrombosis might disrupt the formation of the shield around negatively charged phospholipid molecules at the cell surface.
The beta-adrenergic system is a key regulatory component in HF, and several studies have shown that the adrenergic system is highly activated with advancing stages of HF (26). The fact that the production of beta-adrenergic autoantibodies increased with the development of HTN with LV hypertrophy or diastolic dysfunction (Fig. 3 and Table 1) suggested a potential role in its pathophysiology. Our results are consistent with the prior experimental studies from Matsui et al. (6) and Wallukat et al. (7), suggesting a role of anti-β1 adrenergic antibodies in the development of ventricular hypertrophy. Novel therapies targeting the anti–beta-1-adrenergic receptor antibodies could also offer an opportunity for future research and development in this area (27).
Our study profiles autoantibodies against titin, a key structural myocardial protein (28). Mutations to titin are recognized as an important cause of DCM (29). We observed that autoantibodies to titin differed between the groups of HTN patients with or without LV hypertrophy or diastolic dysfunction (Fig. 3 and Table 1), but they were not the most discriminatory autoantibodies overall. More studies are needed to test different titin antigens because this can influence the sensitivity of the assay.
The different CV autoantibodies in our study were not independent of each other but were linked through a strong network (Fig. 4A). In particular, autoantibodies to troponin I were significantly and directly related to MYH6 and ADRBK1 autoantibodies, whereas EDNR-B autoantibodies displayed the strongest direct correlation with AGTR1 autoantibodies. In a previous study we have shown that IL-18, a cytokine reflecting inflammasome activation, as well as HGF and CXCL9, was associated with cardiac remodeling and dysfunction in patients with HTN (17). Our findings suggested that there could be a link between autoimmunity and inflammasome activation in cardiac maladaptation in early stages of hypertensive heart diseases. This model is an emerging concept in several immune-mediated diseases (30). Moreover, several studies suggest that inflammasome activation can lead to the production of CV autoantibodies that can further cause the progression of disease via proinflammatory, prothrombotic, and cytotoxic effects (31, 32).
The analysis of the repeated biomarkers measurements from two visits showed that the ICC was high for all measured autoantibodies (Table S4). It should be noted, however, that each pair of the baseline and follow-up samples were tested side-by-side on the same chip simultaneously; therefore, the interchip variation was not reflected in this analysis of temporal stability. Over the follow-up period of 4.7 y, we confirmed that all these participants remained in the same category and no target organ damages or worsening of patients’ conditions were observed.
Our study also tested the presence of autoantibodies in the other spectrum of HF, that is, DCM (Fig. S1). Previous studies suggested a role for autoimmunity in the pathogenesis of DCM (30). Latif et al. (3) measured heart-specific autoantibodies including myofibrillar proteins by gel electrophoresis (SDS/PAGE) and Western blotting in patients with DCM or with ischemic heart disease. The study concluded that patients with DCM had a significantly greater frequency and reactivity of IgG autoantibodies against six myocardial proteins than patients with ischemic heart disease. In our study, the nanotechnology-based plasmonic gold platform was very sensitive in detecting circulating autoantibodies in patients with both ischemic and nonischemic cardiomyopathy and could potentiality be used to monitor immunomodulatory therapy and for risk stratification purposes in the future.
In conclusion, we developed a multiplexed specific CV autoantibody detection platform on a nanotechnology-based plasmonic gold chip and successfully applied it in the evaluation of CV autoantibody profiles in HTN patients with cardiac maladaptation for potential diagnostic and prognostic purposes. Autoantibodies to troponin I, annexin-A5, and ADRBK1 emerged as the most strongly associated with LV remodeling and dysfunction in patients with HTN. The panel we have developed could also be promising for application in different fields of cardiology, including myocarditis, dilated cardiomyopathies, pulmonary arterial HTN, and heart transplantation after further optimization and validation.
Materials and Methods
SI Materials and Methods details the materials and methods followed in this paper. Supporting Information contains protocol for the construction of the CV autoantibody detection panel on a plasmonic gold platform, an explanation of the case control study, the statistical methods used for data analysis, a description of the echocardiography, and the test results on DCM patients. Each patient gave informed consent for all experiments. The experiments were approved by Leuven University and Stanford University Institutional Review Boards.
SI Materials and Methods
The 10 antigens used in the multiplexed autoantibodies detection on plasmonic gold chips were purchased from various vendors. Troponin I (purified recombinant troponin I protein) and annexin-A5 (purified recombinant annexin-A5 protein) were purchased from Fitzgerald. α-Chain MYH6 recombinant protein, titin (TTN recombinant protein), and beta 1-adrenegic receptor (ADRBK1 recombinant human protein) were purchased from MyBioSource.com. β-Chain MYH7 human recombinant protein, endothelin receptor A (EDNRA human recombinant protein), endothelin receptor B (EDNRB human recombinant protein), cholinergic receptor muscarinic 2 (CHRM2 human recombinant protein), and angiotensin II receptor 1 (AGTR1 human recombinant protein) were purchased from Abnova. ADRBK1 (human ADRB1 protein) was purchased from Abcam. Troponin I antibodies (mouse troponin I antibody Cardiac) were purchased from Fitzgerald and CHRM2 antibodies (mouse CHRM2 antibody) were from Abnova. Plasmonic gold chips were from Nirmidas. BSA was purchased from Sigma-Aldrich, PBS and FBS were purchased from GE Healthcare Life Sciences, Tween-20 was purchased from Sigma-Aldrich, unlabeled goat anti-human IgG antibody and goat anti-mouse IgG antibody were purchased from Vector Laboratory, and IRDye 800CW NHS-ester was purchased from LI-COR Biosciences.
SI Construction of CV Autoantibody Detection on Plasmonic Gold Platform
To construct a sandwich immunoassay on this platform for specific CV autoantibody quantification we selected 10 commercially available recombinant proteins as target antigens and immobilized them on plasmonic gold chips by robotic array printing (Nano-Plotter 2.1; GESIM) at 3 μM in triplicate spots at specific locations. We tested the performance of each antigen using products from several vendors to identify the best ones for our autoantibody assays.
The chips were then covered by multiarray chambers (Grace Bio-Labs) and up to 16 individual serum samples can be assayed on each chip. The chips were first blocked using 5% BSA (Sigma-Aldrich) in PBS for 1 h. Then, an ultralow volume of 2 μL of each human serum was diluted 1:20 in FBS and incubated over the printed antigen chips for 40 min at room temperature during which the human IgG autoantibodies from the patients’ serum samples would be effectively captured on the target antigen spots. The plasmonic gold chips were then washed three times with PBS with 0.05% Tween (PBST) and subsequently incubated in 2 nM IRDye 800-labeled anti-human IgG secondary antibodies for 20 min. After incubation, the chips were washed three times with PBST, once with PBS, and once with deionized water and dried with compressed air. The abundance of each autoantibody in serum could be quantified by scanning the chip (Odyssey; LI-COR Biosciences) and measuring the signal of IRDye 800 fluorescence of the spots.
For signal normalization purposes and assay quality control, serum samples of one HTN subject with LV remodeling (positive control) and one healthy subject (negative control) were selected and included on each plasmonic gold chip. Each patient’s final result was quantified by an MFI ratio obtained by dividing the patient’s actual MFI of spots by the MFI of the spots of the standard positive-control sample. The same positive-control samples were used for signal normalization for all of the samples measured throughout this work.
To establish the dynamic range and sensitivity of antibody detection on plasmonic gold chips, we generated calibration curves of detection of antibodies to troponin I and CHRM2 using commercial antibodies with known concentrations. The chips with 10 printed CV antigens were first blocked using 5% BSA in PBS for 1 h. Each commercial antibody was serially diluted from concentration 1 ng/mL to 10 fg/mL in FBS and antibody solutions at different concentrations were applied to the multiplexed microarray. After 40 min of incubation, the plasmonic gold chips were washed three times with PBST and subsequently incubated in 2 nM IRDye 800-labeled anti-mouse IgG secondary antibodies for 20 min. After this final incubation, the chips were washed three times with PBST, once with PBS, and once with deionized water and dried with compressed air. The chip was scanned and signals of IRDye 800 fluorescence of the spots at each concentration of antibody were recorded. To determine the consistency and reproducibility of the platform, we repeated the above assay three times on different plasmonic gold chips and on various days. The final calibration curves were constructed by plotting antibody MFI vs. concentration of antibodies.
SI Case Control Study
This study is a subanalysis of the large-scale population study described elsewhere (17, 33). Standardized and validated questionnaires were completed to collect detailed information about each participant’s personal and familial medical history, use of medication, and lifestyle. High-fidelity phenotyping at the examination center included clinical and anthropometric measurements and echocardiography (33). Fasting blood samples were stored in a biobank for later analysis. For measurements of autoantibody we used serum samples. The Ethics Committee approved the population study. All participants gave written informed consent. The exclusion criteria for sample collection were previous history of cancer or systemic inflammatory diseases and age above 80 y. We defined HTN as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or the use of antihypertensive drugs. LV concentric remodeling was relative wall thickness (RWT) exceeding 0.42. LV hypertrophy was a LV mass index exceeding 110 g/m2 in women and 125 g/m2 in men (Table S2). Among all of the tested autoantibodies, only troponin I autoantibodies were associated with GFR (P = 0.024). Due to the cardiac specificity of troponin I autoantibodies, this association was secondary to the deterioration of renal function in patients with more advanced stages of HF. As summarized in Table S2, GFR measurements tended to be lower in the HTN LV+/CV+ group compared with the NTN group (72.6 mL⋅min−1⋅1.73 m−2 vs. 80.0 mL⋅min−1⋅1.73 m−2, P = 0.066).
In the LV+/CV+ group, 9 out of 20 patients reported a previous history of cardiac diseases before the baseline examination. During the follow-up period, nonfatal CV events occurred in 18 patients from this group. The 10th to 90th percentiles of time to events varied from 0.74 to 6.9 y, respectively. Overall, five, six, and seven patients experienced coronary (myocardial infarction and/or PTCA), symptomatic HF, or vascular (stroke or peripheral arteries revascularization) events, respectively.
SI Statistical Methods
For statistical analysis, we used SAS software, version 9.3 and JMP Genomics (SAS Institute, Cary, NC). We normalized the distributions of all analyzed CV autoantibodies by a logarithmic transformation and compared means between groups by a t test or ANOVA test and proportions by the χ2 statistics. Statistical significance was a two-sided significance level of 0.05. We explored correlations between CV antibodies by constructing the partial correlation diagram using JMP Genomics 6.0. This process fits covariance selection models (graphical Gaussian models), in which partial correlations are estimated, and then plots each variable as a node. To identify CV antibodies associated with LV maladaptation in patients with HTN, we used PLS-DA (16). We chose this method for its ability to deal with highly correlated predictors. PLS-DA created several linear combinations (latent factors) of the log-transformed autoantibodies in the way to maximize the covariance between the antibodies and LV remodeling. We identified the minimum number of factors that explained a substantial proportion of variation for both predictor and outcome variables and was not significantly different from the model with the minimum PRESS value. We also applied a logistic model to model the association between selected by PLS-DA autoantibodies and being a case or a control. Age, sex, and body mass index were offered as confounding factors to the model. The P value to enter and stay in the models was set at 0.05. The accuracy of the discrimination model was assessed using the number of correctly classified samples and the ROC plots. The AUC provides a single measure of overall accuracy that is independent of any particular threshold. We applied maximum-likelihood estimation to a mixed-effects model to calculate the ICC. The ICC is commonly used to assess of consistency of quantitative measurements (biomarkers) made in samples obtained during different time points.
SI Echocardiography
The participants refrained from smoking, heavy exercise, and drinking alcohol or caffeine-containing beverages for at least 3 h before echocardiography. The blood pressure during echocardiography was the average of two readings, obtained with a validated OMRON 705IT device (Omron Corp.) at the end of the echocardiographic examination.
Data Acquisition.
One experienced physician (T.K.) did the ultrasound examination (33), using a Vivid7 Pro (GE Vingmed) interfaced with a 2.5- to 3.5-MHz phased-array probe, according to the recommendations of the American Society of Echocardiography (34). With the subjects in partial left decubitus and breathing normally the observer obtained images, together with a simultaneous ECG signal, along the parasternal long and short axes and from the apical four- and two-chamber long-axis views. All recordings included at least five cardiac cycles and were digitally stored for off-line analysis. M-mode echocardiograms of the left ventricle (LV) were recorded from the parasternal long-axis view under control of the 2D image. The ultrasound beam was positioned just below the mitral valve at the level of the posterior chordae tendineae.
To record pulsed-wave Doppler mitral and pulmonary vein (PV) flow velocities from the apical window, the observer positioned the Doppler sample volume at the mitral valve tips and in the right superior PV, respectively. Using TDI (tissue Doppler imaging), the observer recorded low-velocity, high-intensity myocardial signals at a high frame rate (>190 frames per s), while adjusting the imaging angle to ensure a parallel alignment of the ultrasound beam with the myocardial segment of interest. From the apical window, the sonographer placed a 5-mm Doppler sample at the septal, lateral, inferior, and posterior sites of the mitral annulus.
Off-Line Analysis.
One experienced physician (T.K.) analyzed digitally stored images, averaging three heart cycles for statistical analysis, using a workstation running the EchoPac software package (GE Vingmed). The LV internal diameter and interventricular septal and posterior wall thickness were measured at end-diastole from the two-dimensionally guided M-mode tracing. When optimal orientation of M-mode ultrasound beam could not be obtained, the reader performed linear measurements on correctly oriented 2D images. End-diastolic LV dimensions were used to calculate LV mass by an anatomically validated formula. RWT was calculated as the ratio of (interventricular septum + posterior wall)/LV internal diameter at end diastole. LV mass index was LV mass divided by body surface area, calculated as body weight0.425 (in kilograms) × body height0.725 (in centimeters) × 0.007184. LV concentric remodeling was RWT exceeding 0.42. LV hypertrophy was an LV mass index exceeding 110 g/m2 in women and 125 g/m2 in men. We calculated LV EF from LV end-systolic and end-diastolic volumes measured from the apical four- and two-chambers views, using the standard Simpson’s method. We measured left atrial (LA) dimensions in three orthogonal planes: the parasternal long, lateral, and supero-inferior axes. LA volume was calculated using the prolate-elipsoid method and was indexed to body surface area. LA enlargement was a left atrial volume index exceeding 29 mL/m2.
From the transmitral flow signal, we measured peak early diastolic velocity (E), peak late diastolic velocity (A), the E/A ratio, and A flow duration. From the PV flow signal we measured the duration of PV reversal time during atrial systole (AR). From the TDI recordings we measured peaks systolic (s′) and early (e′) and late (a′) diastolic mitral annular velocities, and the e′/ a′ ratio at the four acquisition sites (septal, lateral, inferior, and posterior). We calculated the E/ e′ ratio by dividing transmitral E peak by e′ averaged from the four acquisition sites.
We combined the mitral inflow and TDI velocities to classify the stages of LV diastolic dysfunction at baseline and follow-up as previously described (33, 35). In this study, we selected hypertensive patients with moderate LV diastolic dysfunction, which was defined as having mildly to moderately elevated LV filling pressure (E/ e′ >8.5), and transmitral E/A ratio within the normal age-specific range. We also used the differences in durations between the mitral A flow and the reverse pulmonary vein flow during atrial systole (Ad < ARd + 10) and/or left atrial volume index (≥ 29 mL/m2) to confirm possible elevation of the LV filling pressures in this group. Moreover, this group also included subjects with an elevated E/ e′ ratio and an abnormally low age-specific E/A ratio (combined dysfunction). Previously we observed an increase in all CV events in the diastolic dysfunction group characterized by elevated LV filling pressure (35).
For measurement of 2D strain, the endocardial borders were manually traced at the end-systolic frame of the 2D images from the three long-axis views. The 2D strain software (Q-analysis; GE Vingmed) automatically tracks myocardial speckle motion, creating basal, mid, and apical regions of interest.
Reproducibility.
To determine intraobserver reproducibility, an experienced echocardiographer (T.K.) analyzed the echocardiograms of 17 subjects twice. Intraobserver reproducibility coefficient of a measurement was the 2 SD interval about the mean of the relative differences across pairwise readings. The intraobserver reproducibility was 2.2% for LV internal end-diastolic diameter, 4.6% for LV wall thickness, and 4.3% for LV mass. For tissue Doppler velocities, as reported previously (33), the reproducibility across the four sampling sites ranged from 4.48 to 5.34% for e′ velocities and from 3.96 to 4.52% for a′ velocities.
SI CV Autoantibody Distribution in Subjects with DCM and Healthy Controls
We also measured CV autoantibodies in a group of patients with DCM and left ventricular ejection fraction (LVEF) <35%. We recruited 23 subjects with DCM from Stanford Inherited Cardiomyopathy Centre as well as 30 healthy control subjects. The mean age was 59 ± 13 y, 52% were female, the mean LVEF was 28 ± 4%, and 78% of patients were in New York Heart Association (NYHA) class III. Among comorbidities diabetes mellitus was present in 26% of subjects and chronic kidney disease (greater than stage III) was noted is 34% of subjects. Fig. S1 shows the distribution of the MFI signals of the 10 measured autoantibodies in the sera of DCM subjects as well as in the healthy subjects. We observed that all autoantibodies measured were able to discriminate the two groups of subjects.
Some healthy individuals tested in our study displayed a weak level of detectable circulating CV autoantibodies. It should be noted that any risk factors or pathophysiological process that lead to the damage of cardiomyocytes could activate immune response, which causes an increase in the level of circulating autoantibodies. Adamczyk et al. (36) demonstrated that the prevalence of cardiac troponin I autoantibodies was as high as 12.7% in the cohort of normal donors. The rationale for the presence of autoantibodies to troponin I in the apparently healthy subjects might be related to immunization from the release of small amount of cardiac troponin I as the result of a continual senescence and regeneration of cardiomyocytes throughout life or the release of highly homologous skeletal troponin I from muscular injury (37). This process of clearance of the antigens from the body could be considered as a normal consequence of an adaptive immune response to antigens in healthy individuals. To detect the autoantibodies against cardiac troponin I, these researchers used chemiluminescent microplate immunoassay (36), which might have somewhat lower sensitivity compared with the plasmonic chip used in our study. Similarly, using the high-sensitivity troponin T assay (Roche Troponin T hs STAT), investigators were able to detect circulating troponins in up to 66% of participants recruited from the general population (38). Another study found that the high-sensitive troponin T concentration was below the limit of blank (3 ng/L) only in 6.3% of the general population sample (39).
SI Other Measurements
We used a validated questionnaire to inquire about lifestyle, medical history, use of medications, and smoking and drinking habits. The conventional blood pressure was the average of five consecutive auscultator readings obtained with the subject in the seated position. HTN was defined as a blood pressure of at least 140 mmHg systolic or 90 mmHg diastolic or as the use of antihypertensive drugs. Body mass index was weight in kilograms divided by the square of height in meters.
Acknowledgments
X.L. thanks Youmin Rong for his help with graph making. We thank Janine Sung and Rohit Gupta from Stanford Immune Monitoring Core Laboratory. F.H. thanks Mark Davis and Kirk Knowlton for their advice. H.D. acknowledges gift funds from the Deng family for this work. This work was supported by American Heart Association Grant 13EIA14420025 and a Burroughs Wellcome Foundation Innovation in Regulatory Science Award (to J.C.W.); the Pai Chan Lee research fund (H.D.); and the Stanford Cardiovascular Institute (F.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621457114/-/DCSupplemental.
References
- 1.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Innate immunity and the failing heart: The cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latif N, et al. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS-PAGE and western blotting. J Am Coll Cardiol. 1993;22:1378–1384. doi: 10.1016/0735-1097(93)90546-d. [DOI] [PubMed] [Google Scholar]
- 4.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circ Res. 2013;113:78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei J, et al. The prognostic role of angiotensin II type 1 receptor autoantibody in non-gravid hypertension and pre-eclampsia A meta-analysis and our studies. Medicine (Baltimore) 2016;95:e3494. doi: 10.1097/MD.0000000000003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui S, et al. Active immunization of combined beta1-adrenoceptor and M2-muscarinic receptor peptides induces cardiac hypertrophy in rabbits. J Card Fail. 1999;5:246–254. doi: 10.1016/s1071-9164(99)90009-x. [DOI] [PubMed] [Google Scholar]
- 7.Wallukat G, Blasig IE, Morwinski R, Herrmann HJ, Rohde E. The sera of spontaneously hypertensive rats contain agonistic auto-antibodies against the beta 1-adrenoceptor. J Hypertens. 1995;13:1031–1036. doi: 10.1097/00004872-199509000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Kumar RB, Dai H, Feldman BJ. A plasmonic chip for biomarker discovery and diagnosis of type 1 diabetes. Nat Med. 2014;20:948–953. doi: 10.1038/nm.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, et al. Plasmonic micro-beads for fluorescence enhanced, multiplexed protein detection with flow cytometry. Chem Sci (Camb) 2014;5:4070–4075. [Google Scholar]
- 10.Koh B, et al. Visible to near-infrared fluorescence enhanced cellular imaging on plasmonic gold chips. Small. 2016;12:457–465. doi: 10.1002/smll.201502182. [DOI] [PubMed] [Google Scholar]
- 11.Li X, et al. Multiplexed anti-toxoplasma IgG, IgM, and IgA assay on plasmonic gold chips: Towards making mass screening possible with dye test precision. J Clin Microbiol. 2016;54:1726–1733. doi: 10.1128/JCM.03371-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabakman SM, et al. Plasmonic substrates for multiplexed protein microarrays with femtomolar sensitivity and broad dynamic range. Nat Commun. 2011;2:466. doi: 10.1038/ncomms1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonagh TA, et al. NT-proBNP and the diagnosis of heart failure: A pooled analysis of three European epidemiological studies. Eur J Heart Fail. 2004;6:269–273. doi: 10.1016/j.ejheart.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Kuznetsova T, et al. Additive prognostic value of left ventricular systolic dysfunction in a population-based cohort. Circ Cardiovasc Imaging. 2016;9:e004661. doi: 10.1161/CIRCIMAGING.116.004661. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetsova T, et al. European Project On Genes in Hypertension (EPOGH) Investigators Impact and pitfalls of scaling of left ventricular and atrial structure in population-based studies. J Hypertens. 2016;34:1186–1194. doi: 10.1097/HJH.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 16.SAS . SAS/STAT Users Guide. The PLS Procedure. Version 8. SAS Institute Inc.; Cary, NC: 1999. pp. 2691–2734. [Google Scholar]
- 17.Kuznetsova T, et al. Cytokines profile in hypertensive patients with left ventricular remodeling and dysfunction. J Am Soc Hypertens. 2015;9:975–84.e3. doi: 10.1016/j.jash.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Epstein MM, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol Biomarkers Prev. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donohoe TJ, Schrale RG, Ketheesan N. The role of anti-myosin antibodies in perpetuating cardiac damage following myocardial infarction. Int J Cardiol. 2016;209:226–233. doi: 10.1016/j.ijcard.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 20.Lindahl B, et al. Autoantibodies to cardiac troponin in acute coronary syndromes. Clin Chim Acta. 2010;411:1793–1798. doi: 10.1016/j.cca.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Leuschner F, et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J. 2008;29:1949–1955. doi: 10.1093/eurheartj/ehn268. [DOI] [PubMed] [Google Scholar]
- 22.Shmilovich H, et al. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int J Cardiol. 2007;117:198–203. doi: 10.1016/j.ijcard.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 23.Göser S, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation. 2006;114:1693–1702. doi: 10.1161/CIRCULATIONAHA.106.635664. [DOI] [PubMed] [Google Scholar]
- 24.Schurgers LJ, et al. Circulating annexin A5 predicts mortality in patients with heart failure. J Intern Med. 2016;279:89–97. doi: 10.1111/joim.12396. [DOI] [PubMed] [Google Scholar]
- 25.Koniari I, et al. Antiphospholipid syndrome; its implication in cardiovascular diseases: a review. J Cardiothorac Surg. 2010;5:101. doi: 10.1186/1749-8090-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: A convergence of receptor pharmacology and clinical cardiology. Circ Res. 2011;109:1176–1194. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 27.Patel PA, Hernandez AF. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur J Heart Fail. 2013;15:724–729. doi: 10.1093/eurjhf/hft065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeWinter MM, Zile MR. Could modification of titin contribute to an answer for heart failure with preserved ejection fraction? Circulation. 2016;134:1100–1104. doi: 10.1161/CIRCULATIONAHA.116.023648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayal U, Prasad S, Cook SA. Genetics and genomics of dilated cardiomyopathy and systolic heart failure. Genome Med. 2017;9:20. doi: 10.1186/s13073-017-0410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux-Lombard P, Pagano S, Montecucco F, Satta N, Vuilleumier N. Auto-antibodies as emergent prognostic markers and possible mediators of ischemic cardiovascular diseases. Clin Rev Allergy Immunol. 2013;44:84–97. doi: 10.1007/s12016-010-8233-z. [DOI] [PubMed] [Google Scholar]
- 31.Torre-Amione G, et al. Advanced Chronic Heart Failure CLinical Assessment of Immune Modulation Therapy Investigators Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): A placebo-controlled randomised trial. Lancet. 2008;371:228–236. doi: 10.1016/S0140-6736(08)60134-8. [DOI] [PubMed] [Google Scholar]
- 32.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95:3C–8C, discussion 38C–40C. doi: 10.1016/j.amjcard.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Kuznetsova T, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 34.Gottdiener JS, et al. American Society of Echocardiography American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Kuznetsova T, et al. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;3:e000789. doi: 10.1161/JAHA.114.000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamczyk M, Brashear RJ, Mattingly PG. Circulating cardiac troponin-I autoantibodies in human plasma and serum. Ann N Y Acad Sci. 2009;1173:67–74. doi: 10.1111/j.1749-6632.2009.04617.x. [DOI] [PubMed] [Google Scholar]
- 37.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 38.de Lemos JA, deFilippi CR. Prevalence and significance of detectable troponins as measured by highly sensitive assays in the general population. Coron Artery Dis. 2013;24:705–709. doi: 10.1097/MCA.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 39.Ravassa S, et al. Biomarkers of cardiomyocyte injury and stress identify left atrial and left ventricular remodelling and dysfunction: A population-based study. Int J Cardiol. 2015;185:177–185. doi: 10.1016/j.ijcard.2015.03.046. [DOI] [PubMed] [Google Scholar]