Significance
The production of biofilms is an important strategy used by both bacteria and fungi to colonize surfaces and to enhance resistance to killing by immune cells and antimicrobial agents. We demonstrate that glycoside hydrolases derived from the opportunistic fungus Aspergillus fumigatus and Gram-negative bacterium Pseudomonas aeruginosa can be exploited to disrupt preformed fungal biofilms and reduce virulence. Additionally, these glycoside hydrolases can be used to potentiate antifungal drugs by increasing their hyphal penetration, to protect human cells from fungal-induced injury, and attenuate virulence of A. fumigatus in a mouse model of invasive aspergillosis. The findings of this study identify recombinant microbial glycoside hydrolases as promising therapeutics with the potential for antibiofilm activity against pathogens across different taxonomic kingdoms.
Keywords: biofilm, Aspergillus, Pseudomonas, therapeutics, exopolysaccharide
Abstract
Galactosaminogalactan and Pel are cationic heteropolysaccharides produced by the opportunistic pathogens Aspergillus fumigatus and Pseudomonas aeruginosa, respectively. These exopolysaccharides both contain 1,4-linked N-acetyl-d-galactosamine and play an important role in biofilm formation by these organisms. Proteins containing glycoside hydrolase domains have recently been identified within the biosynthetic pathway of each exopolysaccharide. Recombinant hydrolase domains from these proteins (Sph3h from A. fumigatus and PelAh from P. aeruginosa) were found to degrade their respective polysaccharides in vitro. We therefore hypothesized that these glycoside hydrolases could exhibit antibiofilm activity and, further, given the chemical similarity between galactosaminogalactan and Pel, that they might display cross-species activity. Treatment of A. fumigatus with Sph3h disrupted A. fumigatus biofilms with an EC50 of 0.4 nM. PelAh treatment also disrupted preformed A. fumigatus biofilms with EC50 values similar to those obtained for Sph3h. In contrast, Sph3h was unable to disrupt P. aeruginosa Pel-based biofilms, despite being able to bind to the exopolysaccharide. Treatment of A. fumigatus hyphae with either Sph3h or PelAh significantly enhanced the activity of the antifungals posaconazole, amphotericin B, and caspofungin, likely through increasing antifungal penetration of hyphae. Both enzymes were noncytotoxic and protected A549 pulmonary epithelial cells from A. fumigatus-induced cell damage for up to 24 h. Intratracheal administration of Sph3h was well tolerated and reduced pulmonary fungal burden in a neutropenic mouse model of invasive aspergillosis. These findings suggest that glycoside hydrolases can exhibit activity against diverse microorganisms and may be useful as therapeutic agents by degrading biofilms and attenuating virulence.
The mold Aspergillus fumigatus and the Gram-negative bacterium Pseudomonas aeruginosa are opportunistic pathogens that cause pulmonary infection in immunocompromised patients and individuals who suffer from chronic lung diseases such as cystic fibrosis and bronchiectasis. A. fumigatus is the second most common nosocomial fungal infection (1), and ∼10% of all nosocomial bacterial infections are caused by P. aeruginosa (2). Mortality associated with P. aeruginosa infections is high (3) and has increased with the emergence of multi- and even pan-resistance to antibiotics (3, 4). Similarly, invasive aspergillosis is associated with mortality rates of up to 50% (5), and increasing rates of antifungal resistance have been reported worldwide (6). These factors underscore the urgent need for new effective therapies for these infections.
Although A. fumigatus and P. aeruginosa are members of different taxonomic kingdoms, both produce biofilms that constitute a protective lifestyle for the organism. Biofilms are complex communities of microorganisms that grow embedded in an extracellular matrix composed of DNA, protein, and exopolysaccharide (7). Biofilm formation provides a significant advantage to these organisms because the matrix mediates adherence to host cells (8, 9) and aids in the resistance to both antimicrobial agents (10, 11) and host–immune defenses (12, 13). A. fumigatus biofilm formation depends on the cationic polysaccharide galactosaminogalactan (GAG), a heteroglycan composed of α1,4-linked galactose and N-acetyl-d-galactosamine (GalNAc) that is partially deacetylated (14, 15). In comparison, P. aeruginosa has the genetic capacity to produce three biofilm exopolysaccharides: alginate, Psl and Pel (16). GAG shares several similarities with Pel, which has been identified as a cationic heteroglycan composed of 1,4-linked GalNAc and N-acetyl-d-glucosamine (GlcNAc) (17). Like GAG, the cationic nature of Pel results from partial deacetylation of the polymer (17). Most clinical and environmental isolates of P. aeruginosa use Pel and Psl during biofilm formation (18). Alginate is dispensable for biofilm formation and is only observed in chronic pulmonary infection when strains switch to a mucoid phenotype (18, 19).
Strains of Aspergillus and P. aeruginosa with impaired GAG, or Pel and Psl biosynthesis exhibit attenuated virulence (20, 21), suggesting that targeting these exopolysaccharides may be a useful therapeutic strategy. We previously demonstrated that recombinant glycoside hydrolases PelAh and PslGh, encoded in the pel and psl operons of P. aeruginosa, respectively, target and selectively hydrolyze the Pel and Psl exopolysaccharide components of the Pseudomonas biofilm matrix (22). Treatment with these enzymes rapidly disrupts established biofilms, increasing the susceptibility of P. aeruginosa to human neutrophil killing and potentiation of the antibiotic colistin (22).
Our recent work on Aspergillus has identified a cluster of five genes that encode the proteins necessary for GAG biosynthesis (15). As with P. aeruginosa, we found that the product of one of these genes contains a glycoside hydrolase domain, Sph3h, which is capable of hydrolyzing purified and cell wall-associated GAG (23). In the present study, we assessed the therapeutic potential of Sph3h in disrupting fungal biofilms. We establish that the exogenous addition of Sph3h is capable of rapidly disrupting existing biofilms of this organism at nanomolar concentrations. Additionally, we demonstrate cross-kingdom activity, because the P. aeruginosa glycoside hydrolase, PelAh, was able to disrupt A. fumigatus biofilms. Whereas Sph3h was able to bind Pel, it was unable to disrupt preformed P. aeruginosa Pel-mediated biofilms. Treatment with Sph3h or PelAh increased the susceptibility of wild-type and azole-resistant A. fumigatus strains to lipophilic antifungal drugs. Kinetic studies with labeled posaconazole indicate that the increased susceptibility to antifungals is due to increased penetration of fungal cells by these agents. Both Sph3h and PelAh were nontoxic to mammalian cells and protected epithelial cells from A. fumigatus-induced damage for up to 24 h. Intratracheal delivery of Sph3h was well tolerated by mice and significantly reduced the fungal burden of immunocompromised mice infected with A. fumigatus. Our results suggest that glycoside hydrolases have the potential to be effective antibiofilm therapeutics that can mediate activity against evolutionarily diverse microorganisms.
Results
Sph3h Disrupts Preformed A. fumigatus Biofilms.
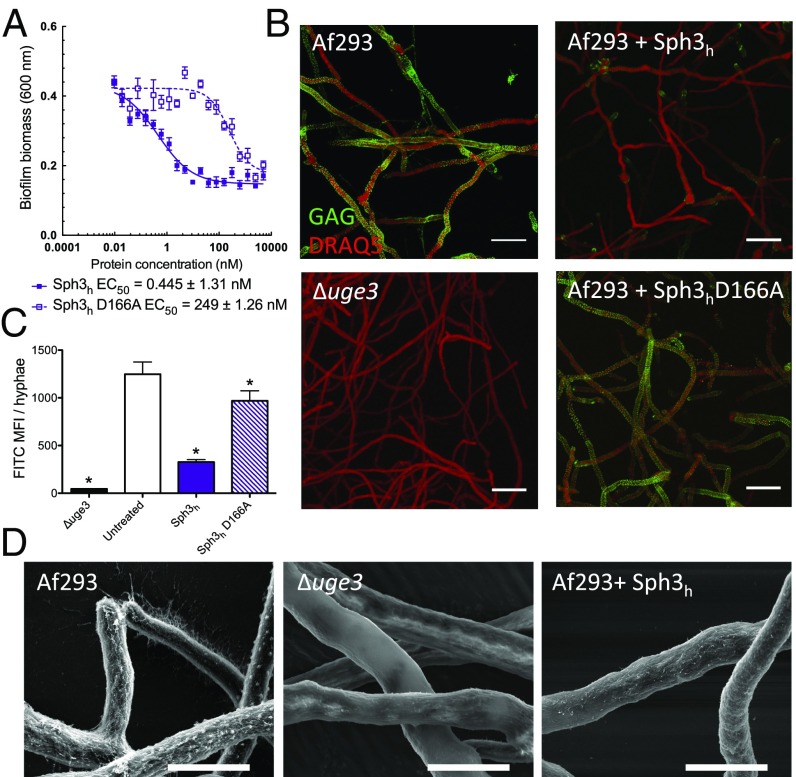
Our previous work demonstrated that Sph3h from A. fumigatus and Aspergillus clavatus can hydrolyze both purified and cell wall-bound GAG on young hyphae (23). We therefore sought to determine whether the degradation of GAG by Sph3h could disrupt established A. fumigatus biofilms. Treatment with Sph3h for 1 h disrupted established A. fumigatus biofilms with an effective concentration for 50% activity (EC50) of 0.45 ± 1.31 nM (Fig. 1A). Biofilm disruption was associated with a marked reduction in hyphae-associated GAG as detected by lectin staining (Fig. 1 B and C) and scanning electron microscopy (Fig. 1D). A catalytic variant Sph3h D166A, which does not mediate GAG hydrolysis (23), displayed a greater than 500-fold reduction in antibiofilm activity (Fig. 1A) and failed to mediate degradation of biofilm-associated GAG (Fig. 1 B and C). Collectively, these data suggest that biofilm disruption is mediated through the enzymatic hydrolysis of GAG.
Fig. 1.
Treatment with Sph3h disrupts A. fumigatus biofilms and degrades GAG on the surface of hyphae. (A) Crystal violet staining of established A. fumigatus biofilms treated with the indicated concentration of each hydrolase. Each data point represents the mean of n = 20 with error bars indicating SE. EC50 indicates the 50% effective concentration ± SE. (B) Effects of the indicated hydrolases on cell wall-associated GAG. Hyphae of the indicated strains grown in the absence of hydrolase treatment (Left) or following exposure to 0.5 μM of the indicated hydrolases (Right). Cell wall-associated GAG was visualized by using FITC-conjugated lectin staining (green) with DRAQ5 as a counterstain (red). The GAG-deficient Δuge3 mutant was included as a negative control. (C) Quantification of lectin-staining from B. Each data point represents the mean fluorescence intensity of at least seven hyphae with error bars indicating SE. * indicates a significant difference (P < 0.05) relative to the untreated A. fumigatus as determined by one-way ANOVA with Dunnett’s multiple comparison test. (D) Scanning electron micrographs of hyphae of the indicated strains grown in the absence of hydrolases (Left and Center) and following exposure to 0.5 μM Sph3h (Right). (Scale bars: B, 20 μm; D, 5 μm.) MFI, mean fluorescence intensity.
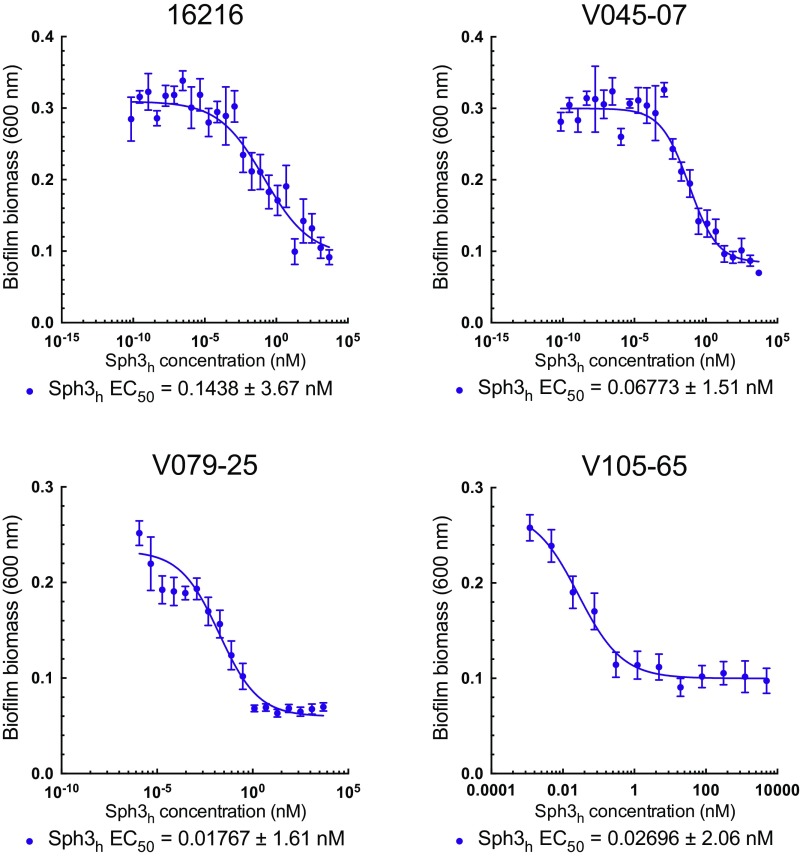
To validate that fungal biofilm disruption by Sph3h is not restricted to the A. fumigatus laboratory strain Af293, the activity of Sph3h was evaluated against four clinical A. fumigatus isolates. Sph3h disrupted biofilms of all isolates tested at EC50 values <0.15 nM (Fig. S1). These results confirm the role of GAG in biofilm formation and indicate that Sph3h exhibits antibiofilm activity across a wide range of A. fumigatus strains.
Fig. S1.
Effects of Sph3h on biofilms formed by clinical isolates of A. fumigatus. Crystal violet staining of pregrown A. fumigatus biofilms treated with the indicated concentration of Sph3h. Data represents the mean of three independent experiments with error bars indicating SE. EC50 reported is ±SE.
The Bacterial Hydrolase PelAh Hydrolyses GAG and Disrupts Fungal Biofilms.
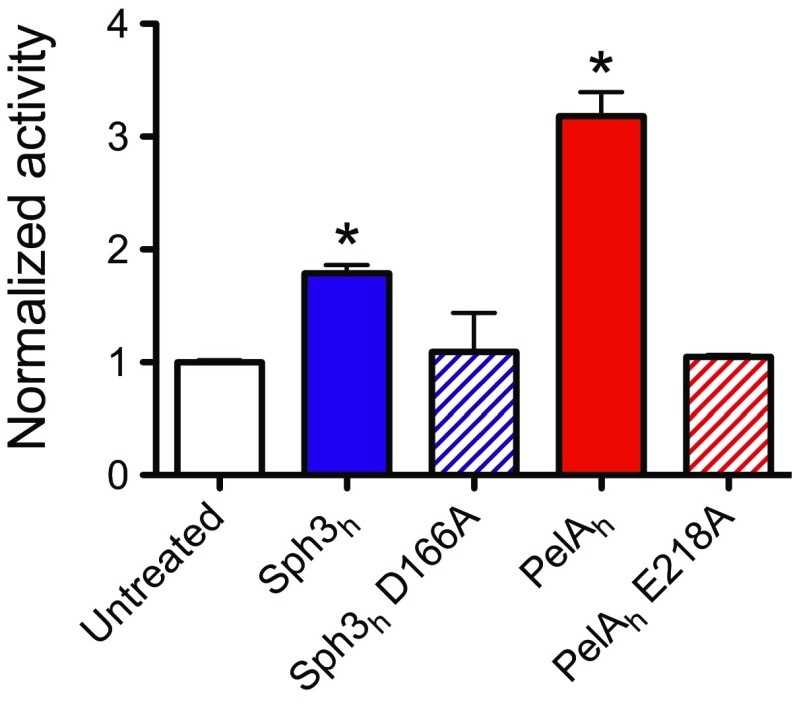
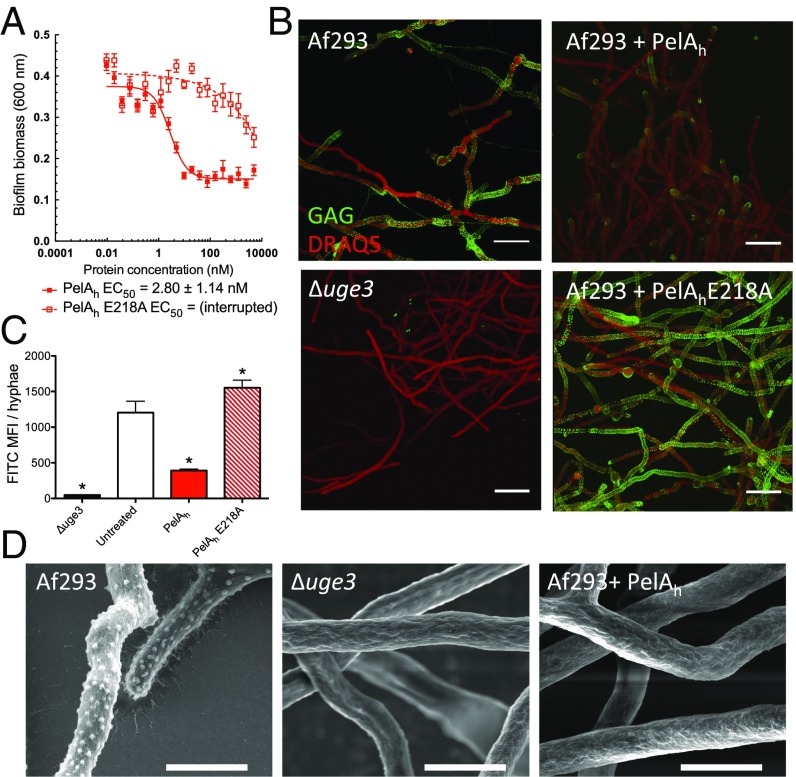
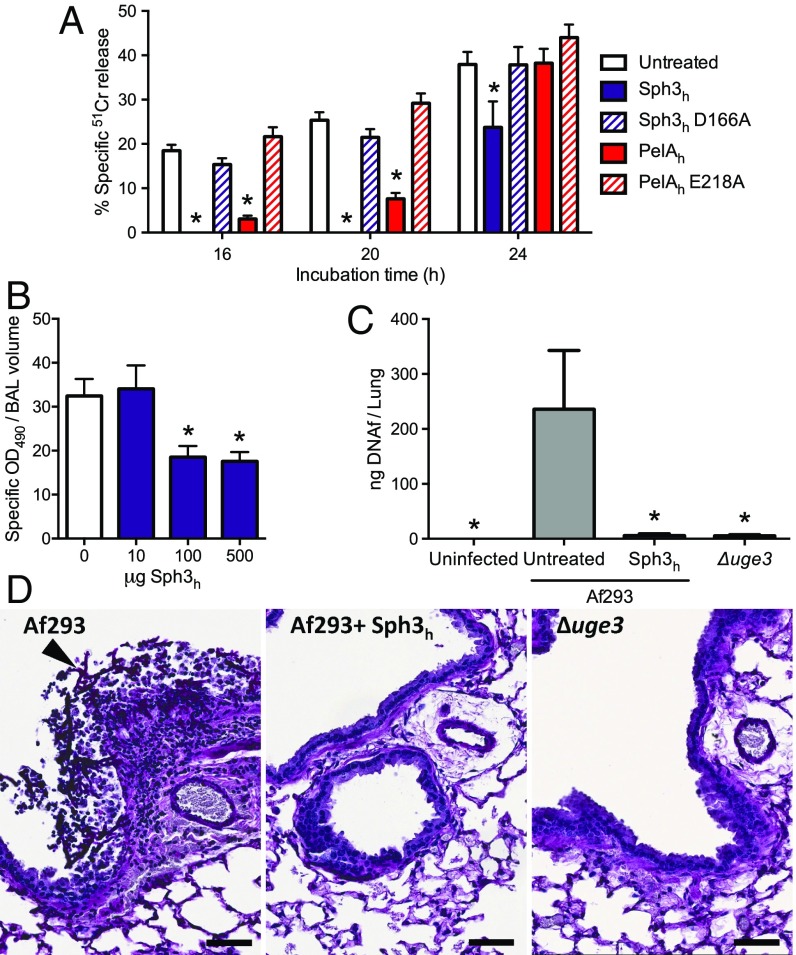
Given that GAG and Pel are both cationic exopolysaccharides containing 1,4-linked GalNAc (14, 17), we hypothesized that PelAh might exhibit activity against GAG. Consistent with this hypothesis, an in vitro reducing sugar assay demonstrated that PelAh was capable of hydrolyzing purified GAG (Fig. S2). Furthermore, using the crystal violet biofilm assay, we found that PelAh disrupted A. fumigatus fungal biofilms with an EC50 value of 2.80 ± 1.14 nM (Fig. 2A). The treatment of A. fumigatus hyphae with PelAh also resulted in a reduction in the amount of cell wall-associated GAG (Fig. 2 B–D) as was observed with Sph3h treatment. The PelAh catalytic variant, PelAh E218A, which is markedly impaired in Pel hydrolysis and is inactive against P. aeruginosa biofilms (22), did not significantly hydrolyze GAG at concentrations as high as 12 μM (Fig. S2). Consistent with this observation, PelAh E218A was also several hundred-fold less active against A. fumigatus biofilms and did not degrade hyphae-associated GAG (Fig. 2 B and C). These results suggest that PelAh disrupts A. fumigatus biofilms through the hydrolysis of biofilm-associated GAG.
Fig. S2.
Sph3h and PelAh can hydrolyze purified GAG. Purified GAG was incubated with 12 μM of the indicated hydrolase for 24 h. GAG hydrolysis was measured through quantification of the release of reducing sugars. The * indicates significant difference (P < 0.01) from the untreated samples by unpaired t test. Each bar represents the mean of two independent experiments.
Fig. 2.
PelAh disrupts A. fumigatus biofilms and degrades GAG. (A) Effects of PelAh on A. fumigatus biofilms. Crystal violet staining of established A. fumigatus biofilms treated with the indicated concentration of PelAh or PelAh E218A. Each data point represents the mean of n = 20 with error bars indicating SE. EC50 reported ± SE. (B) Effects of the indicated hydrolases on cell wall-associated GAG. Established hyphae of the indicated strains were untreated (Left) or exposed to 0.5 μM of the indicated hydrolases (Right). Cell wall-associated GAG was detected by using FITC-conjugated lectin staining (green), with DRAQ5 as a counterstain (red). (C) Mean fluorescent intensity of lectin staining in B. Each data point represents the mean of at least seven hyphae with error bars indicating SE. The * indicates a significant difference (P < 0.05) relative to the untreated A. fumigatus as determined by one-way ANOVA with Dunnett’s multiple comparison test. (D) Scanning electron micrographs of hyphae of the indicated strains grown in the absence of hydrolase treatment (Left and Center) or following treatment with 0.5 μM PelAh (Right). (Scale bars: B, 20 μm; D, 5 μm.) MFI, mean fluorescence intensity.
Sph3h Binds Pel but Does Not Disrupt Established P. aeruginosa Biofilms.
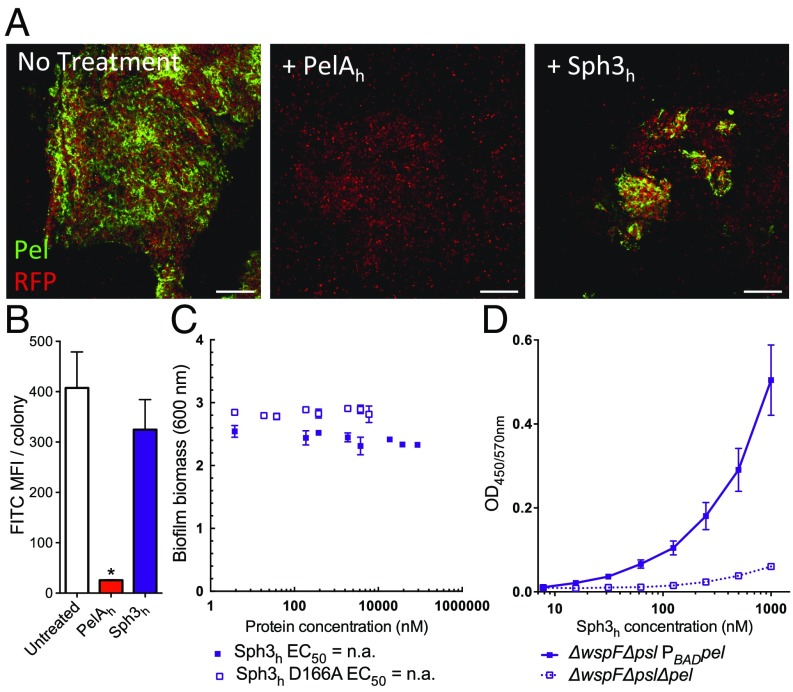
Given that PelAh can hydrolyze GAG and disrupt GAG-mediated biofilms, we hypothesized that Sph3h may exhibit activity against Pel and Pel-mediated biofilms. The inability to purify sufficient quantities of Pel precluded us from using it as a substrate. Therefore, to examine whether Sph3h was capable of hydrolyzing Pel, the enzyme was exogenously applied to biofilms produced by the Pel overproducing P. aeruginosa strain PAO1 ΔwspF Δpsl PBADpel. Treatment of these established biofilms with Sph3h did not affect levels of Pel within the biofilms as visualized by lectin staining (Fig. 3 A and B), nor did it reduce biofilm biomass, even at concentrations exceeding 10 μM (Fig. 3C).
Fig. 3.
Sph3h binds Pel but is inactive against established P. aeruginosa biofilm. (A) Established biofilms of RFP-producing P. aeruginosa overexpressing the Pel operon (red) were untreated (Left) or exposed to 0.5 μM of the indicated hydrolases (Center and Right). Biofilm-associated Pel was detected by using FITC-conjugated Wisteria fluoribunda lectin staining (green). (Scale bars: 20 μm.) (B) Mean fluorescent intensity of lectin staining in A. Each data point represents the mean of at least four P. aeruginosa colonies with error bars indicating SE. * indicates a significant difference (P < 0.001) relative to the untreated P. aeruginosa as determined by one-way ANOVA with Dunnett’s multiple comparison test. (C) Effects of Sph3h on Pel-mediated P. aeruginosa biofilms. Crystal violet staining of established biofilms of P. aeruginosa overexpressing the Pel operon incubated with the indicated concentrations of Sph3h or Sph3h D166A. Each data point represents the mean of n = 3 with error bars indicating SE. EC50 reported ± SE. (D) Sph3h binding to Pel polysaccharide. Microtiter plates were coated with culture supernatants of the indicated P. aeruginosa strains and the binding of Sph3h was determined by using an anti-Sph3h antibody. Each data point represents the mean of three independent experiments with error bars indicating SE. MFI, mean fluorescence intensity.
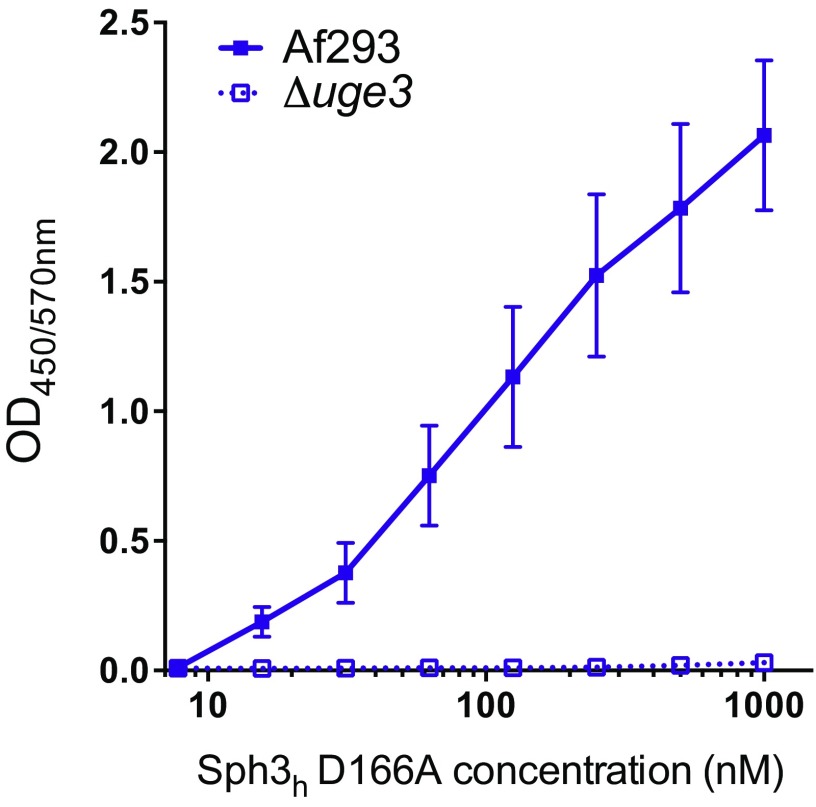
Because Sph3h did not hydrolyze Pel within P. aeruginosa biofilms, we tested whether the enzyme was capable of recognizing and binding this polysaccharide. Using an ELISA-based binding assay, we observed dose-dependent binding of Sph3h to culture supernatants from the Pel overproducing P. aeruginosa strain, but not from supernatants of the Pel-deficient strain PAO1 ΔwspF Δpel Δpsl (Fig. 3D). These data suggest that the inability of Sph3h to disrupt Pel-mediated biofilms is likely a consequence of an inability to hydrolyze Pel rather than being unable to bind the polysaccharide. Dose-dependent binding of the inactive Sph3h D166A variant to GAG-containing culture supernatants was also observed (Fig. S3), suggesting that binding of hydrolases to exopolysaccharides is insufficient to disrupt established biofilms in the absence of enzymatic cleavage of the polymer.
Fig. S3.
Sph3h D166A binds to GAG. Microtiter plates were coated with culture supernatants of the indicated A. fumigatus strains, and the binding of the indicated concentrations of Sph3h D166A was determined as above. Data represents the mean of three independent experiments with error bars indicating SE.
Sph3h and PelAh Potentiate Antifungals by Enhancing Their Intracellular Penetration.
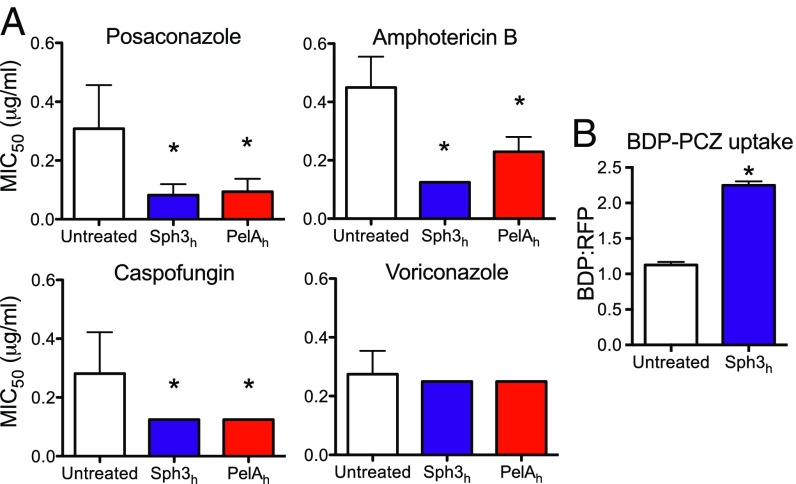
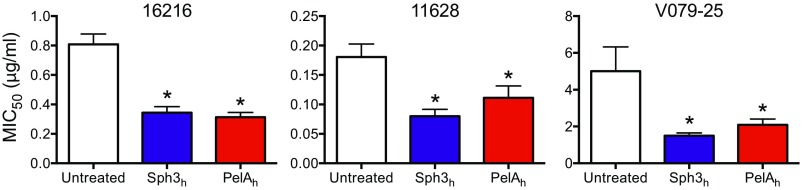
The Pel polysaccharide enhances resistance to several antibiotics including aminoglycosides and colistin (22, 24, 25). Because biofilm formation by A. fumigatus is associated with increased resistance to a number of antifungal agents (26–28), we hypothesized that GAG may have an analogous function to Pel in enhancing resistance to antifungal agents. To test this hypothesis, we investigated whether Sph3h or PelAh could potentiate the activity of commonly used antifungal drugs. Treatment of established fungal biofilms with either enzyme resulted in a significant reduction in the MIC50 of the azole posaconazole, the polyene amphotericin B, and the echinocandin caspofungin (Fig. 4A). Sph3h or PelAh treatment produced a similar increase in sensitivity to posaconazole for both azole-sensitive and azole-resistant strains of A. fumigatus (Fig. S4). Susceptibility to voriconazole, a smaller and more polar azole, was unaffected by treatment with either glycoside hydrolase (Fig. 4A). Because both posaconazole and voriconazole have the same intracellular target, these findings suggest that cationic GAG mediates antifungal resistance by hindering cellular uptake of large, nonpolar molecules such as posaconazole. To investigate this hypothesis, the effect of Sph3h on intracellular penetration of posaconazole was examined by using posaconazole conjugated to the fluorophore BODIPY (BDP-PCZ). Previous work has established that BDP-PCZ displays similar cellular and subcellular pharmacokinetics to unmodified posaconazole (29). Fluorometric studies revealed that Sph3h treatment resulted in higher accumulation of BDP-PCZ within A. fumigatus hyphae (Fig. 4B). This finding indicates that GAG protects A. fumigatus from the action of lipophilic antifungals by limiting their penetration into hyphae.
Fig. 4.
Glycoside hydrolases increase sensitivity of A. fumigatus to antifungal agents. (A) Established biofilms of wild-type A. fumigatus strain Af293 were treated with the indicated concentrations of antifungals with or without 0.5 μM of the indicated hydrolase and the viability of the resulting biofilms was then measured by using the XTT metabolic assay. Susceptibility to antifungals was quantified by determining the antifungal concentration resulting in a 50% reduction in fungal metabolic activity (MIC50) compared with untreated controls. Bars represent the mean of at least n = 4 with error bars indicating SE. (B) Effects of hydrolase therapy on antifungal uptake. A. fumigatus hyphae were treated with 1 μM Sph3h then exposed to 2 μg/mL BDP-PCZ. Uptake of BDP-PCZ was quantified via fluorometry. Each bar represents the mean of three independent experiments with error bars indicating SE. The * indicates a significant difference (P < 0.05) relative to untreated control samples as determined by one-way ANOVA with Dunnett’s multiple comparison test in A, or two-tailed Student’s t test in B. MIC, minimum inhibitory concentration.
Fig. S4.
Susceptibility of azole-resistant isolates of A. fumigatus to posaconazole when treated with Sph3h or PelAh. Susceptibility to antifungals was quantified by determining the antifungal concentration resulting in a 50% reduction in fungal metabolic activity (MIC50) compared with untreated controls. The * indicates a significant difference (P < 0.05) from untreated control samples as determined by one-way ANOVA with Dunnett’s multiple comparison test. Each bar represents the mean of at least three independent experiments, with error bars indicating SE. MIC, minimum inhibitory concentration.
Recombinant Sph3h and PelAh Protect Epithelial Cells from Damage by A. fumigatus.
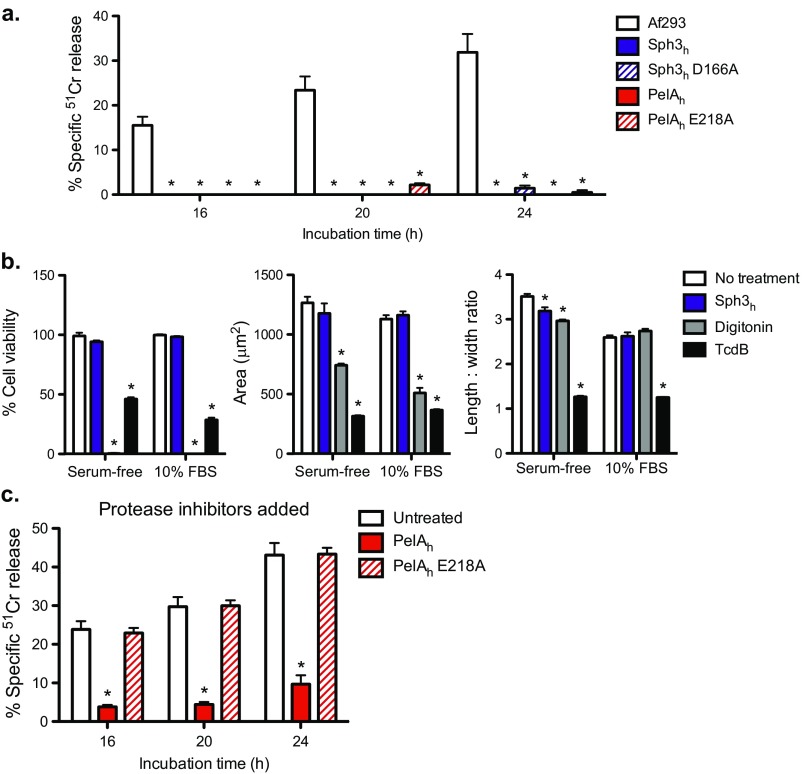
A. fumigatus GAG-mediated adherence is required for A. fumigatus to damage A549 pulmonary epithelial cells in vitro (20). We therefore tested whether treatment with either Sph3h or PelAh could protect epithelial cells from fungal-induced injury by using an established chromium (51Cr) release damage assay (30). We first established that the enzymes were not cytotoxic and that the addition of Sph3h or PelAh to uninfected A549 cell monolayers did not cause detectable cellular damage (Fig. S5A), a finding verified with the IMR-90 human lung fibroblast cell line (Fig. S5B). These data are consistent with the lack of cytotoxicity previously reported for PelAh (22). Next, we assessed whether Sph3h or PelAh were able to protect A549 cell monolayers from damage by A. fumigatus. Sph3h significantly reduced epithelial cell injury for up to 24 h (Fig. 5A). Treatment with PelAh also protected epithelial cells from A. fumigatus-induced damage (Fig. 5A). The protective effect of PelAh was shorter than that observed with Sph3h, and was lost before 24 h of treatment. The addition of protease inhibitors extended PelAh-mediated epithelial cell protection to 24 h (Fig. S5C), suggesting that the decrease in PelAh-mediated protection was likely due to proteolytic degradation of the recombinant protein. Epithelial cell protection was not observed with the catalytic variants, PelAh E218A or Sph3h D166A, suggesting that the hydrolytic activity of the enzymes is required for protection (Fig. 5A).
Fig. S5.
Sph3h and PelAh are nontoxic to mammalian cell lines in vitro. (A) Chromium-loaded A549 pulmonary epithelial cells were incubated with conidia of wild-type A. fumigatus or 0.5 μM of the indicated hydrolases. Epithelial cell damage was determined by measurement of the amount of chromium released into supernatant at the indicated time points. Each bar represents the mean of at least n = 3, with error bars indicating SE. (B) Potential toxic and morphological effects of the Sph3h on IMR-90 fibroblasts were assayed as previously described. (Left) IMR-90 fibroblast cell viability assay using PrestoBlue reagent. All data were normalized to a no treatment control (100%). IMR-90 cellomics assay to measure the area (Center) and length-to-width ratio (Right) of the cells using CellTracker Orange CMRA. The C. difficile toxin TcdB was used as a positive control in cell morphology assays, and the detergent digitonin was used as a negative control in cell viability assays. Each data point represents the mean from n = 3 from cellomic and PrestoBlue measurements in microtiter plate well, with error bars indicating SE. (C) Chromium-loaded A549 cells were incubated with conidia in the presence or absence of 0.5 μM of the indicated hydrolases and supplemented with 0.1% (vol/vol) protease inhibitor mixture. Each bar represents the mean of four independent experiments performed in triplicate, with error bars indicating SE. The * indicates a significant difference (P < 0.05) compared with cells infected with A. fumigatus alone (A and C) or no treatment (B), as determined by two-way ANOVA with Dunnett’s multiple comparison test.
Fig. 5.
Effects of hydrolases on A. fumigatus-induced airway epithelial cell damage and in vivo pulmonary infection. (A) 51Cr-loaded A549 pulmonary epithelial cells were incubated with conidia of wild-type A. fumigatus in the presence or absence of 0.5 μM concentrations of the indicated hydrolases. Epithelial cell damage was determined by measurement of the amount of 51Cr released into supernatant at the indicated time points. Each bar represents the mean of at least five independent experiments performed in duplicate with error bars indicating SE. (B) Pulmonary injury as measured by lactose dehydrogenase activity of the bronchoalveolar lavage fluid from BALB/c mice treated intratracheally or not with the indicated quantities of Sph3h and killed 7 d after treatment. Data represents the mean of at least n = 5, with error bars representing SE. (C) Fungal burden of neutropenic mice as determined by quantitative PCR following 4 d of infection with the indicated A. fumigatus strain with or without treatment with a single dose of 500 μg of Sph3h. Data represents the mean of at least n = 12, from two independent experiments with error bars indicating SE. The * indicate a significant difference P < 0.01 for A and C, and < 0.05 for B, relative to untreated controls by using a two-way ANOVA for A and a one-way ANOVA for B and C with a Dunnett’s multiple comparison test. (D) Histopathological analysis of lung sections of mice from C stained with periodic acid Schiff reagent. Arrow indicates hyphal lesion. (Scale bars: 20 μm.)
Intratracheal Sph3h Is Well Tolerated, and Attenuates Fungal Virulence in an Immunocompromised Mouse Model of Pulmonary Aspergillosis.
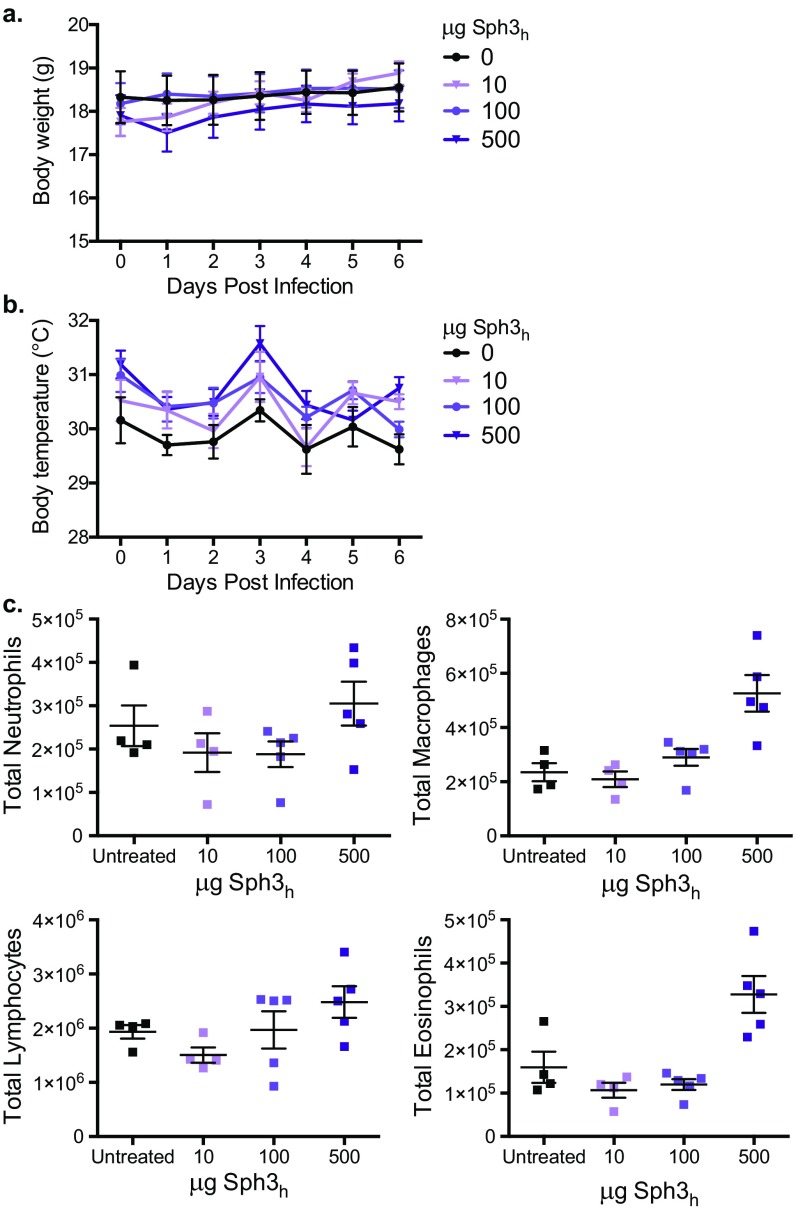
Given the ability of Sph3h to protect epithelial cells for more than 24 h, this hydrolase was selected for evaluation in vivo. BALB/c mice treated intratracheally with doses up to 500 μg of Sph3h exhibited no signs of stress, weight loss, or change in body temperature after treatment (Fig. S6 A and B). Additionally, no significant increase in pulmonary injury or inflammation between treated and untreated mice were observed as measured by bronchoalveolar lavage lactate dehydrogenase activity and total pulmonary leukocyte populations (Fig. 5B and Fig. S6C). Collectively these results suggest that a single intratracheal dose of Sph3h is well tolerated by mice.
Fig. S6.
Intratracheal administration of Sph3h is well tolerated in BALB/c mice. Body weight (A) and surface body temperature (B) of immunocompetent BALB/c mice administered an intratracheal injection of the indicated amount of Sph3h and monitored daily. Data represents the mean of at least n = 4, with error bars indicating SE. No significance compared with untreated controls, two-way ANOVA with Sidak’s multiple comparisons test. (C) Total pulmonary leukocyte populations of the mice after 7 d posttreatment, as determined by flow cytometry. No significance compared with untreated controls, Kruskal–Wallis test with Dunn’s multiple comparisons test.
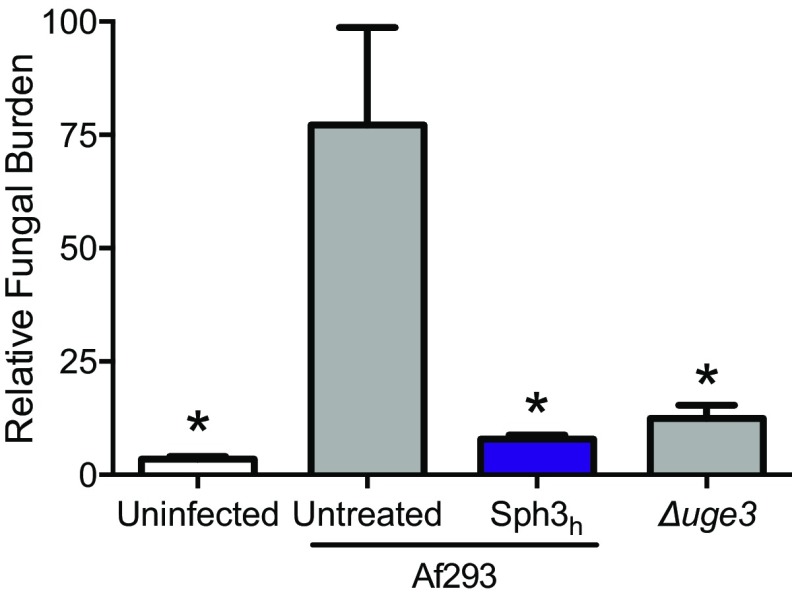
To determine the ability of Sph3h to attenuate virulence of A. fumigatus, neutropenic BALB/c mice were infected intratracheally with A. fumigatus conidia with or without the coadministration of 500 μg of Sph3h. Four days after infection, mice infected with A. fumigatus and treated with Sph3h had a significantly lower pulmonary fungal burden to untreated, infected mice as measured by both fungal DNA (Fig. 5C) and pulmonary galactomannan content (Fig. S7). The fungal burden of the Sph3h-treated mice was similar to that observed with mice infected with the GAG-deficient hypovirulent strain Δuge3 (20). Consistent with the fungal burden data, histopathologic examination of lung sections revealed the presence of fungal lesions in untreated, infected mice, but no detectable lesions in the lungs of infected mice treated with Sph3h, or those infected with conidia of the Δuge3 mutant (Fig. 5D). These findings suggest that Sph3h-mediated degradation of GAG can limit the growth of A. fumigatus in vivo, to the same degree as is observed with GAG-deficient organisms.
Fig. S7.
Sph3h coadministration attenuates A. fumigatus invasive infection in BALB/c mice. Fungal burden of neutropenic mice as determined by galactomannan quantification following 4 d of infection with the indicated A. fumigatus strain with or without treatment with a single dose of 500 μg of Sph3h. Data represents the mean of at least n = 12, from two independent experiments with error bars indicating SE. *P < 0.001 compared with untreated mice infected with wild-type A. fumigatus using a one-way ANOVA and Dunnett’s multiple comparison test.
Discussion
In this study, we demonstrate that the fungal glycoside hydrolase Sph3h is able to degrade preformed A. fumigatus biofilms. This study is an example of the use of a glycoside hydrolase to disrupt a fungal biofilm. Further, we establish that the glycoside hydrolase PelAh displays activity against biofilms formed by organisms across different microbial kingdoms. Both glycoside hydrolases potentiated the penetration and activity of antifungal agents in vitro, exhibited no toxicity against mammalian cells, and protected epithelial cells from A. fumigatus-induced damage. Pulmonary administration of Sph3h was well tolerated and limited fungal growth in an immunocompromised mouse model, suggesting that these enzymes are promising therapeutic agents for the treatment of fungal disease.
The mechanism by which the biofilm matrix enhances A. fumigatus resistance to antifungals is poorly understood. The effect of hydrolase treatment on the antifungal sensitivity of A. fumigatus provides some insight into this question and establishes a role for GAG in biofilm-associated antifungal resistance. Multiple observations suggest that GAG enhances antifungal resistance by acting as a barrier to antifungal penetration of hyphae. First, glycoside hydrolase degradation of GAG enhanced the activity of multiple antifungals with different mechanisms of action. Second, the activity of posaconazole, but not voriconazole, was enhanced although both azoles target the same enzyme, CYP51A. These hydrolases also display similar activity against azole-resistant and azole-sensitive strains. The cationic nature of GAG may explain the differential effects on voriconazole compared with other antifungals. The GAG barrier would be predicted to be most effective against large, lipophilic or cationic antimicrobial agents, and thus therapeutic hydrolases may be most effective as adjuvants for lipophilic antifungals. Previous studies have reported that the enzymatic degradation of neutral α-glucans of A. fumigatus did not enhance susceptibility to antifungals (31), further supporting our hypothesis that exopolysaccharide charge plays a role in mediating antibiotic resistance. Similarly, hydrolysis of cationic Pel exopolysaccharide by PelAh enhances the activity of the polycationic antibacterial colistin (22). Interestingly, degradation of biofilm-associated extracellular DNA (eDNA) has been reported to enhance A.fumigatus susceptibility to caspofungin and amphotericin B, although the effects on posaconazole and voriconazole susceptibility were not reported in the study (26). Recent work has suggested that Pel anchors eDNA within P. aeruginosa biofilms through charge–charge interactions (17). Given the similarities between Pel and GAG, it is possible that GAG-mediated binding of eDNA may also contribute to enhancing antifungal resistance.
The results of these studies add to an emerging body of evidence that fungal biofilms share structural (32–35) and functional (26, 36, 37) similarity with those formed by pathogenic bacteria. The finding that glycoside hydrolases can display activity against the exopolysaccharides and biofilms of both fungi and bacteria provides evidence that these similarities could potentially be exploited for the development of therapeutics active against both organisms. Additionally, the similarity between the exopolysaccharides of P. aeruginosa and A. fumigatus, coupled with the interspecies activity of their glycoside hydrolases, suggest the intriguing possibility that exopolysaccharide interactions may occur between organisms during multispecies biofilm formation. Cocolonization with P. aeruginosa and A. fumigatus is not uncommon in patients with chronic pulmonary disease such as cystic fibrosis (38). Although studies of the formation of mixed fungal-bacterial biofilms during pulmonary infection are limited, a recent study of patients with chronic lung disease reported that antibacterial therapy for P. aeruginosa was associated with a reduction in fungal colonization, suggesting the possibility of microbial cooperation (39). Further studies examining the role of cross-species exopolysaccharide and exopolysaccharide-modifying enzyme interactions are required to establish a role for cooperative biofilm interactions in pulmonary disease.
Whereas PelAh exhibited cross-species activity and disrupted preformed fungal biofilms, Sph3h bound Pel, but was unable to disrupt established Pel-mediated biofilms. This difference in activity may reflect differences in the composition or conformation of each polysaccharide because GAG is a heteropolymer of GalNAc and galactose whereas Pel is comprised of GalNAc and GlcNAc. It is likely that these differences influence the ability of Sph3h and PelAh to hydrolyze the polymer. The inability of Sph3h to degrade preformed P. aeruginosa biofilms may suggest that mature Pel adopts a configuration or undergoes postsynthetic modification that renders it incompatible with the catalytic active site of Sph3h and resistant to cleavage. Detailed studies of these enzymes to determine the mechanisms underlying their differential activity against Pel will require purified polysaccharide, which is not available.
Both Sph3h and PelAh were found to be noncytotoxic, and a single dose of intratracheal Sph3h was well tolerated by BALB/c mice. Coadministration of Sph3h with wild-type conidia to neutropenic mice greatly reduced fungal outgrowth within the lungs of these mice. Together these results provide proof-of-concept that the glycoside hydrolases can be used to improve the outcome of fungal infection, with minimal side effects and toxicity. These findings will pave the way for future work to evaluate the utility of these agents as antifungal therapeutics including detailed pharmacokinetic and toxicity studies, and the evaluation of these enzymes for the treatment of established fungal infections alone and in combination therapy with lipophilic antifungal agents such as posaconazole or amphotericin B.
Methods
Strains and Culture Conditions.
Strains used in this study are detailed in Table S1, and detailed culture conditions are described in SI Methods.
Table S1.
A list of fungal and bacterial strains used in this study
| Organism | Strain or genotype | Source |
| A. fumigatus | Af293 (wild–type) | Paul T. Magee |
| Δuge3 | 20 | |
| Af293 pRFP | 29 | |
| 16216 (wild-type) | William W. Hope | |
| 11628 (wild-type) | William W. Hope | |
| V045-07 (wild-type) | Paul E. Verweij | |
| V079-25 (wild-type) | Paul E. Verweij | |
| V107-65 (wild-type) | Paul E. Verweij | |
| P. aeruginosa | PAO1 ΔwspFΔpslPBADpel | 17 |
| PAO1 ΔwspFΔpslPBADpel pRFP | This study |
Recombinant Hydrolase Expression and Purification.
Hydrolases were expressed and purified as described (22, 23).
Treatment of A. fumigatus with Glycoside Hydrolases.
To visualize the effects of hydrolases on cell wall-associated GAG, hyphae were treated with recombinant hydrolases and stained with fluorescein-conjugated soybean agglutinin as described (23), with minor modifications. Hyphae were counterstained with a 1:1,000 dilution of DRAQ5 (eBioscience) in PBS for 5 min before paraformaldehyde (PFA) fixation. Complete image acquisition and processing methods can be found in SI Methods. To study the effects of hydrolases on biofilms, 104 conidia were grown in Brian media in polystyrene, 96-well plates for 19 h at 37 °C and 5% CO2 and then treated with the indicated concentration of glycoside hydrolase in PBS for 1 h at room temperature. Biofilms were then gently washed, stained with 0.1% (wt/vol) crystal violet and destained with 100% ethanol for 10 min. The optical density of the destain fluid was measured at 600 nm (OD600).
Scanning Electron Microscopy.
Complete sample preparation and processing methods are detailed in SI Methods.
Treatment of P. aeruginosa with Glycoside Hydrolases.
For biofilm disruption, static P. aeruginosa cultures were grown for 22 h at 30 °C, at which point the planktonic cells were aspirated, and LB + 0.5% arabinose + 0.5 μM glycoside hydrolase was added for an additional 3 h. For the detection of Pel, samples were incubated with 30 μg/mL fluorescein-conjugated Wisteria fluoribunda lectin for 2 h at 4 °C, fixed with 8% (wt/vol) PFA for 20 min at 4 °C and imaged as detailed in SI Methods. The ability of hydrolases to disrupt established biofilms were studied as described (22).
Quantification of Hydrolase Binding to Culture Supernatants.
Details of culture supernatant production and hydrolase binding quantification can be found in SI Methods.
Effects of Glycoside Hydrolases on Antifungal Susceptibility of A. fumigatus.
Fungal biofilms were prepared in tissue culture treated with 24-well plates in RPMI medium 1640 (Life Technology) buffered with Mops [3-(N-Morpholino) Propane-Sulfonic Acid] (RPMI-Mops) (Fisher) for 9 h at 37 °C, 5% CO2. Serial dilutions of antifungal compounds with or without 0.5 μM Sph3h or PelAh were added to wells, and the plates were incubated at 37 °C and 5% CO2 for 15 h. Fungal viability was measured by using the sodium 3′-{1-[(phenylamino)-carbony]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)benzene-sulfonic acid hydrate (XTT) metabolic assay as described (40). The concentration of antifungal resulting in a 50% decrease in viability (MIC50) was used as a measure of antifungal effect.
Fluorometric Quantification of Hyphal Uptake of BDP-PCZ.
A total of 2.5 × 104 conidia of red fluorescent protein (RFP)-expressing A. fumigatus were grown in each well of a 96-well black, clear-bottom plate for 8 h at 37 °C, 5% CO2. Hyphae were treated with 1 μM Sph3h in PBS for 90 min at 37 °C, 5% CO2 then exposed to 2 μg/mL BDP-PCZ for 10 min. The plate was then read by using an Infinite M1000 fluorescent plate reader with excitation wavelengths of 532 and 488 nm for RFP and BDP-PCZ, respectively. Background fluorescence was subtracted from both RFP and BDP-PCZ signals, and the BDP-PCZ signal was then normalized to total RFP fluorescence for each well.
Effects of Glycoside Hydrolases on A. fumigatus-Induced Epithelial Cell Damage.
A549 pulmonary epithelial cell damage by A. fumigatus was tested by using the 51Cr release assay as described (20, 30). Recombinant hydrolases were added to the A549 cultures at the time of infection at a final concentration of 0.5 μM.
Characterization of Pulmonary Damage by Sph3h.
All procedures involving mice were approved by the Animal Care Committees of the McGill University Health Centre. Female BALB/c mice 5–6 wk of age were anesthetized with isoflurane and administered a single endotracheal injection of 500 μg of Sph3h in 50 μL PBS and monitored daily for 7 d. Mice were then euthanized by CO2 overdose, and their airways lavaged with 1 mL of PBS that was administered and collected through a needle inserted in the trachea. A total of two lavages were performed and pooled. The presence of LDH in the BAL fluid was used as an indicator of pulmonary damage; LDH activity was measured in the fluid by using a commercial assay (Promega), as per manufacturer’s instructions.
Effects of Sph3h in a Severely Immunocompromised Mouse Model of Invasive Pulmonary Aspergillosis.
Mice were immunosuppressed with cortisone acetate and cyclophosphamide as described (20, 41). Mice were infected with an endotracheal injection of 5 × 103 A. fumigatus conidia, resuspended in either PBS alone, or in combination with 500 μg of Sph3h. Mice were monitored daily, and moribund animal were euthanized. At 4 d after infection, mice were euthanized and their lungs were harvested. For fungal burden analysis, lungs were homogenized in 5 mL of PBS containing protease inhibitor mixture (Roche), and aliquots were stored at −80 °C until use. Pulmonary fungal burden was determined as described (15), and as detailed in SI Methods.
SI Methods
Growth Conditions.
Unless specified, fungal strains were grown on yeast-extract peptone dextrose (YPD) agar (Fisher Scientific) at 37 °C for 6 d, and the conidia were harvested by gentle washing with PBS + 0.01% (vol/vol) Tween 20. P. aeruginosa strains were grown in Luria–Bertani (LB) media to stationary phase overnight, at which point cultures were diluted to an OD600 of 0.05 in LB broth supplemented with 0.5% (wt/vol) arabinose and grown at 30 °C, as indicated. P. aeruginosa strains were modified by transformation with a plasmid containing a constitutively expressing RFP.
Confocal Image Acquisition and Modifications.
A. fumigatus.
Samples were acquired on a Zeiss LSM780 confocal fluorescent microscope by using a Plan-Apochromat 63×/1.40 Oil DIC M27 objective lens at a resolution of 1,024 × 1,024, using 488- and 633-nm lasers to excite fluorescein and DRAQ5, respectively. The channels were acquired in tandem, with fluorescein being detected for 493–585 nm, and DRAQ5 for 661–759 nm. Z stacks were acquired with a spacing of 0.74 μm. Stacks were composited into three-dimensional renderings with the “3D Project” function of the ImageJ software by using the “Brightest Point” Projection Method. A 20-μm scale bar was then added to each image before being exported as a .jpg file format and assembled into the final figure in Microsoft PowerPoint.
P. aeruginosa.
Samples were acquired on a Zeiss LSM780 confocal fluorescent microscope by using a Plan-Apochromat 63×/1.40 Oil DIC M27 objective lens at a resolution of 1,024 × 1,024, using 488- and 561-nm lasers to excite fluorescein and RFP, respectively. The channels were acquired separately, with fluorescein being detected for 490–577 nm and RFP for 577–691 nm. Z stacks were acquired with a spacing of 0.74 μm. Stacks were combined by using the “Extended Depth of Focus” algorithm of Zeiss Zen software. A 20-μm scale bar was then added to each image by using ImageJ before being exported as a .jpg file format. The brightness was increased 54% for all images in Microsoft PowerPoint before being assembled into the final figure.
Introduction of Constitutively Expressed RFPs into P. aeruginosa.
A plasmid containing the gene for the monomeric second-generation RFP protein mCherry under the control of a constitutive promoter (42) was transformed into P. aeruginosa via chemical transformation.
Scanning Electron Microscopy.
Conidia were grown for 9 h in Dulbecco's modified Eagle's medium (DMEM) at 37 °C, 5% CO2 on glass coverslips, washed once with Ham's F-12K (Kaighn's) Medium, and incubated with 500 μL in F-12K Medium with or without 0.5 μM hydrolase for 3 h at 37 °C, 5% CO2. Coverslips were washed twice with PBS, then fixed with 2.5% (vol/vol) gluteraldehyde in 0.1 M sodium cacodylate buffer at 4 °C overnight, sequentially dehydrated in ethanol, and critical-point dried (Leica Microsystems EM CPD030 Critical Point Dryer Model). Samples were then sputter coated with Palladium (Leica Microsystems EM ACE600 High Resolution Coater) and imaged with a field-emission scanning electron microscope (FEI Inspect F-50 FE-SEM).
Culture Supernatant Production.
A. fumigatus conidia were inoculated into Brian media at 104 conidia per mL and grown at 37 °C for 72 h shaking at 200 rpm in a Forma Benchtop Orbital Shaker, Model 420 (Thermo Scientific). Supernatants were collected by filtration through Miracloth. P. aeruginosa cultures were grown at 30 °C for 24 h shaking at 200 rpm in a Forma Benchtop Orbital Shaker, Model 420 (Thermo Scientific). Cultures were then centrifuged at 311 × g for 10 min, and supernatants were filtered by using 0.44-μm syringe filters. Culture supernatants were stored at −20 °C until use.
Hydrolase Binding Quantification.
Undiluted culture supernatants were incubated on Immulon 2HB high-binding 96 well microtiter plates overnight at 4 °C. Wells were washed 3× with wash buffer [PBS + 0.05% (vol/vol) Tween-20] and blocked for 30 min at 4 °C in blocking buffer [1% (wt/vol) BSA in wash buffer]. Wells were washed 1× with wash buffer and incubated with the indicated concentrations of Sph3h diluted in blocking buffer for 3 h at 4 °C. Wells were washed 3× with wash buffer and incubated with polyclonal rabbit anti-Sph3h (custom-produced by Cedarlane Laboratories) diluted 1/100 in blocking buffer for 1.5 h at 4 °C. Wells were then washed 3× with wash buffer and incubated with donkey–anti-rabbit secondary antibody conjugated to horseradish peroxidase diluted 1/2,000 in blocking buffer for 1 h at 4 °C. Wells were then washed 4× with wash buffer and incubated with TMB substrate (ThermoFisher) for 15 min at room temperature. The reaction was stopped with the addition of 1 M H2SO4 and absorbance read at 450 nm with a 570-nm correction.
In Vitro Galactosaminogalactan Hydrolysis Assays.
The capacity of the glycoside hydrolases to degrade purified galactosaminogalactan was evaluated by using a reducing sugar assay as described (23).
Cell Viability and Morphology Assays.
Potential toxic and morphological effects of the glycoside hydrolases on IMR-90 fibroblasts were performed as described (22).
Daily Monitoring of Mice.
Mice were monitored daily for signs of stress (ruffled fur, inactivity, hunched posture), and body weights and temperatures were taken. Body weight was measured by using a top-loading balance, whereas surface body temperature was taken on the abdomen by using a digital infrared thermometer.
Pulmonary Leukocyte Recruitment.
Lungs were harvested and washed in PBS before being minced and digested for 60 min at 37 °C in RPMI media 1640 (Life Technology) containing 5% (vol/vol) FBS and 150 U/mL collagenase (Sigma). The resulting suspension was passed through a 70-μm cell strainer to obtain a single cell suspension, at which point the erythrocytes were lysed by using ACK buffer and the remaining cells were resuspended in PBS. Approximately 106 cells were resuspended in 1 mL of PBS containing 1 μL of fixable viability dye (eBioscience) and incubated for 30 min at 4 °C. Cells were then washed in PBS + 2% FBS (staining buffer), and Fc receptors were blocked by incubating with unlabeled anti-CD16/32 antibodies (FcBlock; BD Pharmingen) for 15 min at 4 °C. Cell surface components were stained for 30 min at 4 °C by using the following fluorescently labeled antibodies (all purchased from BD Biosciences): CD3-fluorescein isothiocyanate (clone 17A2), CD11c-allophycocyanin (clone HL3), CD11b-phycoerythrin-CF594 (clone M1/70), CD45-allophycocyanin-Cy7 (clone 30-F11), CD19-phycoerythrin-Cy7 (clone 1D3), SiglecF-brilliant violet 421 (clone E50-2440), and Ly6G-phycoerythrin (clone 1A8). Cells were washed with staining buffer and fixed with 2% (wt/vol) paraformaldehyde in PBS for 15 min at 4 °C. Cells were washed and resuspended in PBS before data acquisition. Data were acquired on an LSR Fortessa flow cytometer by using FACSDiva software (BD Biosciences). Data were further analyzed by using FlowJo software version 10 (FlowJo, LLC). Immune cell subsets were defined as follows: neutrophils, CD45+ Ly6G+ CD11c− CD11b+; alveolar macrophages, CD45+ CD11c+ siglecF+ CD11bneg/low; lymphocytes, CD45+ CD11c− Ly6G− CD3+ or CD45+ CD11c− Ly6G− CD19+; eosinophils, CD45+ CD11c− siglecF+. Total cell numbers of each population were calculated by using the BD CountBright absolute counting beads during data acquisition.
Pulmonary Fungal Burden by Galactomannan Content.
Pulmonary galactomannan content was determined by using the Platelia Aspergillus immunoassay kit (Bio-Rad), according to manufacturer’s instructions.
Pulmonary Fungal DNA Quantification.
Total DNA was extracted from mouse lung homogenates by using the High Pure PCR Template Preparation Kit (Roche Diagnostics), as per manufacturer’s instructions. To quantify fungal DNA, real-time PCR was performed on 150 ng of total lung DNA by using TaqMan Universal PCR Master Mix (Roche Diagnostics). Fungal DNA was quantified by using primers targeting the Aspergillus 18S ribosomal RNA gene (forward: GGCCCTTAAATAGCCCGGT; reverse: TGAGCCGATAGTCCCCCTAA; labeled probe: 6-FAM-AGCCAGCGGCCCGCAAATG-MGB) by comparing to a standard curve of A. fumigatus DNA. Quantitative PCR was run as follows: 2 min at 50 °C; 10 min at 95 °C; and 50 cycles of 15 s at 95 °C plus 1 min at 60 °C.
Histopathological Examination.
Lungs were inflated with 10% buffered formalin (Fisher Scientific) and immersed in formalin overnight to fix. Lungs were then embedded in paraffin and 4-μm-thick sections were stained with periodic acid Schiff (PAS) stain.
Statistical Analysis.
A nonlinear least-squares fitting to a dose–response model was used to calculate EC50 values. Data are presented and statistical significance calculated as indicated. All graphs were generated and statistical analyses were performed by using Prism v6.0 (GraphPad Software).
Acknowledgments
Research described in this paper is supported by Canadian Institutes of Health Research (CIHR) Grants 81361 (to P.L.H. and D.C.S.), 123306 (to D.C.S.), and 43998 (to P.L.H.) and Cystic Fibrosis Canada (CFC) (D.C.S. and P.L.H). B.D.S. has been supported by graduate scholarships from CFC and CIHR. N.C.B. has been supported in part by graduate scholarships from the Natural Sciences and Engineering Research Council of Canada; Mary H. Beatty and Dr. James A. and Connie P. Dickson Scholarships from the University of Toronto; CFC; and The Hospital for Sick Children. P.B. has been supported in part by a CFC Postdoctoral Fellowship and a Banting Fellowship from CIHR. P.L.H. is the recipient of a Canada Research Chair. D.C.S. is supported by a Chercheur-Boursier Award from the Fonds de Recherche Quebec Santé.
Footnotes
Conflict of interest statement: A patent has been filed describing the utility of the glycoside hydrolases as antibiofilm therapeutics (CA2951152 A1, WO2015184526 A1). B.D.S., P.B., N.C.B., P.L.H., and D.C.S. are listed as inventors.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702798114/-/DCSupplemental.
References
- 1.Bajwa S, Kulshrestha A. Fungal infections in intensive care unit: Challenges in diagnosis and management. Ann Med Health Sci Res. 2013;3:238–244. doi: 10.4103/2141-9248.113669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, et al. EPIC International Advisory Committee The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 4.Maltezou HC. Metallo-β-lactamases in Gram-negative bacteria: Introducing the era of pan-resistance? Int J Antimicrob Agents. 2009;33:405.e1–405.e7. doi: 10.1016/j.ijantimicag.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, et al. Hidden killers: Human fungal infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 6.Verweij PE, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Mann EE, Wozniak DJ. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev. 2012;36:893–916. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravelat FN, et al. Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol. 2010;12:473–488. doi: 10.1111/j.1462-5822.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheppard DC. Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr Opin Microbiol. 2011;14:375–379. doi: 10.1016/j.mib.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Seidler MJ, Salvenmoser S, Müller FMC. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–4136. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhede M, Bjarnsholt T, Givskov M, Alhede M. Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Adv Appl Microbiol. 2014;86:1–40. doi: 10.1016/B978-0-12-800262-9.00001-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee MJ, et al. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 2015;11:e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine T, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MJ, et al. Deacetylation of fungal exopolysaccharide mediates adhesion and biofilm formation. MBio. 2016;7:e00252–16. doi: 10.1128/mBio.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin MJ, Nivens DE, Weadge JT, Howell PL. Biosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, Alginate, Pel, and Psl. Front Microbiol. 2011;2:167. doi: 10.3389/fmicb.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennings LK, et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc Natl Acad Sci USA. 2015;112:11353–11358. doi: 10.1073/pnas.1503058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colvin KM, et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritt B, O’Brien L, Winn W. Mucoid Pseudomonas in cystic fibrosis. Am J Clin Pathol. 2007;128:32–34. doi: 10.1309/KJRPC7DD5TR9NTDM. [DOI] [PubMed] [Google Scholar]
- 20.Gravelat FN, et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 2013;9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, et al. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2012;65:366–376. doi: 10.1111/j.1574-695X.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker P, et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci Adv. 2016;2:e1501632. doi: 10.1126/sciadv.1501632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamford NC, et al. Sph3 is a glycoside hydrolase required for the biosynthesis of galactosaminogalactan in Aspergillus fumigatus. J Biol Chem. 2015;290:27438–27450. doi: 10.1074/jbc.M115.679050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan W, et al. Aminoglycoside resistance of Pseudomonas aeruginosa biofilms modulated by extracellular polysaccharide. Int Microbiol. 2010;13:207–212. doi: 10.2436/20.1501.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colvin KM, et al. The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011;7:e1001264. doi: 10.1371/journal.ppat.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajendran R, et al. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot Cell. 2013;12:420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajendran R, et al. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother. 2011;55:2092–2097. doi: 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mowat E, et al. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J Antimicrob Chemother. 2008;62:1281–1284. doi: 10.1093/jac/dkn402. [DOI] [PubMed] [Google Scholar]
- 29.Campoli P, et al. Pharmacokinetics of posaconazole within epithelial cells and fungi: Insights into potential mechanisms of action during treatment and prophylaxis. J Infect Dis. 2013;208:1717–1728. doi: 10.1093/infdis/jit358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes Bezerra LM, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: Internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103:2143–2149. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]
- 31.Beauvais A, et al. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 32.Martins M, et al. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia. 2010;169:323–331. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krappmann S, Ramage G. A sticky situation: Extracellular DNA shapes Aspergillus fumigatus biofilms. Front Microbiol. 2013;4:159. doi: 10.3389/fmicb.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuxman Bass JI, et al. Extracellular DNA: A major proinflammatory component of Pseudomonas aeruginosa biofilms. J Immunol. 2010;184:6386–6395. doi: 10.4049/jimmunol.0901640. [DOI] [PubMed] [Google Scholar]
- 35.Vilain S, Pretorius JM, Theron J, Brözel VS. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl Environ Microbiol. 2009;75:2861–2868. doi: 10.1128/AEM.01317-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012;55:80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang WC, et al. Extracellular DNA shields against aminoglycosides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2013;57:2352–2361. doi: 10.1128/AAC.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paugam A, et al. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med Mycol. 2010;48 Suppl 1:S32–S36. doi: 10.3109/13693786.2010.503665. [DOI] [PubMed] [Google Scholar]
- 39.Baxter CG, et al. Intravenous antibiotics reduce the presence of Aspergillus in adult cystic fibrosis sputum. Thorax. 2013;68:652–657. doi: 10.1136/thoraxjnl-2012-202412. [DOI] [PubMed] [Google Scholar]
- 40.Pierce CG, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheppard DC, et al. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–1911. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brannon MK, et al. Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell Microbiol. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]