Significance
Theoretical predictions and subsequent experimental observations of high-temperature superconductivity in dense hydrogen-rich compounds have reinvigorated the field of superconductivity. A systematic computational study of the hydrides of lanthanum and yttrium over a wide composition range reveals hydrogen-rich structures with intriguing electronic properties under pressure. Electron–phonon coupling calculations predict the existence of new superconducting phases, some exhibiting superconductivity in the range of room temperature. Moreover, the calculated stabilities indicate the materials could be synthesized at pressures that are currently accessible in the laboratory. The results open the prospect for the design, synthesis, and recovery of new high-temperature superconductors with potential practical applications.
Keywords: high pressure, superconductivity, hydrides, structure search
Abstract
A systematic structure search in the La–H and Y–H systems under pressure reveals some hydrogen-rich structures with intriguing electronic properties. For example, LaH10 is found to adopt a sodalite-like face-centered cubic (fcc) structure, stable above 200 GPa, and LaH8 a C2/m space group structure. Phonon calculations indicate both are dynamically stable; electron phonon calculations coupled to Bardeen–Cooper–Schrieffer (BCS) arguments indicate they might be high-Tc superconductors. In particular, the superconducting transition temperature Tc calculated for LaH10 is 274–286 K at 210 GPa. Similar calculations for the Y–H system predict stability of the sodalite-like fcc YH10 and a Tc above room temperature, reaching 305–326 K at 250 GPa. The study suggests that dense hydrides consisting of these and related hydrogen polyhedral networks may represent new classes of potential very high-temperature superconductors.
Extending his original predictions of very high-temperature superconductivity of high-pressure metallic hydrogen (1), Ashcroft later proposed that hydrogen-rich materials containing main group elements might exhibit superconductivity at lower pressures, as the hydrogen in these structures may be considered “chemically precompressed” (2). These proposals, which were based on the Bardeen–Cooper–Schrieffer (BCS) (3) phonon-mediated theory of superconductivity, have motivated many theoretical and experimental efforts in the search for high-temperature superconductivity in hydrides at elevated pressures (4–10). Theory has predicted the stability of a variety of dense hydride structures for which BCS arguments give superconducting transition temperatures, Tcs, that are very high (11–18).
In recent times, compression of hydrogen sulfides has provided a new incentive in hydride superconductivity, one in which theory played an important role. First, theoretical calculations predicted H2S to have a Tc of ∼80 K at pressures above 100 GPa (19). Compression of H2S led to the striking discovery of a superconducting material with a Tc of 203 K at 200 GPa (20). Moreover, the critical temperature exhibits a pronounced isotope shift consistent with BCS theory. It was proposed that the superconducting phase is not stoichiometric H2S but SH3, with a calculated Tc of 194 K at 200 GPa including anharmonic effects (21, 22). A subsequent experiment (23) suggested that the superconducting phase is cubic SH3, in agreement with a theoretical study that gave Tc = 204 K within the harmonic approximation (24). Compression of another hydride, PH3, was reported to reach a Tc of ∼100 K at high pressures (25). Subsequent theoretical calculations predicted possible structures and calculated Tcs close to the experimental results (26–28). The experimental picture for these materials remains not entirely clear, and the synthesis of the superconducting phases, which has been reproduced for hydrogen sulfide, is path-dependent (23).
There is great experimental and theoretical interest in searching for related materials with both higher Tc and potentially broader ranges of stability. To date, simple hydrides with the highest predicted superconducting Tcs are MgH6 (271 K at 300 GPa) (29), CaH6 (235 K at 150 GPa) (17), and YH6 (264 K at 120 GPa) (30). In searching for other high-Tc superconducting hydrides, here we investigated theoretically possible high-pressure crystal structures of La–H and Y–H. We predict the existence of new stable hydride phases of these elements, with remarkably high Tcs at attainable pressures. The search for low-energy crystalline structures of La–H was performed using particle swarm optimization methodology implemented in the CALYPSO code (31, 32). This method has been applied successfully to a wide range of crystalline systems ranging from elemental solids to binary and ternary compounds (33–35) and has proven to be a powerful tool for predicting crystal structures at high pressures (36–39). Structure searches were performed in the pressure range of 150–300 GPa using models consisting of 1–4 formula units. In general, the structure search was terminated after the generation of 1,500 structures. Structural optimizations, enthalpies, electronic structures, and phonons were calculated using density-functional theory (DFT). Structure relaxations were performed using DFT using the Perdew–Burke–Ernzerhof (40) generalized gradient approximation. Phonon dispersion and electron–phonon coupling (EPC) calculations were performed with density functional perturbation theory. Ultrasoft pseudopotentials for La and H were used with a kinetic energy cutoff of 80 Ry. A q mesh of 6 × 6 × 6 and k mesh of 24 × 24 × 24 for fcc-LaH10 structure in the first Brillouin zone (BZ) was used in the EPC calculations. The superconductivity calculations were performed with the Quantum-ESPRESSO package (17)·
Results
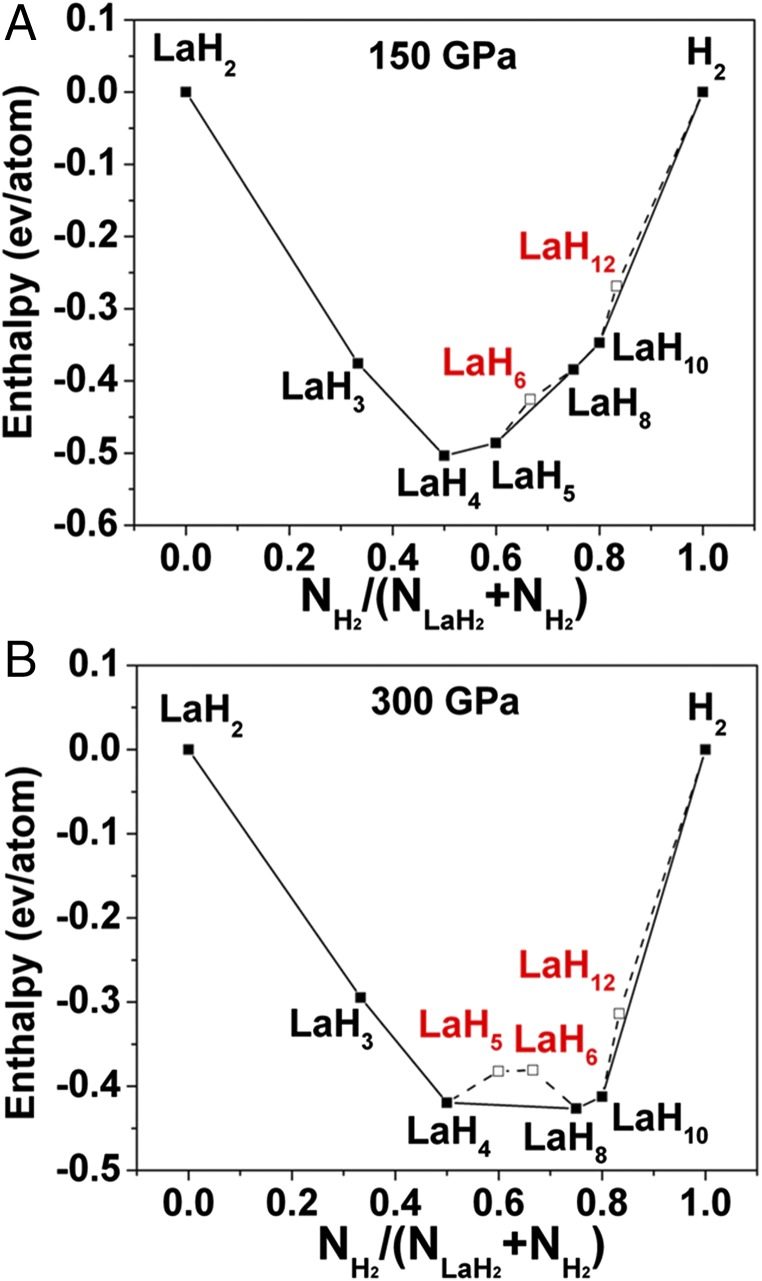
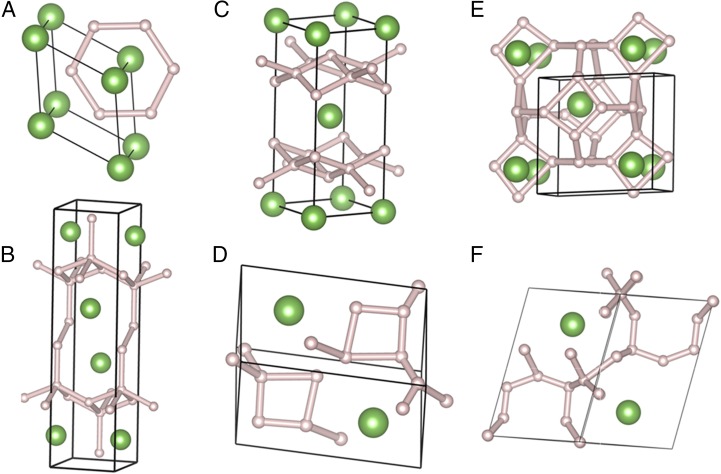
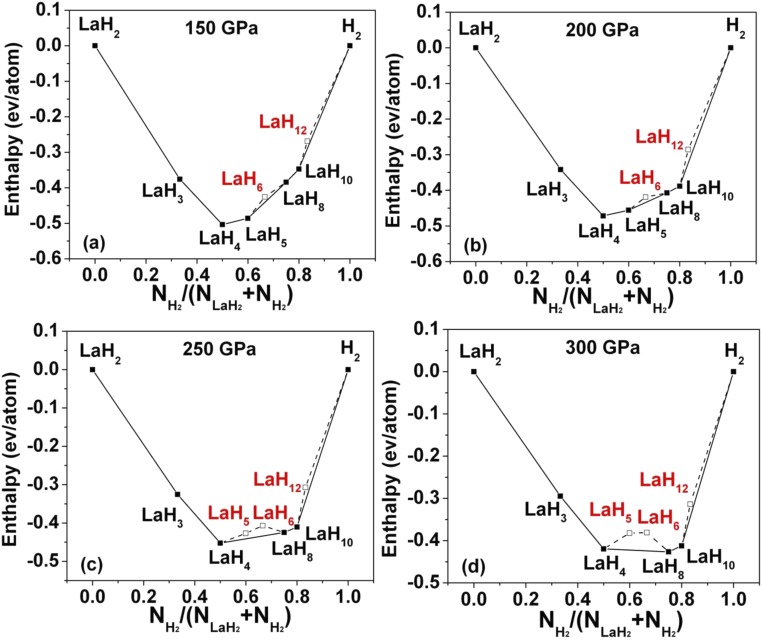
We began with structure searches at ambient pressure for LaH2. The fcc structure is found to be stable at low pressure, in agreement with experiment. Thinking the high-pressure regime would be most productive for new stoichiometries, we moved directly to 150 and 300 GPa. We first performed structure prediction at 150 and 300 GPa for LaHx (x = 1–12). LaH2, LaH3, LaH4, LaH5, LaH8, and LaH10 are found to be stable at 150 GPa (Fig. 1). Note that this figure is plotted with LaH2 as the lanthanum-rich endpoint, because this composition has the most negative enthalpy/atom at every pressure considered. Fig. 2 shows the computed geometries for the most stable phases we found at each stoichiometry, for n = 2–8. Interestingly, the stable phase predicted for LaH6 is not sodalite-type but an R-3m structure. The convex hull shows that LaH6 is not stable from 150 to 300 GPa (Fig. S1); LaH5 in a P-1 structure is stable from 150 to 200 GPa. LaH2 adopts a C2/m structure with a H–H distance of 1.53 Å, LaH3 adopts a Cmcm structure with H–H distance of 1.42 Å, and LaH8 has a C2/m structure with H–H distance of 1.02 Å (all at 300 GPa).
Fig. 1.
Formation enthalpy of predicted structures in the La–H system at (A) 150 GPa, (B) 300 GPa. The x axis is the concentration of hydrogen pairs in the stoichiometric compositions considered as described by the generic formula (H2)x(LaH2)1−x (0 < x < 1). The enthalpies are shown relative to the ground states of H2 and LaH2 at each pressure.
Fig. 2.
Predicted lanthanum hydride structures. (A) LaH2, (B) LaH3, (C) LaH4, (D) LaH5, (E) LaH6, and (F) LaH8. Large and small spheres represent La and H atoms.
Fig. S1.
Formation enthalpy of predicted structures in the La–H system at (A) 150, (B) 200, (C) 250, and (D) 300 GPa. The x axis is the concentration of hydrogen pairs in the stoichiometric compositions considered as described by the generic formula (H2)x(LaH2)1−x (0 < x < 1). The enthalpies are shown relative to the ground states of H2 and LaH2 at each pressure.
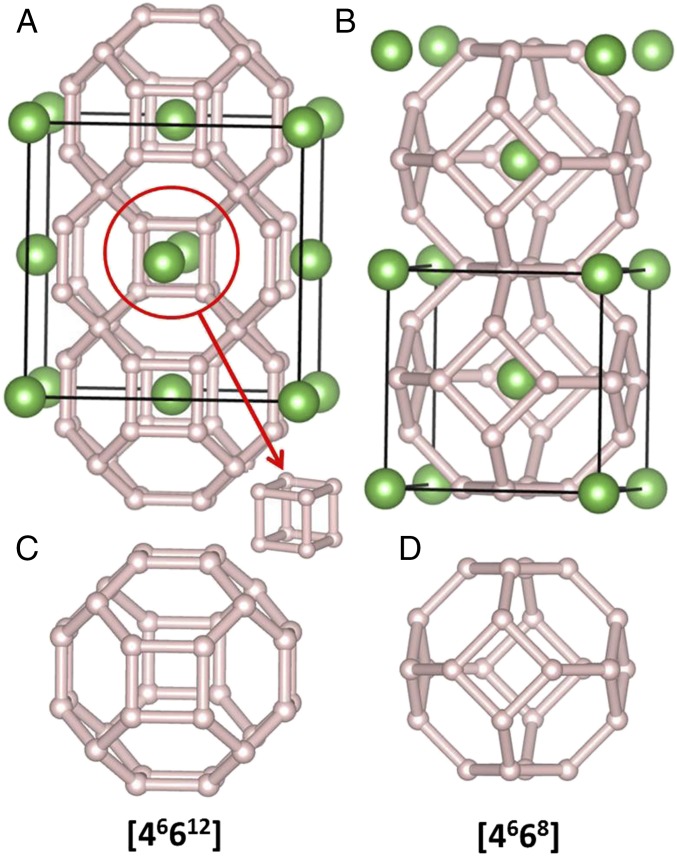
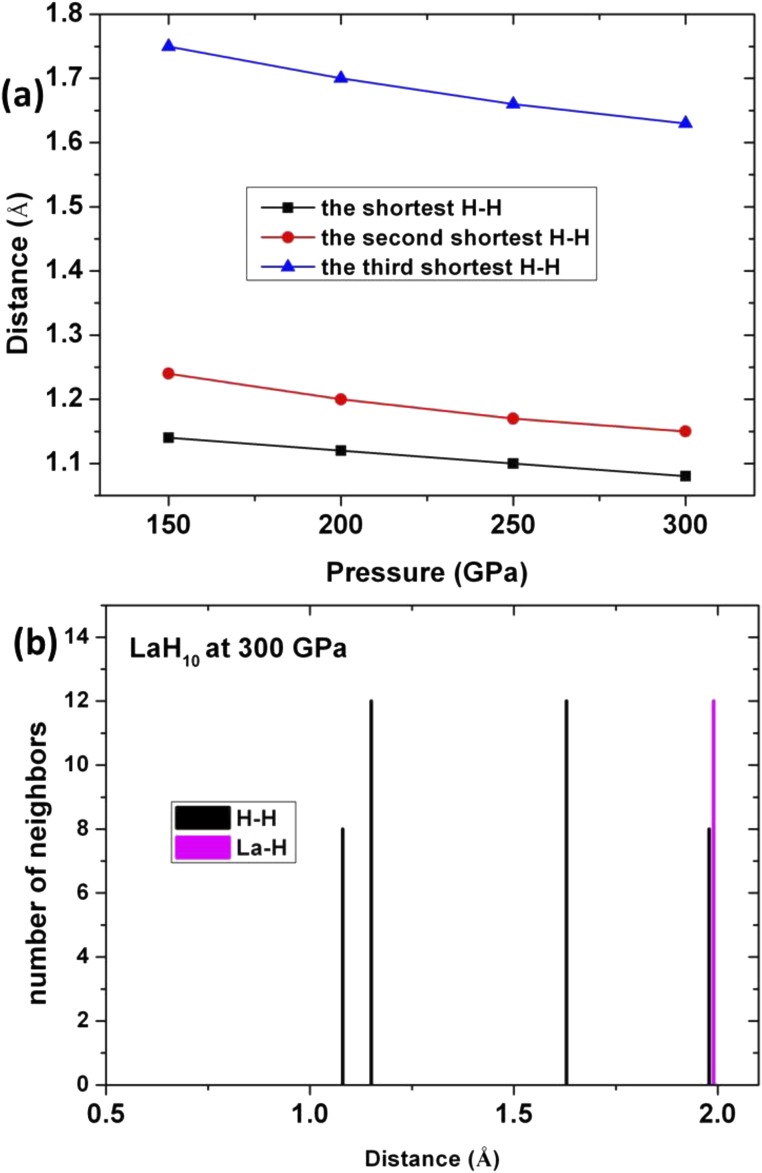
Most interestingly, we find that LaH10 adopts a sodalite-like structure with the La atoms arrayed on an fcc rather than a bcc lattice (Fig. 3). To illustrate the difference between fcc LaH10 and previous sodalite-like bcc CaH6/YH6 structure, Fig. 3B shows the sodalite-like LaH6 structure. The fcc-LaH10 structure contains [46612] polyhedra which contain hydrogen cubes (Fig. 3 A and C), whereas conventional sodalite is built up of [4668] polyhedra (Fig. 3 B and D). The H network, made of different atoms, is known in the clathrate and zeolite community as AST (41). The La atoms sit at the 4b Wyckoff position (0, 0, 0), the H atoms at the 32f position (0.12, 0.38, 0.12) and 8c position (0.25, 0.25, 0.75). The shortest H–H distance is 1.1 Å (250 GPa), which is close to H–H distance predicted for atomic metallic hydrogen near 500 GPa (1 Å) (1). In contrast, the H–H distance in sodalite-type CaH6 is 1.24 Å at 150 GPa.
Fig. 3.
Crystal structure of sodalite-like LaH10 (A) and LaH6 (B) at 300 GPa. In the LaH10 structure, the red circle highlights the cube hydrogen units. The bottom shows the [46612] (C) and [4668] (D) hydrogen polyhedra in these structures.
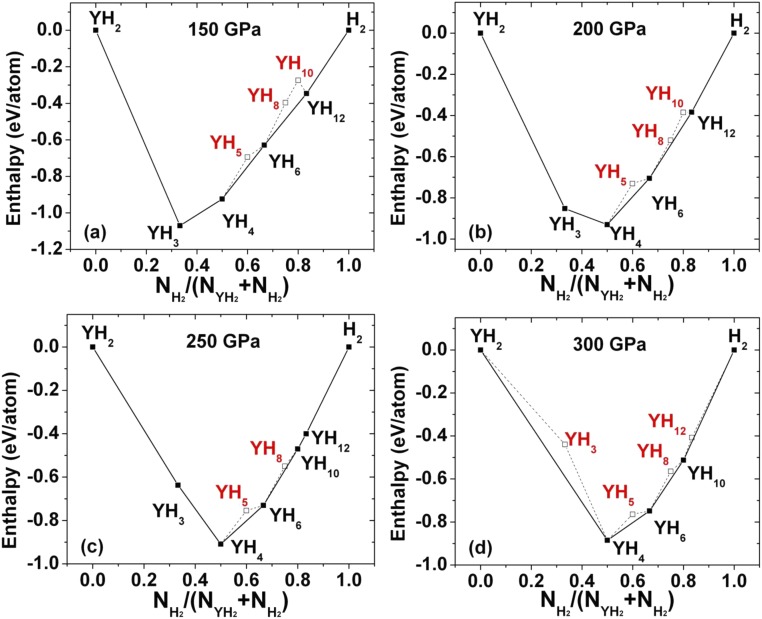
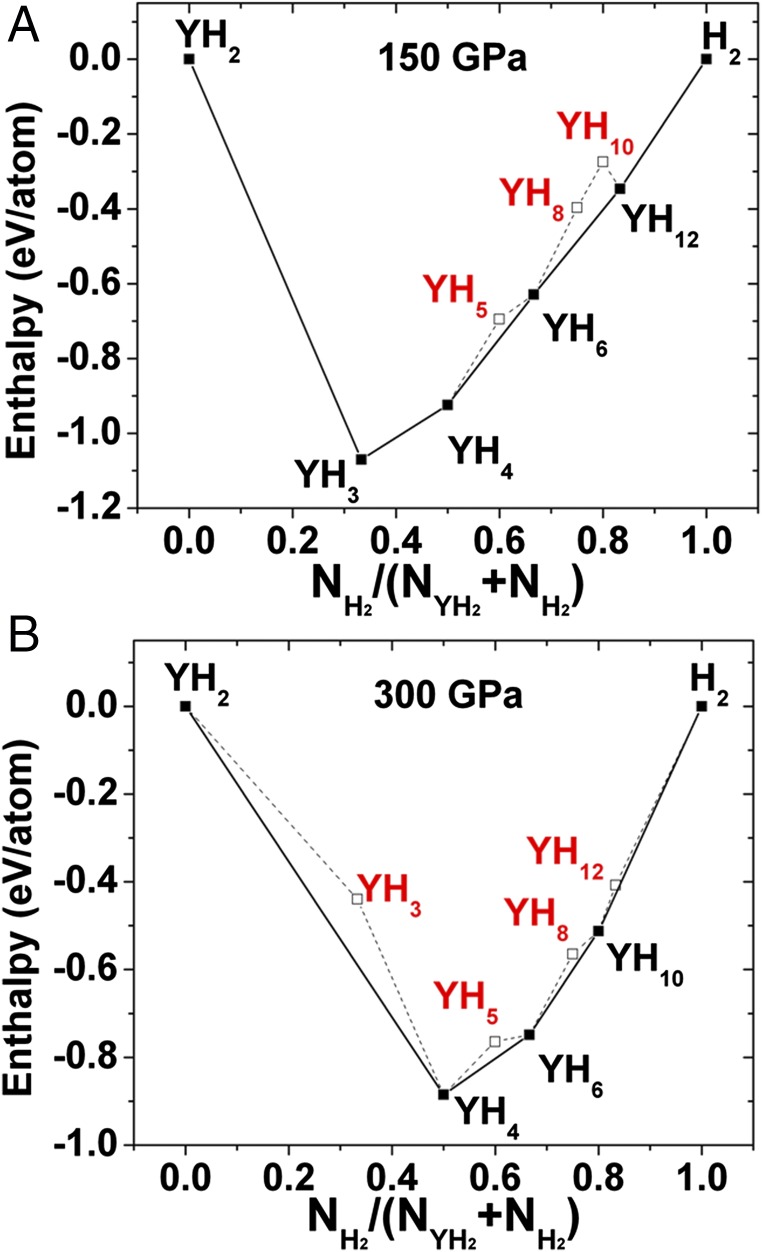
The above structures of LaH10 encouraged us to explore the Y–H system at similar pressures (Fig. S2). Previously, YH3 was predicted to adopt an fcc structure of Y, with atomic H located in the tetrahedral and octahedral interstitial sites. The compound was predicted to be superconducting with a maximum Tc is 40 K near 18 GPa (μ* = 0.1) (16). Another theoretical study suggested two energetically competing hydrogen-rich polymorphs YH4 and YH6 (30). At 120 GPa, both are predicted to be superconductors with maximum Tc of 95 K and 264 K for YH4 and YH6, respectively. Our calculations indicate that YH6 is stable in the sodalite structure up to 300 GPa (Fig. 4 and Fig. S2). As expected, YH10 adopts the same structure as LaH10 over a range of pressures. We predict that YH10 is the energetically stable phase from 250 to 300 GPa, and is dynamically stable down to 220 GPa.
Fig. S2.
Formation enthalpy of predicted structures in the Y–H system at (A) 150, (B) 200, (C) 250, and (D) 300 GPa. The enthalpies are shown relative to the ground states of H2 and YH2 at each pressure.
Fig. 4.
Formation enthalpy of predicted structures in the Y–H system at (A) 150 GPa, (B) 300 GPa. The enthalpies are shown relative to the ground states of H2 and YH2 at each pressure.
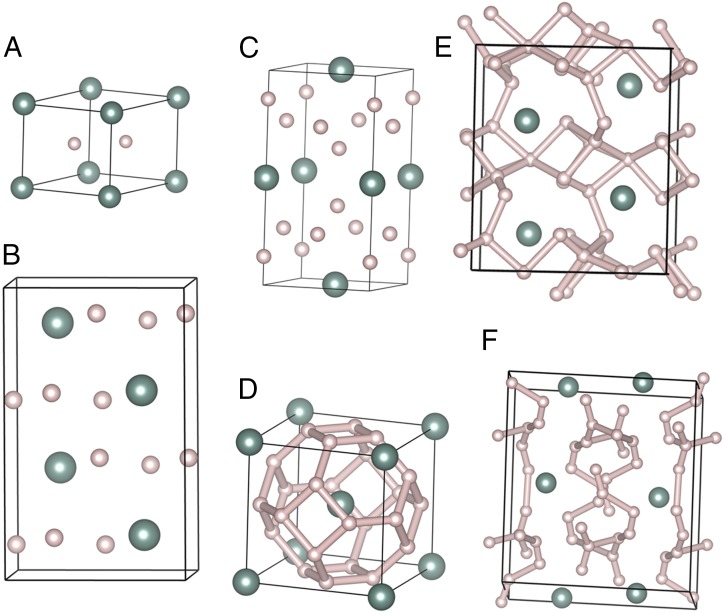
Fig. 5 shows computed YHn geometries for the most stable phases found at each stoichiometry, for n = 2, 3, 4, 6, 8, and 12. The YH2 adopts a P6/mmm structure with H–H distances of 1.46 Å at 300 GPa. The YH3 is stable from 150 to 250 GPa and adopts a Pnma structure with H–H distances of 1.54 Å at 300 GPa (Fig. S2). The YH4 adopts an I4/mmm structure with H–H distances of 1.37 Å at 300 GPa. It is interesting to see that YH6 has the same sodalite structure as CaH6 from 150 to 300 GPa with H–H distances of 1.19 Å at 300 GPa and is stable from 150 to 300 GPa. We found YH8 is unstable from 150 to 300 GPa with a Cc structure (Fig. S2). The YH12 is stable from 50 to 250 GPa and is a C2/c structure with shortest H–H distances of 0.79 Å at 250 GPa.
Fig. 5.
Predicted yttrium hydride structures. (A) YH2, (B) YH3, (C) YH4, (D) YH6, (E) YH8, and (F) YH12. Large and small spheres represent Y and H atoms.
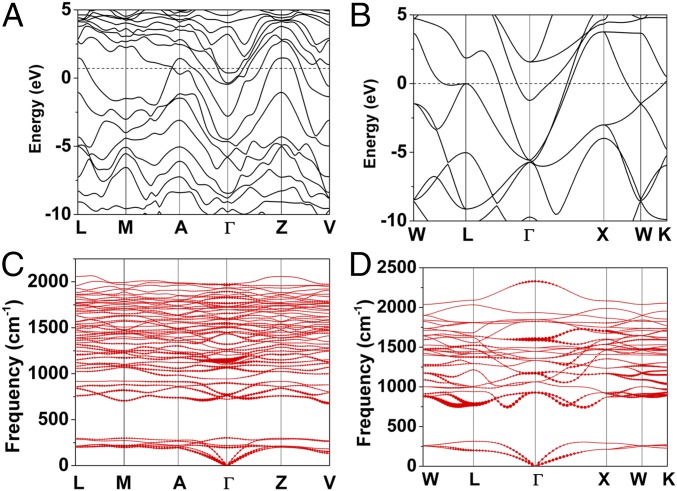
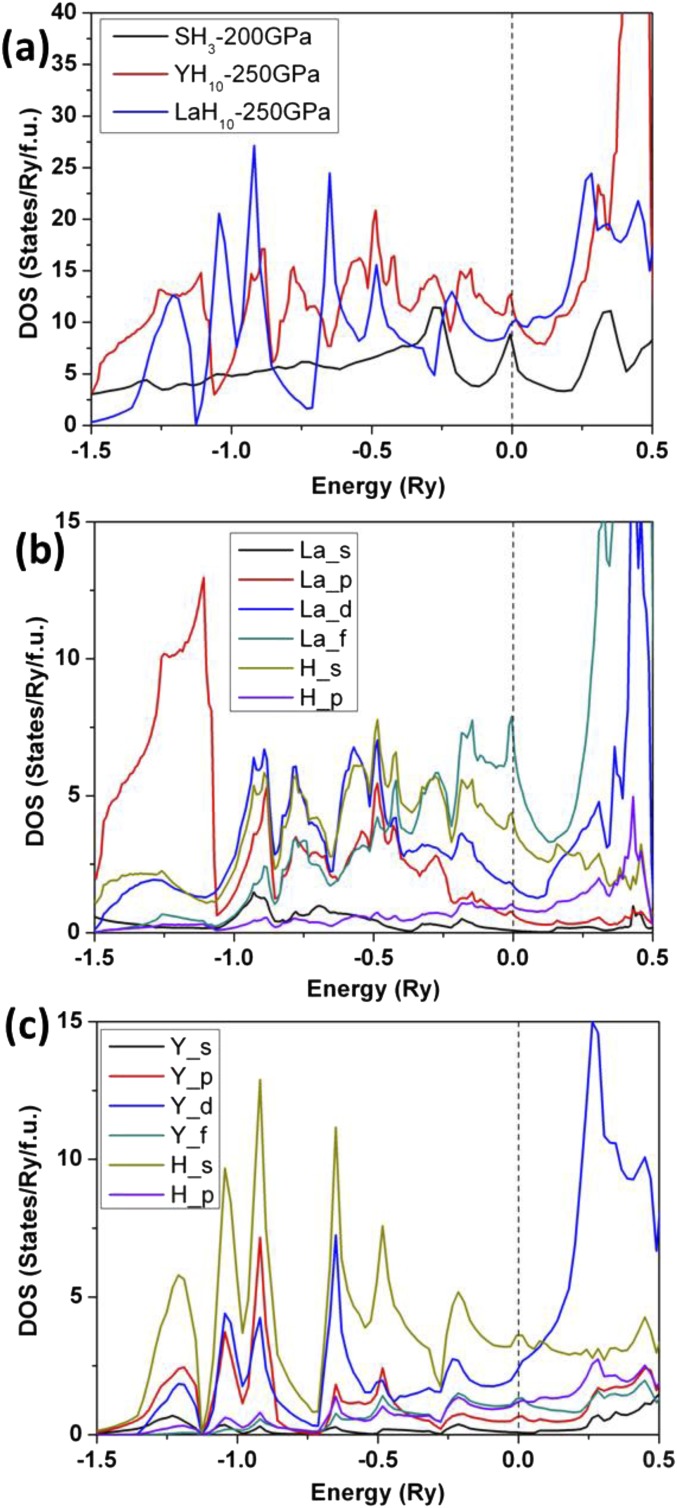
Before calculating possible superconducting properties we analyze the electronic band structures of the C2/m-LaH8 and fcc-LaH10 structures. The band dispersion shows the metallic character of both structures at these pressures (Fig. 6). The fcc LaH10 is a good metal with several bands crossing the Fermi level along many directions. This fact manifests itself in a noticeable density of electronic states at the Fermi level: 10.0 states/Ry, which is a factor of 1.4 higher than that previously found in SH3 (42) at an optimal pressure of 200 GPa (Fig. S3). Remarkably, not only the d electrons of La and s electrons of H contribute to N(Ef) but also f electrons of La; moreover, the latter contribution is dominant. This is due to the fact that external pressure destabilizes La 6s and La 5d orbitals to a greater extent than La 4f: the first two have five and two nodes in their radial wave functions, respectively, whereas the latter has none. This is in sharp contrast with the YH10 system, where only the d electrons of Y and s electrons of H are the main contributors to N(Ef). Phonon calculations reveal no imaginary frequencies for LaH8 and LaH10 over a wide pressure range, indicating dynamic stability. Specifically, we find that the structure shown for LaH10 is dynamically stable down to 210 GPa. The EPC calculations for different phonon modes indicate that there are no significant contributions in particular directions (Fig. 6 C and D). Note that there are no high-frequency vibrations, consistent with the absence of molecular H2 entities. The highest frequency for the structure here (2,000 cm−1) can be compared to the 2,600 cm−1 calculated for atomic metallic hydrogen in the Cs-IV structure at 500 GPa (43); the lower frequency for the former suggests still weaker H–H interactions in the hydride.
Fig. 6.
Calculated electronic band structures of (A) LaH8 and (B) LaH10 at 300 GPa. The phonon spectrum for (C) LaH8 and (D) LaH10 at 300 GPa. Red solid circles (Bottom) show the EPC with the radius proportional to the respective coupling strength.
Fig. S3.
Calculated total and partial DOS. (A) The total density for SH3 at 200 GPa, LaH10 at 250 GPa, and YH10 at 250 GPa. (B and C) The partial density for LaH10 and YH10 at 250 GPa.
Electron-coupling calculations for LaH4 give a relatively small λ of 0.43. The estimated Tc is 5–10 K at 300 GPa with the typical choice of the Coulomb potential of μ* = 0.1–0.13. Using the McMillan equation (44), we calculated Tc using the spectral function [α2F(ω)], again with μ* = 0.1–0.13, as previously used in superconductivity calculations for Y (15), La (45, 46), SH3 (24), CaH6 (17), YH6 (30), and other superconductors.
| [1] |
Here, λ is the first reciprocal moment of α2F(ω),
| [2] |
and ω(q) is the weight of a q (wave vector of crystal vibrations) point in the first BZ. The EPC spectral function α2F(ω) is expressed in terms of the phonon linewidth γqj arising from EPC,
| [3] |
In this equation, Nf is the electronic density of electron states at the Fermi level. The linewidth γqj of a phonon mode j at wave vector q, arising from EPC is given by
| [4] |
where the sum is over the first BZ, with ΩBZ as the volume of the BZ, and ξkn are the energies of bands measured with respect to the Fermi ξF level at point k. Here, gjkn,k+qm is the electron–phonon matrix element for scattering from an electron in band n at wave vector k state to band m at wave vector k+q via a phonon with wave vector q.
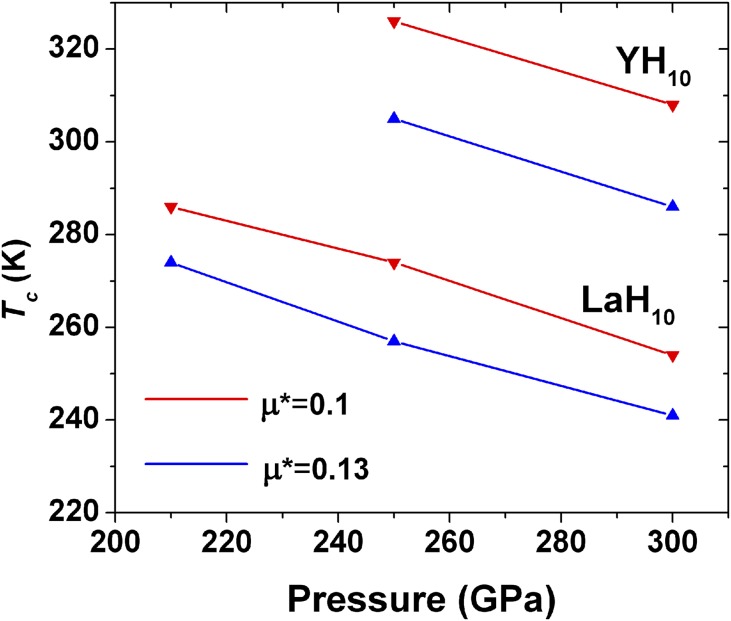
The calculated electron-coupling parameter for LaH8 at 300 GPa is relatively large (λ = 1.12), and the calculated Tc is 114–131 K, assuming μ* = 0.1–0.13 (Eq. 1). That result encouraged us to calculate the Tc of LaH10. Previous theoretical studies also found that sodalite-structured hydrides (e.g., CaH6 and YH6) were associated with calculated high-Tc superconductivity. In the LaH10 structure, the predicted shortest H–H distance is 1.1 Å (Fig. S4) at these pressures, indicating some H…H interactions and no clear distinction between stretching and bending vibrations. As a result, all H vibrations effectively participate in the EPC process, which appears to enhance high superconductivity. The calculated EPC is quite large (λ = 2.2). Tc was estimated from α2F(ω) by numerically solving the Eliashberg equations (17, 30) with μ* = 0.1–0.13. Coulomb repulsion is taken into account in terms of μ* scaled to a cutoff frequency. At 250 GPa, the estimated Tc of LaH10 is 257–274 K with μ* = 0.1–0.13. The calculated Tc is found to decrease with increasing pressure (Fig. 7 and Table S1). The predicted Tc for YH10 is very high (Fig. 7) using similar EPC calculations. At 250 GPa, the λ is 2.56 and gives a Tc of 305–326 K with μ* = 0.1–0.13 based on numerically solving the Eliashberg equations. The Tc of YH10 increased by ∼30 K relative to YH6 (30) despite the fact that λ decreases (from 2.93 to 2.56). But, this change is offset by the higher average ωlog calculated for YH10 compared with YH6 (1,282 K versus 1,124 K), as a result of the higher hydrogen content in the former. The information of all predicted structures for La–H and Y–H is summarized in Table S2.
Fig. S4.
(A) Distance between H atoms in LaH10 as a function of pressure. (B) Histogram of H–H and La–H distances in LaH10 at 300 GPa.
Fig. 7.
Calculated Tc of LaH10 and YH10 as a function of pressure.
Table S1.
Tc of La–H and Y–H systems at different pressures calculated using the McMillan and Eliashberg equations
| Compound | Pressure, GPa | λ | wlog, K | Tc, K; μ* = 0.1 McMillan | Tc, K; μ* = 0.13 McMillan | Tc, K; μ* = 0.1 Eliashberg | Tc, K; μ* = 0.13 Eliashberg |
| LaH4 | 300 | 0.43 | 1,624 | 10 | 5 | 10 | 5 |
| LaH8 | 300 | 1.12 | 1,591 | 131 | 114 | 150 | 138 |
| LaH10 | 210 | 3.41 | 848 | 238 | 219 | 286 | 274 |
| LaH10 | 250 | 2.29 | 1,253 | 232 | 212 | 274 | 257 |
| LaH10 | 300 | 1.78 | 1,488 | 215 | 196 | 254 | 241 |
| YH10 | 250 | 2.58 | 1,282 | 265 | 244 | 326 | 305 |
| YH10 | 300 | 2.06 | 1,511 | 255 | 233 | 308 | 286 |
Table S2.
Lattice parameters and atomic positions for predicted La–H and Y–H systems
| Compound | Pressure, GPa | Space group | Lattice parameters, Å, degree | Atomic positions |
| LaH2 | 300 | P6/mmm | a = b = 2.65 | La 0.000 0.000 0.000 |
| c = 2.58 | H 0.666 0.333 0.500 | |||
| α = 90 | H 0.333 0.666 0.500 | |||
| β = 90 | ||||
| γ = 120 | ||||
| LaH3 | 300 | Cmcm | a = 2.62 | La −0.000 0.885 0.250 |
| B = 9.99 | La 0.500 0.385 0.250 | |||
| C = 2.65 | La −0.000 0.114 0.750 | |||
| α = 90 | La 0.500 0.614 0.750 | |||
| β = 90 | H 0.500 0.808 0.750 | |||
| γ = 90 | H 0.500 0.962 0.750 | |||
| H −0.000 0.462 0.750 | ||||
| H 0.500 0.037 0.250 | ||||
| H 0.000 0.537 0.250 | ||||
| H −0.000 0.751 0.750 | ||||
| H 0.500 0.251 0.750 | ||||
| H −0.000 0.248 0.250 | ||||
| H 0.500 0.748 0.250 | ||||
| H −0.000 0.691 0.250 | ||||
| H 0.500 0.191 0.250 | ||||
| H −0.000 0.308 0.750 | ||||
| LaH4 | 300 | I4/mmm | a = b = 2.53 | La 0.000 0.000 0.000 |
| c = 5.87 | La 0.500 0.500 0.500 | |||
| α = 93.33 | H 0.500–0.000 0.750 | |||
| β = 93.33 | H 0.000 0.500 0.750 | |||
| γ = 93.33 | H 0.500 0.000 0.250 | |||
| H 0.000 0.500 0.250 | ||||
| H 0.500 0.500 0.831 | ||||
| H 0.000 0.000 0.331 | ||||
| H 0.500 0.500 0.168 | ||||
| H 0.000 0.000 0.668 | ||||
| LaH6 | 300 | R-3m | a = b = c = 3.53 | La 0.776 0.776 0.776 |
| α = 93.33 | La 0.223 0.223 0.223 | |||
| β = 93.33 | H 0.291 0.709 0.000 | |||
| γ = 93.33 | H 0.000 0.291 0.709 | |||
| H 0.709 0.000 0.291 | ||||
| H 0.709 0.291–0.000 | ||||
| H −0.000 0.709 0.291 | ||||
| H 0.291–0.000 0.709 | ||||
| H 0.727 0.272 0.500 | ||||
| H 0.500 0.727 0.272 | ||||
| H 0.272 0.500 0.727 | ||||
| H 0.272 0.727 0.500 | ||||
| H 0.500 0.272 0.727 | ||||
| H 0.727 0.500 0.272 | ||||
| LaH8 | 300 | C2/m | a = b = 3.42 | La 0.491 0.491 0.778 |
| c = 8.40 | La 0.508 0.508 0.221 | |||
| α = β = 135.1887 | H 0.731 0.233 0.413 | |||
| γ = 60.2893 | H 0.766 0.268 0.586 | |||
| H 0.268 0.766 0.586 | ||||
| H 0.233 0.731 0.413 | ||||
| H 0.738 0.738 0.133 | ||||
| H 0.998 0.998 0.639 | ||||
| H 0.932 0.932 0.874 | ||||
| H 0.241 0.241 0.419 | ||||
| H 0.701 0.701 0.670 | ||||
| H 0.261 0.261 0.866 | ||||
| H 0.001 0.001 0.360 | ||||
| H 0.067 0.067 0.125 | ||||
| H 0.758 0.758 0.580 | ||||
| H 0.298 0.298 0.329 | ||||
| H 0.252 0.747–0.000 | ||||
| H 0.747 0.252–0.000 | ||||
| LaH10 | 300 | Fm-3m | a = 4.78 | La 0.500 0.500 0.500 |
| b = 4.78 | H 0.639 0.120 0.120 | |||
| c = 4.78 | H 0.120 0.639 0.120 | |||
| α = 90 | H 0.120 0.120 0.639 | |||
| β = 90 | H 0.120 0.120 0.120 | |||
| γ = 90 | H 0.360 0.879 0.879 | |||
| H 0.879 0.360 0.879 | ||||
| H 0.879 0.879 0.879 | ||||
| H 0.879 0.879 0.360 | ||||
| H 0.250 0.250 0.250 | ||||
| H 0.750 0.750 0.750 | ||||
| YH2 | 300 | P6/mmm | a = b = 2.53 | Y 0.000 0.000 0.000 |
| b = 2.4 | H 0.333 0.666 0.500 | |||
| α = 90 | H 0.666 0.333 0.500 | |||
| β = 90 | ||||
| γ = 120 | ||||
| YH3 | 300 | Pnma | a = 5.8888 | Y 0.143 0.750 0.675 |
| b = 3.6288 | Y 0.356 0.250 0.175 | |||
| c = 2.9464 | Y 0.856 0.250 0.324 | |||
| α = 90 | Y 0.643 0.750 0.824 | |||
| β = 90 | H 0.906 0.502 0.836 | |||
| γ = 90 | H 0.593 0.497 0.336 | |||
| H 0.093 0.002 0.163 | ||||
| H 0.406 0.997 0.663 | ||||
| H 0.093 0.497 0.163 | ||||
| H 0.406 0.502 0.663 | ||||
| H 0.906 0.997 0.836 | ||||
| H 0.593 0.002 0.336 | ||||
| H 0.866 0.750 0.324 | ||||
| H 0.633 0.250 0.824 | ||||
| H 0.133 0.250 0.675 | ||||
| H 0.366 0.750 0.175 | ||||
| YH4 | 300 | I4/mmm | a = b = 2.52 | Y 0.500 0.500 0.500 |
| c = 5.15 | Y −0.000–0.000–0.000 | |||
| α = 90 | H −0.000 0.000 0.354 | |||
| β = 90 | H 0.500 0.500 0.854 | |||
| γ = 90 | H −0.000 0.000 0.645 | |||
| H 0.500 0.500 0.145 | ||||
| H 0.500 0.000 0.750 | ||||
| H 0.000 0.500 0.750 | ||||
| H 0.500 0.000 0.250 | ||||
| H 0.000 0.500 0.250 | ||||
| YH6 | 300 | Im-3m | a = 3.37 | Y 0.500 0.500 0.500 |
| b = 3.37 | Y 0.000 0.000 0.000 | |||
| c = 3.37 | H 0.000 0.750 0.500 | |||
| α = 90 | H 0.500 0.000 0.250 | |||
| β = 90 | H 0.000 0.500 0.250 | |||
| γ = 90 | H 0.000 0.250 0.500 | |||
| H 0.250 0.000 0.500 | ||||
| H 0.750 0.000 0.500 | ||||
| H 0.500 0.250 0.000 | ||||
| H 0.000 0.500 0.750 | ||||
| H 0.500 0.000 0.750 | ||||
| H 0.500 0.750 0.000 | ||||
| H 0.750 0.500 0.000 | ||||
| H 0.250 0.500 0.000 | ||||
| YH8 | 300 | Cc | a = b = 3.23 | Y 0.458 0.793 0.264 |
| c = 4.89 | Y 0.793 0.458 0.764 | |||
| α = β = 90.86 | H 0.779 0.944 0.606 | |||
| γ = 120.79 | H 0.533 0.337 0.422 | |||
| H 0.961 0.241 0.069 | ||||
| H 0.802 0.971 0.949 | ||||
| H 0.241 0.388 0.561 | ||||
| H 0.788 0.458 0.161 | ||||
| H 0.132 0.123 0.264 | ||||
| H 0.975 0.302 0.445 | ||||
| H 0.944 0.779 0.106 | ||||
| H 0.337 0.533 0.922 | ||||
| H 0.241 0.961 0.569 | ||||
| H 0.971 0.802 0.449 | ||||
| H 0.388 0.241 0.061 | ||||
| H 0.458 0.788 0.661 | ||||
| H 0.123 0.132 0.764 | ||||
| H 0.302 0.975 0.945 | ||||
| YH10 | 300 | Im-3m | a = 4.6 | Y 0.000–0.000–0.000 |
| b = 4.6 | H 0.750 0.750 0.750 | |||
| c = 4.6 | H 0.250 0.250 0.250 | |||
| α = 90 | H 0.379 0.862 0.379 | |||
| β = 90 | H 0.862 0.379 0.379 | |||
| γ = 90 | H 0.379 0.379 0.379 | |||
| H 0.379 0.379 0.862 | ||||
| H 0.620 0.137 0.620 | ||||
| H 0.137 0.620 0.620 | ||||
| H 0.620 0.620 0.137 | ||||
| H 0.620 0.620 0.620 | ||||
| YH12 | 300 | C2/c | a = b = 4.42 | Y 0.708 0.291 0.250 |
| c = 3.21 | Y 0.291 0.708 0.750 | |||
| α = β = 70.41 | H 0.308 0.942 0.139 | |||
| γ = 81.15 | H 0.073 0.034 0.009 | |||
| H 0.657 0.767 0.244 | ||||
| H 0.872 0.692 0.921 | ||||
| H 0.059 0.382 0.728 | ||||
| H 0.514 0.695 0.158 | ||||
| H 0.057 0.691 0.360 | ||||
| H 0.965 0.926 0.490 | ||||
| H 0.232 0.342 0.255 | ||||
| H 0.307 0.127 0.578 | ||||
| H 0.617 0.940 0.771 | ||||
| H 0.304 0.485 0.341 | ||||
| H 0.691 0.057 0.860 | ||||
| H 0.926 0.965 0.990 | ||||
| H 0.342 0.232 0.755 | ||||
| H 0.127 0.307 0.078 | ||||
| H 0.940 0.617 0.271 | ||||
| H 0.485 0.304 0.841 | ||||
| H 0.942 0.308 0.639 | ||||
| H 0.034 0.073 0.509 | ||||
| H 0.767 0.657 0.744 | ||||
| H 0.692 0.872 0.421 | ||||
| H 0.382 0.059 0.228 | ||||
| H 0.695 0.514 0.658 |
Discussion
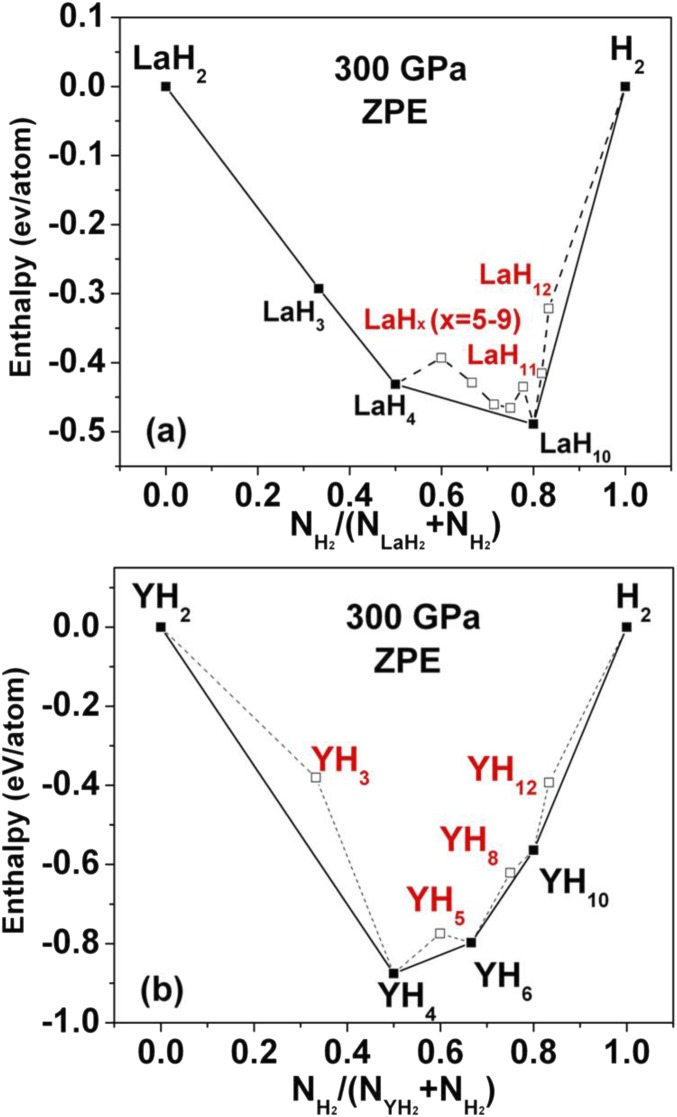
We now examine the above results, including various assumptions and comparisons with previous studies. Because zero-point energy (ZPE) has been shown to play an important role in determining the stability of hydrogen-rich materials, we consider its effect. We recomputed the formation enthalpies of different phases in La–H and Y–H systems at 300 GPa by including ZPE (Fig. S5). The phases LaH10 and YH10 are still stable; therefore, the above conclusions are not altered by considering zero-point vibrations. The authors of ref. 30 focused on the YH6 stoichiometry, and the authors did not consider hydrogen compositions higher than 1:8. To understand the role of the La atoms in stabilization of clathrate hydrogen structure, we computed the “formation volume” VLaH10 − VH10 − VLa, where VLaH10 is the volume of LaH10, VH10 is the volume of hydrogen in the sodalite-like structure, and VLa is the volume of La at 300 GPa. We found the formation volume to be negative: −4.66 A3 per primitive cell. This suggests that the La atoms help to stabilize the clathrate hydrogen structure.
Fig. S5.
Formation enthalpy of predicted structures in the (A) La–H and (B) Y–H system at 300 GPa including ZPE.
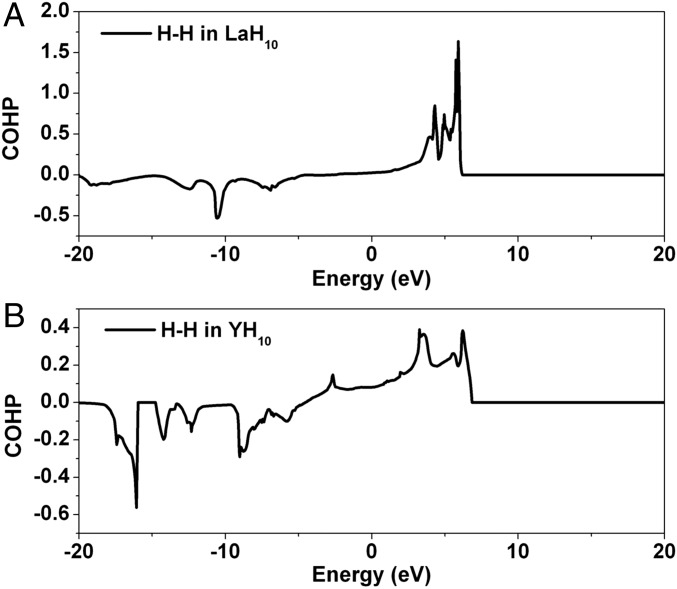
We also investigated the bonding in these hydrides by a crystal orbital Hamiltonian population (COHP) analysis. The COHP provides an atom-specific measure of the bonding character of states in a given energy region (47). A negative COHP indicates bonding and positive COHP indicates antibonding. As Fig. 8 shows, predictably most of the states below the Fermi level are H–H bonding for LaH10 and YH10. The interesting difference is that some H–H antibonding states are occupied in the yttrium hydride.
Fig. 8.
COHP for pairs of H–H in (A) LaH10 and (B) YH10 at 300 GPa.
We comment on the reliability of the present predictions of very high-Tc superconductivity by comparing our results with existing experimental data and additional BCS calculations for related superconductors. We calculated a Tc of 6–7 K for fcc La under ambient pressure using μ* = 0.1–0.13, which is close to previous theoretical (46) and experimental work (Tc = 6 K) (48), and consistent with high-pressure behavior (Tc = 13 K at 20 GPa) (45, 48). Likewise, our method gives the Tc of elemental Y under pressure to be 15–16 K using μ* = 0.1–0.13, which is also consistent with previous theoretical (15) and experimental studies (Tc = 17 K at 89 GPa) (49). All of our calculations were carried out using the harmonic approximation. It is well known that in many hydrides anharmonicity tends to lower Tc (50). For the well-studied SH3 system, however, such an effect is weak: Tc is lowered from its harmonic 204 K value (24) only to 194 K (21) at 250 GPa, and both are close to the reported experiment Tc at that pressure (20).
There is a good reason to think that hydrogen-rich hydrides should behave similarly to pure hydrogen. Recent first-principles calculations of metallic hydrogen in structures with space groups I41/amd and Cmca-4 predict anharmonic behavior that differs from calculated results for hydrides with relatively low hydrogen content (51, 52). Whereas in the I41/amd the inclusion of anharmonicity slightly lowers Tc (i.e., from 318 to 300 K at 500 GPa), the situation is drastically different in the Cmca-4 phase, where the calculated Tc increases by a factor of 2 with anharmonicity introduced, such that the phase is predicted to be a superconductor above 200 K. In this context it is also worth noticing that anharmonic vibrations may enhance the electron–phonon matrix elements, e.g., in the case of disordered materials (53).
In summary, exploration of La–H and Y–H systems at high pressures reveals stable hydrogen-rich phases with calculated unusually high superconducting temperatures. To be realistic, we should be careful about these high Tcs. Although well-calibrated on low Tc elemental superconductors, more high-Tc compounds need to be studied––we only have SH3 mentioned above (with its attendant ambiguities––not in the Tc, measured, but in the structure). The predicted stabilities of the phases are in the range of current high-pressure techniques, although the megabar pressures required present challenges for combined synthesis and characterization. On the other hand, it may also be possible to synthesize these and related materials metastably at lower pressure. For instance, one might reason from the fact that Y (54) itself has a distorted fcc structure that it may be possible to produce sodalite-like YH10 at a metal–hydrogen interface. The exploration, theoretical and experimental, of high hydrides of heavy metals is thus a promising field (55–58).
SI Text
Table S1 presents calculated Tcs of LaHn (n = 4, 8, and 10) and YH10 at high pressures for different models and parameters. It is seen that the McMillan and Eliashberg equations lead to similar results for small λ. For larger λ, however, the Eliashberg equation predicts higher Tc values. Table S2 contains the lattice parameters and atomic coordinates for the predicted structures.
We compare the calculated density of states (DOS) for LaH10, YH10, and SH3 in Fig. S3. In the case of SH3, we found a DOS at the Fermi level of 7.1 states/Ry which is close to the value of 6.93 states/Ry found in the previous work (42). The calculated N(Ef) of LaH10 and YH10 at 250 GPa is larger than that of SH3 at 200 GPa. For LaH10, both the d and f electrons of La as well as the s electrons of H contribute to N(Ef). Indeed, the f-electron contribution is dominant, which is in sharp contrast with the YH10 system, where only the d electrons of Y and s electrons of H are the main contributors to N(Ef).
The distances between H atoms in LaH10 as a function of pressure are shown in Fig. S4. Each La atom is coordinated with 12 hydrogen atoms. H atoms are 4-coordinated, with bond distances of 1.08 and 1.15 Å (see also Fig. 3). Finally, the formation enthalpy of predicted La–H and Y–H structures at 300 GPa including ZPE is shown in Fig. S5.
Acknowledgments
We are grateful to the reviewers for their comments on the manuscript. This research was supported by EFree, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award DE-SC0001057. The infrastructure and facilities used are supported by the US Department of Energy/National Nuclear Security Administration (Grant DE-NA-0002006, Capital/Department of Energy Alliance Center).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704505114/-/DCSupplemental.
References
- 1.Ashcroft NW. Metallic hydrogen: A high-temperature superconductor? Phys Rev Lett. 1968;21:1748. doi: 10.1103/PhysRevLett.92.187002. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft NW. Hydrogen dominant metallic alloys: High temperature superconductors? Phys Rev Lett. 2004;92:187002. doi: 10.1103/PhysRevLett.92.187002. [DOI] [PubMed] [Google Scholar]
- 3.Bardeen J, Cooper LN, Schrieffer JR. Microscopic theory of superconductivity. Phys Rev. 1957;106:162. [Google Scholar]
- 4.Eremets MI, Trojan IA, Medvedev SA, Tse JS, Yao Y. Superconductivity in hydrogen dominant materials: Silane. Science. 2008;319:1506–1509. doi: 10.1126/science.1153282. [DOI] [PubMed] [Google Scholar]
- 5.Chen XJ, et al. Superconducting behavior in compressed solid SiH4 with a layered structure. Phys Rev Lett. 2008;101:077002. doi: 10.1103/PhysRevLett.101.077002. [DOI] [PubMed] [Google Scholar]
- 6.Zurek E, Hoffmann R, Ashcroft NW, Oganov AR, Lyakhov AO. A little bit of lithium does a lot for hydrogen. Proc Natl Acad Sci USA. 2009;106:17640–17643. doi: 10.1073/pnas.0908262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strobel TA, et al. High-pressure study of silane to 150 GPa. Phys Rev B. 2011;83:144102. [Google Scholar]
- 8.Shamp A, Zurek E. Superconducting high-pressure phases composed of hydrogen and iodine. J Phys Chem Lett. 2015;6:4067–4072. doi: 10.1021/acs.jpclett.5b01839. [DOI] [PubMed] [Google Scholar]
- 9.Struzhkin VV, et al. Synthesis of sodium polyhydrides at high pressures. Nat Commun. 2016;7:12267. doi: 10.1038/ncomms12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamp A, Zurek E. Superconductivity in hydrides doped with main group elements under pressure. Novel Supercond Mat. 2017;3:14–22. [Google Scholar]
- 11.Feng J, et al. Structures and potential superconductivity in at high pressure: En route to “metallic hydrogen”. Phys Rev Lett. 2006;96:017006. doi: 10.1103/PhysRevLett.96.017006. [DOI] [PubMed] [Google Scholar]
- 12.Tse JS, Yao Y, Tanaka K. Novel superconductivity in metallic SnH4 under high pressure. Phys Rev Lett. 2007;98:117004. doi: 10.1103/PhysRevLett.98.117004. [DOI] [PubMed] [Google Scholar]
- 13.Gao G, et al. Superconducting high pressure phase of germane. Phys Rev Lett. 2008;101:107002. doi: 10.1103/PhysRevLett.101.107002. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, et al. Superconductivity at approximately 100 K in dense SiH4(H2)2 predicted by first principles. Proc Natl Acad Sci USA. 2010;107:15708–15711. doi: 10.1073/pnas.1007354107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Hu QM, Yang R. Predicted suppression of the superconducting transition of new high-pressure yttrium phases with increasing pressure from first-principles calculations. Phys Rev Lett. 2012;109:157004. doi: 10.1103/PhysRevLett.109.157004. [DOI] [PubMed] [Google Scholar]
- 16.Kim DY, Scheicher RH, Ahuja R. Predicted high-temperature superconducting state in the hydrogen-dense transition-metal hydride YH3 at 40 K and 17.7 GPa. Phys Rev Lett. 2009;103:077002. doi: 10.1103/PhysRevLett.103.077002. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Tse JS, Tanaka K, Iitaka T, Ma Y. Superconductive sodalite-like clathrate calcium hydride at high pressures. Proc Natl Acad Sci USA. 2012;109:6463–6466. doi: 10.1073/pnas.1118168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao G, et al. Theoretical study of the ground-state structures and properties of niobium hydrides under pressure. Phys Rev B. 2013;88:184104. [Google Scholar]
- 19.Li Y, Hao J, Liu H, Li Y, Ma Y. The metallization and superconductivity of dense hydrogen sulfide. J Chem Phys. 2014;140:174712. doi: 10.1063/1.4874158. [DOI] [PubMed] [Google Scholar]
- 20.Drozdov AP, Eremets MI, Troyan IA, Ksenofontov V, Shylin SI. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature. 2015;525:73–76. doi: 10.1038/nature14964. [DOI] [PubMed] [Google Scholar]
- 21.Errea I, et al. High-pressure hydrogen sulfide from first principles: A strongly anharmonic phonon-mediated superconductor. Phys Rev Lett. 2015;114:157004. doi: 10.1103/PhysRevLett.114.157004. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, et al. Dissociation products and structures of solid H2S at strong compression. Phys Rev B. 2015;93:020103. [Google Scholar]
- 23.Einaga M, et al. Crystal structure of the superconducting phase of sulfur hydride. Nat Phys. 2016;12:835–838. doi: 10.1038/nphys3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan D, et al. Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci Rep. 2014;4:6968. doi: 10.1038/srep06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drozdov AP, Eremets MI, Troyan IA. 2015. Superconductivity above 100 K in PH3 at high pressures. arXiv:1508.06224. [DOI] [PubMed]
- 26.Shamp A, et al. Decomposition products of phosphine under pressure: PH2 stable and superconducting? J Am Chem Soc. 2016;138:1884–1892. doi: 10.1021/jacs.5b10180. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Li Y, Gao G, Tse JS, Naumov II. Crystal structure and superconductivity of PH3 at high pressures. J Phys Chem C. 2016;120:3458–3461. [Google Scholar]
- 28.Flores-Livas JA, et al. Superconductivity in metastable phases of phosphorus-hydride compounds under high pressure. Phys Rev B. 2016;93:020508. [Google Scholar]
- 29.Feng X, Zhang J, Gao G, Liu H, Wang H. Compressed sodalite-like MgH6 as a potential high-temperature superconductor. RSC Adv. 2015;5:59292–59296. [Google Scholar]
- 30.Li Y, et al. Pressure-stabilized superconductive yttrium hydrides. Sci Rep. 2015;5:9948. doi: 10.1038/srep09948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Lv J, Zhu L, Ma Y. Crystal structure prediction via particle-swarm optimization. Phys Rev B. 2010;82:094116. [Google Scholar]
- 32.Wang Y, Lv J, Zhu L, Ma Y. CALYPSO: A method for crystal structure prediction. Comput Phys Commun. 2012;183:2063–2070. [Google Scholar]
- 33.Liu H, Wang H, Ma Y. Quasi-molecular and atomic phases of dense solid hydrogen. J Phys Chem C. 2012;116:9221–9226. [Google Scholar]
- 34.Zhu L, et al. Substitutional alloy of Bi and Te at high pressure. Phys Rev Lett. 2011;106:145501. doi: 10.1103/PhysRevLett.106.145501. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, et al. Two-dimensional boron-nitrogen-carbon monolayers with tunable direct band gaps. Nanoscale. 2015;7:12023–12029. doi: 10.1039/c5nr03344f. [DOI] [PubMed] [Google Scholar]
- 36.Miao MS. Caesium in high oxidation states and as a p-block element. Nat Chem. 2013;5:846–852. doi: 10.1038/nchem.1754. [DOI] [PubMed] [Google Scholar]
- 37.Zhu L, Liu H, Pickard CJ, Zou G, Ma Y. Reactions of xenon with iron and nickel are predicted in the Earth’s inner core. Nat Chem. 2014;6:644–648. doi: 10.1038/nchem.1925. [DOI] [PubMed] [Google Scholar]
- 38.Yong X, et al. Crystal structures and dynamical properties of dense CO2. Proc Natl Acad Sci USA. 2016;113:11110–11115. doi: 10.1073/pnas.1601254113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Naumov II, Hemley RJ. Dense hydrocarbon structures at megabar pressures. J Phys Chem Lett. 2016;7:4218–4222. doi: 10.1021/acs.jpclett.6b02001. [DOI] [PubMed] [Google Scholar]
- 40.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 41.Amri M, Clarkson GJ, Walton RI. Anisotropic thermal expansion of SiO2 and AlPO4 clathrasils with the AST-type structure. J Phys Chem C. 2010;114:6726–6733. [Google Scholar]
- 42.Papaconstantopoulos D, Klein B, Mehl M, Pickett W. Cubic H3S around 200 GPa: An atomic hydrogen superconductor stabilized by sulfur. Phys Rev B. 2015;91:184511. [Google Scholar]
- 43.McMahon JM, Ceperley DM. Ground-state structures of atomic metallic hydrogen. Phys Rev Lett. 2011;106:165302. doi: 10.1103/PhysRevLett.106.165302. [DOI] [PubMed] [Google Scholar]
- 44.Dynes R. McMillan’s equation and the Tc of superconductors. Solid State Commun. 1972;10:615–618. [Google Scholar]
- 45.Nixon LW, Papaconstantopoulos DA, Mehl MJ. Electronic structure and superconducting properties of lanthanum. Phys Rev B. 2008;78:214510. [Google Scholar]
- 46.Bağci S, Tutuncu H, Duman S, Srivastava G. Phonons and superconductivity in fcc and dhcp lanthanum. Phys Rev B. 2010;81:144507. [Google Scholar]
- 47.Dronskowski R, Bloechl PE. Crystal Orbital Hamilton Populations (COHP). Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J Phys Chem. 1993;97:8617–8624. [Google Scholar]
- 48.Tissen VG, Ponyatovskii EG, Nefedova MV, Porsch F, Holzapfel WB. Effect of pressure on the superconducting Tc of lanthanum. Phys Rev B: Condens Matter. 1996;53:8238–8240. doi: 10.1103/physrevb.53.8238. [DOI] [PubMed] [Google Scholar]
- 49.Hamlin J, Tissen V, Schilling J. Superconductivity at 17 K in yttrium metal under nearly hydrostatic pressures up to 89 GPa. Phys Rev B. 2006;73:094522. [Google Scholar]
- 50.Errea I. Approaching the strongly anharmonic limit with ab initio calculations of materials––Vibrational properties colloquium. Eur Phys J B. 2016;89:237. [Google Scholar]
- 51.Borinaga M, et al. Anharmonic enhancement of superconductivity in metallic molecular Cmca-4 hydrogen at high pressure: A first-principles study. J Phys: Condens Matter. 2016;28:494001. doi: 10.1088/0953-8984/28/49/494001. [DOI] [PubMed] [Google Scholar]
- 52.Borinaga M, Errea I, Calandra M, Mauri F, Bergara A. Anharmonic effects in atomic hydrogen: Superconductivity and lattice dynamical stability. Phys Rev B. 2016;93:174308. [Google Scholar]
- 53.Garland J, Bennemann K, Mueller F. Effect of lattice disorder on the superconducting transition temperature. Phys Rev Lett. 1968;21:1315. [Google Scholar]
- 54.Samudrala GK, Tsoi GM, Vohra YK. Structural phase transitions in yttrium under ultrahigh pressures. J Phys Condens Matter. 2012;24:362201. doi: 10.1088/0953-8984/24/36/362201. [DOI] [PubMed] [Google Scholar]
- 55.Zaleski-Ejgierd P, Labet V, Strobel TA, Hoffmann R, Ashcroft NW. WH(n) under pressure. J Phys: Condens Matter. 2012;24:155701. doi: 10.1088/0953-8984/24/15/155701. [DOI] [PubMed] [Google Scholar]
- 56.Zaleski-Ejgierd P, Hoffmann R, Ashcroft NW. High pressure stabilization and emergent forms of PbH4. Phys Rev Lett. 2011;107:037002. doi: 10.1103/PhysRevLett.107.037002. [DOI] [PubMed] [Google Scholar]
- 57.Zhong X, et al. Tellurium hydrides at high pressures: High-temperature superconductors. Phys Rev Lett. 2016;116:057002. doi: 10.1103/PhysRevLett.116.057002. [DOI] [PubMed] [Google Scholar]
- 58.Kim DY, Scheicher RH, Pickard CJ, Needs RJ, Ahuja R. Predicted formation of superconducting platinum-hydride crystals under pressure in the presence of molecular hydrogen. Phys Rev Lett. 2011;107:117002. doi: 10.1103/PhysRevLett.107.117002. [DOI] [PubMed] [Google Scholar]