Significance
Research into modern amphibian origins is increasingly focusing on the limbless caecilians, a poorly studied group whose pre-Cenozoic fossils are limited to two species. We describe tiny fossils from the Triassic of Colorado with a mixture of traits found in caecilians and extinct Permian–Triassic temnospondyls: Stereospondyli. Computed 3D tomography shows how skull bones organized around internal structures, and we suggest how these may have become fused or simplified in caecilians. The fossils’ association with burrows highlights ecological diversity of Triassic amphibians as well as when and how burrowing evolved in the stereospondyl ancestors of caecilians. Our narrative for research on amphibian origins highlights the importance of stereospondyls, the most numerous and anatomically diverse amphibian group of the Triassic.
Keywords: burrow, Gymnophiona, temnospondyl, tetrapod, Triassic
Abstract
The origin of the limbless caecilians remains a lasting question in vertebrate evolution. Molecular phylogenies and morphology support that caecilians are the sister taxon of batrachians (frogs and salamanders), from which they diverged no later than the early Permian. Although recent efforts have discovered new, early members of the batrachian lineage, the record of pre-Cretaceous caecilians is limited to a single species, Eocaecilia micropodia. The position of Eocaecilia within tetrapod phylogeny is controversial, as it already acquired the specialized morphology that characterizes modern caecilians by the Jurassic. Here, we report on a small amphibian from the Upper Triassic of Colorado, United States, with a mélange of caecilian synapomorphies and general lissamphibian plesiomorphies. We evaluated its relationships by designing an inclusive phylogenetic analysis that broadly incorporates definitive members of the modern lissamphibian orders and a diversity of extinct temnospondyl amphibians, including stereospondyls. Our results place the taxon confidently within lissamphibians but demonstrate that the diversity of Permian and Triassic stereospondyls also falls within this group. This hypothesis of caecilian origins closes a substantial morphologic and temporal gap and explains the appeal of morphology-based polyphyly hypotheses for the origins of Lissamphibia while reconciling molecular support for the group’s monophyly. Stem caecilian morphology reveals a previously unrecognized stepwise acquisition of typical caecilian cranial apomorphies during the Triassic. A major implication is that many Paleozoic total group lissamphibians (i.e., higher temnospondyls, including the stereospondyl subclade) fall within crown Lissamphibia, which must have originated before 315 million years ago.
The stem caecilian fossil record is restricted to two species: the well-known Eocaecilia micropodia (1, 2) from the Early Jurassic of Arizona and Rubricaecilia monbarroni (3) from the Early Cretaceous of North Africa. Consequently, an ∼70-Ma gap exists between Eocaecilia and early Permian tetrapods inferred previously to represent early stem caecilians (4–7). This prominent gap in the tetrapod fossil record hinders efforts to place caecilians into a broader phylogenetic context of tetrapod phylogeny and to identify patterns in the early derivation of the caecilian Bauplan. Although molecular (8–12) and morphological (4, 5) studies support that caecilians belong to a monophyletic Lissamphibia that descended from a tympanate ancestor (5), the early evolutionary history of the group remains a complete mystery. The fossils reported here from the Upper Triassic of Colorado substantially reduce this gap and allow caecilians to be placed with better confidence into tetrapod phylogeny.
Results
Systematic Paleontology.
Tetrapoda Haworth, 1825; Temnospondyli Zittel, 1888; Stereospondyli Zittel, 1887; Chinlestegophis jenkinsi gen. et sp. nov.
Etymology.
Jenkins’s amphibian-serpent from the Chinle. “Chinle” for the Triassic Chinle Formation; “stego-” (Greek) meaning cover or roof, but commonly applied to temnospondyl amphibians and other early tetrapods; “-ophis” (Greek) meaning serpent. The species name honors paleontologist Farish Jenkins, whose work on the Jurassic Eocaecilia inspired the present study.
Holotype.
Denver Museum of Nature & Science (DMNH) 56658, partial skull with lower jaw and disarticulated postcrania (Fig. 1 A–D). Discovered by B.J.S. in 1999 in the Upper Triassic Chinle Formation (“red siltstone” member), Main Elk Creek locality, Garfield County, Colorado (DMNH loc. 1306). The tetrapod assemblage is regarded as middle–late Norian in age (Revueltian land vertebrate faunachron) (13). More specific locality information is on file at DMNH and US Bureau of Land Management.
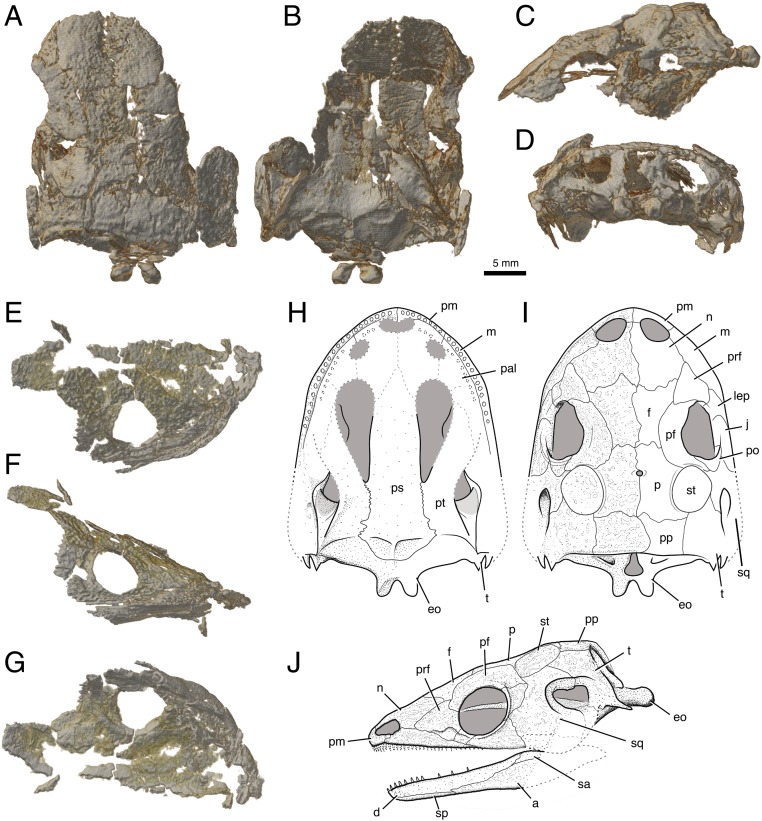
Fig. 1.
Skulls of C. jenkinsi gen. et sp. nov., DMNH 56658 (A–D) and DMNH 39033 (E–G). Specimens are shown in dorsal (A and E), ventral (B and G), lateral (C and F), and occipital (D) views. A reconstruction of the skull based on the two specimens is shown in ventral (H), dorsal (I), and left lateral (J) views. All are to scale. a, angular; d, dentary; eo, exoccipital; f, frontal; j, jugal; lep, lateral exposure of palatine; m, maxilla; n, nasal; p, parietal; pal, palatine; pf, postfrontal; pm, premaxilla; po, postorbital; pp, postparietal; prf, prefrontal; ps, parasphenoid; pt, pterygoid; sa, surangular; sp, splenial; sq, squamosal; st, supratemporal; t, tabular.
Referred Material.
DMNH 39033, anterior skull, and partial lower jaw preserved in burrow fill (Fig. 1 E–G). Discovered, also by B.J.S., in 1997 in the Upper Triassic Chinle Formation (red siltstone member) of Eagle County, Colorado (DMNH loc. 692).
Diagnosis.
Small stereospondyl with a combination of brachyopoid and caecilian characteristics. Unique features include the following: lateral line sulcus restricted to suborbital margins of jugal and postorbital; parietal–tabular narrowly contact [may be shared with Compsoceros (14)]; postfrontal anterior process long, forming the majority of the dorsomedial border of the orbit; finger-like process of prefrontal interlocks with notch on postfrontal. Shared features with stereospondyls include the following: parasphenoid strongly sutured to pterygoid, supratemporal excluded from otic notch, secondary upper tooth row. A shared feature with stereospondyls and caecilians is opisthotics fused to exoccipitals. Shared features with brachyopoids and caecilians include lacrimal fused to maxilla and two small posterior processes (“horns”) on the occipital exposure of the tabular, just posterior to otic notch (as in chigutisaurids). Shared features with Rileymillerus and some other small temnospondyls comprise palatine exposed laterally in ventral margin of the orbit [LEP (lateral exposure of palatine)]. Shared features with Rileymillerus and caecilians include the following: orbits small and laterally directed. Shared features with caecilians include double tooth row on mandible; quadrate completely anterior to ear; broad, parallel-sided parasphenoid cultriform process >20% skull width; occipital condyles extend far beyond posterior edge of skull roof; and pterygoquadrate. Shared features with some other temnospondyls but not caecilians include large, laterally directed otic notch.
General Features.
The holotype and attributed specimen of Chinlestegophis are both incomplete but preserve enough overlapping information to be attributed to the same species and to reconstruct most of the skull (Fig. 1). The skull of Chinlestegophis is less than 3 cm long, larger than most modern caecilians, but much smaller than other contemporary amphibians. Despite its small size, the orbits are small and laterally positioned, suggesting caecilian-like reduction in the size of the eye. A well-developed otic notch is present, indicating that Chinlestegophis had a fully tympanic ear, like many total group amphibians but unlike modern caecilians. The quadrate articulation is displaced far anterior to the ear, as in caecilians, possibly an adaptation for jaw opening in confined spaces.
The skull roof is generally conservative and compares well with the plesiomorphic total group amphibian condition. A few features are noteworthy, however. No separate lacrimal bone exists, and instead the nasolacrimal duct passes through the dorsal lamina of the maxilla (SI Appendix, Fig. S4). We interpret this as a fusion of the maxilla and lacrimal to form an incipient caecilian maxillopalatine. The topological associations of this feature vary between caecilians and batrachians, but the condition in Chinlestegophis is uniquely shared by caecilians and some brachyopoid temnospondyls (14–16), therefore making its expression phylogenetically important. Specifically, batrachians either do not enclose the nasolacrimal duct (frogs) (17) or incorporate the enclosing lacrimal into the prefrontal bone (salamanders) (18, 19), whereas caecilians incorporate the passage for the duct (which is modified into the tentacular foramen) into the maxillopalatine (20–22).
In the temporal region, there is a small, round supratemporal that is only loosely articulated to its surrounding calvarial elements. This bone is morphologically and topologically identical to an element identified as the “tabular” in Eocaecilia (2). In light of their position in the broader context of temnospondyl phylogeny, we suggest a reinterpretation of this element as a supratemporal, a possibility that was also acknowledged by Jenkins et al. (2). As in Eocaecilia, the element is not joined by interdigitating sutures to its neighboring bones but instead overlies ventral lappets of its surrounding calvarial bones [see p. 291 in Jenkins et al. (2)]. Its tentative attribution to the tabular in Eocaecilia was likely confounded by the formation of a fused os basale in this region, making the homologies of the calvariae difficult to interpret in the absence of separate tabular and occipital bones. The homologies are clarified by the holotype of Chinlestegophis, which preserves separate supratemporal, tabular, and occipital elements.
Palate and Suspensorium.
The palate and suspensorium are well preserved in the type specimen and are atypical of other contemporaneous temnospondyl amphibians. The cultriform process is extremely broad and parallel-sided rather than narrow and tapering between large suborbital vacuities that typify most other temnospondyls. The opisthotic and exoccipitals are fused into an incipient os basale, which is sutured tightly to the pterygoid and parasphenoid. The occipital elements thereby form a single, unpaired element in the holotypic specimen (rather than having left and right complements). The occipital condyles are distinctly paired, saddle-shaped, being broadly concave, and protrude well behind the posterior edge of the skull roof.
The lower jaw is incompletely preserved posteriorly in both specimens. A nearly full early tetrapod complement of bones is present in the lower jaw, although there are no signs of a separate splenial and postsplenial. Consequently, unlike Eocaecilia, there is no pseudodentary or pseudangular (SI Appendix, Fig. S3). However, the coronoids bear a single row of teeth similar in size to the marginal dentition, producing a combined double tooth row on the mandible. The teeth are small in size and densely packed but are simple, isodont cones, rather than multicusped.
Postcrania.
Disarticulated postcranial elements are preserved in association with the holotypic skull of Chinlestegophis, including a pectoral girdle, limb bone, vertebrae, and ribs (SI Appendix, Fig. S5). These elements are of typical Triassic temnospondyl morphology (14, 16). Both clavicles have a broad triangular ventral surface with a slender dorsal stalk. The interclavicle is a long, narrow diamond with a pronounced stalk and ventral keel bordered by the clavicular facets. The ventral surfaces of the interclavicle and clavicles are sculptured. Several neural arches and ribs are preserved in articulation. Neural arches preserve prominent transverse processes as well as a high, broad neural spine. The corresponding centra do not appear to be present, even in cases where several neural arches are preserved in articulation. Ribs are elongate and curved, with distinct tuberculum and capitulum. No uncinate processes are present.
Phylogenetic Relationships.
We conducted a phylogenetic analysis of total group lissamphibians to assess the relationships of Chinlestegophis and Eocaecilia among Paleozoic and early Mesozoic total group lissamphibians. To accomplish this, we constructed a combined matrix using taxon and character sampling from a recent analysis of broader total group lissamphibian relationships (23) with another recent analysis designed to test lissamphibian monophyly and the caecilian affinities of Eocaecilia (4). Redundant characters were removed from the analysis, and new character states were scored from direct observation of specimens wherever possible and from the literature where necessary (SI Appendix). After an initial run that confirmed the placement of caecilians and Chinlestegophis within temnospondyls (SI Appendix, Fig. S6), nontemnospondyl “lepospondyls” were removed from the combined dataset to improve computing time. The final trimmed matrix of 76 taxa and 345 morphological characters was analyzed using Bayesian posterior probability in MrBayes v. 3.1.2 and using parsimony in PAUP* 4.0a151.
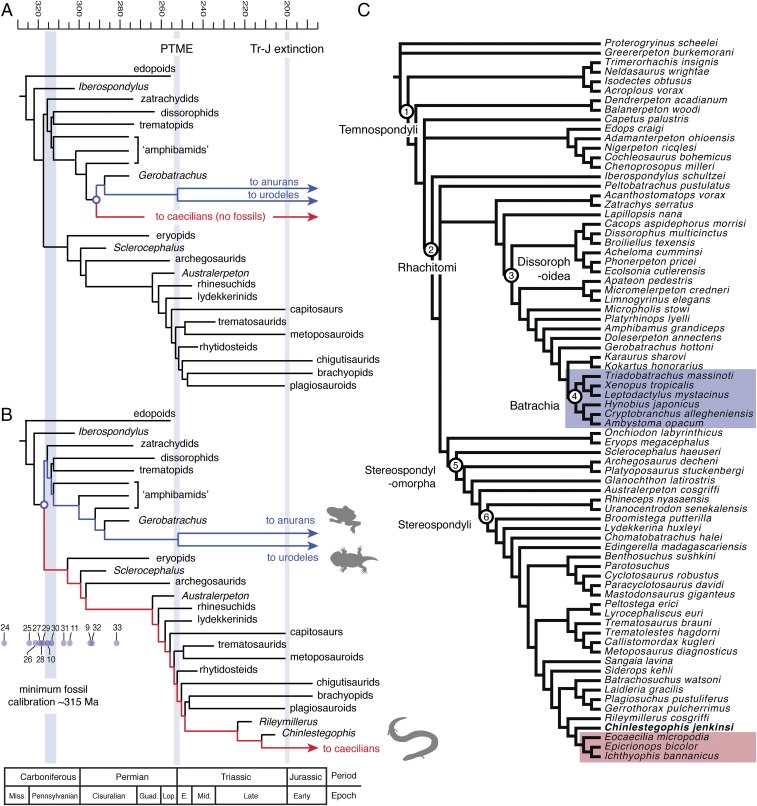
Chinlestegophis is found to be the closest relative to the most exclusive clade that includes the Jurassic Eocaecilia plus extant caecilians in both the Bayesian and parsimony analyses (Fig. 2 and SI Appendix, Fig. S7). Batrachians (represented by the Triassic frog Triadobatrachus and two modern frogs and three modern salamanders) are found within dissorophoids as in previous analyses (4–6), with the batrachian-like Gerobatrachus hottoni, Doleserpeton annectens, and Amphibamus grandiceps forming successive outgroups. Most surprisingly, though, our study found that a substantial number of Paleozoic and early Mesozoic total group lissamphibians (temnospondyls) belong to the lissamphibian crown and identified large numbers of total group caecilians as well as total group batrachians. This result is driven by clear stepwise acquisition of caecilian apomorphies as exhibited by Chinlestegophis and Eocaecilia within late Paleozoic and early Mesozoic stereospondyls (Figs. 2 and 3). This contrasts with some prior studies that inferred a close relationship between caecilians and amniote-like lepospondyls (6) on the likely basis of convergent fossorial specializations (4, 36–38) or a close relationship between caecilians and batrachians to the exclusion of some Paleozoic total group lissamphibians on the basis of small size and reduced exoskeleton. In contrast to these prior hypotheses, the relationship between caecilians, Chinlestegophis, and stereospondyls is supported by numerous highly specific fusions or reductions of elements before the accomplishment of small body size or fossorial habitus as well as other highly specific features of overall cranial organization.
Fig. 2.
Time-calibrated phylogeny of temnospondyls illustrating the major divergences of Lissamphibia and its subgroups. (A) The traditional “dissorophoid hypothesis” for the origins of Lissamphibia implies a shallow divergence but with long ghost lineages spanning at least two mass extinctions. (B) Our hypothesis suggests a deep divergence with a caecilian total group that is well populated by fossil stem taxa, including the stereospondyls. (C) Detailed topology of the consensus Bayesian tree (see SI Appendix). Blue lineages represent the batrachian (frog and salamander) stem, whereas red lineages represent the caecilian stem. Numbered scale at top is in Ma. Light-blue interval to the left represents the minimum fossil calibration age for the divergence of crown Lissamphibia during the early–late Pennsylvanian (∼315 Ma) (based on the Moscovian records of Platyrhinops and Amphibamus). Lavender circles are estimated molecular divergence dates for crown Lissamphia from refs. 9–11, 24–33, which are curated on the Time Tree of Life database (www.timetree.org). Silhouettes of amphibians are from the open source PhyloPic.org. PTME, Permo-Triassic mass extinction; Tr-J, Triassic–Jurassic mass extinction.
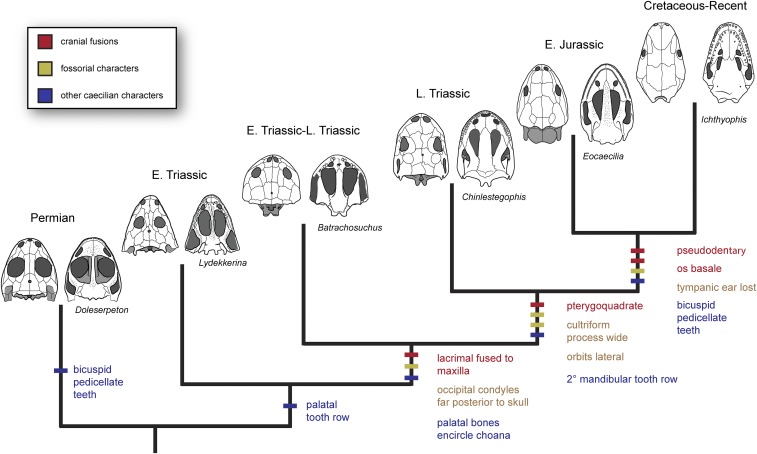
Fig. 3.
Hypothesis of morphological innovations and character transformations along the caecilian stem. Representative skulls are shown in dorsal (left) and ventral (right) views. Characters are color-coded by cranial fusions (red), fossorial characters (yellow), and other classic “caecilian” characters (blue). Some cranial fusions may represent additional adaptations for fossoriality but are treated here separately. Exemplary taxa are shown in stratigraphic order (left, oldest; right, youngest): the Permian dissorophoid Doleserpeton [redrawn from Sigurdsen and Bolt (34)]; the Early Triassic stereospondyl Lydekkerina [redrawn from Jeannot et al. (35)]; the Middle Triassic brachyopoid Batrachosuchus (based on observation of the holotype of Batrachosuchus browni); d, the Late Triassic C. jenkinsi n. gen. et sp.; the Early Jurassic stem caecilian Eocaecilia [redrawn from Jenkins et al. (2)]; and the crown caecilian Ichthyophis [redrawn from Jenkins et al. (2)]. Skulls are not drawn to scale.
Most strikingly, our phylogenetic results show the stepwise fusion of the cranial skeleton of caecilians into a series of compound bones (Fig. 3), including the maxillopalatine (comprising lacrimal, maxilla, and palatine), pseudodentary (comprising the dentary, coronoid, splenial, and anterior Meckel’s cartilage, but with a varying number of ossifications in different species), pseudangular (comprising the angular, articular, and prearticular), pterygoquadrate (comprising the pterygoid and quadrate), and os basale (comprising the exoccipitals, opisthotics, prootics, basisphenoid, and parasphenoid) (20–22). Early members of the caecilian stem show steps toward the formation of a maxillopalatine (via fusion of the lacrimal and maxilla) and os basale (via fusion of the exoccipitals and opisthotics). Chinlestegophis differs from more basal stem caecilians in exhibiting both an incipient os basale that incorporates the posterodorsal braincase and, perhaps, an incipient pterygoquadrate based on the structure of the suspensorium and apparent absence of the quadratojugal. This trend is further continued by Eocaecilia, which exhibits a complete os basale, pseudodentary, and pseudangular. A full maxillopalatine is seen only within the caecilian crown.
Discussion
Lissamphibian Monophyly: Controversy and Consensus.
Our study finds an unexpected phylogenetic position for caecilians within early tetrapods. This result is a major departure from current phylogenetic hypotheses of caecilian origins and demands further rigorous testing. However, we note that the phylogenetic relationships of caecilians have never before been tested within a larger sampling of total group lissamphibians and do not find strong support for any particular position of caecilians within early tetrapod diversity. Previous studies have placed caecilians (exemplified by Eocaecilia) in one of two disparate positions: within recumbirostran or lysorophian lepospondyls (6, 39) or within dissorophoid temnospondyls (4, 5). The former hypothesis, which proposes a polyphyletic Lissamphibia, is supported by general similarities in the gestalt of caecilians and the recumbirostran Rhynchonkos or the elongate lysorophian Brachydectes (40, 41). However, recent studies of lepospondyl and caecilian braincases have found that much of this similarity has been overstated, and resemblances between the two groups may be due to miniaturization and shared fossorial lifestyle rather than common descent (36–38, 42). Prior placement of caecilians within dissorophoids is due to a combination of strong phylogenetic support for a dissorophoid origin of batrachians (6) in combination with soft tissue support for a monophyletic Lissamphibia (5). However, nondissorophoid members of the lissamphibian total group have been systematically excluded from studies of caecilian origins. As a result, the possibility that some Paleozoic or Triassic total group lissamphibians may be stem caecilians has never been formally tested in a phylogenetic framework until now. Our hypothesis shares some features with both hypotheses: A richly populated caecilian stem group is hypothesized as in the polyphyly hypothesis, whereas reciprocal monophyly of lissamphibians and amniotes is preserved as in the dissorophoid hypothesis and most molecular hypotheses. Our hypothesis differs, however, in the pattern, tempo, and chronology of morphological evolution in the caecilian stem during the Triassic.
In this light, our phylogenetic results are less surprising; prior analyses have been unequipped both to identify caecilian characteristics in nondissorophoid total group lissamphibians and, conversely, to identify characteristics of nondissorophoid total group lissamphibians in caecilians. Future studies must account for this by more comprehensive sampling of underrepresented Paleozoic tetrapod groups and their morphologic characters.
Evolution of Fossoriality in Caecilians.
Stepwise change in sensory anatomy, possibly associated with a shift from semiaquatic to fossorial lifestyle, is also observed clearly along the caecilian stem. Chinlestegophis and its close relatives exhibit early signs of this process with the loss of most cranial sensory line canals as well as a shift in the position of the orbits from the dorsal to lateral surface of the skull. Eocaecilia approaches the crown condition, with a reduction in orbit size, loss of an external tympanic ear, and complete bony covering of the pineal organ. The timing of the integration of the nasolacrimal duct into the caecilian tentacle organ is uncertain; a sulcus associated with the opening of the nasolacrimal duct in the orbit is present in both Chinlestegophis and Eocaecilia in a similar position to the tentacular sulcus of the basal caecilian Epicrionops petersi [figure 10 in Jenkins et al. (2)]. Whether this sulcus in Chinlestegophis housed a tentacle organ or simply the primitive course of the nasolacrimal duct is uncertain, but its combination with the reduction of interpterygoid vacuities in Chinlestegophis may together indicate that the m. retractor bulbi had already lost its role in orbital retraction, unlike other temnospondyls (43), possibly freeing it for a caecilian-like function in tentacle retraction.
These stepwise changes in sensory structure morphology are consistent with stepwise acquisition of fossorial morphology and ichnology among amphibious stereospondyls. The occurrence of one specimen of Chinlestegophis within a burrow is strongly suggestive that this taxon may have been at least facultatively fossorial, but whether Chinlestegophis was a secondary burrow occupant, like the Early Triassic stereospondyl Broomistega (44), or was actively engaged in headfirst burrowing as in modern caecilians cannot be determined at this time. However, the consolidation of the skull, reduction of the orbits, and anteriorization of the jaw articulation suggests that Triassic stem group caecilians were increasingly specialized for life and feeding in confined spaces. Subsequent loss of a tympanic middle ear, reduction of the orbits, and closure of the pineal foramen in Eocaecilia (10) may indicate a shift from facultative to obligate burrowing in the latest Triassic or Early Jurassic. Notably, Chinlestegophis retains a circumorbital sulcus, which likely housed a cranial lateral line canal as is seen in some larval aquatic caecilians (45), suggesting that caecilian fossoriality does not preclude emergence of the behavior within a semiaquatic or aquatic environment.
Divergence Timing of Lissamphibia and Subgroups.
By populating the Paleozoic caecilian and batrachian stem lineages, our phylogenetic results confidently move the minimum fossil calibration date of the caecilian–batrachian split from the late–early Permian (∼280 Ma) further back to the early–late Carboniferous (∼315–307 Ma). Late Carboniferous stem batrachians are represented by Amphibamus, Platyrhinops, and other taxa (46). Coeval stem caecilians might include Carboniferous eryopoids and earliest Permian stereospondylomorphs represented by Sclerocephalus and Archegosaurus (47). This is slightly younger than the median molecular clock estimate for the divergence of Gymnophiona and Batrachia, which is early–late Carboniferous (8–11) (Fig. 2). The diversity of crown lissamphibians in these early assemblages suggests either a much earlier date for the origin of the lissamphibian crown or a rapid diversification of crown lissamphibians during the early-to-late Carboniferous transition (ca. 324 Ma). We consider this a striking example of the profound effect that the changing state of morphological phylogenies can have on molecular clock calibration, and we expect that these calibration dates, and our confidence in them, will change as our understanding of total group lissamphibian phylogeny continues to improve. We also note that the timeline implied by an early-to-late Carboniferous caecilian–batrachian divergence lends credibility to some molecular clock estimates that place the frog–salamander divergence somewhat later in the earliest Permian (8, 10–12). Such estimates suggest a scenario in which the divergence of modern lissamphibian orders was gradual and that the derived morphology encompassed by the Early Triassic anurans Triadobatrachus (48) and Czatkobatrachus (49) evolved over the full duration of the Permian. If so, the tempo of early lissamphibian evolution may need to be reconsidered.
Materials and Methods
High-Resolution X-Ray Computed Tomography (HRXCT).
Both specimens were scanned using a General Electric EVS-RS9 micro-CT at the HSC Small Animal Imaging Facility, University of Utah, Salt Lake City. Scanner settings were 100 kV and 60 µA with a voxel size of 38.9 µm. Resulting DICOMs were extracted as image stacks using RadiAnt and cropped using ImageJ. Stacks were segmented and rendered as 3D models in Amira 5.4.0 (Visage Imaging). The 3D movies presenting the volumized data are available online (SI Appendix, Part H: HRXCT Movies).
Phylogenetic Methods.
A combined data matrix using temnospondyl taxa sampled from Schoch (23) and Maddin et al. (4) was constructed and analyzed to assess the positions of Chinlestegophis and caecilians in the larger context of tetrapod phylogeny. With the additions of Chinlestegophis and Rileymillerus, we performed an initial pilot run on the Maddin et al. (4) data only, which showed no relation between these taxa and lepospondyls (SI Appendix, Fig. S6). To evaluate effects of including stereospondyl taxa and characters, we combined the matrices but omitted most nontemnospondyl outgroups (except for Proterogyrinus and Greererpeton) to improve computing time. Thus, 76 taxa and 345 morphological characters were analyzed using Bayesian posterior probability in MrBayes v. 3.1.2 (50) and using parsimony in PAUP* 4.0a151 (51). The parsimony analysis was performed using the tree bisection–reconnection branch-swapping algorithm of PAUP*. All characters had equal weight, and none were ordered. For the Bayesian analysis, we used the default Mk model (52) in MrBayes with variable character rates and running a Markov chain Monte Carlo for 10 million generations, sampling the posterior distribution every 100 generations. For the parsimony analysis, a heuristic search (random addition sequence with 10,000 replicates) was performed and recovered 882 most parsimonious trees (tree score, 1,514 steps; consistency index, 0.2642; retention index, 0.6858). The resulting consensus trees are presented in SI Appendix, Fig. S7.
Supplementary Material
Acknowledgments
The authors thank Robert Douglass, Heather Finlayson, Jacqueline Lungmus, and Tyler Schlotterbeck for assistance in the field; Harley Armstrong and Vanessa Caranese, Colorado Bureau of Land Management; and Jason Anderson and Hillary Maddin for helpful discussion. HRXCT was performed at the University of Utah HSC Cores Research Facility. The specimens are reposited at the Denver Museum of Nature & Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706752114/-/DCSupplemental.
References
- 1.Jenkins PA, Walsh DM. An early Jurassic caecilian with limbs. Nature. 1993;365:246–250. [Google Scholar]
- 2.Jenkins FA, Walsh DM, Carroll RL. Anatomy of Eocaecilia micropodia, a limbed caecilian of the Early Jurassic. Bull Mus Comp Zool. 2007;158:285–365. [Google Scholar]
- 3.Evans SE, Sigogneau‐Russell D. A stem‐group caecilian (Lissamphibia: Gymnophiona) from the Lower Cretaceous of North Africa. Palaeontol. 2001;44:259–273. [Google Scholar]
- 4.Maddin HC, Jenkins FA, Jr, Anderson JS. The braincase of Eocaecilia micropodia (Lissamphibia, Gymnophiona) and the origin of Caecilians. PLoS One. 2012;7:e50743. doi: 10.1371/journal.pone.0050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddin HC, Anderson JS. Evolution of the amphibian ear with implications for lissamphibian phylogeny: Insight gained from the caecilian inner ear. Fieldiana Life Earth Sci. 2012;5:59–76. [Google Scholar]
- 6.Anderson JS, Reisz RR, Scott D, Fröbisch NB, Sumida SS. A stem batrachian from the Early Permian of Texas and the origin of frogs and salamanders. Nature. 2008;453:515–518. doi: 10.1038/nature06865. [DOI] [PubMed] [Google Scholar]
- 7.Carroll RL. The Palaeozoic ancestry of salamanders, frogs, and caecilians. Zool J Linn Soc. 2007;150:1–140. [Google Scholar]
- 8.Roelants K, et al. Global patterns of diversification in the history of modern amphibians. Proc Natl Acad Sci USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang P, Wake DB. Higher-level salamander relationships and divergence dates inferred from complete mitochondrial genomes. Mol Phylogenet Evol. 2009;53:492–508. doi: 10.1016/j.ympev.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.San Mauro D. A multilocus timescale for the origin of extant amphibians. Mol Phylogenet Evol. 2010;56:554–561. doi: 10.1016/j.ympev.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Pyron RA. Divergence time estimation using fossils as terminal taxa and the origins of Lissamphibia. Sys Biol. 2011;60:466–481. doi: 10.1093/sysbio/syr047. [DOI] [PubMed] [Google Scholar]
- 12.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol. 2011;61:543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Small BJ, Martz JW. A new aetosaur from the Upper Triassic Chinle Formation of the Eagle Basin, Colorado USA. Geol Soc Lond Spec Publ. 2013;379:393–412. [Google Scholar]
- 14.Schoch RR, Milner AR. Temnospondyli II: Stereospondyli. In: Wellnhofer P, editor. Handbuch der Paläoherpetologie. Verlag; Munich: 2000. pp. 1–203. [Google Scholar]
- 15.Bolt JR, Chatterjee S. A new temnospondyl amphibian from the Late Triassic of Texas. J Paleontol. 2000;74:670–683. [Google Scholar]
- 16.Yates AM, Warren AA. The phylogeny of the ‘higher’ temnospondyls (Vertebrata: Choanata) and its implications for the monophyly and origins of the Stereospondyli. Zool J Linn Soc. 2000;128:77–121. [Google Scholar]
- 17.Stephenson EM. The anatomy of the head of the New Zealand frog. Leiopelma Trans Zool Soc Lond. 1951;27:255–305. [Google Scholar]
- 18.Gao KQ, Shubin NH. Late Jurassic salamanders from northern China. Nature. 2001;410:574–577. doi: 10.1038/35069051. [DOI] [PubMed] [Google Scholar]
- 19.Vater M. Is the prefrontal bone in Alpine newt (Triturus alpestris Laurenti, 1768) of dual origin? J Anat. 2007;211:290–295. doi: 10.1111/j.1469-7580.2007.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wake MH, Hanken J. Development of the Skull of Dermophis mexicanus (Amphibia: Gymnophiona), with comments on skull kinesis and amphibian relationships. J Morphol. 1982;173:203–223. doi: 10.1002/jmor.1051730208. [DOI] [PubMed] [Google Scholar]
- 21.Müller H, Oommen OV, Bartsch P. Skeletal development of the direct-developing caecilian Gegenophis ramaswamii (Amphibia: Gymnophiona: Caeciliidae) Zoomorphology. 2005;124:171–188. [Google Scholar]
- 22.Müller H. Ontogeny of the skull, lower jaw, and hyobranchial skeleton of Hypogeophis rostratus (Amphibia: Gymnophiona: Caeciliidae) revisited. J Morphol. 2006;267:968–986. doi: 10.1002/jmor.10454. [DOI] [PubMed] [Google Scholar]
- 23.Schoch RR. The evolution of major temnospondyl clades: An inclusive phylogenetic analysis. J Syst Palaeontology. 2013;11:673–705. [Google Scholar]
- 24.Pyron RA. A likelihood method for assessing molecular divergence time estimates and the placement of fossil calibrations. Syst Biol. 2010;59:185–194. doi: 10.1093/sysbio/syp090. [DOI] [PubMed] [Google Scholar]
- 25.Okajima Y, Kumazawa Y. Mitogenomic perspectives into iguanid phylogeny and biogeography: Gondwanan vicariance for the origin of Madagascan oplurines. Gene. 2009;441:28–35. doi: 10.1016/j.gene.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Hugall AF, Foster R, Lee MS. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Syst Biol. 2007;56:543–563. doi: 10.1080/10635150701477825. [DOI] [PubMed] [Google Scholar]
- 27.Alfaro ME, et al. Nine exceptional radiations plus high turnover explain species diversity in jawed vertebrates. Proc Natl Acad Sci USA. 2009;106:13410–13414. doi: 10.1073/pnas.0811087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen XX, Liang D, Wen JZ, Zhang P. Multiple genome alignments facilitate development of NPCL markers: A case study of tetrapod phylogeny focusing on the position of turtles. Mol Biol Evol. 2011;28:3237–3252. doi: 10.1093/molbev/msr148. [DOI] [PubMed] [Google Scholar]
- 29.Kumazawa Y. Mitochondrial genomes from major lizard families suggest their phylogenetic relationships and ancient radiations. Gene. 2007;388:19–26. doi: 10.1016/j.gene.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 30.Shen XX, Liang D, Zhang P. The development of three long universal nuclear protein-coding locus markers and their application to osteichthyan phylogenetics with nested PCR. PLoS One. 2012;7:e39256. doi: 10.1371/journal.pone.0039256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Wake MH. A mitogenomic perspective on the phylogeny and biogeography of living caecilians (Amphibia: Gymnophiona) Mol Phylogenet Evol. 2009;53:479–491. doi: 10.1016/j.ympev.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Hedges SB, Kumar S. The Timetree of Life. Oxford Univ Press; Oxford: 2009. [Google Scholar]
- 33.Marjanović D, Laurin M. Fossils, molecules, divergence times, and the origin of lissamphibians. Syst Biol. 2007;56:369–388. doi: 10.1080/10635150701397635. [DOI] [PubMed] [Google Scholar]
- 34.Sigurdsen T, Bolt JR. The Lower Permian amphibamid Doleserpeton (Temnospondyli: Dissorophoidea), the interrelationships of amphibamids, and the origin of modern amphibians. J Vert Paleont. 2010;30:1360–1377. [Google Scholar]
- 35.Jeannot AM, Damiani R, Rubidge BS. Cranial anatomy of the Early Triassic stereospondyl Lydekkerina huxleyi (Tetrapoda: Temnospondyli) and the taxonomy of South African lydekkerinids. J Vert Paleont. 2006;26:822–838. [Google Scholar]
- 36.Maddin HC, Olori JC, Anderson JS. A redescription of Carrolla craddocki (Lepospondyli: Brachystelechidae) based on high-resolution CT, and the impacts of miniaturization and fossoriality on morphology. J Morphol. 2011;272:722–743. doi: 10.1002/jmor.10946. [DOI] [PubMed] [Google Scholar]
- 37.Szostakiwskyj M, Pardo JD, Anderson JS. Micro-CT study of Rhynchonkos stovalli (Lepospondyli, Recumbirostra), with description of two new genera. PLoS One. 2015;10:e0127307. doi: 10.1371/journal.pone.0127307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardo JD, Szostakiwskyj M, Anderson JS. Cranial morphology of the brachystelechid ‘microsaur’ Quasicaecilia texana Carroll provides new insights into the diversity and evolution of braincase morphology in recumbirostran ‘microsaurs’. PLoS One. 2015;10:e0130359. doi: 10.1371/journal.pone.0130359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson JS. Incorporating ontogeny into the matrix: A phylogenetic evaluation of developmental evidence for the origin of modern amphibians. In: Anderson JS, Sues H-D, editors. Major Transitions in Vertebrate Evolution. Indiana Univ Press; Bloomington: 2007. pp. 182–227. [Google Scholar]
- 40.Nussbaum RA. The evolution of a unique dual jaw‐closing mechanism in caecilians:(Amphibia: Gymnophiona) and its bearing on caecilian ancestry. J Zool. 1983;199:545–554. [Google Scholar]
- 41.Vallin G, Laurin M. Cranial morphology and affinities of Microbrachis, and a reappraisal of the phylogeny and lifestyle of the first amphibians. J Vert Paleont. 2004;24:56–72. [Google Scholar]
- 42.Pardo JD, Szostakiwskyj M, Ahlberg PE, Anderson JS. Hidden morphological diversity among early tetrapods. Nature. doi: 10.1038/nature22966. in press. [DOI] [PubMed] [Google Scholar]
- 43.Witzmann F, Werneburg I. The palatal interpterygoid vacuities of temnospondyls and the implications for the associated eye and jaw musculature. Anat Rec (Hoboken) 2017 doi: 10.1002/ar.23582. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez V, et al. Synchrotron reveals Early Triassic odd couple: Injured amphibian and aestivating therapsid share burrow. PLoS One. 2013;8:e64978. doi: 10.1371/journal.pone.0064978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hetherington TE, Wake MH. The lateral line system in larval Ichthyophis (Amphibia: Gymnophiona) Zoomorphology. 1979;93:209–225. [Google Scholar]
- 46.Clack JA, Milner AR. Morphology and systematics of the Pennsylvanian amphibian Platyrhinops lyelli (Amphibia: Temnospondyli) Earth Environ Sc Trans Roy Soc Edinburgh. 2009;100:275–295. [Google Scholar]
- 47.Schoch RR, Milner AR. Temnospondyli. In: IP Wellnhofer., editor. Handbuch der Paläoherpetologie. Verlag; Munich: 2014. pp. 1–150. [Google Scholar]
- 48.Rage JC, Roček Z. Redescription of Triadobatrachus massinoti (Piveteau, 1936) an anuran amphibian from the early Triassic. Palaeontogr Abt A. 1989;206:1–16. [Google Scholar]
- 49.Evans SE, Borsuk-Bialynicka M. A stem-group frog from the Early Triassic of Poland. Acta Palaeontol Pol. 1998;43:573–580. [Google Scholar]
- 50.Ronquist F, van der Mark P, Huelsenbeck JP. Bayesian phylogenetic analysis using MrBayes. In: Vandamme AM, Salemi M, Lemey P, editors. The Phylogenetic Handbook. 2nd Ed. Cambridge Univ Press; Cambridge, UK: 2009. pp. 210–266. [Google Scholar]
- 51.Swofford D. PAUP* Phylogenetic Analysis Using Parsimony v. 4.0a151. Sinauer Associates; Sunderland, MA: 1999. [Google Scholar]
- 52.Lewis PO. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol. 2001;50:913–925. doi: 10.1080/106351501753462876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.