Abstract
Overtreatment is pervasive in medicine and leads to potential patient harms and excessive costs in healthcare. Although evidence-based medicine (EBM) is often derided as practice by rote algorithmic medicine, the appropriate application of key EBM principles in clinical decision making is fundamental to preventing overtreatment and promoting high-value, individualized patient-centered care. Specifically, this article discusses the importance of: 1) using absolute rather than relative estimates of benefits to inform treatment decisions; 2) considering the time horizon to benefit of treatments; 3) balancing potential harms and benefits; and 4) using shared decision making by physicians to incorporate the patient’s values and preferences into treatment decisions. Here, we illustrate the application of these principles to considering the decision of whether or not to recommend intensive glycemic control to patients in order to minimize microvascular and cardiovascular complications in type 2 diabetes mellitus. Through this lens, this example will illustrate how an EBM approach can be used to individualize glycemic goals and prevent overtreatment, and can serve as a template for applying EBM to inform treatment decisions for other conditions to optimize health and individualize patient care.
Keywords: Diabetes Mellitus, Evidence-Based Medicine, Medical Overuse, Diabetes Complications, Clinical Decision-Making, Review [Publication Type]
INTRODUCTION: WHAT IS OVERTREATMENT?
Overtreatment is defined by the Institute of Medicine as the use of a treatment even when the potential harms exceed the possible benefits.1 Overtreatment is pervasive in medicine and is not limited to a single therapeutic category.2 Overuse, which encompasses both overtesting and overtreatment, accounts for nearly 20% of the estimated $750 billion of wasteful spending in health care in the United States.3 Additionally, many physicians in the U.S. recognize that their own patients are receiving too much medical care that is potentially harmful. In a nationally representative survey, 42% of U.S. primary care physicians thought their patients were receiving too much care in their own practices.4 Even more were concerned about overly aggressive practice styles among medical subspecialists (61%).4
Despite the potential for harm, the enormous costs, and increasing awareness, overtreatment nonetheless remains prevalent due to many system-level factors including financial incentives, malpractice concerns, performance metrics, practice culture, and time constraints.3–14 Although legal, policy, and health system reforms are needed, front-line action in parallel by individual physicians is also critical to addressing the epidemic of overtreatment. Applying the principles of evidence-based medicine (EBM) to the clinical decision making process is a key strategy that physicians can use both at the bedside and in guideline development and policy decisions, to prevent overtreatment in their immediate spheres of influence. This review article will outline an EBM framework to guide clinical decision making to enable individualized medical treatment decisions concordant with the aims of patient-centered and personalized healthcare, increasing areas of national priority.15, 16
WHAT IS EVIDENCE-BASED MEDICINE?
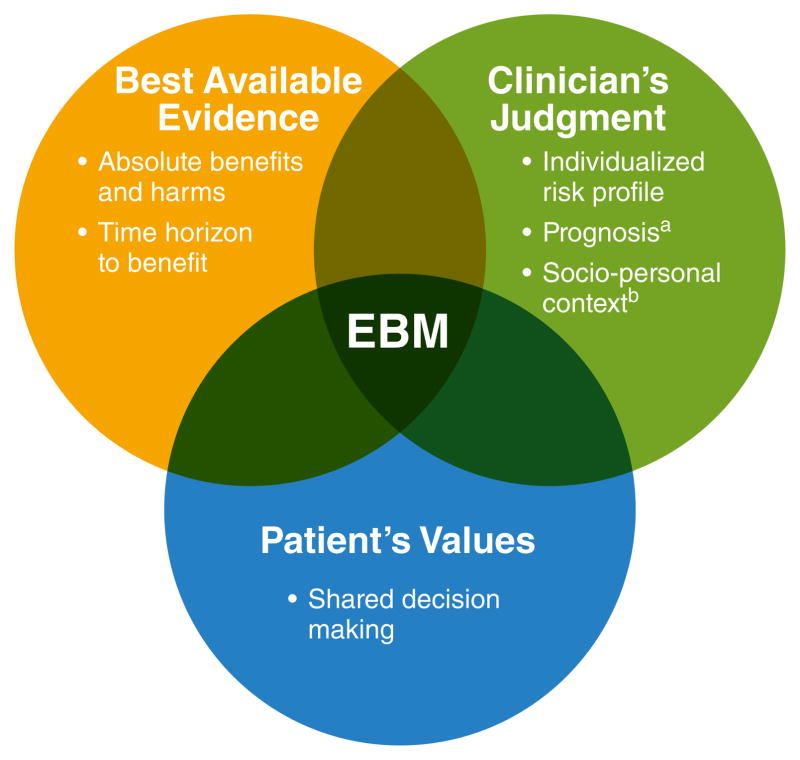
In the words of the late David Sackett, a founding father of evidence-based medicine, EBM is the “conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients” [emphasis added].17 The practice of EBM does not consist of universal, rote application of clinical guidelines. Rather, EBM requires physicians to assess and understand what the best available evidence is, use his or her clinical judgment to apply this knowledge to the treatment of individual patients, and do so in the context of the patient’s values and preferences (Figure 1). Thus, the practice of EBM is not ‘cookbook’ medicine, but is a complex and nuanced approach requiring mastery of three separate but overlapping domains of knowledge.
Figure 1. Evidence-Based Medicine Framework for Clinical Decision Making.
Adapted from a conceptual framework put forth by Sacket et al.17
aEstimated based on age, comorbidities, and functional status
bIncludes an individual’s lifestyle, social support, financial circumstances, and workload capacity
There are two key reasons why practicing EBM is not synonymous with the delivery of algorithmic ‘cookbook’ medicine. First, many recommendations and guidelines are poorly substantiated by current evidence and as such, should not be broadly disseminated and indiscriminately applied.18 For example, among the American College of Cardiology (ACC) and the American Heart Association (AHA) practice guidelines issued between 1984 and 2008, only 11% of the 2,711 recommendations were classified as level of evidence A (i.e. supported by multiple randomized trials or a meta-analysis).19 Furthermore, among the 1,305 class I ACC/AHA guideline recommendations, which indicate general consensus that a particular health care service is useful and effective, only 19% have level of evidence A.19 As such, approximately 20% of class I ACC/AHA guideline recommendations were downgraded, reversed, or omitted within a decade, suggesting questionable durability in the absence of strong evidence.20 The proliferation of recommendations based on a weak or non-existent evidence base is not only an issue for practice guidelines, but is a phenomenon observed among popular online ‘evidence-based’ resources. For example, approximately two-thirds of the 9,400 graded recommendations in UpToDate are supported by weak evidence (i.e. absence of clinical trials or robust observational studies).18
The second and more compelling reason why EBM is not synonymous with ‘cookbook’ medicine is that even for recommendations based on strong levels of evidence, decisions in clinical practice are still ultimately value judgements – i.e., decisions ultimately hinge on the physician’s and patient’s assessment of whether or not a treatment is actually worth it after considering the potential harms and benefits enumerated in the scientific literature.17, 18 Science can be used to inform clinical decisions, but cannot definitively inform value judgements since the significance of potential benefits and harms of a therapy are in the eye of the beholder and will differ across individuals. Thus, the EBM triad requires a bottom-up approach that incorporates the best available evidence with both the physician’s expertise and judgement and the patient’s values and preferences.
AN EBM APPROACH TO OVERCOMING OVERTREATMENT AND PERSONALIZING THERAPEUTIC DECISIONS
Distilled to its simplest form, an EBM approach provides guidance for making the best therapeutic decision for an individual patient through the understanding and application of four key principles:
Absolute estimates of benefit: What are the absolute (and not relative) benefits of a particular therapy for patient-centered outcomes?
Time horizon to benefit: How long must a patient be on a particular therapy in order to reap the potential benefit, compared to their current life expectancy?
Balance of benefits versus harms: Do the potential absolute benefits outweigh the potential harms of therapy for the specific patient under consideration?
Shared decision making: Is the decision to treat consistent with the patient’s values and preferences?
Case Example: Should Physicians Recommend Intensive Glycemic Control in Patients With Type 2 Diabetes Mellitus?
To illustrate how EBM principles should be applied to clinical decision making, we present the case example of considering whether to recommend intensive glycemic control in patients with type 2 diabetes mellitus (henceforth referred to as ‘diabetes’) to reduce microvascular and cardiovascular complications. While using an EBM approach is not unique to specific diseases, diabetes is an ideal disease model to illustrate how an EBM approach can prevent the harms of overtreatment because: 1) diabetes is highly prevalent;21 2) the strategy of intensive glycemic control has been thoroughly investigated, and there is a large body of evidence available on the benefits and harms of this treatment strategy; 3) the decision to pursue glycemic control is influenced less by external factors (i.e., financial incentives and malpractice concerns) and more by physicians’ clinical decision making compared to other conditions (e.g. percutaneous coronary intervention for stable angina);22, 23 and 4) applying an EBM approach to the treatment of hyperglycemia in type 2 diabetes highlights the discordance between the current prevailing paradigm of treatment versus what is actually supported by the best available current evidence.
ASSESSING THE ABSOLUTE ESTIMATES OF TREATMENT BENEFIT FOR PATIENT-CENTERED OUTCOMES
To understand why absolute estimates of benefit are the preferred measure of treatment efficacy, it is first necessary to understand the limitations of relative risks. Imagine a hypothetical trial assessing the benefit of drug X versus placebo in preventing myocardial infarction over a one-year treatment period. If the outcome occurs in 10% of individuals on drug X (risk 1) versus 15% taking placebo (risk 2), the relative risk (RR) of a myocardial infarction for individuals prescribed drug X is 0.67 (risk 1/risk 2) and the corresponding relative risk reduction (RRR) is 33% (100*(1-RR)). In this example, the absolute risk reduction (ARR) for myocardial infarction among individuals treated with drug X is 5% (risk 2–risk 1) and the number needed to treat (NNT) is 20 (1/ARR). For this same hypothetical trial, if the risk of myocardial infarction for those prescribed drug X is only 1% and the risk in the placebo group is only 1.5%, the RR remains a deceptively impressive 0.67; but the ARR is now only 0.5%, and the NNT is 200, indicating a minimally effective therapy. Thus, reports of the RR or RRR do not provide physicians any information on the magnitude of treatment benefit. Relying on these measures alone makes it impossible to assess the clinical relevance of the treatment, and may lead to erroneously inflated estimates about the potential benefit.
In contrast, absolute estimates of treatment benefit in the form of ARR or NNT more accurately convey the treatment effect size and potential clinical significance of treatment. In the hypothetical trial above, a NNT of 20 means that one would need to treat 20 individuals (with characteristics within the parameters of the trial’s inclusion and exclusion criteria) with drug X for one year to prevent one additional myocardial infarction compared to placebo. Notably, a NNT of 20 also implies that for every 20 people treated with drug X, on average, 19 people derive no benefit with respect to preventing a myocardial infarction compared to being prescribed placebo over this time period. While both the ARR and NNT are valid measures to convey the absolute benefit of therapy, the NNT more clearly illustrates that the benefits of treatment are not shared equally by every individual in the treatment group, and that most individuals in this example will not derive any benefit after one year of therapy, even for what many would consider a clinically meaningful effect size.
There are important limitations to the interpretation and application of absolute risks. First, absolute risks are estimated at the population level. As the evolutionary biologist, Stephen Jay Gould, astutely stated in his essay, The Median isn’t the Message, “variation is the hard reality, not a set of imperfect measures for a central tendency.”24 Thus, ARR and NNT convey average estimates of benefit in a group, and cannot predict the potential benefit for an individual patient. Physicians must therefore also consider an individual’s risk profile for the outcome of interest based on established risk factors. Assessing the potential benefits (and harms) of treatment in the context of individuals’ risks is essential to the optimal selection of individuals for treatment. Second, treating individuals who are either healthier or sicker than those included in studies can significantly alter the harm-benefit profile of a therapy.25 This phenomenon commonly referred to as indication drift, can markedly reduce the effectiveness of therapies. Lastly, absolute measures of risk (as well as relative risks) are estimated only for specific durations of time on treatment, and as such, do not capture the full effect of time.26 Consequently, ARR and NNT provide limited information to guide treatment decisions beyond the duration reported in clinical studies. Despite these limitations, the absolute risk is essential to understanding the magnitude of potential benefit, and is the first step in deciding whether the treatment is valuable for an individual patient.
What are the estimated benefits of intensive glycemic control in type 2 diabetes?
Large Relative Benefits in Preventing (Mostly Surrogate) Outcomes
Intensive glycemic control is defined in randomized controlled trials as treatment strategies resulting in a hemoglobin A1c (HbA1c) value between 6.4–7.0%; and conventional glycemic control is defined as treatment strategies resulting in a modestly higher HbA1c, between 7.9 and 8.4%.27–31 The relative benefits of intensive versus conventional glycemic control in diabetes are summarized in Table 1. In the landmark UKPDS trial, the relative benefits of 10 years of intensive glycemic control for individuals with newly diagnosed diabetes were only demonstrated for intermediate markers of microvascular complications (i.e., progression of retinopathy on eye exam, loss of reflexes or change in biothesiometer readings, and microalbuminuria), but not for actual meaningful clinical manifestations of microvascular disease (i.e., vision loss, symptomatic neuropathy, amputation, or end-stage renal disease requiring dialysis).27 Other trials have similarly failed to show improvement in these more clinically meaningful endpoints.32, 33
Table 1.
Relative Benefits of Intensive Glycemic Control in Type 2 Diabetes Mellitus
| Outcome | Relative risk reduction | Source |
|---|---|---|
| Retinopathy * | 29% per 0.9% ↓ A1c | UKPDS27 |
| Neuropathy † | 19% per 0.9% ↓ A1c | UKPDS27 |
| Microalbuminuria‡ | 33% per 0.9% ↓ A1c | UKPDS27 |
| Non-fatal myocardial infarction | 15% per 1.0% ↓ A1c | Boussageon et al.31 |
Surrogate outcome defined as one microaneurysm or more in one eye or worse retinopathy, and progression of retinopathy as a two-step change in Early Treatment of Diabetic Retinopathy Study grade
Surrogate outcome defined as loss of both ankle or both knee reflexes, or mean biothesiometer reading from both toes > 25 volts
Surrogate outcome defined as urinary albumin concentration greater than 50 mg/L
The evidence for the relative benefits of intensive glycemic control for preventing cardiovascular disease is even less well substantiated. Although the benefits for reducing surrogate microvascular outcomes have been consistently observed in trials,31, 34 the 15% relative risk reduction for the cardiovascular outcome of non-fatal myocardial infarction (MI) has not been consistently observed. Although three of the four landmark trials on intensive glycemic control (UKPDS, VADT, and ADVANCE) did not show a statistically significant reduction in non-fatal MIs,27–30, 35 the results of a meta-analysis more clearly supported this 15% relative reduction.31 However, it should be noted that intensive glycemic control has not been shown in parallel to also reduce cardiovascular disease-specific mortality (RR 1.11, 95% CI, 0.86–1.43) or all-cause mortality (RR 1.04, 95% CI, 0.91–1.19), calling into question the reliability and significance of the reduced risk in non-fatal MIs.31 Additionally, intensive glycemic control does not reduce the risk of strokes.36 Thus, while diabetes remains a strong prognostic marker for cardiovascular disease, achieving intensive glycemic control does not modify the risk of cardiovascular disease.
Of note, two recent multicenter diabetes outcomes trials of empagliflozin and liraglutide demonstrated clinically significant reductions in both cardiovascular disease-specific and all-cause mortality.37, 38 While potentially representing important advances in the therapy of type 2 diabetes, the observed mortality reduction in these trials was likely not mediated through the mechanism of intensive glycemic control for several reasons. First, while not explicitly designed to test the effect of intensive glycemic control, there was only a modest reduction in HbA1c of 0.4% compared to approximately 1% reduction achieved in other trials, none of which showed reductions in cardiovascular or all-cause mortality.31, 37, 38 Second, the modest37 to nonexistent reductions38 in large vessel atherosclerotic-mediated cardiovascular outcomes (MIs and strokes) were disproportionately smaller in magnitude than the observed reduction in cardiovascular disease-specific mortality in these trials. These results suggest that these medications may be working through mechanisms other than preventing the progression of atherosclerosis, which is the implicated causal pathway between hyperglycemia of diabetes and cardiovascular disease-specific mortality.39 Lastly, the improvement in cardiovascular mortality in both of these trials emerged far earlier than expected (within the first 3 months for empagliflozin and 6 months for liraglutide) given the natural history of the progression of atherosclerosis due to hyperglycemia.39 Taken together, these trials suggest that the reductions in mortality observed with these medications are not attributed to intensive glycemic control, but rather are due to medication-specific effects on other unidentified mechanistic pathways.
Modest Absolute Benefits of Intensive Glycemic Control for Diabetes
There is no direct evidence of an absolute benefit of intensive glycemic control on patient-centered outcomes such as clinically apparent microvascular disease or mortality despite several well-conducted clinical trials with up to 15 years of follow-up.33 Consequently, to estimate the absolute benefits of glycemic control, we must rely instead on simulation studies that model the potential benefits of glycemic control on clinical outcomes, extrapolating the observed effects on intermediate outcomes from landmark trials over a much longer timespan than is feasible in those trials. This approach represents the best available evidence regarding the absolute benefits of intensive glycemic control with respect to clinically relevant outcomes that patients and physicians care about. To this end, Vijan and colleagues developed a Markov simulation model to estimate the absolute benefits of intensive glycemic control using simulated individuals with characteristics based on adults with diabetes sampled in the National Health and Nutrition Examination Study (NHANES), a nationally representative population survey in the US.40 The authors modeled the risk of progression from the onset of asymptomatic diabetes to intermediate microvascular outcomes and the cardiovascular outcome of non-fatal MI using the relative risk reductions with intensive glycemic control observed in clinical trials (Table 1). The authors then estimated the likelihood of progression from these intermediate outcomes to end-stage complications (e.g., visual loss, amputation, end-stage renal disease) and death using findings from other randomized controlled trials and US population-based observational studies.41–46
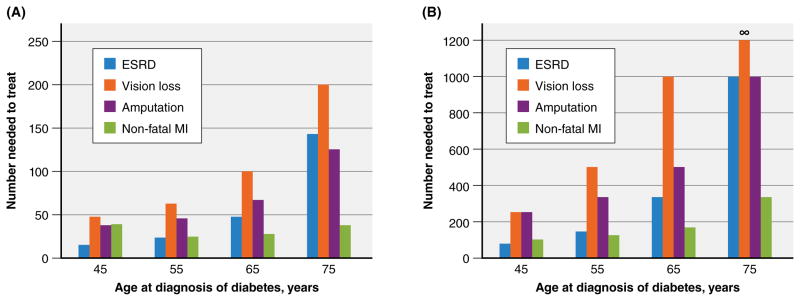
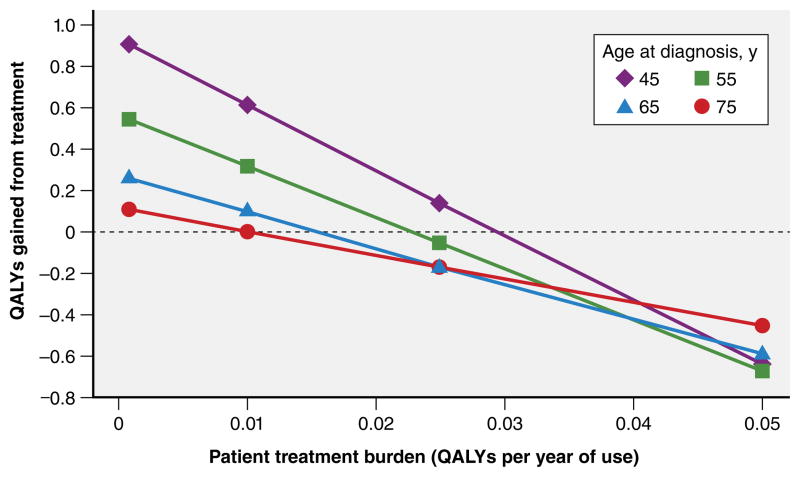
Using this model, the authors simulated two treatment scenarios commonly encountered in clinical practice to estimate the absolute benefits of intensive glycemic control on clinically apparent microvascular and cardiovascular complications of diabetes. The first scenario assesses the benefit of lifelong oral pharmacotherapy to achieve intensive glucose control. It consists of a newly diagnosed person with diabetes with an HbA1c of 8.5% who is prescribed metformin and experiences a reduction in the HbA1c level of 1.5 points to 7.0%, which remains at this level over the course of the person’s life. The second scenario assesses the benefit of augmenting an oral pharmacotherapy regimen with insulin. In this scenario, insulin therapy is initiated for the same patient in the first treatment scenario after ‘failing’ 10 years of oral antihyperglycemic therapy with a rise in HbA1c level back to 8.5%. Insulin therapy reduces the HbA1c level to 7.5% and remains at this level over the remainder of the person’s life. The lifetime absolute benefits of intensive glycemic control for each scenario, depending on the age at which diabetes is initially diagnosed, are shown in Figure 2. In both scenarios, the potential benefit of intensive glycemic control greatly declines with the age at which an individual is diagnosed. For an average 75 year old with newly diagnosed diabetes initiating metformin (Figure 2A), the NNT for preventing end-stage renal disease (ESRD), vision loss, and amputation over that person’s lifetime are 143, 200, and 125 respectively. For an average 55 year old who ‘failed’ oral antihyperglycemic therapy and was subsequently initiated on insulin 10 years later for ‘uncontrolled’ diabetes (HbA1c of 8.5% on oral therapy), the NNT are well above 100 for each microvascular endpoint (Figure 2B). In other words, greater than 99% of adults 65 years of age or older initiated on insulin therapy to achieve intensive glycemic control will not derive any benefit with respect to clinically apparent microvascular complications within their lifetime.
Figure 2. Estimated Lifetime Absolute Benefits of Intensive Glycemic Control for Preventing Microvascular Outcomes.
A. Treatment Scenario 1: initiation of metformin at diagnosis, with HbA1c reduction from 8.5% to 7.0%
B. Treatment Scenario 2: initiation of insulin 10 years after ‘failing’ initial oral therapy, with HbA1c reduction from 8.5% to 7.5%
Abbreviations: ESRD, end-stage renal disease; MI, myocardial infarction. The number needed to treat values were calculated from the estimated absolute risk reductions based on Vijan and colleagues’ Markov simulation model.40 For scenario 2, the absolute risk reduction for prevention vision loss among 75 year olds with new-onset diabetes was 0%, thus yielding a number needed to treat of infinity.
Although the NNT for preventing non-fatal MIs are considerably more favorable compared to preventing patient-centered microvascular outcomes across all age groups for both scenarios, the confidence behind these estimates are limited due to the increase in cardiovascular mortality observed in the ACCORD trial, as well as the nonsignificant increase seen in meta-analyses.28, 34, 36
These findings illustrate the stark differences in using relative versus absolute risks to inform treatment decisions. Even when relative risk reductions for surrogate microvascular and cardiovascular outcomes are the same across individuals, the absolute risk reductions for corresponding clinically meaningful outcomes may be vastly different for individuals with different baseline risk profiles and life expectancies. Relying on relative risks can vastly overestimate the benefits from treatment, and is the reason why absolute risks are the preferred method to inform therapeutic decisions.
ASSESSING THE TIME HORIZON TO BENEFIT FOR INTENDED THERAPIES
As discussed above, a major limitation of absolute measures of risk is that they do not capture the full effect of time. Rather, absolute measures of risk quantify the benefits of treatment in a given time period. Consequently, absolute measures of risk cannot be used to quantify the potential benefits of postponing an outcome. For example, consider a hypothetical trial of adults with a 100 year follow-up period where the primary outcome is mortality. Because adult human life expectancies rarely exceed 100 years, all trial subjects will die within the follow-up period irrespective of any intervention aimed at reducing mortality in this study. Thus, the observed absolute risk reduction for the intervention will be 0 (with a NNT of infinity) at the conclusion of the trial. In this extreme example, relying solely on the ARR or NNT as measures of effect size may fail to capture a potential benefit of treatment if therapy postponed the inevitable outcome of death for a clinically meaningful amount of time (e.g., perhaps subjects receiving the intervention postponed death by 10 years, even if they inevitably died within the 100 year follow up period).
Similarly, absolute measures of risk also do not consider the time horizon to benefit—that is, the time that must be accrued on treatment until a meaningful benefit emerges. This is a more commonly encountered issue than postponement of an outcome since study follow-up periods tend to be limited in duration. For a treatment with a sufficiently long time horizon to benefit, individuals with a life expectancy less than the time horizon to treatment benefit will not reap the potential benefits of treatment. Thus, these individuals will be exposed to the potential upfront harms of therapy without any chance of benefit, a classic scenario of overtreatment. To avoid these potential harms, only individuals whose anticipated life expectancy exceeds the estimated time horizon to benefit should be considered as candidates for treatment.
The time horizon to benefit can be estimated from clinical studies by calculating the difference in the area under the survival curves, though these measures are infrequently quantified and reported. Alternately, the time horizon to benefit can be qualitatively estimated by assessing Kaplan-Meier survival curves for the intervention and control groups to identify the point at which the two curves diverge.47 This divergence point provides an estimate of the minimum time horizon to benefit for a given treatment. To illustrate how to estimate the time horizon to benefit using survival curves and understand its implications, we will return to the example of diabetes.
Time Horizon to Benefit for Intensive Glycemic Control in Diabetes
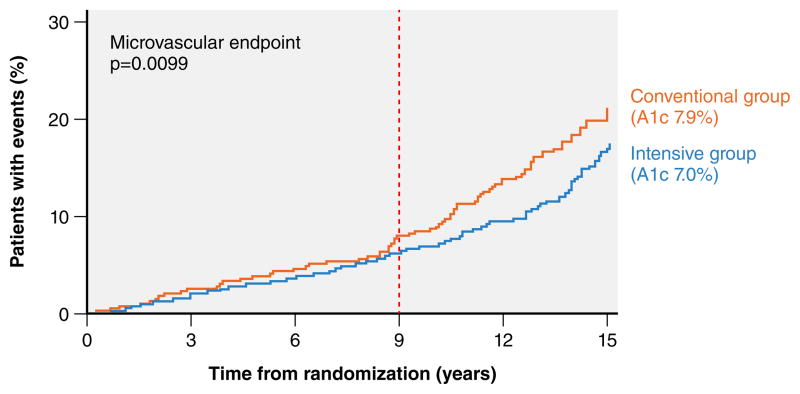
Based on the Kaplan-Meier curve from the UKPDS trial, it takes approximately 9 years of intensive glycemic control to yield an observable reduction in composite microvascular events in the treatment group (vertical dashed line in Figure 3). However, it is important to note that the composite microvascular outcome reported in the UKPDS trial consists mostly of intermediate outcomes that may not directly harm patients (i.e. need for photocoagulation, vitreous hemorrhage, or renal failure defined as an elevated creatinine >2.8 mg/dL). The potential for intensive glycemic control to prevent more meaningful microvascular outcomes (i.e. vision loss or ESRD requiring dialysis) is estimated to take 2 or more decades to manifest.48 Of note, even with an additional 10 years of follow-up in the UKPDS trial (for a total of 25 years), improvements in these endpoints have not yet been reported.49 This suggests that the legacy effect observed for surrogate microvascular endpoints seen after a decade of early intensive glycemic control in patients with newly diagnosed diabetes may not translate into reductions in patient-centered microvascular outcomes.
Figure 3. Time horizon to benefit for intensive glycemic control for intermediate microvascular outcomes.
The composite microvascular endpoint was defined as retinopathy requiring photocoagulation, presence of vitreous hemorrhage, and or fatal or non-fatal renal failure defined as an elevated creatinine >2.8 mg/dL. Reprinted from the Lancet, 352(9131), 837–853, Copyright ©1998, with permission from Elsevier.27
Despite the long time horizon to benefit for intensive glycemic control, many patients prescribed intensive glycemic control nonetheless have life expectancies less than 20 years, and potentially even less than 9 years, the earliest time interval after which one could expect to observe benefits for intermediate microvascular outcomes.50 This is a clear example of overtreatment – individuals who are initiated on intensive glycemic control who have a life expectancy of less than 9 years are exposed to the potential harms of treatment without any reasonable chance of benefit. Consequently, a pragmatic approach to avoiding the harms of overtreatment would be to defer intensive glycemic control in individuals with a life expectancy that is clearly less than 9 years. For example, individuals with advanced cancers, ESRD, advanced dementia, cirrhosis, and end-stage heart failure and lung disease are unlikely to benefit from intensive glycemic control.
There are also several highly prevalent but less commonly recognized conditions that also indicate poor prognosis and limited life expectancy. After a first hospitalization for heart failure with either preserved or reduced ejection fraction, individuals have a 70% 5-year mortality rate.51, 52 Likewise, after the first hospitalization for chronic obstructive pulmonary disease (COPD) exacerbation, individuals have an 80% 9-year mortality.53 Thus, initiating intensive glycemic control during or after an index hospitalization for either heart failure or COPD is unlikely to yield any benefit beyond that of a conventional glycemic control strategy.
Individuals with multimorbidity and impaired functional status also have limited life expectancy due to the cumulative burden of illness, though prognosis can be challenging to assess in these complex patients. The Lee Schonberg Index is a validated tool that quantifies the 4-year and 10-year risk of mortality among community dwelling adults 50 years of age or older (http://eprognosis.ucsf.edu/leeschonberg.php).54, 55 This index assesses the cumulative contribution of age, selected comorbidities and health behaviors (diabetes, cancer, heart disease, lung disease, body mass index < 25, and current smoker), and impairments in functioning (bathing, managing finances, ambulating, and pushing or pulling heavy objects) on the risk of mortality using a point score ranging from 0 to 25, with higher scores corresponding to higher risk of death. The Lee Schonberg Index has excellent discrimination (C-statistic of 0.83) and calibration (less than a 4% difference between predicted and observed mortality rates across all risk levels).55
In a nationally representative longitudinal cohort, the Lee Schonberg Index identified that 25% of community dwelling individuals 50 years of age or older have a greater than 50% risk of death within 10 years.55 Thus, 1 in 4 middle-aged and older adults in the US are unlikely to live 10 years, let alone 20 years, in order to reap the benefits of intensive glycemic control. This is reflected in the exceedingly low lifetime absolute benefits for intensive glycemic control estimated for individuals who have high point scores on the Lee Schonberg Index. In a decision analysis study, Huang and colleagues developed a simulation model based on data from the UKPDS trial and found that among older adults with diabetes, those with greater multimorbidity and functional impairment are far less likely to benefit from intensive glucose control.56 For example, for individuals aged 60 to 64 years who have new-onset diabetes and only 1 point on the Lee Schonberg Index, the NNT to prevent ESRD, blindness, and amputation over one’s lifetime are approximately 125, 83, and 77 respectively. In contrast, the lifetime NNT for the same scenario, except for patients who have significant multimorbidity and functional impairment (Lee Schonberg Index score of 15 points), are infinity (ARR of 0%), 2,700, and 1,125 respectively.56 Thus, only the few older adults with diabetes and almost no functional limitations will derive benefit from intensive glucose control. Those with substantial multimorbidity and functional limitations have an infinitesimally small probability of deriving any benefits from this treatment strategy.
ASSESSING THE BALANCE BETWEEN POTENTIAL BENEFITS VERSUS HARMS OF TREATMENT
Overtreatment may arise when one focuses only on the benefits of therapy without any consideration of potential harms, since this would lead to always favoring treatment even if the benefit of treatment is extremely small. Thus, it is critical to understand and assess the potential harms of treatment, and to incorporate this knowledge into treatment decisions. The potential harms of therapy are quantified through estimates of the absolute risk increase and the number needed to harm (NNH) for specific adverse effects related to treatment. Awareness of both the NNT and NNH for a given treatment can help physicians and patients alike make more informed decisions about whether the potential for benefits is worth taking the risks.
There are several challenges in assessing the balance between the NNT and NNH for treatments. First, since each individual beneficial or harmful effect will have varying impact on quality of life, it may be difficult to directly compare benefits and harms. Consider, for example, the potential benefit of preventing a stroke versus the potential harm of causing a bleed with anticoagulation therapy for atrial fibrillation. Compared to major bleeding, having a stroke is considered more detrimental to quality of life since major bleeding is more likely to be transient and reversible than neurologic deficits from a stroke.57 Second, treatments may have a myriad of beneficial and harmful effects; consequently, it can be difficult to simultaneously assess the net balance of benefits and harms. Third, the NNT and NNH are reported for selected benefits and harms that are discrete in nature (i.e. present or absent), and are not designed to take into account the effect of treatment on other measures of quality of life (i.e. treatment burden) that are also important to patients. By only considering the value of treatment by the NNT and NNH, important information may be lost on other dimensions of therapy.
An approach to overcome these limitations is to quantify the balance between the potential benefits and harms of therapy by estimating the net quality-adjusted life years (QALYs) associated with treatment. This measure of overall health impact incorporates both the quantity and quality of life related to all potential benefits and harms of therapy. QALYs integrate the general quality of life on a scale from 0 to 1, with values closer to 1 indicating higher quality of life, based on an individual’s probability of being in various disease and treatment complication states each year.58, 59 Estimated QALYs, when available, can thus allow for direct comparisons of health impact between different treatments.
In the absence of studies evaluating the net QALYs gained from therapy, the NNT can be used to estimate the magnitude of benefit for a treatment. Acknowledging that the NNT does not fully account for many factors, including the clinical significance of the outcome (i.e. microalbuminuria versus ESRD), the cost, convenience, and invasiveness of the therapy (i.e. metformin versus insulin), and the number needed to harm (NNH) for various adverse effects, one may roughly interpret that a NNT greater than 15 is associated with a small net treatment benefit, and a NNT ≤ 5 is typically associated with a meaningful net benefit.60
Balancing the Harms and Benefits of Intensive Glycemic Control in Diabetes
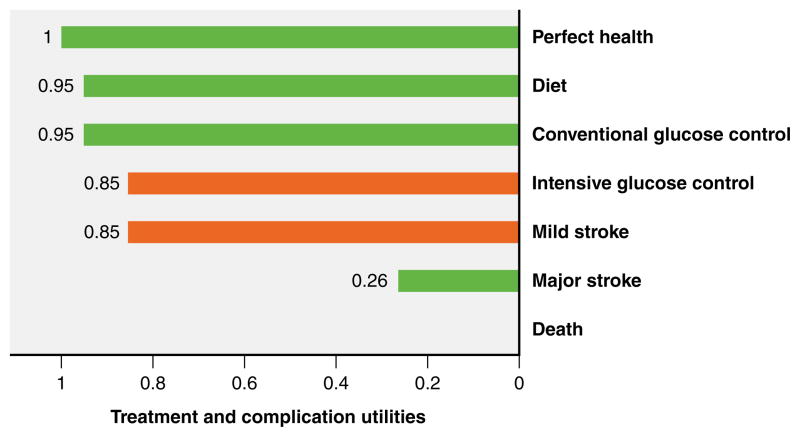
With respect to diabetes, there are several important potential harms to consider before prescribing antihyperglycemic therapy. While risks are unique to each therapy, the most common side effects of intensive glycemic control reported in clinical trials were severe hypoglycemia and weight gain, both attributable to higher rates of insulin use among these patients.31, 33 Severe hypoglycemia occurred in 4.9% of those on intensive glycemic control versus 2.0% among those on conventional glycemic control (absolute risk increase of 2.9%; NNH of 35).31 Additionally, intensive glycemic control increased body weight by approximately 3%.33 Another important but often overlooked harm of intensive glycemic control is the potential decrease in a person’s overall quality of life due to the burden of the treatment itself. Due to the burden of polypharmacy, multiple insulin injections, frequent blood glucose monitoring, and increased frequency of health care visits, an intensive glycemic control strategy per the UKPDS trial protocol was perceived by individuals to have a median quality of life utility value of 0.85 (mean of 0.67 ± 0.34) on a scale where perfect health is 1 and death is 0.61 A utility of 0.85 – also referred to as a disutility of 0.15 – means individuals equate living 10 years with intensive glycemic control the same as living 8.5 years in perfect health. Notably, the decrease in quality of life due to intensive glycemic control is perceived by patients to be of the same magnitude as that due to a mild stroke (Figure 4).
Figure 4. Patients’ Perceptions of Quality of Life for Selected Diabetes Treatments and Complications.
Median quality of life utility values for various treatments and complications were derived from Huang et al.61 The orange bars for intensive glucose control and mild stroke are added for emphasis to highlight that on average, patients perceive these different states as having the same magnitude in the decrease in quality of life.
In order to balance the potential benefits of intensive glycemic control with the potential harms, Vijan and colleagues used a Markov simulation model as described previously to estimate the net lifetime QALYs gained with therapy. The authors assigned quality of life utility values to each disease state along the spectrum from asymptomatic diabetes to death based on prior literature to estimate net QALYs gained or lost.40, 62, 63 The overall treatment burden was estimated across a range of disutilities for intensive glycemic control for a scenario where an individual was prescribed a pharmacologic therapy that reduced the HbA1c from 8.5% to 7.5%. In this scenario, treatment burden has a profoundly detrimental impact on the net QALYs gained from treatment (Figure 5). If an individual experiences no treatment burden whatsoever (i.e. a treatment burden of 0), antihyperglycemic therapy in this scenario would yield only modest net benefits across all four age groups with a net lifetime gain of 0.9 QALYs for an average 45 year old to 0.1 QALYs for an average 75 year old. However, a treatment burden as little as 0.04 negates any benefit of intensive glycemic control for all age groups, and actually results in loss of quality of life. Therefore, the value of intensive glycemic control depends on an individual’s experienced or perceived treatment burden.
Figure 5. Balancing Benefits and Harms of Antihyperglycemic Treatment.
Net lifetime quality-adjusted life years gained or lost by a treatment that leads to a 1% reduction in the hemoglobin A1C level (from 8.5% to 7.5%) across four age groups and different views of the burden of treatment (disutility value for treatment ranging from 0 to 0.05). This figure was reproduced with permission from JAMA Internal Medicine, 2014, 174(8), 1227–1234.40 Copyright ©2014, American Medical Association.
These findings should be interpreted in the context of certain limitations. First, the authors’ optimistic assumptions of a 15% risk reduction in non-fatal myocardial infarction events and relatively low estimates of treatment disutilities (capped at 0.05, much lower than the previously reported median disutility of 0.15 for intensive glycemic control in Figure 4) biases the results in favor of intensive glycemic control. The actual net loss in quality of life is likely to be greater than this study suggests. Second, as discussed earlier, the net benefits of intensive glycemic control are estimated for the average patient for each age group. Individuals with greater risk for microvascular and cardiovascular disease (e.g., tobacco use, hypertension, high cholesterol) compared to the average patient with diabetes (mean blood pressure of 130/80 mm/Hg and mean low-density lipoprotein cholesterol of 100 mg per deciliter)64 are more likely to derive benefits from intensive glycemic control. However, individuals with a lower than average risk profile will have even less to potentially gain from therapy than the modeled scenarios suggest.
ASSESSING WHETHER TREATMENT IS CONSISTENT WITH PATIENT VALUES AND PREFERENCES: SHARED DECISION MAKING
Shared-decision making (SDM) is defined as a partnership between physicians and patients to make preference-sensitive decisions together, guided by the best available evidence and the patient’s risks, prognosis, and socio-personal context.65 Preference-sensitive therapeutic decisions indicate that there is clinical equipoise regarding two or more treatment options, such that a person’s values and preferences can meaningfully shift the decision towards one therapeutic option. SDM is not indicated for every clinical decision. For decisions where there is overwhelming support for a particular treatment such that the benefits clearly outweigh the harms (i.e. insulin in diabetic ketoacidosis), a more parentalistic model of decision-making is preferred.
There are four key steps to implementing SDM in clinical practice: 1) explain to the individual that there is clinical equipoise with respect to how best to treat the disease; 2) present information on the different choices based on the best available evidence; 3) support deliberation between the various therapeutic choices by discussing the person’s values and their socio-personal context (e.g., lifestyle, social support, financial means, workload capacity) to effectively implement different treatments; and 4) formulate a decision (Table 2).65–67 For SDM to be effective physicians must both provide information in an unbiased manner and support the decision making process. In a population-based survey in the US, nearly everyone (96%) reported that they would like to be offered choices and to participate in the decision making process (steps 1–3 above); however, half of respondents preferred their physician to make the final decision (step 4).68 In these situations, a physician can still engage individuals in SDM through empathic conversation, followed by choosing a therapeutic option that best matches the person’s values and the individual’s “effort, time, and energy to effectively implement treatment within their lives and routine.” 69–71
Table 2.
Shared Decision Making Model for Clinical Practice
| Treatment decision scenario: Should a patient with diabetes with a hemoglobin A1C value of 8.5% despite taking three oral antihyperglycemic medications initiate insulin to target an A1C of 7.5%? | |||
|---|---|---|---|
|
| |||
| Step 1: Explain equipoise | Step 2: Present information | Step 3: Support deliberation by empathic conversation | Step 4: Formulate a decision |
|
| |||
|
Explain equipoise:
Regarding your diabetes, let’s talk about how best
to move forward. There is good evidence to support two different
treatment strategies to managing your diabetes. These strategies
have different pros and cons. As such, people differ in what they
believe is most
important. Invitation for SDM: To figure out the best approach for you, shall we discuss the details for each treatment approach? |
Present options:
The two different treatment options include starting insulin to
lower your blood sugar versus continuing your current diabetes
medications and monitoring your blood sugars every 3 to 6 months as
we have been doing. Explain the details of the treatment, which here in this example, includes insulin administration and need for frequent blood glucose monitoring. Explain potential benefits and harms of therapy using absolute measures of risk (with the use of visual aids if available), and the time horizon of benefit. |
Open-ended
questions: -Having discussed the two treatment options, what from your perspective, is most important to you? -What are the most important aspects of insulin that you are factoring into your decision? -What do you think of the possible benefits of starting insulin? -What do you think of the potential harms of starting insulin? Specific probes to assess how the treatment may fit within a patient’s lifestyle and routine: -What is your typical meal schedule like? -What barriers do you foresee in injecting insulin or checking your blood sugars regularly within your current routine? -Are there others at home who can help? |
Clarify roles:
Some people prefer to make the decision together. Others
prefer to defer the decision entirely to their doctor, while some
prefer to make the decision themselves. Which do you
prefer? Finalizing a decision: Patient is decider: Are you ready to make a decision? Would you like more time to more carefully think it over or discuss it with your friends or family? Doctor is involved in the decision: Understanding what you value and how you seem to weigh the pros and cons, I recommend we should proceed with… Review the decision: To ensure that we are on the same page, you [we] have decided that proceeding with […] is the best approach for you. Is that correct? |
|
| |||
| Throughout the shared decision making conversation | |||
|
Check
understanding periodically using ask, tell,
ask in a nonjudgmental way: To make sure I am doing
a good job explaining things to you, can you please tell me what we
discussed about […]; I know we discussed a
lot of information and it can be hard to keep everything straight,
so let’s review what you understand about
[…] Defer closure by patients who prefer to defer the decision entirely to the clinician before expressing their values and opinions: I would be pleased to make the decision for you, but for me to make the best decision about how to move forward it is important I understand how you value each treatment. | |||
The practice of SDM has many benefits, including improving patient’s knowledge regarding treatment options, reducing patient decisional conflict, stimulating patients to take a more active role in the decision making process, and leading patients to make more conservative treatment choices compared to their physicians.72, 73 For example, among patients with stable coronary disease, more complete SDM discussions were strongly associated with fewer patients choosing to undergo angiography and possible percutaneous coronary intervention.73 Despite these benefits, the practice of SDM is suboptimal in current clinical practice, with few physicians providing a balanced explanation of the pros and cons of therapeutic options, and fewer exploring patients’ preferences and values.73–75 This may be in part because implementing SDM in clinical practice is challenging, and because the optimal strategies for practice of SDM have yet to be identified and represent a growing area of research. To facilitate SDM, the use of decision aids (either in electronic, pamphlet, or video format) can help clarify the decision, present evidence-based information regarding the potential benefits and harms of different therapeutic options, and elicit patient’s values.72 The use of visual aids, such as icon arrays, have been shown to improve a patient’s understanding of probabilistic information on the benefits and harms of treatment.76
Shared Decision Making in Diabetes
Given the very modest potential gains from antihyperglycemic treatment (Figure 2), an individual’s treatment burden can potentially negate any benefit gained from intensive glycemic control (Figure 5). Consequently, there is clinical equipoise regarding treating to an HbA1c of 6.4–7.0% among adults with diabetes. Thus, it is essential to elicit patients’ values, preferences, and socio-personal context and use this information to make treatment decisions. To translate the principles of shared decision making into a pragmatic clinical model, we outline a SDM approach in Table 2 through the lens of a treatment decision scenario of whether or not to initiate insulin. This SDM model is informed by the conceptual framework by Charles and colleagues, and extends on principles from two other pragmatic SDM approaches.65–67, 77 To our knowledge, no decision aid for setting glycemic goals in diabetes currently exists. However, once the decision has been made to treat diabetes, the Diabetes Medication Choice decision aid (https://diabetesdecisionaid.mayoclinic.org) can help physicians prescribe the optimal medication that best aligns with the patient’s preferences and socio-personal context,71, 78 since the best available evidence shows that there are no meaningful differences between the available antihyperglycemic drugs for reducing cardiovascular or all-cause mortality.79
OVERTREATMENT IN TYPE 2 DIABETES
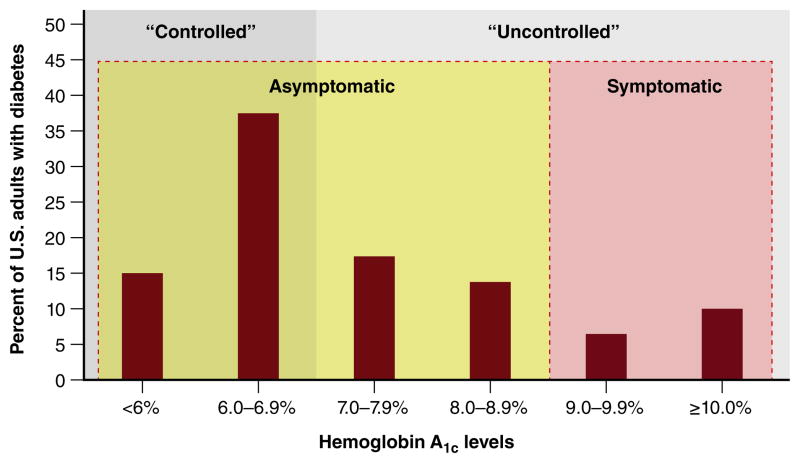
Current evidence strongly supports that there is a potential epidemic of overtreatment with antihyperglycemic therapies in diabetes. A considerable number of individuals are being treated aggressively despite the fact that the potential harms of therapy exceed potential benefits. Nationally representative data from three different waves of NHANES from 1999 to 2004 show that glycemic control, as measured through HbA1c levels, have trended down among American adults with diabetes.80 As of 2012, over 50% of people with diabetes have achieved HbA1c levels less than 7%, which by standard convention is considered ‘well controlled’ (Figure 6). However, achieving ‘tight’ glycemic control is not an end in and of itself, and may represent overtreatment if the patients who have achieved HbA1c levels less than 7% have minimal potential for benefit, are exposed to greater risk of harm, and/or have life expectancies shorter than the time horizon to benefit.
Figure 6. Conceptual Model for Classifying Hemoglobin A1c Distribution Among U.S. Adults with Diabetes, NHANES 2011–2012.
Abbreviations: NHANES, National Health and Nutrition Examination Survey. The current paradigm of glucose management in type 2 diabetes is to classify adults as being “controlled” (solid shaded grey area) if the hemoglobin A1C level is less than 7%, and “uncontrolled” if the hemoglobin A1C level is greater than 7% (solid shaded light grey area). Since this classification does not define whether treating glucose to lower levels is beneficial for an individual patient, we propose classifying diabetes as asymptomatic and symptomatic (shaded areas indicated by dashed lines), such that for asymptomatic patients a shared decision making discussion between the patient and clinician should occur first before reflexively treating glucose to lower levels. Although we dichotomize this distinction at 9% at the population level, patients may have symptoms related to hyperglycemia at lower values of hemoglobin A1C.
A closer examination of the data indeed shows that many of these individuals who have achieved ‘tight’ glycemic control are likely being overtreated – those with the most potential for harm and the least potential to benefit are being treated too intensively. Using NHANES data, Lipska and colleagues found that nearly 60% (1.8 million) of the 3 million older patients with diabetes in the US with limited life expectancy [i.e., health classified as being “complex, intermediate” (≥ 3 chronic conditions or ≥ 2 instrumental activities of daily living impairments) or “complex, poor” (dialysis dependent or ≥ 2 activities of daily living impairments)] nonetheless have HbA1c levels less than 7%.81 Furthermore, among these 1.8 million older adults, nearly 60% are using insulin and/or sulfonylureas, two classes of antihyperglycemic therapies with the greatest potential for harms.81 This implies that many of these multimorbid and frail older adults are unlikely to experience the potential benefits of intensive glycemic control, but nonetheless are exposed to the potential harms of therapy, including hypoglycemia and decreased quality of life due to the treatment burden itself.
In the Veteran Affairs healthcare system the pattern of overtreatment is similar. Approximately 50% of older veterans who are prescribed insulin and/or a sulfonylurea and are at high risk for hypoglycemia (≥ 75 years of age; serum creatinine ≥ 2 mg/dL; and/or dementia) have achieved HbA1c levels less than 7%.82 While the authors were unable to assess the rate of severe hypoglycemia in this high risk cohort, others have shown that hospitalizations for hypoglycemia among older adults with diabetes are now more common in the US than hospitalizations for hyperglycemia (105 vs. 70 admissions per 100,000 person-years),83 with patients with the lowest HbA1c levels being at greatest risk.84
Lastly, in a national cohort of more than 30,000 individuals with diabetes enrolled in private and Medicare Advantage insurance plans in the US, nearly 20% of those with a HbA1c level less than 7% were individuals with advanced clinical complexity (≥75 years of age, or high comorbidity burden) received intensive antihyperglycemic therapy. These individuals had nearly double the probability of severe hypoglycemia (3.0%) compared to those receiving standard antihyperglycemic therapy (1.7%).85 Taken together, these data suggest that we, as a collective profession, are substantially overtreating diabetes without regard for absolute benefits, harms, or time horizon to benefit.
Aside from making the appropriate initial recommendation of whether or not to pursue intensive glycemic control, another approach to overcoming overtreatment of diabetes is to deintensify antihyperglycemic medications when HbA1c levels are at or below goal by either reducing medication doses or discontinuing therapy altogether if warranted. However, medication deintensification is uncommon in clinical practice. Among older veterans who are actively being treated for diabetes, medication deintensification rates are fairly low (<30%), even among patients with extremely ‘tight’ glycemic control (HbA1c levels < 6%) and limited life expectancy, as predicted by multimorbidity and age.86
There are several reasons for this clinical inertia. First, a substantial proportion of physicians believe that intensive glycemic control is beneficial even among older adults at high risk for adverse effects. In a national survey of primary care physicians, nearly 40% believed that a hypothetical older adult with diabetes at high risk for hypoglycemia (HbA1c of 6.4%, chronic kidney disease, and treated with a sulfonylurea) would benefit from an HbA1c level lower than 7.0%.87 Similarly, almost half of providers (45%) worried that this individual would be harmed by an HbA1c level above 7.0%.87 Second, practice guidelines for diabetes focus primarily on glycemic goals and have been slow to incorporate EBM and patient-centered principles into revised recommendations. In the 2015 American Diabetes Association (ADA) Standards of Medical Care in Diabetes (93 pages) and the ADA and European Association for the Study of Diabetes position statement (20 pages), there was not a single mention of the absolute magnitude of potential harms or benefit of intensive glycemic control.88 Furthermore, practice guidelines for diabetes are relatively blind to context. In a review of 28 different practice guidelines for setting glycemic goals in type 2 diabetes, only 60% considered comorbidities, 40% considered socio-personal context (e.g., financial means, caregiver support), and 40% considered patient preference.89 Overall, the synthesis of best available evidence to incorporate information on absolute benefits and harms and on patient preferences remains suboptimal, and may unintentionally lead to uniform rather than personalized application of glycemic control goals to patient care, even when the harms of therapy outweigh the potential benefits in many individuals with diabetes.
CONCLUSION: AN EBM APPROACH IS NEEDED TO PREVENT OVERTREATMENT
In summary, Figure 1 illustrates an EBM framework that can be used for clinical decision making to inform treatment decisions. To promote personalized care and overcome overuse, it is essential to incorporate best available evidence (balancing absolute harms and benefits, while incorporating the time horizon to benefit) with the physician’s judgment (individualizing the evidence based on a patient’s risk profile, prognosis and context) and the patient’s preferences and values (via shared decision making). What EBM does not entail is eschewing clinical significance and patient preferences for statistical significance, nor does it entail the uniform application of therapies based on measures of average population tendencies, especially when the level of evidence is weak. In contrast, an EBM approach calls for making personalized treatment recommendations with full consideration given to a patient’s individual socio-personal context and values.
Applying this EBM framework to the example of managing hyperglycemia in type 2 diabetes highlights the clinical equipoise in setting glycemic goals for diabetes—that no single HbA1c level is appropriate for all patients. As such, we should abandon the notion that HbA1c levels less than or equal to 7% are ‘well controlled’ and greater than 7% are ‘uncontrolled.’ This arbitrary dichotomy does not adequately portray whether we are optimizing the benefits of treatment, quality of life, and value for individuals since most people with diabetes in the ‘uncontrolled’ range (i.e. HbA1c >7%) are nonetheless asymptomatic (Figure 6); and achieving ‘tight’ glycemic control does not meaningfully reduce cardiovascular complications. Rather, an EBM approach would consist of treating to achieve adequate glycemic control to prevent symptomatic disease (e.g. polyuria, polydipsia), followed by further consideration of more intensive treatment if a clinical assessment suggests that the potential absolute benefits outweigh the harms, as with any other cardiovascular risk factor. This assessment would encompass a thorough understanding of the patient’s risks, prognosis (i.e. age, comorbidities, and functional status), and socio-personal context (e.g., lifestyle, social support, workload capacity), as well as engaging with the patient to elicit perceived or experienced treatment burden, and values and preferences for care. Above all else, it is imperative for physicians to remember that the fundamental goal is to help individuals who have diabetes make the best therapeutic decision to improve their overall health and quality of life, not to prevent diabetes-related complications by any means possible.
The EBM approach we present here is not limited to the consideration of intensive glycemic control in type 2 diabetes, but rather, is intended to serve as a framework to inform treatment decisions more broadly. Furthermore, this EBM framework also enables physicians to directly advance the overall goal of the national ‘precision medicine’ initiative—to individualize treatments to achieve the best health outcomes for each person.15 To achieve this goal, further advances in genomics and other biomedical knowledge will undoubtedly be instrumental; however, physicians currently have the tools available at their disposal to personalize care for many important treatment decisions without further medical innovation. As such, the purpose of this review is a call to action for physicians, medical educators, researchers, and policy leaders to apply the principles of EBM to individualize treatment decisions to optimize the health and well-being of patients.
Acknowledgments
The authors would like to thank Lei Xuan, PhD, Division of Outcomes and Health Services Research, UT Southwestern Medical Center, for her assistance in analyzing the National Health and Nutrition Examination Study data used in Figure 6. All persons who have made substantial contributions to the manuscript, but who do not fulfill authorship criteria, are named with their specific contributions in the Acknowledgements section of the manuscript; and all persons named in the Acknowledgements section have provided the corresponding author with written permission to be named in the manuscript.
SOURCES OF FUNDING
Drs. Makam and Nguyen received funding from the UT Southwestern KL2 Scholars Program (NIH/NCATS KL2 TR0001103). Dr. Makam was also supported by a NIH/NIA K23 award (K23 AG052603). Dr. Nguyen was also supported by the Agency for Healthcare Research and Quality-funded UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418).
Footnotes
DISCLOSURES
None.
References
- 1.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 2.Korenstein D, Falk R, Howell EA, Bishop T, Keyhani S. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172:171–178. doi: 10.1001/archinternmed.2011.772. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Saunders R, Stuckhardt L, McGinnis JM, editors. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): 2013. [PubMed] [Google Scholar]
- 4.Sirovich BE, Woloshin S, Schwartz LM. Too Little? Too Much? Primary care physicians’ views on US health care: a brief report. Arch Intern Med. 2011;171:1582–1585. doi: 10.1001/archinternmed.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong PL, Saunders RS, Olsen LA, editors. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington (DC): 2010. [PubMed] [Google Scholar]
- 6.Bishop TF, Federman AD, Keyhani S. Physicians’ views on defensive medicine: a national survey. Arch Intern Med. 2010;170:1081–1083. doi: 10.1001/archinternmed.2010.155. [DOI] [PubMed] [Google Scholar]
- 7.Carrier ER, Reschovsky JD, Katz DA, Mello MM. High physician concern about malpractice risk predicts more aggressive diagnostic testing in office-based practice. Health Aff (Millwood) 2013;32:1383–1391. doi: 10.1377/hlthaff.2013.0233. [DOI] [PubMed] [Google Scholar]
- 8.Carrier ER, Reschovsky JD, Mello MM, Mayrell RC, Katz D. Physicians’ fears of malpractice lawsuits are not assuaged by tort reforms. Health Aff (Millwood) 2010;29:1585–1592. doi: 10.1377/hlthaff.2010.0135. [DOI] [PubMed] [Google Scholar]
- 9.Tilburt JC, Wynia MK, Sheeler RD, Thorsteinsdottir B, James KM, Egginton JS, Liebow M, Hurst S, Danis M, Goold SD. Views of US physicians about controlling health care costs. JAMA. 2013;310:380–388. doi: 10.1001/jama.2013.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174:1640–1648. doi: 10.1001/jamainternmed.2014.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linder JA, Doctor JN, Friedberg MW, Reyes Nieva H, Birks C, Meeker D, Fox CR. Time of day and the decision to prescribe antibiotics. JAMA Intern Med. 2014;174:2029–2031. doi: 10.1001/jamainternmed.2014.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makam AN, Auerbach AD, Steinman MA. Blood culture use in the emergency department in patients hospitalized for community-acquired pneumonia. JAMA Intern Med. 2014;174:803–806. doi: 10.1001/jamainternmed.2013.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makam AN, Auerbach AD, Steinman MA. Blood culture use in the emergency department in patients hospitalized with respiratory symptoms due to a nonpneumonia illness. J Hosp Med. 2014;9:521–524. doi: 10.1002/jhm.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makam AN, Nguyen OK. Use of cardiac biomarker testing in the emergency department. JAMA Intern Med. 2015;175:67–75. doi: 10.1001/jamainternmed.2014.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–1584. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 17.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djulbegovic B, Guyatt GH. Evidence-based practice is not synonymous with delivery of uniform health care. JAMA. 2014;312:1293–1294. doi: 10.1001/jama.2014.10713. [DOI] [PubMed] [Google Scholar]
- 19.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC., Jr Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA. 2009;301:831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- 20.Neuman MD, Goldstein JN, Cirullo MA, Schwartz JS. Durability of class I American College of Cardiology/American Heart Association clinical practice guideline recommendations. JAMA. 2014;311:2092–2100. doi: 10.1001/jama.2014.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 22.Lin GA, Dudley RA, Redberg RF. Why physicians favor use of percutaneous coronary intervention to medical therapy: a focus group study. J Gen Intern Med. 2008;23:1458–1463. doi: 10.1007/s11606-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med. 2007;167:1604–1609. doi: 10.1001/archinte.167.15.1604. [DOI] [PubMed] [Google Scholar]
- 24.Gould SJ. The median isn’t the message. Virtual Mentor. 2013;15:77–81. doi: 10.1001/virtualmentor.2013.15.1.mnar1-1301. [DOI] [PubMed] [Google Scholar]
- 25.Djulbegovic B, Paul A. From efficacy to effectiveness in the face of uncertainty: indication creep and prevention creep. JAMA. 2011;305:2005–2006. doi: 10.1001/jama.2011.650. [DOI] [PubMed] [Google Scholar]
- 26.Christensen PM, Kristiansen IS. Number-needed-to-treat (NNT)--needs treatment with care. Basic Clin Pharmacol Toxicol. 2006;99:12–16. doi: 10.1111/j.1742-7843.2006.pto_412.x. [DOI] [PubMed] [Google Scholar]
- 27.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 28.Action to Control Cardiovascular Risk in Diabetes Study G. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD Investigators V. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 30.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med. 2009;150:803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- 34.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ. 2011;343:d6898. doi: 10.1136/bmj.d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV Investigators V. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Gutierrez R, Montori VM. Glycemic Control for Patients With Type 2 Diabetes Mellitus: Our Evolving Faith in the Face of Evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504–512. doi: 10.1161/CIRCOUTCOMES.116.002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS Investigators LT. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 39.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med. 2014;174:1227–1234. doi: 10.1001/jamainternmed.2014.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey LL, Ballard DJ, Frohnert PP, Chu CP, O’Fallon WM, Palumbo PJ. Chronic renal failure in non-insulin-dependent diabetes mellitus. A population-based study in Rochester, Minnesota. Ann Intern Med. 1989;111:788–796. doi: 10.7326/0003-4819-111-10-788. [DOI] [PubMed] [Google Scholar]
- 42.Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):766–785. [PubMed] [Google Scholar]
- 43.Ferris FL., 3rd How effective are treatments for diabetic retinopathy? JAMA. 1993;269:1290–1291. [PubMed] [Google Scholar]
- 44.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 45.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I Collaborative Study G. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 46.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P Irbesartan in Patients with Type D, Microalbuminuria Study G. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Leipzig RM, Walter LC. Incorporating lag time to benefit into prevention decisions for older adults. JAMA. 2013;310:2609–2610. doi: 10.1001/jama.2013.282612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann Intern Med. 1997;127:788–795. doi: 10.7326/0003-4819-127-9-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 49.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 50.Leal J, Gray AM, Clarke PM. Development of life-expectancy tables for people with type 2 diabetes. Eur Heart J. 2009;30:834–839. doi: 10.1093/eurheartj/ehn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 52.Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, Chalmers JW, Capewell S, McMurray JJ. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 53.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 55.Cruz M, Covinsky K, Widera EW, Stijacic-Cenzer I, Lee SJ. Predicting 10-year mortality for older adults. JAMA. 2013;309:874–876. doi: 10.1001/jama.2013.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11–19. doi: 10.7326/0003-4819-149-1-200807010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson R, Parkin D, Eccles M, Sudlow M, Robinson A. Decision analysis and guidelines for anticoagulant therapy to prevent stroke in patients with atrial fibrillation. Lancet. 2000;355:956–962. doi: 10.1016/S0140-6736(00)90012-6. [DOI] [PubMed] [Google Scholar]
- 58.Naglie G, Krahn MD, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: Part 3--Estimating probabilities and utilities. Med Decis Making. 1997;17:136–141. doi: 10.1177/0272989X9701700203. [DOI] [PubMed] [Google Scholar]
- 59.Pletcher MJ, Pignone M. Evaluating the clinical utility of a biomarker: a review of methods for estimating health impact. Circulation. 2011;123:1116–1124. doi: 10.1161/CIRCULATIONAHA.110.943860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chong CA, Tomlinson G, Chodirker L, Figdor N, Uster M, Naglie G, Krahn MD. An unadjusted NNT was a moderately good predictor of health benefit. J Clin Epidemiol. 2006;59:224–233. doi: 10.1016/j.jclinepi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30:2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12:219–230. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 63.Matza LS, Boye KS, Yurgin N, Brewster-Jordan J, Mannix S, Shorr JM, Barber BL. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res. 2007;16:1251–1265. doi: 10.1007/s11136-007-9226-0. [DOI] [PubMed] [Google Scholar]
- 64.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 65.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49:651–661. doi: 10.1016/s0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 66.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, Edwards A, Barry M. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: Concepts, evidence, and practice. Patient Educ Couns. 2015;98:1172–1179. doi: 10.1016/j.pec.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 68.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Politi MC, Dizon DS, Frosch DL, Kuzemchak MD, Stiggelbout AM. Importance of clarifying patients’ desired role in shared decision making to match their level of engagement with their preferences. BMJ. 2013;347:f7066. doi: 10.1136/bmj.f7066. [DOI] [PubMed] [Google Scholar]
- 70.Hargraves I, LeBlanc A, Shah ND, Montori VM. Shared Decision Making: The Need For Patient-Clinician Conversation, Not Just Information. Health Aff (Millwood) 2016;35:627–629. doi: 10.1377/hlthaff.2015.1354. [DOI] [PubMed] [Google Scholar]
- 71.Serrano V, Rodriguez-Gutierrez R, Hargraves I, Gionfriddo MR, Tamhane S, Montori VM. Shared decision-making in the care of individuals with diabetes. Diabet Med. 2016;33:742–751. doi: 10.1111/dme.13143. [DOI] [PubMed] [Google Scholar]
- 72.Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Thomson R, Trevena L, Wu JH. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 73.Rothberg MB, Sivalingam SK, Kleppel R, Schweiger M, Hu B, Sepucha KR. Informed Decision Making for Percutaneous Coronary Intervention for Stable Coronary Disease. JAMA Intern Med. 2015;175:1199–1206. doi: 10.1001/jamainternmed.2015.1657. [DOI] [PubMed] [Google Scholar]
- 74.Couet N, Desroches S, Robitaille H, Vaillancourt H, Leblanc A, Turcotte S, Elwyn G, Legare F. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18:542–561. doi: 10.1111/hex.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowler FJ, Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions?: Results of a national survey. JAMA Intern Med. 2013;173:1215–1221. doi: 10.1001/jamainternmed.2013.6172. [DOI] [PubMed] [Google Scholar]
- 76.Zipkin DA, Umscheid CA, Keating NL, Allen E, Aung K, Beyth R, Kaatz S, Mann DM, Sussman JB, Korenstein D, Schardt C, Nagi A, Sloane R, Feldstein DA. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270–280. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 77.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 78.Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, Perestelo-Perez LI, Stroebel RJ, Yawn BP, Yapuncich V, Breslin MA, Pencille L, Smith SA. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169:1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 79.Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. JAMA. 2016;316:313–324. doi: 10.1001/jama.2016.9400. [DOI] [PubMed] [Google Scholar]
- 80.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 81.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175:356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174:259–268. doi: 10.1001/jamainternmed.2013.12963. [DOI] [PubMed] [Google Scholar]
- 83.Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, Huang ES, Desai MM, Gill TM, Krumholz HM. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–1124. doi: 10.1001/jamainternmed.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lipska KJ, Warton EM, Huang ES, Moffet HH, Inzucchi SE, Krumholz HM, Karter AJ. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. 2013;36:3535–3542. doi: 10.2337/dc13-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive Treatment and Severe Hypoglycemia Among Adults With Type 2 Diabetes. JAMA Intern Med. 2016;176:969–978. doi: 10.1001/jamainternmed.2016.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sussman JB, Kerr EA, Saini SD, Holleman RG, Klamerus ML, Min LC, Vijan S, Hofer TP. Rates of Deintensification of Blood Pressure and Glycemic Medication Treatment Based on Levels of Control and Life Expectancy in Older Patients With Diabetes Mellitus. JAMA Intern Med. 2015:1–8. doi: 10.1001/jamainternmed.2015.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caverly TJ, Fagerlin A, Zikmund-Fisher BJ, Kirsh S, Kullgren JT, Prenovost K, Kerr EA. Appropriate Prescribing for Patients With Diabetes at High Risk for Hypoglycemia: National Survey of Veterans Affairs Health Care Professionals. JAMA Intern Med. 2015:1–3. doi: 10.1001/jamainternmed.2015.5950. [DOI] [PubMed] [Google Scholar]
- 88.McCormack JP, Martin SA, Newman DH. Comment on Inzucchi et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149. Diabetes Care. 2015;38:e141–142. doi: 10.2337/dc15-0074. [DOI] [PubMed] [Google Scholar]
- 89.Wyatt KD, Stuart LM, Brito JP, Carranza Leon B, Domecq JP, Prutsky GJ, Egginton JS, Calvin AD, Shah ND, Murad MH, Montori VM. Out of context: clinical practice guidelines and patients with multiple chronic conditions: a systematic review. Med Care. 2014;52(Suppl 3):S92–S100. doi: 10.1097/MLR.0b013e3182a51b3d. [DOI] [PubMed] [Google Scholar]