Abstract
Background aims
In this study, we demonstrate long-term persistence of human mesenchymal stromal cells (hMSCs) after intracoronary injection in a large animal model of pulmonary hypertension (PH).
Methods
Commercially available placenta-derived hMSCs were used. Experiments were conducted on 14 female Yorkshire swine. Four animals served as controls, and 10 underwent pulmonary vein (PV) banding. After 12 ± 2 weeks, PH and PV dysfunction were confirmed by right heart catheterization and cardiac magnetic resonance imaging. hMSCs were injected in the marginal branch of the right coronary artery. Tissues were harvested 6, 9 or 24 days after infusion.
Results
After 12 ± 2 weeks after PV banding, all subjects had increased mean pulmonary artery pressure (13.6 ± 3.6 versus 30.8 ± 4.5 mm Hg, P < 0.007) and a decrease in right ventricular ejection fraction from 51.7 ± 5.7% versus 30.5 ± 11.3% (P = 0.003). Intracoronary injection of hMSCs was well tolerated. Up to 24 days after hMSC injection, immunohistochemistry revealed extravascular viable human CD105+ mononuclear cells in the right ventricle (RV) that were Ki67+. This was confirmed by fluorescence in situ hybridization. CD45+ porcine inflammatory cells were identified, commonly seen adjacent to areas of healing microscopic infarction that likely dated to the time of the original hMSC injection. Anti-CD31 staining produced strong signals in areas of injected hMSCs. Immunohistochemistry staining for vascular cell adhesion molecule-1 showed upregulation in the clusters, where mononuclear cells were located.
Conclusions
hMSCs injected via intracoronary infusion survived up to 24 days and demonstrated proliferative capacity. hMSCs can persist long term in the RV and are potential cell source for tissue repair in RV dysfunction.
Keywords: differentiation, human mesenchymal stromal cells, intracoronary injection, pulmonary hypertension, right ventricular remodeling
Introduction
Mesenchymal stromal cells (MSCs) can differentiate into multiple cell types and have been proposed as a cell-based therapy for cardiac repair in numerous studies. In vitro studies have indicated that MSCs derived from placenta of fetal origin possess greater therapeutic potential compared with MSCs isolated from other tissues and organs [1,2]. Several studies support the ability of these MSCs either to repair or regenerate damaged myocardium [3–5], although their long-term durability in tissue is controversial [6,7]. The safety of MSC delivery to the left ventricle after acute myocardial infarction has also been established, via intracoronary, intramyocardial, or intravenous routes of administration [8,9]. Preclinical studies have also suggested that cell therapy with MSCs has substantial potential to treat right ventricular (RV) remodeling and dysfunction resulting from pulmonary hypertension (PH) and other diseases of the lung [10,11]. To translate these findings to patients, studies to evaluate the distribution and survival of MSCs in adult large animal models of PH with established RV dysfunction are warranted. The aim of the present study was to investigate the long-term survival and to characterize the proliferation and differentiation potential of human MSCs (hMSCs) after intracoronary injection in a swine pulmonary vein (PV) banding model of PH and RV remodeling.
Methods
This study was approved by the Harvard Medical Area Institutional Animal Care and Use Committee and was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals. Additional methods are in the Supplementary Data.
Study design
Fourteen female Yorkshire pigs were included in the study. Surgical banding of the inferior PV was used to create the model of post-capillary PH with RV dysfunction (n = 10). The animals were housed for 12 ± 2 weeks and allowed to develop PH gradually. PH, defined as a mean pulmonary artery pressure >25 mm Hg, was confirmed by right heart catheterization, and RV function was assessed by cardiac magnetic resonance imaging (cMRI). Intracoronary hMSC injection using the largest marginal branch of the right coronary artery (RCA) that perfused the majority of the RV was performed and animals were sacrificed after 6,9 or 24 days. Four animals served as controls to optimize the hMSC intracoronary injection procedure and for histological analysis.
Peri-procedural anesthesia
Vital signs were monitored continuously while depth of anesthesia was maintained. Animals were sedated with subcutaneous Telazol (4.4 mg/kg) and xylazine (2.2 mg/kg). They were orally intubated and mechanically ventilated with 40% oxygen, 10 mL/kg tidal volume at 15 respirations per minute. General anesthesia was maintained with 1.5–2.5% isoflurane. For the thoracotomy procedure, animals were pretreated with intravenous lidocaine (2.0–4.0 mg/kg bolus and 50 µg/kg/min) drip and amiodarone (10–12 mg/kg, followed by 0.5–3.5 mg/kg/h) and received a 25–50 mcg/h fentanyl patch in the postoperative period.
Post-capillary PV banding
The procedure was performed in female piglets (10 kg) using the technique described previously by Aguero et al. [12]. Briefly, each animal was placed in the left lateral decubitus position, and an anterolateral thoracotomy was performed through the right fifth intercostal space. Once exposed, the right inferior venous confluence was carefully isolated with a right angle dissector, and cotton umbilical tape (Ethicon) was passed around the vein and tied loosely. The ribs were then approximated with reabsorbable pericostal sutures, and the wound was closed with absorbable sutures. Animals were recovered and allowed to develop PH gradually with somatic growth.
hMSC preparation
Commercially available human placenta–derived MSCs, passage 1 (Zenbio, catalog number: CA-10, lot number: ZB0003) were expanded to passage 5. Cells were cultured in Dulbecco’s Modified Eagle Medium (Life Technologies) supplemented with 10% fetal bovine serum (Biowest) and 1% penicillin/streptomycin (Life Technologies) at 37°C, 5% CO2. Aliquots of cells were cryopreserved in 10% dimethylsulfoxide/Dulbecco’s Modified Eagle Medium/vapor phase nitrogen tank. Aliquots were thawed once and suspended in 0.1% bovine serum albumin (BSA)/saline solution (Sigma-Aldrich). Cell number and viability were assessed using trypan blue exclusion (Life Technologies), and cell number was determined using an automated cell counter (TC20, BioRad). Cells were resuspended in sterile 0.1% BSA/saline solution at a concentration of 2.5 million cells/mL; a total number of 107 hMSCs were prepared in a syringe and kept on ice. Cells were infused into the coronary artery within 2 h after preparation. One control pig received the same volume of vehicle only (0.1% BSA/saline).
hMSC infusion technique and angiographic evaluation
Vascular sheaths were placed in the femoral artery and vein. The animals were heparinized with a bolus of 250 IU/kg heparin sulfate (SAGENT Pharmaceutical), and additional heparin was given to achieve an activated clotting time of 200–300 s. Hemodynamics were measured using a 7.0-Fr Swan-Ganz catheter (Baxter Healthcare). Right atrial pressure; pulmonary artery systolic, diastolic, and mean pressures; and pulmonary capillary wedge position pressures were recorded. Cardiac output was measured using the thermodilution method. After initial right coronary angiography, a 0.014-inch coronary guide wire was advanced into the largest RCA marginal branch that supplies the right ventricle. An over-the-wire (OTW) balloon sized to the vessel diameter was placed in the proximal part of the marginal branch, and the balloon was inflated at 4–6 atm. Contrast injections via the OTW balloon lumen were performed to ensure the complete occlusion of the vessel by the balloon. A total of 107 hMSCs were infused through the OTW balloon lumen in four separate injections of 2.5 million cells/mL. One milliliter of suspended cells was injected slowly through the balloon lumen while inflated over 3 min. Between injections the balloon was deflated for 3 min, and coronary angiography was performed to assure vessel patency.
Fluorescent microspheres injection
Before tissue harvest, fluorescent microspheres (FMs) with a nominal size of 15 µm and an excitation/ emission maximum of (645/680) (LifeTechnologies) were injected in the largest marginal branch of the RCA to localize the region of the previously injected MSCs. The FM beads were supplied at 1.0 × 106 beads/ mL; 700-µL microspheres were mixed with 700 µL of 0.1% BSA/saline in a 1.5-mL Eppendorf tube. Just before tissue harvest, 1 mL of the FM BSA/saline solution was slowly injected through the lumen of the OTW balloon catheter; the balloon remained inflated for 2 min post-injection. Intracoronary injected FMs were restricted to the same area perfused by the marginal branch used to inject the hMSC.
Tissue harvest and macroscopic ex vivo fluorescence reflectance imaging
Animals were euthanized 5–10 min after FM injection via 180 mg/kg Euthasol intravenously. The hearts were excised, sliced and incubated in 10% formalin for 3 days. Formalin fixed heart tissues were analyzed by fluorescence reflectance imaging with an image station 4000MMPro (Kodak) 3–6 days after tissue harvest. Near infrared fluorescence images were obtained in the 680-nm channels (excitation filter: 630 nm; emission filter: 700 nm) with progressive exposure time for 30 min (heart sections) [13]. Measurements were performed in all affected slices. Data were quantified for perfusion analysis by measuring mean signal intensity as counts per pixel in regions of interest traced manually.
cMRI
The cMRI studies were performed on a whole-body clinical 3T scanner (3T Siemens Skyra Hardware). Studies were done at baseline (before PV banding) and at 12 ± 2 weeks post-surgery. The images were acquired in the short axis, long axis and four chamber views of the heart. Right ventricular structure and function (ejection fraction and end-diastolic, end-systolic, and stroke volumes) were evaluated as well as enlargement (end-diastolic transversal diameter) and hypertrophy (wall thickness) [14]. For measurements of ventricular function and mass, breath-hold-balanced steady-state free precession MRI, cine images were obtained [15]. Endocardial tracings were performed for volume measurement; wall mass was defined as the ventricular wall enclosed by endocardial and epicardial contours. Segmentation of the RV on the short axis cine was done in the end diastolic phase in approximately 20 slices from apex to base [16]. Offline imaging analysis was performed using Qmass MR 7.4 enterprise solutions (Medis Medical Imaging Systems).
Immunohistochemistry and immunofluorescence
Formalin fixed heart tissues were embedded in paraffin, sectioned (5-µm thickness) and stained with hematoxylin and eosin or Masson’s trichrome as described previously. hMSCs and porcine inflammatory cells were identified in RV myocardium by immunohistochemistry as described previously. Briefly, sections were deparaffinized and rehydrated, and antigen retrieval was performed by high-temperature heating of sections (≥92°C) in citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and sections were incubated with either 5% normal goat serum or normal horse serum for 30 min at room temperature. Sections were then incubated with anti-CD105, anti-Ki67, anti-caspase-3 or anti-CD45 antibodies overnight at 4°C followed by incubation with biotinylated horse anti-mouse immunoglobulin (Ig)G (H + L) or biotinylated goat anti-Rabbit IgG (H + L) secondary antibodies for 45 min at room temperature. Sections were enhanced with streptavidin-horseradish peroxidase (Dako), developed in AEC substrate chromogen (Dako), counterstained with hematoxylin and mounted with HistoMount (Thermo Fisher Scientific). For immunofluorescence studies, after incubation with primary antibodies, sections were incubated with AlexaFluor555 conjugated goat anti-rabbit IgG (1:500 dilution) or AlexaFluor488 conjugated goat anti-mouse IgG (1:200 dilution). Prolong Gold anti-fade reagent including DAPI (Invitrogen) was added for nuclear visualization. Sections were imaged by confocal microscopy (Fluoview FV 1000, Olympus) (Table I).
Table I.
Primary antibodies used in study.
| Antibody | Host species |
Clonality | Catalogue No. |
IHC dilution |
IF dilution |
Supplier | Species reactivity |
|---|---|---|---|---|---|---|---|
| CD105 | Rabbit | Poly | Ab74462 | 1:40 | 1:30 | Abcam | Human |
| Ki67 | Mouse | Mono | M 7240 | 1:50 | 1:30 | Dako | Human |
| CD45 | Mouse | Mono | MCA1222 | 1:25 | AbD Serotec | Porcine | |
| Cleaved Caspase-3 | Rabbit | Poly | 9662 | 1:3000 | Cell Signaling Technology | Human | |
| Desmin | Mouse | Mono | M 0760 | 1:50 | Dako | Human | |
| Connexin 43 | Rabbit | Poly | PA1-37496 | Ready to use | Thermo Fisher Scientific | Human | |
| CD31 (PECAM-1) | Rat | Mono | DIA-310 | 1:20 | Dianova | Porcine | |
| Anti-VCAM-1 | Rabbit | Mono | Ab134047 | 1:500 | Abcam | Human and porcine |
IF, immunofluorescence; IHC, immunohistochemistry.
Fluorescence in situ hybridization
A two-color species-differentiation fluorescence in situ hybridization (FISH) probe was generated by nick translation of species-specific repeated DNA sequences: human Cot-1 (Invitrogen) was labeled with SpectrumGreen, and porcine ID Block-It Cot-1 (Empire Genomics) was labeled with SpectrumOrange. The mixture was hybridized to control specimens from both species to confirm specific hybridization. Tissue slides were fixed in methanol/acetic acid fixative (3:1) for 60 min. The slides were dehydrated in graded ethanol (70%, 90%, 100%) at 25°C for 2 min each and air dried. The probe (8–10 µL) was added to the slide and a cover slip was placed. The slides and probe were co-denatured in a HYBrite at 73°C for 2 min and then hybridized at 37°C overnight in a humidified chamber. The slides were washed in 0.4 × SSC/ 0.3% NonidetP-40 (Igepal, CA 630) at 72°C for 2 min and 2 × SSC/0.1% NonidetP-4 at 25°C for 1 min. DAPI II is applied and the slides are analyzed by fluorescent microscopy.
Cell counting and vessel quantification
Cell counter (Fiji Is Just ImageJ software) was used to quantify the cells. Single images of porcine right ventricle at 40 × objective (400 × magnification) were used. In each section, different groups of cells were marked up manually (e.g., Myocytes [red], hMSCs [blue] and Caspase 3 positive cells [brown]).
Vessel cross-sections per unit area were quantified at 40 × magnification in cardiac tissue, stem cell infiltrated cardiac tissue and granulation tissue using Image J 1.47v software. Each CD31-positive endothelial cell cluster in contact with the selected field was counted as an individual vessel in addition to the morphologically identifiable vessels with a lumen.
Statistical analysis
Continuous variables are presented as mean ± SD, and the differences between the variables were assessed using paired Student’s t-test or Wilcoxon signed-rank test for paired samples. A P value <0.05 was considered significant. All statistical analysis were performed using STATA 14 software (StataCorp).
Results
Evidence of PH and RV remodeling and dysfunction
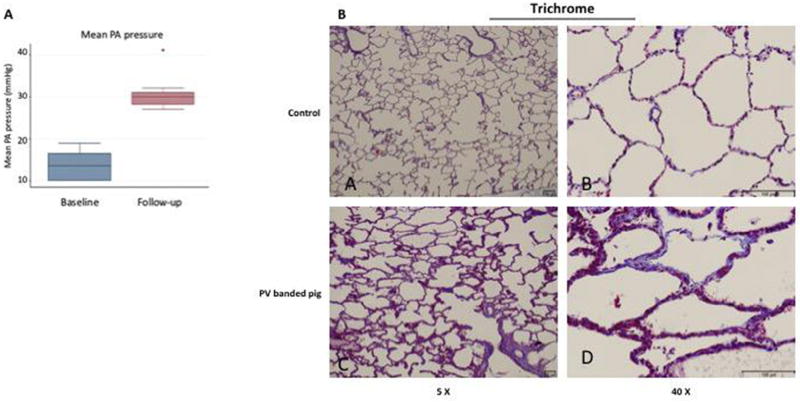
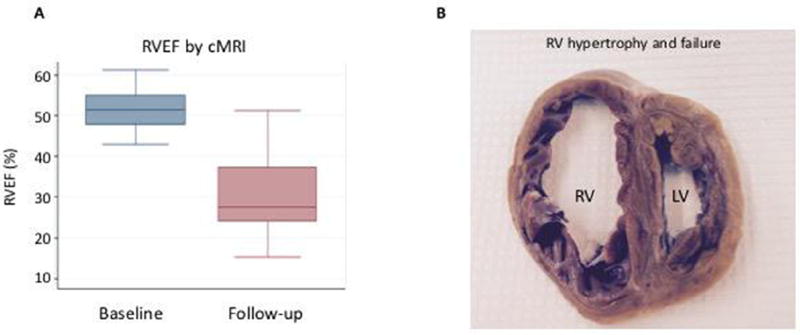
Fourteen animals were included in this study. Two swine did not undergo the PV banding procedure and served as controls for the histological analyses. One control pig underwent intracoronary injection of 1 million hMSCs (0.25 million cells/mL), and a second control animal was injected vehicle alone (0.1% BSA/saline) with sacrifice after 3 days to serve as a positive or negative control, respectively. Two additional swine were used to optimize the surgical and cell delivery technique. Ten swine underwent the PV banding procedure. One pig died immediately after surgery, and one expired at week 11 before the final hemodynamic measurements could be obtained. All other animals (n = 8) survived to the end of the study and developed PH as demonstrated by hemodynamic measurements (Figure 1A, Table II). At 12 ± 2 weeks after PV banding, the mean pulmonary artery pressure (13.6 ± 3.6 versus 30.8 ± 4.5 mm Hg, P < 0.007), transpulmonary gradient (8.1 ± 2.9 versus 16.6 ± 8.9 mm Hg, P = 0.024) and RV end-diastolic pressure (4.5 ± 1.9 versus 7.3 ± 2.1 mm Hg, P = 0.011) were increased significantly compared with baseline values consistent with PH and RV pressure overload. There was also evidence of pulmonary arteriole hypertrophic remodeling and pulmonary vascular fibrosis (Figure 1B). RV structure and function was also evaluated by cMRI, which was performed at baseline and at 12 ± 2 weeks when PH was established. Compared with baseline, there was a significant decrease in the RV ejection fraction (51.7 ± 5.7 versus 30.5 ± 11.3%, P = 0.003) (Figure 2A) with a concomitant increase in RV end-systolic volume (18.3 ± 3.2 versus 92.0 ± 30.1 mL, P = 0.011) and RV end-diastolic volume (37.7 ± 4.4 versus 132.2 ± 37.0 mL, P < 0.011) (Table II). Pathological examination of the RV demonstrated RV dilation and hypertrophic remodeling (Figure 2B). Thus, PH with RV dysfunction was present at the time of hMSC intracoronary delivery.
Figure 1.
PH and vascular remodeling. (A) Mean pulmonary artery (PA) pressures were measured by right heart catheterization at the time of pulmonary vein banding (baseline, n = 10) and 12 ± 2 weeks later just before cell injection (follow-up, n = 8). *P = 0.007. (B) Pulmonary arteriole remodeling and collagen deposition was assessed by Masson’s trichrome staining in control non-diseased pigs (A,B) and PV-banded pigs with PH (C,D).
Table II.
Cardiopulmonary hemodynamics.
| Baseline (n = 10) |
Follow-up (n = 8) |
P value | |
|---|---|---|---|
| mPAP (mm Hg) | 13.6 ± 3.6 | 30.8 ± 4.5 | 0.007 |
| PCWP (mm Hg) | 5.5 ± 2.0 | 14.1 ± 5.7 | 0.011 |
| RA (mm Hg) | 3.1 ± 2.4 | 3.9 ± 1.9 | 0.40 |
| RV systole (mm Hg) | 24.3 ± 4.4 | 45.0 ± 7.2 | 0.011 |
| RV diastole (mm Hg) | 4.5 ± 1.9 | 7.3 ± 2.1 | 0.011 |
| TPG (mm Hg) | 8.1 ± 2.9 | 16.6 ± 8.9 | 0.024 |
| Cardiac output (L/min) | 1.7 ± 0.40 | 7.4 ± 2.0 | 0.011 |
| Systolic BP (mm Hg) | 84.8 ± 5.0 | 111.7 ± 14.8 | 0.016 |
| Diastolic BP (mm Hg) | 49.1 ± 4.0 | 68.6 ± 13.5 | 0.014 |
Values are mean ± SD.
BP, blood pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RA, right atrium; RV, right ventricle; TPG, transpulmonary gradient.
Figure 2.
RV remodeling. (A) RV ejection fraction (RVEF) was evaluated by cMRI at the time of PV banding (baseline, n = 10) and 12 ± 2 weeks later just before cell injection (follow-up, n = 8). *P = 0.003. (B) Cross-section through the heart of a PV-banded pig at week 16 demonstrating RV hypertrophy and dilation consistent with RV failure. RV, right ventricle; LV, left ventricle.
Intracoronary hMSC delivery and delineating region of interest
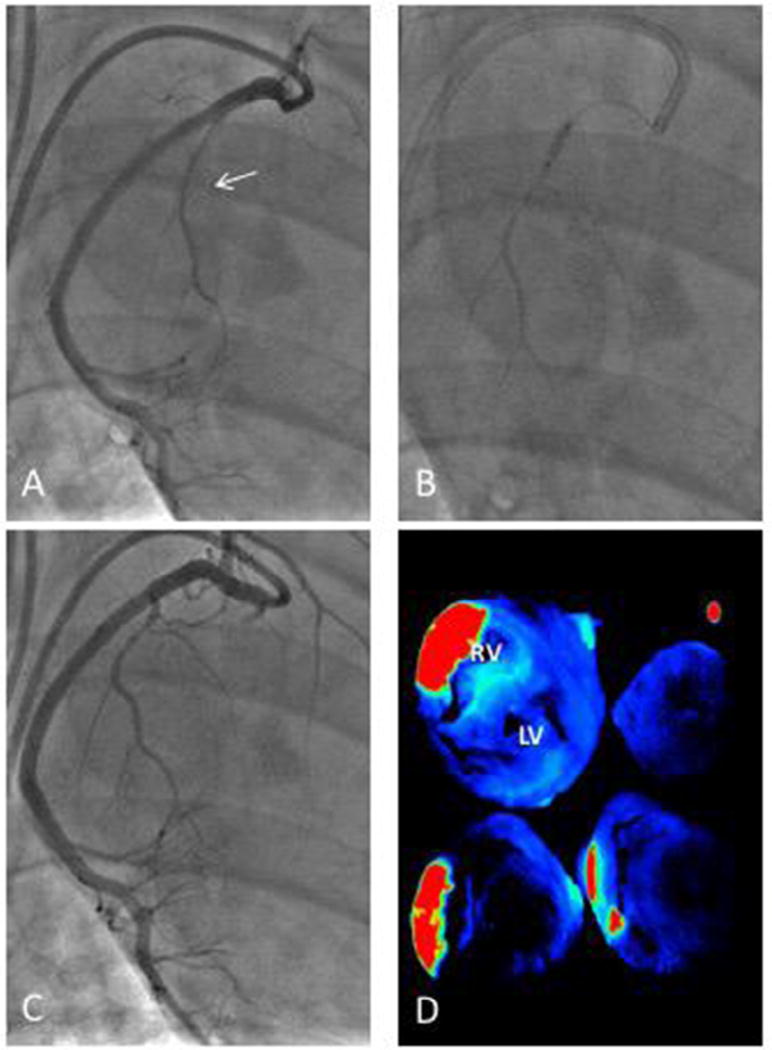
Intracoronary delivery of hMSCs was performed using the largest RCA marginal branch (Figure 3A – C). After intracoronary delivery of hMSCs, study subjects were sacrificed at day 6 (n = 2), 9 (n = 3) or 24 (n = 2). Before harvesting the heart, fluorescent beads were injected into the same RCA marginal branch used for hMSC injection to mark the region of myocardium perfused by this vessel. Intracoronary injection of fluorescent microspheres did not cause any significant changes in coronary blood flow, and no electrocardiogram changes suggesting microvascular plugging were observed. Examination of the heart by ex vivo fluorescence reflectance imaging demonstrated that the microspheres were restricted to the RV, and there was no off-target transit to the left ventricle. The intensity of the bead fluorescence allows visualization of the relevant perfused regions of the porcine myocardium. The quantitative data shows significantly high maximum signal intensities (red) in the area of interest compared with the other areas of myocardium (blue) with a significant difference between myocardium perfused by the RCA marginal branch and other territories (1881.3 ± 429.8 versus 85.6 ± 14.8 arbitrary units, P = 0.027) (Figure 3D).
Figure 3.
Intracoronary cell injection technique via the RCA marginal branch. (A) Intracoronary cell delivery was performed after initial coronary angiography identified the largest right coronary artery (RCA) marginal branch (arrow). (B) The marginal branch was wired with a 0.014-inch coronary guide wire and a balloon inflated to occlude blood flow for cell delivery. (C) After cell delivery, blood flow was normal in the coronary artery. (D) Selective delivery of cells to the right ventricle (RV) using this technique was confirmed by intracoronary injection of fluorescent microbeads in the marginal and using ex vivo fluorescence reflectance imaging of RV sections. Red and green indicate higher fluorescence while blue is low fluorescence. A representative image is shown. LV, left ventricle.
hMSCs are retained and proliferate in the RV
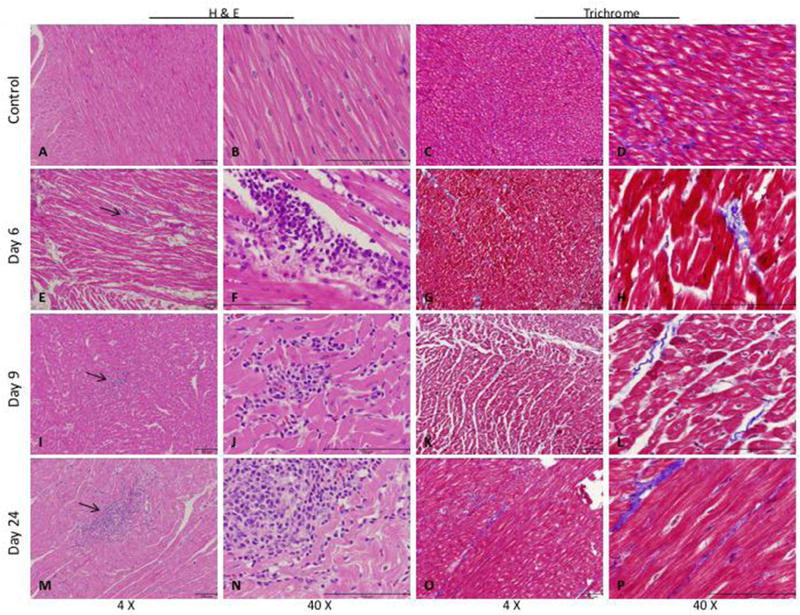
Histological analysis of the right ventricle of PV banded animals demonstrated RV myocyte hypertrophy with increased myocardial interstitial fibrosis (Figure 4). There was no significant difference in cardiac interstitial fibrosis between time points despite functional disease progression. At all three time points after cell delivery (days 6, 9 and 24) and in all animals, we identified collections of human CD105+ mononuclear cells in the regions highlighted by the fluorescent beads. These cells were present scattered throughout the entire transmural thickness of the myocardium and were most commonly seen as well-defined clusters within the interstitium, adjacent to viable myocardium (Figures 4 and 5A). There were also areas of subacute infarction in the same general distribution as the beads and the CD105+ cells, with healing morphology consistent with injury that occurred at the time of hMSC injection. However, the clear majority of these infarcts did not show any associated CD105+ cells. FISH assay was used to confirm the presence of isolated human cells and clusters of human cells in the RV (Figure 6).
Figure 4.
Cell collections in the right ventricle (RV) after hMSC delivery. (A–D) RV section from a control pig injected with physiological saline as a negative control. RV section from pigs with PH 6 days after hMSC injection (E–H), 9 days hMSC injection (I–L) or 24 days post-hMSC injection (M–P). Arrows indicate areas of cell collections/cell islands. Representative images are shown.
Figure 5.
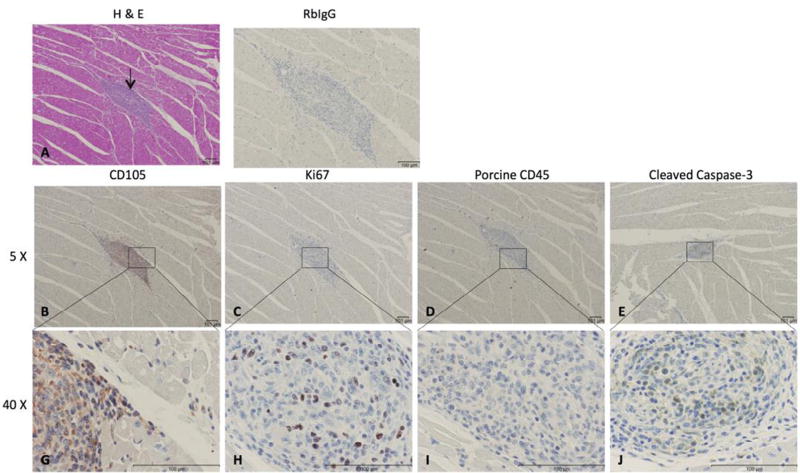
Cell islands in the right ventricle (RV) 24 days after delivery of hMSCs. (A) Hematoxylin and eosin–stained sections of the RV; arrows indicate cell islands 24 days post-hMSC delivery. (B,G) Cell collection of CD105+ mononuclear cells. (C,H) Immunohistochemistry (IHC) with antihuman Ki67 proliferative marker. (D,I) Negative IHC staining for porcine inflammatory cells in area of cell collection using CD45 cell marker. (E,J) IHC using caspase-3 antibody showing positive cytoplasmic localization. Representative images are shown.
Figure 6.
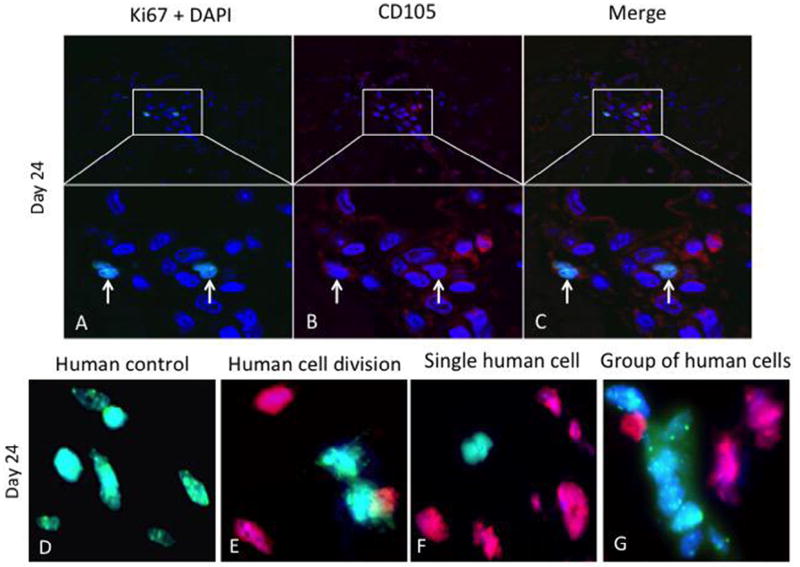
hMSCs are retained and proliferate in the right ventricle (RV). Double immunofluorescence staining of RV sections for (A) 4′-6-diamidino-2-phenylindole (DAPI) for nuclear staining (blue) and Ki67 (green), (B) CD105 (red) from PV-banded pigs 24 days after intracoronary injection of hMSCs. (C) Merged sections show co-localization of CD105 and Ki67, indicating proliferation of hMSCs. Arrows show positive immunostaining. Representative images are shown. FISH was performed on RV sections to confirm the presence of human cells. (E) Human control hybridized with combined green (human) and red (pig) species-specific Cot-1 FISH probe. (F) Two adjacent human (daughter) cells in the final stages of cell division (telophase/cytokinesis). (G) An individual human cell (green) and (H) a group of human cells (green) are seen in the RV. Representative images are shown.
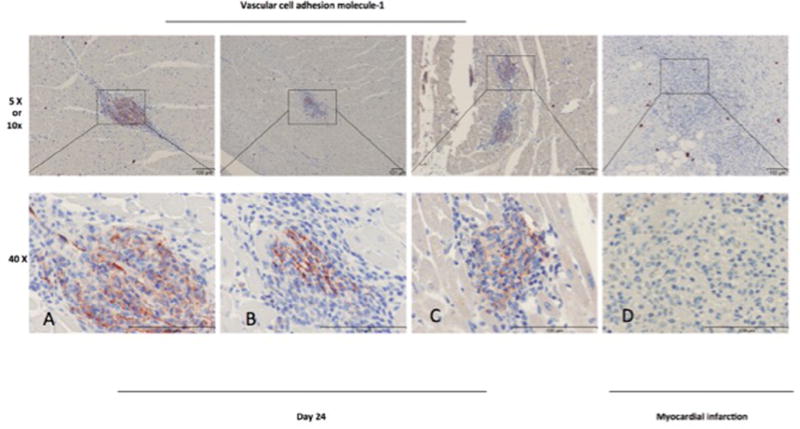
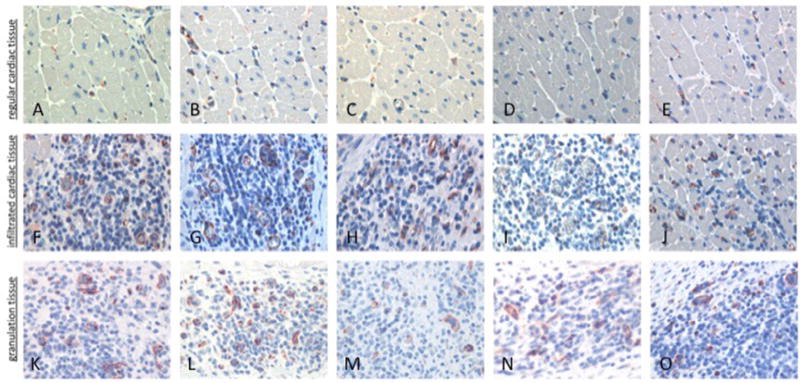
Immunohistochemical staining with the proliferation marker Ki67 showed cell division within the areas of the CD105 collections (18.6% of human CD105+ mononuclear cells were proliferating) (Figure 5B,G); Ki67+ cells were most commonly seen in the 24-day time point samples (Figure 5C,H). We quantified the number of hMSCs relative to the number of surrounding myocyte nuclei revealing a ratio of 0.5:1 in focal areas of CD105+ mononuclear cells (Figure 5B); the count of Ki67+ cells and caspase-3+ cells relative to total hMSCs showed a ratio of 0.3:1 for both markers. Although we observed negligible cross reactivity with the porcine Ki67, this did not affect our findings because we focused on the foci of hMSCs in RV. Proliferation of hMSCs was also revealed by FISH assay, as demonstrated in Figure 6. Similarly, in these same collections of cells, porcine CD45+ cells were present (Figure 5D,I), and caspase-3 immunohistochemical staining is present, indicating scattered ongoing apoptosis (Figure 5E,J). Double immunofluorescent staining with anti-human Ki67 demonstrated that some of the CD105+ cells were proliferating. At day 24, double-labeled cells (CD105 and Ki67) were distributed within the area of hMSCs cluster (Figure 6A – C). FISH demonstrated clusters of human cells as well as individual human cells in the RV tissue. Importantly, there was overlap of hMSCs clusters revealed by FISH assay with CD105+/Ki67+ cells. Human cells in the final stages of cell division (telophase/cytokinesis) were also identified (Figure 6D – G). Porcine CD45+ cells were also observed in all samples but tended to localize to regions adjacent to areas of healing microscopic infarction (Figure 7) and were not typically present in the areas with CD105+ cells. None of the cells in the region of CD105+/Ki67+ cells showed cardiac-specific differentiation, as assessed by desmin and connexin 43 immunohistochemistry (results not shown). Anti– vascular cell adhesion molecule-1 (VCAM-1) staining showed upregulation of VCAM-1 in the clusters where most of the mononuclear cells were located, indicating cytokine production happens during endothelial activation. In contrast, we did not see an increased VCAM-1 in infarcted areas (Figure 8). Evidence for neoangiogenesis within the porcine right ventricle after hMSC injection was determined using an anti-CD31 antibody that showed strong signal in areas of hMSC clusters (Figure 9). The absolute amount of CD31 positive vessels in the three areas revealed a mean of 19.6 vessels in cardiac tissue without hMSCs (Figure 9A – E); 27.2 vessels in areas of cardiac tissue with hMSC infiltration (Figure 9F–J); and 25 vessels in granulation tissue with hMSC infiltration (Figure 9K – O).
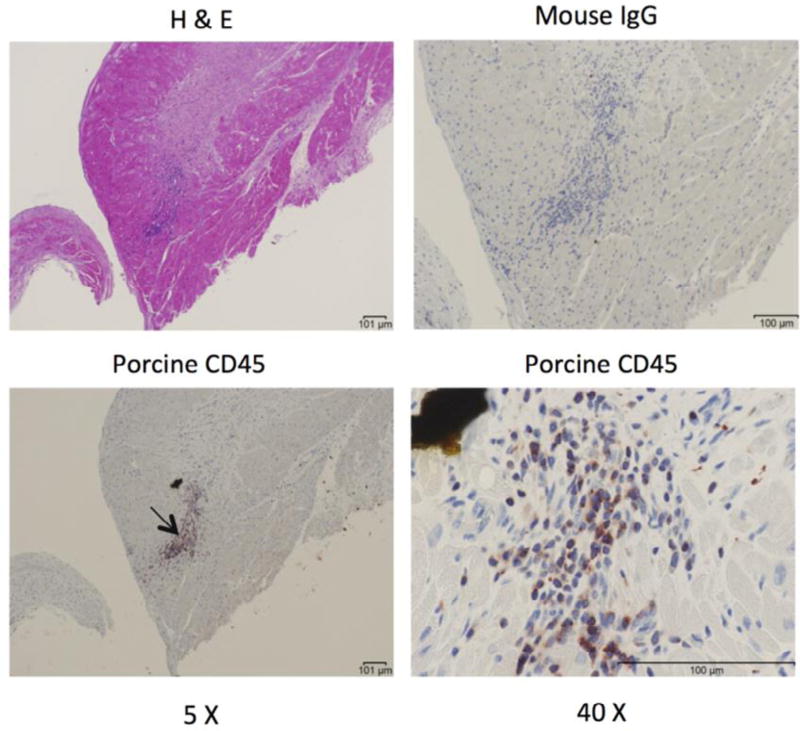
Figure 7.
Inflammatory cells in the right ventricle (RV). Porcine CD45+ inflammatory cells (arrow) were identified adjacent to areas of microscopic infarction by immunohistochemistry of the RV. Representative images are shown. H&E, hematoxylin and eosin.
Figure 8.
VCAM-1: immunohistochemistry staining of paraffin-embedded porcine right ventricle tissue 24 days post-hMSC injection (A–C) and infarcted porcine right ventricle tissue (D).
Figure 9.
CD31: comparison of vessel cross-sections from porcine right ventricle in a PH-diseased pig 9 days after hMSC injection. (A– E) Cardiac tissue without hMSC infiltration; (F–J) cardiac tissue with hMSC infiltration; (K–O) infarction/granulation tissue.
Discussion
In this study, we found evidence of long-term survival of hMSCs in the RV myocardium in a porcine model of pulmonary hypertension after directed intracoronary delivery. The pulmonary hypertension model used here has been described previously [12]; the associated RV remodeling significantly alters RV function with ventricular enlargement, and pressure and volume overload, resulting in RV hypertrophy and (mal) adaptive remodeling. The objective of the present study was to demonstrate that selective coronary infusion of hMSCs using an RCA marginal branch results in persistence and proliferation of cells of human stem cell origin in porcine RV myocardium in a disease model. This was confirmed using the human-specific CD105 monoclonal antibody, a marker for hMSCs [17]. Our results demonstrate multiple foci of CD105+ mono-nuclear cells, with associated proliferation as shown by Ki67 immunostaining. In fact, Ki67+ cells were more frequently observed 24 days after cell delivery than at earlier time points. Several studies conducted with hMSCs have shown inflammatory reactions after cell delivery [18]. We also observed an increase of CD45+ inflammatory porcine cells in the RV myocardium. Because these animals were not immune suppressed, it is unclear whether the inflammation is due to the injection of hMSCs or to ischemic tissue injury, although we favor the latter. Nevertheless, it appears that the proinflammatory response is not a limiting factor for long-term survival of hMSCs in this model, despite the presence of caspase-3+ cells, suggesting ongoing apoptosis. It has previously been demonstrated that human embryonic stem cells can differentiate into cardiomyocytes, although the efficiency of this process is low [19]. In this study, neither desmin nor connexin 43 staining could be identified among the clusters of CD105+ cells. This suggests that our hMSCs are immature and do not exhibit cardiomyocte differentiation.
CD31, or platelet endothelial cell adhesion molecule-1 (PECAM-1), is found in large quantities on the surface of endothelial cells and is less abundant on platelets and leukocytes. It plays a role between adjacent endothelial cells during angiogenesis [20]. We observed an increased expression of CD31, mostly in the area of small vessels with immature endothelium, suggesting active neoangiogenesis (Figure 9).
Our current investigation is the first study to demonstrate that intracoronary injected hMSCs are viable up to 24 days post-injection in an adult large animal model of PH with RV remodeling. We believe that a subset of hMSCs have long-term engraftment capability. Previous studies [21] may have inadvertently not included engraftment capable hMSCs or inhibited their growth by pre-injection procedures. Our cell delivery technique (multiple injections after balloon occlusion) could play an important role in cell passage through vessel walls, and their persistence in the RV, comparing to single-bolus cell-therapy techniques [6,7]. This approach could allow the cells to remain in the cardiac tissue long enough to adhere to endothelium and migrate into the extravascular space. Balloon occlusion could also provide enough transient ischemia to allow for a mild injury that would “condition” the local environment promoting donor cell survival and retention [8,9]. Another consideration is that only a subset of hMSCs retain long-term engraftment capability. Previous studies may have had limited numbers of cells that retained proliferative potential, or the techniques inhibited their growth because of pre- or peri-injection procedures.
Immunosuppressive therapy was not required to maintain cell viability. MSCs are believed to possess the capacity to modulate both innate and adaptive immune responses. Most of the current data available for human MSCs are derived from in vitro studies, whereas in vivo data are primarily available from pre-clinical trials. Interestingly, several in vitro and in vivo studies reported that evasion from immune recognition is intrinsic for MSCs but not for other cells derived from the MSC donors [22–25]. MSCs derived from human placenta have been shown to express MHC-I but not MHC-II [25], which may explain a suppression of the host immunological response to the allograft.
One study performed in a neonatal swine model of PV banding injected 106 hMSCs to the RV myocardium immediately after banding and before the development of disease. These investigators found improvement in RV parameters after 30 days; however, they identified relatively few hMSCs retained in the myocardium at day 28 [26]. It is plausible that our findings differ based on the age (neonatal vs. adult) and disease state (none vs. PH with RV remodeling) of the animals studied; the dose of cells administered and route of injection, and the use of immune suppression, which our study did not use. Other investigators also could not demonstrate long-term survival of hMSCs after intramyocardial injection of cells. The persistence of CD105+ cells in this report may be related to the selective intracoronary route of hMSCs administration leading to a larger number of viable cells being directed to the RV myocardium or to the ongoing remodeling in the RV in this model. Intracoronary cell injection is the most practical and translatable method to deliver hMSCs to the heart tissue [27]. This method is less invasive than other administration routes, and the cells can be selectively directed to the entire myocardium at risk [28,29]. Although durability of injected cells is likely to have a beneficial impact in cardiac regenerative therapies [30,31], therapeutic efficacy may not require long-term persistence. Interestingly, improved left ventricle contractile function in large animal models has been documented in other studies, even though the original stem cells could not be demonstrated at later time points [32,33], suggesting a paracrine effect.
The present study is limited by the small sample size, although our findings were consistent between study subjects. Despite histological characterization, high-specificity/high-sensitivity markers for detecting hMSCs in formalin-fixed, paraffin-embedded pig tissue are not available; we suspect that many of the mononuclear cells in the interstitial clusters of injected animals are donor-derived hMSCs, suggesting that a larger number of cells may be retained. Finally, the current study did not specifically address the therapeutic effect of these cells because we did not include a group that received sham hMSCs and so cannot comment about any potential benefits of the adoptively transferred cells on RV function. Further studies with a larger number of animals and different cohorts of treated versus untreated study subjects with PH may provide additional insights into the fate and distribution of hMSCs. These types of studies will also examine the potential therapeutic benefits of injected hMSCs and may determine the relative importance of retained proliferating hMSCs in this large animal model of established PH with RV remodeling.
Acknowledgments
This work was supported by an unrestricted regenerative medicine research grant provided by United Therapeutics Corporation. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Roza Badr Eslam receives funds from the Austrian Science Fund (FWF), Erwin Schroedinger, grant J3522-B13.
We thank Lori Foley and Ryan Caulfield from the ICE Laboratory, Cardiovascular Medicine Division, and Eugenia Shvartz from the Cardiovascular Medicine Division, Brigham and Women’s Hospital, Boston, Massachusetts; and J. Luis Guerrero from the Surgical Cardiovascular Laboratory, Massachusetts General Hospital, Boston, Massachusetts, for their technical expertise. Additionally, we thank Anita Hawkins from Cytogenetic Core facility at Brigham and Women’s Hospital, Boston, Massachusetts.
Footnotes
Disclosure of interest: The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Zhu Y, Song X, Wang J, Li Y, Yang Y, Yang T, et al. Placental mesenchymal stem cells of fetal origin deposit epigenetic alterations during long-term culture under serum-free condition. Expert Opin Biol Ther. 2015;15(2):163–80. doi: 10.1517/14712598.2015.960837. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Yang Y, Zhang Y, Hao G, Liu T, Wang L, et al. Placental mesenchymal stem cells of fetal and maternal origins demonstrate different therapeutic potentials. Stem Cell Res Ther. 2014;5(2):48. doi: 10.1186/scrt436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;(2):CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Martin-Rendon E, Snowden JA, Watt SM. Stem cell-related therapies for vascular diseases. Transfus Med. 2009;19(4):159–71. doi: 10.1111/j.1365-3148.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 5.Harvey E, Fisher SA, Doree C, Taggart DP, Martin-Rendon E. Current evidence of the efficacy of cell-based therapies in heart failure. Circ J. 2015;79(2):229–36. doi: 10.1253/circj.CJ-14-1415. [DOI] [PubMed] [Google Scholar]
- 6.Ding Z, Burghoff S, Buchheiser A, Kogler G, Schrader J. Survival, integration, and differentiation of unrestricted somatic stem cells in the heart. Cell Transplant. 2013;22(1):15–27. doi: 10.3727/096368912X640466. [DOI] [PubMed] [Google Scholar]
- 7.Gyongyosi M, Hemetsberger R, Wolbank S, Kaun C, Posa A, Marian T, et al. Imaging the migration of therapeutically delivered cardiac stem cells. JACC Cardiovasc Imaging. 2010;3(7):772–5. doi: 10.1016/j.jcmg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Noiseux N, Marquis-Gravel G, Mansour S, Shahzad U, Stewart DJ, Yau TM. The current state of stem cell therapeutics: Canadian approaches in the international context. Can J Cardiol. 2014;30(11):1361–9. doi: 10.1016/j.cjca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Fiarresga A, Mata MF, Cavaco-Goncalves S, Selas M, Simoes IN, Oliveira E, et al. Intracoronary delivery of human mesenchymal/stromal stem cells: insights from coronary microcirculation invasive assessment in a swine model. PLoS ONE. 2015;10:e0139870. doi: 10.1371/journal.pone.0139870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8(3):223–72. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177(7):701–11. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, et al. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol. 2014;307(8):H1204–15. doi: 10.1152/ajpheart.00246.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi M, Erdem SS, Weissleder R, Lichtman AH, McCarthy JR, Libby P. Imaging granzyme B activity assesses immune-mediated myocarditis. Circ Res. 2015;117(6):502–12. doi: 10.1161/CIRCRESAHA.115.306364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breuckmann F, Nassenstein K, Bucher C, Konietzka I, Kaiser G, Konorza T, et al. Systematic analysis of functional and structural changes after coronary microembolization: a cardiac magnetic resonance imaging study. JACC Cardiovasc Imaging. 2009;2(2):121–30. doi: 10.1016/j.jcmg.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Blazquez R, Sanchez-Margallo FM, Crisostomo V, Baez C, Maestre J, Alvarez V, et al. Intrapericardial delivery of cardiosphere-derived cells: an immunological study in a clinically relevant large animal model. PLoS ONE. 2016;11:e0149001. doi: 10.1371/journal.pone.0149001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment—freely available software for cardiovascular image analysis. BMC Med Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 19.Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink RJ, Kuijk E, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005;23(6):772–80. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 20.Muller AM, Hermanns MI, Skrzynski C, Nesslinger M, Muller KM, Kirkpatrick CJ. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72(3):221–9. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 21.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27(9):1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 22.Andree H, Nickel P, Nasiadko C, Hammer MH, Schonemann C, Pruss A, et al. Identification of dialysis patients with panel-reactive memory T cells before kidney transplantation using an allogeneic cell bank. J Am Soc Nephrol. 2006;17(2):573–80. doi: 10.1681/ASN.2005030299. [DOI] [PubMed] [Google Scholar]
- 23.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero C, Pérez-Simón JA. Immunomodulatory effect of mesenchymal stem cells. Braz J Med Biol Res. 2010;43:425–30. doi: 10.1590/s0100-879x2010007500033. [DOI] [PubMed] [Google Scholar]
- 25.Li CD, Zhang WY, Li HL, Jiang XX, Zhang Y, Tang PH, et al. Mesenchymal stem cells derived from human placenta suppress allogeneic umbilical cord blood lymphocyte proliferation. Cell Res. 2005;15(7):539–47. doi: 10.1038/sj.cr.7290323. [DOI] [PubMed] [Google Scholar]
- 26.Wehman B, Sharma S, Pietris N, Mishra R, Siddiqui OT, Bigham G, et al. Mesenchymal stem cells preserve neonatal right ventricular function in a porcine model of pressure overload. Am J Physiol Heart Circ Physiol. 2016;310(11):H1816–26. doi: 10.1152/ajpheart.00955.2015. [DOI] [PubMed] [Google Scholar]
- 27.Keith MC, Tang XL, Tokita Y, Li QH, Ghafghazi S, Moore Iv J, et al. Safety of intracoronary infusion of 20 million C-kit positive human cardiac stem cells in pigs. PLoS ONE. 2015;10(4):e0124227. doi: 10.1371/journal.pone.0124227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128(2):122–31. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58(9):977–86. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Bartunek J, Behfar A, Vanderheyden M, Wijns W, Terzic A. Mesenchymal stem cells and cardiac repair: principles and practice. J Cardiovasc Transl Res. 2008;1(2):115–19. doi: 10.1007/s12265-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 31.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 32.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102(32):11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290–3. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]