Abstract
The developing embryo is a remarkable example of self-organization, where functional units are created in a complex spatio-temporal choreography. Recently, human embryonic stem cells (ESCs) have been used to recapitulate in vitro the self-organization programs that are executed in the embryo in vivo. This represents a unique opportunity to address self-organization in humans that is otherwise not addressable with current technologies. In this essay, we review the recent literature on self-organization of human ESCs, with a particular focus on two examples: formation of embryonic germ layers and neural rosettes. Intriguingly, both activation and elimination of TGFβ signaling can initiate self-organization, albeit with different molecular underpinnings. We discuss the mechanisms underlying the formation of these structures in vitro and explore future challenges in the field.
Introduction
The developing embryo has the extraordinary ability to consistently and reproducibly distribute its cells into the various tissues that give rise to the fully formed organism. This is the result of self-organizing processes where a seemingly homogenous group of cells spontaneously organizes into an overall patterned structure (Figure 1A). From the earliest stages of development, countless examples of self-organization exist in vivo, perhaps best studied in teleosts and amphibians (Movie 1), but also apparent in mammalian systems (Figure 1B–C). However, a mechanistic understanding of these processes is lacking, due to the difficulty of integrating multiple events (such as signaling, morphogenetic forces, and movements) over different time and size scales.
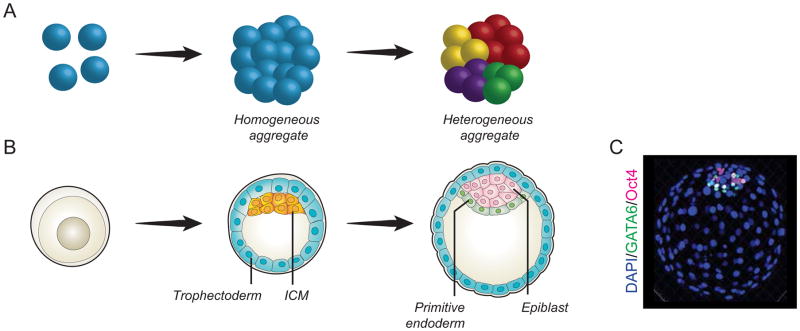
Figure 1.
A) Self-organization is the process by which a population of seemingly homogeneous cells (“homogenous aggregate”) gives rise to cells with heterogeneous properties (“heterogeneous aggregate”). B–C) The developing embryo self-organizes to give rise to cells of different lineages: after a first distinction between trophectoderm (blue) and ICM (orange), cells in the ICM further differentiate into hypoblast (the precursor of primitive endoderm, green) and epiblast (which will give rise to the embryo proper, pink).
The study of self-organization in vivo is challenging and limited to a few model organisms that can be easily imaged and manipulated. In mammals, especially humans, in utero development represents an experimental barrier that has been difficult to overcome. Recently, the use of embryonic stem cells (ESCs) to recapitulate early developmental processes in vitro has started to shed some light on the mechanisms of self-organization. This has highlighted key parameters that control tissue morphogenesis and has provided valuable insights into how cells give rise to higher order architectures.
One of the simplest examples of in vitro self-organization occurs when ESCs aggregate into spherical structures known as embryoid bodies (EBs). EBs can differentiate and give rise to the three germ layers (ectoderm, mesoderm, endoderm), albeit with variability in the localization and relative amounts of each cell type between different EBs. Upon activation of the Wnt pathway, cells in mouse ESC-derived EBs can spontaneously undergo gastrulation-like morphogenetic movements and establish a spatially restricted region that resembles the primitive streak [1], thus mimicking the anterior-posterior (A–P) organization of the gastrulating embryo. In addition, small clumps of ESCs embedded in matrigel can grow into spheres that polarize and cavitate in the center, mirroring the cavitation in the peri-implantation embryo in both mouse [2], and human ESCs (Etoc, et al. unpublished).
More complex developmental processes have also been reproduced in vitro, further confirming the surprising self-organization abilities of ESCs and pluripotent stem cells (PSCs). For example, cell aggregates that have differentiated to retinal epithelium can evaginate to form optic vesicles and subsequently invaginate to give rise to optic cups thus generating highly ordered structures similar to those observed in vivo [3, 4]. More recently, the self-organizing properties of ESCs and iPSCs have also been harnessed to generate three-dimensional organ-like structures (so-called “organoids”) [5].
However, despite the plethora of self-organization examples in biological systems, the molecular control of self-organization remains in its infancy. How does a uniform group of cells become an architecturally complex organized structure? Recent studies, including our own, point to a highly dynamic integration of processes such as cell polarity, signal sensing, and selective competence of cells to integrate and respond to signals. In this review, we use two examples, the formation of germ layers on micropatterns and the generation of neural rosettes, to discuss architectural and molecular aspects of self-organization. We further explore the limitations of current models and touch upon challenges and future directions at the intersection of stem cell biology, bioengineering, and physics.
Self-organization of human embryonic germ layers
A remarkable example of self-organization within the mammalian embryo occurs at the onset of gastrulation, when each pluripotent epiblast cell is allocated to one of the three germ layers: ectoderm, mesoderm, and endoderm. This process is tightly coordinated in time and space and begins with the formation of a transient structure, the primitive streak, at the posterior end of the embryo [6, 7]. As cells ingress through the primitive streak, they acquire a mesendodermal fate. Cells that do not go through the streak become ectoderm (Figure 2A). A correct spatial patterning of the germ layers is critical for the formation of the organism’s body plan. Early BMP4 signals deriving from the extra-embryonic tissue set up a cascade of signaling in the epiblast via activation of Wnt, which induces Nodal that in turn maintains BMP4 signals. Integration of signaling activities and cell movement ultimately leads to gastrulation [6].
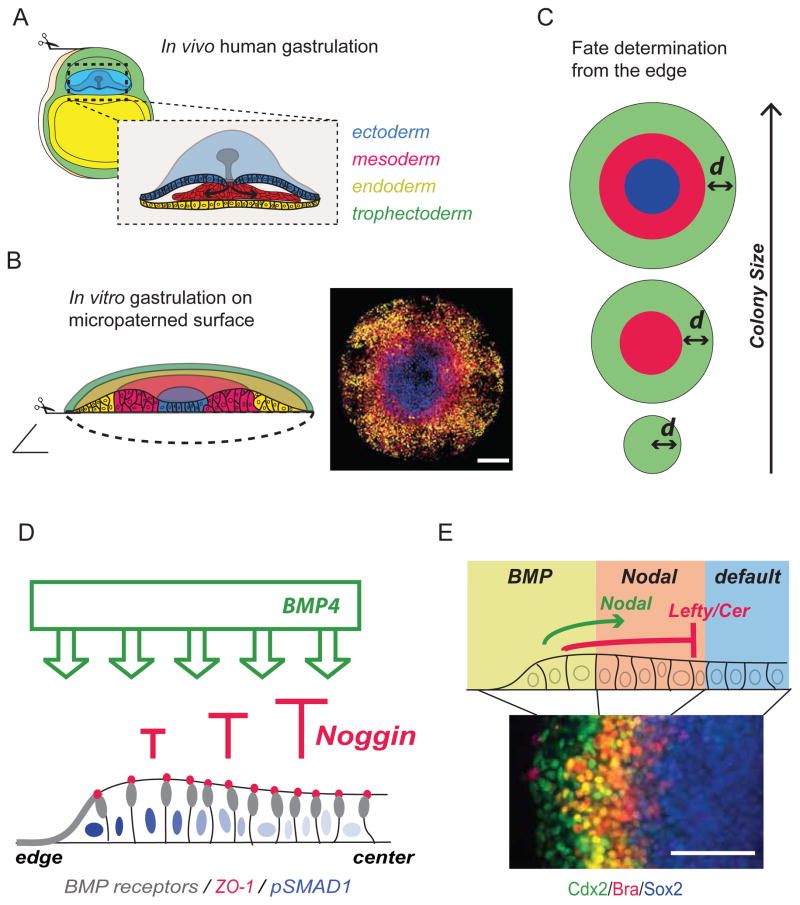
Figure 2.
Self-organization of embryonic germ layers. A) Schematic of a gastrulating human embryo (~day post fertilization 15) showing the epiblast (light blue), primitive endoderm (yellow), and trophectoderm (green). Inset: Higher magnification representation of the epiblast. Gastrulation starts by formation of the primitive streak (gray). Cells ingress through the streak to form definitive endoderm (yellow) and mesoderm (red). Cells that do not go through the streak become ectoderm (dark blue). B) In vitro gastrulation on micropatterned surfaces. Human ESC are plated on 1mm circular substrates. 48 hrs after BMP4 presentation, cell fates are radially organized (from center to edge) into ectoderm (blue), mesoderm (red), endoderm (yellow), and trophectoderm (green). A schematic representation of fate organization is shown on the left, while a picture of differentiating cells expressing markers of the three germ layers is shown on the right. Scale bar 200 um. C) Schematic of micropatterned colonies of different sizes: from top to bottom, 1000, 500 and 200 μm diameter. Green: trophectoderm, red: mesendoderm, blue: ectoderm. As the colony size decreases, only the outer fate is induced via an edge-sensing mechanism. D) Interplay between BMP4 and Noggin in micropatterned colonies. BMP4 is applied homogeneously. BMP receptors (gray) are apically localized in cells at the edge and laterally localized in cells in the center of the colony, below adherens junction (labeled by ZO-1, red). Noggin is expressed in response to BMP4 and contributes to inhibition of BMP4 signal at the center of the colony (red arrows). As a result, BMP4 signaling as detected by pSMAD1 intensity, is higher at the edge than at the center (blue nuclei). E) The domain of maximal BMP4 signaling will give rise to trophectoderm. These cells produce Nodal and its inhibitors that diffuse inward toward the center of the colony. The cells that integrate both Nodal and BMP4 will generate mesendoderm, while the cells that do not respond to either BMP4 or Nodal will give rise to ectoderm. Scale bar 100 um.
We have recently discovered a surprising and unexpected self-organization ability of human ESCs cultured in micropatterns of circular geometry, and presented with a single BMP4 signal (human gastrulation in a dish, Figure 2B) [8]. 48 hours after BMP4 application, pluripotent cells differentiate in radially symmetrical patterns of ectoderm, mesoderm, endoderm, and extra embryonic tissue from the center to the edge respectively (Figure 2B). Moreover, mesendodermal cells undergo epithelial-mesenchymal transition (EMT), closely paralleling the organization of the primitive streak [8].
At least two theoretical concepts can explain the observed spatial patterning. First, a “reaction-diffusion” model based on signaling suggests that spatial patterns are formed by following a Turing-like mechanism [9]. According to this model, patterns arise by extrinsic signals as the result of discrete morphogens that induce, directly or indirectly, their own inhibitors, which diffuse faster than the morphogens themselves. This generates spatial segregation of independent fates [10], and has been linked to several developmental contexts including germ-layer formation in zebrafish [11]. In support of this hypothesis, knockdown of pairs of BMP or Nodal inhibitors has been shown to abolish spatial patterning [8]. We recently proposed a second concept, “edge-sensing”. According to this model, cell fate decisions are based on changes in the intrinsic response of cells as a function of their distance from the edge, relying on forces and architecture rather than diffusion of ligands and inhibitors. This is supported by the observation that reducing the colony size leads only to induction of the outer fate (Figure 2C) [8].
We found that human ESCs use a combination of both mechanisms (Etoc et al, unpublished). Edge sensing is created within a colony by TGFβ receptor subcellular re-localization. As pluripotent cells proliferate and increase density, cells at the center of the colony gradually re-localize their receptors from the apical to the lateral membrane, immediately below tight junctions, thus losing their capacity to respond to apically presented BMP4 (Figure 2D) [12]. Reaction-diffusion, instead, is created by BMP4-mediated induction of inhibitors such as Noggin (Etoc et al., unpublished) that progressively reduce the effective BMP4 concentration sensed by the cells (Figure 2D). As a result of these two dynamic effects – receptor re-localization and secretion of BMP4 inhibitors – BMP4 signaling is abolished at the center and only cells at the outer edge of the colony respond to BMP4 and acquire a trophectodermal fate (Figure 2E).
At the same time, Nodal and its inhibitors are produced by the peripheral domain at the edge of the colony in response to BMP4. From the edge, Nodal signaling spreads inward, toward the center (Etoc et al., unpublished). Because the domain of Nodal signaling is more extended than that of BMP4 response, some cells only integrate Nodal signaling but no BMP4 (Figure 2E). These cells become mesendodermal precursors and express Brachyury (mesoderm) or Sox17 (endoderm) between the center and the outer edge. Finally, the cells at the center of the colony that do not receive either BMP4 or Nodal acquire ectodermal fate following the default mechanism (Figure 2E) [13]. While asymmetry in response to TGFβ ligands in micropatterns is controlled by edge-sensing and reaction-diffusion, its relevance to the in vivo situation remains to be tested. However, this represents a novel and unexpected mechanism for TGFβ signaling, as well as an exciting model for patterning of the gastrulating embryo.
Self-organization of neural rosettes
Following gastrulation, the ectoderm develops into, among other tissues, a layer of polarized cells that form the neuroepithelium and will give rise to the central nervous system (CNS). The nascent neuroepithelium is subdivided into regional identities based on its position in the embryonic A–P axis, as well as in the medio-lateral plane, which will become the dorso-ventral (D–V) axis. However, all regions share fundamental epithelial characteristics such as apico-basal polarity, pseudo-stratification, tight and adherens junctions, apical mitosis, and basal lamina. The neural tube arises from the folding of the neuroepithelium along the D–V axis. Following folding, the apical surface of the neuroepithelium lines the inner side of the neural tube, while the basal surface, lined by a basement membrane, faces the outside (Figure 3A). After neural tube closure, the neuroepithelial cells will elongate to form radial glial (RG) cells (Figure 3B), the master progenitors of the CNS, while the neural tube will form a fluid-filled cavity, the ventricles. RG cells retain their epithelial characteristics throughout embryonic life; hence, the initial transition from non-polarized cells to polarized epithelium is a requirement for the generation of every CNS neural cell type in all vertebrates.
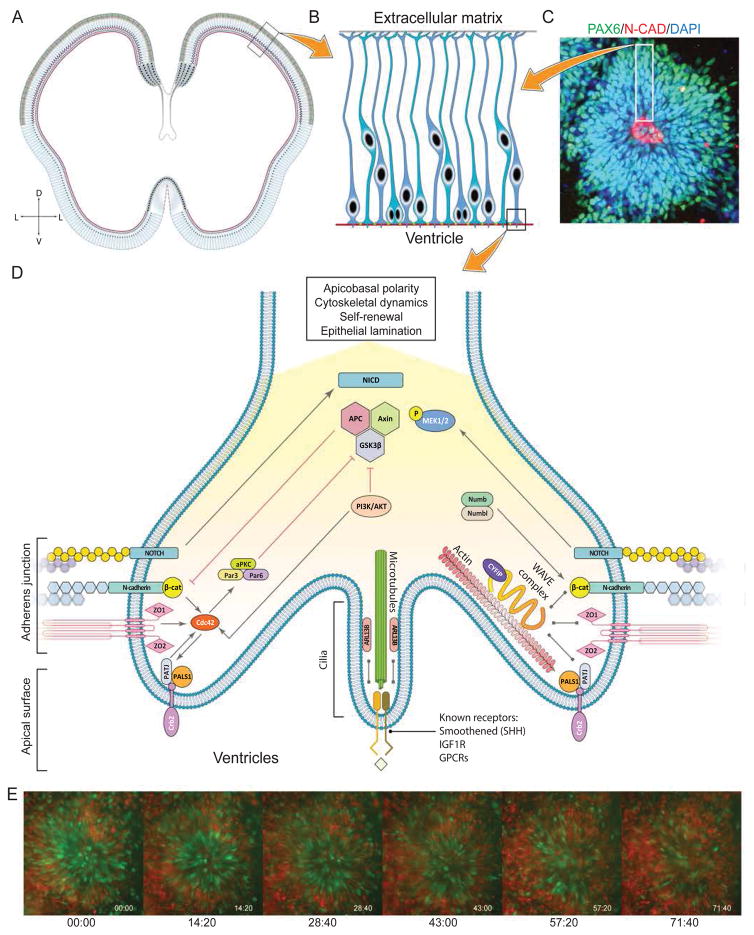
Figure 3.
Self-organization of neural rosettes. A) Schematic of the cortical neuroepithelium in a post-conception week 5 human fetus. The neuroepithelium is comprised of radial glial cells. B) Higher magnification view of the boxed area in A representing a schematic of radial glial cells lining the ventricle. The apical surface is closest to the ventricle while the basal surface is away from it and in contact with the extracellular matrix. C) Rosette structure at day 22 after neural induction in human ESCs. The neuroepithelium is identified by expression of PAX6 (green), while the central lumen structure (corresponding to the ventricles in vivo) is lined by N-Cadherin (red), a component of adherens junction. This organization resembles the in vivo situation. D) Schematic of radial glia end feet and high magnification view of the boxed region in B. The various pathways involved in establishment and maintenance of apico-basal polarity are displayed, as reviewed in Table 1. E) Human ESC derived neuroepithelium dynamically self-organizes into proliferative and non-proliferative zones. The pictures are still frames from Movie 2 and depict a human ESC line engineered with FUCCI markers. Green cells in this montage represent actively proliferating radial glia, while the red cells are mostly post-mitotic neurons. Initially green cells are spread across the entire rosette. Over the course of 48 hours, radial glia come to lie next to the rosette lumen forming a proliferative zone resembling the “ventricular zone” of the embryonic cortex. Conversely, the post-mitotic neurons come to lie on the outside of the rosette; this basal part resemble the “cortical plate” of the embryonic cortex.
It has been shown that ESC-derived neural progenitors - the in vitro equivalent of RG cells - can recapitulate the early sequence of events from gastrulation to neural tube closure, either in monolayer or 3D organoid cultures of both mouse and human ESCs [5, 14–16]. Folding of the neuroepithelium in vitro gives rise to structures called neural rosettes (Figure 3C). Neural rosettes are also found in vivo in childhood tumors of the neuroectoderm as well as teratomas, suggesting that they are a fundamental property of primitive neuroepithelium regardless of the development or disease context. Neural rosettes can also be derived in vitro from primitive neuroepithelium generated by inhibition of TGFβ signaling from human ESCs [13, 17, 18] (Figure 3C).
What are the mechanisms that underlie establishment of apical-basal polarity in the neuroepithelium? While a complete mechanistic picture is lacking, there are clues emerging from studies in mouse embryos and neural rosettes. It appears that cilia are essential for establishing the initial direction of neuroepithelial polarity (apical=ventricles, basal=meninges) as knockout of the ciliary-enriched GTPase Arl13b at an early stage results in inversion of the polarity [19]. In addition, multiple proteins associated with adherens junctions (Crumbs, N-cadherin, β-catenin), polarity proteins (such as aPKC, Cdc42, Par3, Par6) [20], and the WAVE complex, which connects the cell membrane to the cytoskeleton [21], are required for proper neuroepithelial polarity, as demonstrated by a series of mutation, knockdown, or knockout studies (Table 1). Indeed, adherens junction proteins (such as N-cadherin) as well as tight junction proteins (zona occludens 1; ZO1) are found in the center of rosette structures (Figure 3C), the correlate of the neural tube-derived ventricles, as are Crumbs and polarity complex members (Crb2, aPKC, Par3, Par6) and centriolar markers (pericentrin, ASPM) [18, 22, 23]. Unlike early embryonic germ layer specification, described above, the role of geometric confinement in establishment of neuroepithelial properties has not been studied to date. Many of the factors listed above are known to be required for maintenance of polarity as interdependent modules, with all modules being required for maintenance of polarity. While many apical polarity modules have been identified, much less is known about basal polarity modules, and their cross-interactions with apical modules. The further study of human ESC-derived neural rosettes will provide a better understanding of polarity and self-organization principles in the developing brain, potentially uncovering species-specific differences between humans and model systesm.
Table 1.
List of genes affecting neuroepithelial polarity
| Gene/Signaling pathway | Mechanism of action | Expression | Cellular/developmental context | Reference(s) |
|---|---|---|---|---|
| Arl13b | GTPase that maintains ciliary structure and function. | Apical surface | Embryonic mouse cortex | [19] |
| N-cadherin | Component of adherence junctions, promotes neuroepithelial continuity and WNT/β-catenin signaling. | Apical surface | Embryonic mouse cortex | [29–31] |
| Crb2 | Stabilizes the polarity complex (aPKC/Par6/Cdc42) and promotes notch signaling. | Apical surface | Neural rosettes | [22, 23] |
| β-catenin | Component of adherence junctions, promotes radial glial self-renewal. Is activated by N-cadherin via AKT. Also part of WNT pathway and promotes self-renewal of radial glia. | Apical surface | Embryonic mouse cortex | [29, 30, 32, 33] |
| aPKC | Component of polarity complex that is essential for adherens junction integrity and apical polarity. | Apical surface | Embryonic mouse cortex | [34] |
| Cdc42 | A GTPase that is also part of the polarity complex. Required for adherens junction integrity, apical localization of polarity complexes, and radial glial identity. | Apical surface | Embryonic mouse cortex | [35, 36] |
| Hungtingtin | Scaffolding protein shown to be necessary for neuroepithelial polarization in vitro. Interacts with the metalloprotease ADAM10 to regulate N-cadherin cleavage. | Apical surface | Neural rosettes | [37] |
| Numb/Numb-like | Required for adherens junction integrity and polarization by mediating basolateral insertion of cadherins. | Apical surface | Embryonic mouse cortex | [38] |
| APC | Required for polarized extension of radial glial endfeet. Regulates β-catenin in the cortex. Also stabilizes microtubules dynamics in radial glia to promote scaffolding function. | Cell soma Basal surface |
Embryonic mouse cortex | [39] |
| CYFIP1 | Component of adherens junctions and part of the WAVE complex, which links actin skeleton to multiple cell surface receptors. | Apical surface | Embryonic mouse cortex Human iPSC neural rosettes |
[21] |
| GSK3beta | Cytoplasm | Embryonic mouse cortex | [32, 40] | |
| Lgl | Regulator of Notch signaling. Involved in maintenance of apical junctional complex and interacts with aPKC. Required for asymmetrical localization of Numb and regulates proliferation. | Basal surface | Embryonic mouse cortex | [41] |
| FGF | Microlumen | Apical surface | Zebrafish lateral line | [42] |
| Notch | Apical surface | Neural rosettes Embryonic mouse cortex |
[15] |
Challenges and future directions
In order to understand the complex local interactions underlying self-organization, several complementary approaches need to be implemented. As self-organization is a dynamic process, live cell-tracking analysis in space and time is required to shed light on the underlying morphogenetic movements. This can be accomplished, for example, by genome editing to mark selective cell type specific markers (Figure 2B) or cell cycle reporters such as the FUCCI line (Figure 3E and Movie 2) [24]. A major question that needs to be addressed is how different territories are created from seemingly homogenous cells. From classical studies of model organisms, at least two mechanisms have been suggested: 1) the sorting of “salt and pepper” cell populations [25], and 2) the establishment of spatially precise fate allocation mechanisms [26]. Moreover, mathematical models that merge dynamic information at the subcellular, cellular, and tissue level are required for a more complete understanding of these processes. These models will need to integrate large data into comprehensive readouts and provide a quantitative evaluation of their mechanistic basis.
We suggest that the formation of self-organizing structures occurs in three stages: initiation, amplification, and maintenance. First, an initial symmetry-breaking event introduces asymmetry within a seemingly homogeneous structure. In the context of gastrulating micropatterned colonies, this is represented by edge-sensing mechanisms that direct the epithelization of the cells at the colony center. In the case of neuroepithelial rosettes, this may manifest in apical localization of the cilia by a currently unknown mechanism. Second, the early asymmetry is subsequently amplified and propagated to the entire structure. For micropatterned colonies, this is mediated by morphogen/inhibitor combinations that ultimately pattern the tissue from the edge to the center using a reaction-diffusion mechanism. In the case of the neuroepithelium, the initial break in cellular polarity is propagated to the surrounding cells that will line the lumen. Third, the self-organized structure needs to be actively maintained to avoid dissolution of its organization. In micropatterned colonies, hemophilic interactions between differentiated cells of similar type are likely to stabilize differentiated domains. In rosettes, the polarized structures are maintained by adherens junctions, which serve as scaffolds for complexes that mediate proliferative and neurogenic cell divisions (Figure 3E).
This generic classification of self-organization allows to define specific questions pertaining to its genesis: What triggers the initial symmetry-breaking? What is the molecular mechanism underlying this event? Is the exogenous presentation of these factors sufficient for symmetry breaking? What are the feedbacks mechanism(s) that reinforce the initial cue? How are mechanical forces and signaling integrated to generate self-organizing units? What are the mechanisms of dynamic stabilization of self-organized structures that ultimately give rise to functional organs during embryogenesis? And finally, how are self-organizing principles integrated across germ layers to generate the overall embryonic structures? Recent studies using in vitro models and in vivo genetic approaches are beginning to provide some of the answers to these questions [4, 27]. The dynamic nature of embryogenesis involves ever-changing environments of compressive and geometric forces, which likely contribute to self-organization as well. While modeling these events is currently challenging, the cellular and molecular mechanisms underlying these forces will pave the way for a more developmentally relevant framework of understanding of self-organization.
Finally, despite the progress in the in vitro generation of germ layer and neural rosette self-organized structures described above, the establishment of the embryological polarity (ie A–P and D–V axis) has yet to be accomplished in vitro. The development of novel tools such as microfluidics [28] and optogenetics (Etoc et al., unpublished) will allow the asymmetric presentation of active components with precise spatio-temporal coordinates. Ultimately, this understanding will impact the basic knowledge of embryonic development and will inevitably provide the basis for a rational development of clinical applications.
Supplementary Material
Self-organization of the developing Xenopus embryo.
Dynamic self-organization of human neural rosettes.
References
- 1.ten Berge D, et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell. 2008;3(5):508–18. doi: 10.1016/j.stem.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedzhov I, Zernicka-Goetz M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell. 2014;156(5):1032–44. doi: 10.1016/j.cell.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakano T, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10(6):771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 6.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10(2):91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 7.Nowotschin S, Hadjantonakis AK. Cellular dynamics in the early mouse embryo: from axis formation to gastrulation. Curr Opin Genet Dev. 2010;20(4):420–7. doi: 10.1016/j.gde.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warmflash A, et al. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014;11(8):847–54. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1952;237(641):37–72. [Google Scholar]
- 10.Muller P, et al. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336(6082):721–4. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubrulle J, et al. Response to Nodal morphogen gradient is determined by the kinetics of target gene induction. Elife. 2015;4 doi: 10.7554/eLife.05042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nallet-Staub F, et al. Cell density sensing alters TGF-beta signaling in a cell-type-specific manner, independent from Hippo pathway activation. Dev Cell. 2015;32(5):640–51. doi: 10.1016/j.devcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77(2):273–81. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 14.Elkabetz Y, et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22(2):152–65. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Main H, et al. Notch signaling maintains neural rosette polarity. PLoS One. 2013;8(5):e62959. doi: 10.1371/journal.pone.0062959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–30. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, et al. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15(3):477–86. S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higginbotham H, et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16(8):1000–7. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Famulski JK, Solecki DJ. New spin on an old transition: epithelial parallels in neuronal adhesion control. Trends Neurosci. 2013;36(3):163–73. doi: 10.1016/j.tins.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon KJ, et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell. 2014;15(1):79–91. doi: 10.1016/j.stem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boroviak T, Rashbass P. The apical polarity determinant Crumbs 2 is a novel regulator of ESC-derived neural progenitors. Stem Cells. 2011;29(2):193–205. doi: 10.1002/stem.567. [DOI] [PubMed] [Google Scholar]
- 23.Ohata S, et al. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 2011;69(2):215–30. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Sakaue-Sawano A, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132(3):487–98. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173(4):395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- 26.Cohen M, Briscoe J, Blassberg R. Morphogen interpretation: the transcriptional logic of neural tube patterning. Curr Opin Genet Dev. 2013;23(4):423–8. doi: 10.1016/j.gde.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Muguruma K, et al. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10(4):537–50. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Sorre B, et al. Encoding of temporal signals by the TGF-beta pathway and implications for embryonic patterning. Dev Cell. 2014;30(3):334–42. doi: 10.1016/j.devcel.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, et al. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell. 2010;18(3):472–9. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, et al. AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev. 2013;8:7. doi: 10.1186/1749-8104-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304(1):22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Kim WY, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12(11):1390–7. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutch CA, et al. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS One. 2010;5(8):e12376. doi: 10.1371/journal.pone.0012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai F, et al. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133(9):1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 35.Cappello S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9(9):1099–107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci U S A. 2006;103(44):16520–5. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo Sardo V, et al. An evolutionary recent neuroepithelial cell adhesion function of huntingtin implicates ADAM10-Ncadherin. Nat Neurosci. 2012;15(5):713–21. doi: 10.1038/nn.3080. [DOI] [PubMed] [Google Scholar]
- 38.Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10(7):819–27. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 39.Yokota Y, et al. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61(1):42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan-Smith M, et al. GSK-3 signaling in developing cortical neurons is essential for radial migration and dendritic orientation. Elife. 2014;3:e02663. doi: 10.7554/eLife.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klezovitch O, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18(5):559–71. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durdu S, et al. Luminal signalling links cell communication to tissue architecture during organogenesis. Nature. 2014;515(7525):120–4. doi: 10.1038/nature13852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Self-organization of the developing Xenopus embryo.
Dynamic self-organization of human neural rosettes.