Abstract
Herbal and dietary supplements (HDS) are used increasingly both in the United States and worldwide and HDS induced liver injury in the U.S. has increased proportionally. Current challenges in the diagnosis and management of HDS-induced liver injury were the focus of a 2-day research symposium sponsored by the American Association for the Study of Liver Disease and the National Institutes of Health. HDS-induced liver injury now accounts for 20% of cases of hepatotoxicity in the United States based on research data. The major implicated agents include anabolic steroids, green tea extract, and multi-ingredient nutritional supplements (MINS). Anabolic steroids marketed as bodybuilding supplements typically induce a prolonged cholestatic, but ultimately self-limiting liver injury that has a distinctive serum biochemical as well as histological phenotype. Green tea extract and many other products, in contrast, tend to cause an acute-hepatitis like injury. Currently, however, the majority of cases of HDS-associated liver injury are due to MINS, and the component responsible for the toxicity is usually unknown or can only be suspected. HDS-induced liver injury presents many clinical and research challenges, in diagnosis, identification of the responsible constituents, treatment and prevention. Also important are improvements in regulatory oversight of non-prescription products to guarantee their constituents and insure purity and safety. The confident identification of injurious ingredients within HDS will require strategic alignments among clinicians, chemists, and toxicologists. The ultimate goal should be to prohibit or more closely regulate potentially injurious ingredients and thus promote public safety.
Introduction
Herbal and Dietary Supplements (HDS) are used commonly around the world, either in place of or to supplement conventional (Western) medical therapies. There is little dispute that some HDS have been responsible for causing liver injury. Indeed, the issue of HDS-related hepatotoxicity is a growing concern particularly with the evidence in both the United States and Europe that the use of these products appears to be increasing. (1–4)
The authors have chosen to use the phrase “Herbal and Dietary Supplements”, or HDS, to refer to any supplement that might be implicated in causing liver injury. These products would include herbal or botanical supplements, products such as vitamins, minerals, amino acids and proteins that are used to supplement the diet, as well as performance enhancing supplements that may contain chemically synthesized and illicit anabolic steroids.
Because of the special problems surrounding liver injury from HDS, a clinical research workshop to consider the issues was organized, sponsored jointly by the National Institutes of Health (NIH) and the American Association for the Study of Liver Disease (AASLD). The symposium focused mainly on liver injury from HDS in the US, although speakers from around the world were invited to put the problem into perspective. Topics included current uses of HDS, regulation, current rates and features of liver injury attributable to HDS, mechanisms of injury, problems in identification, purity and standardization of HDS and future directions in diagnosis, prevention, treatment and control of HDS related hepatotoxicity.
HDS and their Use in the United States
As stated, we use the term “HDS” to refer to the broad spectrum of supplements, including vitamins, minerals, dietary elements, food components, natural herbs, herbal preparations and synthetic compounds that are used to supplement the diet and that could induce liver injury. (4) They are generally obtained without prescription and taken without specific medical advice or monitoring. In contrast to conventional drugs, however, the safety and efficacy of HDS are not always well defined.
In the U.S., dietary supplements are regarded as foods rather than as drugs, and are assumed to be safe, unless proven otherwise. The Food and Drug Administration (FDA) regulates dietary supplements under the 1994 Dietary Supplement Health and Education Act (DSHEA). (5) The Act designates that manufacturers are required to submit notification of a new dietary ingredient (NDI) to the FDA for any ingredients introduced after October 15, 1994, providing information on safety prior to marketing the product. These premarketing requirements do not apply to dietary ingredients legally marketed before that date. Unlike the requirement for drugs, however, documentation of efficacy need not be reported. Manufacturers are prohibited from making medical claims for efficacy in treating diseases or conditions, such as hypertension or hyperlipidemia. They are, however, able to make non-specific claims of function, such as enhancing energy, wellness, liver health, sexual enjoyment, or weight control.
Population surveys indicate that one-third to one-half of the adult U.S. population take dietary supplements. (1–2) Users are more likely to be women, non-Hispanic whites and to be more financially secure than are those who do not use these products. Supplement sales in the United States have increased in recent years from $9.6 billion in 1994 to $36.7 billion in 2014. (6,7) The most common products taken are vitamins and minerals.
Reliable and readily accessible information on most products is provided by the National Institutes of Health (NIH) websites maintained by the Office of Dietary Supplements (ODS) at www.ods.nih and the National Center for Complementary and Integrative Health (NCCIH) at www.NCCIH.factsheets. A descriptive categorization of HDS used by the U.S. Drug Induced Liver Injury Network (DILIN) is shown in Table 1.
Table 1.
The Drug Induced Liver Injury Network Categorization for HDS
| Category | Examples |
|---|---|
| Vitamins | Vitamin C, Niacin, Folate |
| Minerals and Elements | Iron, Calcium, Potassium |
| Named Botanical or Herbal products | Green Tea Extract, Ginseng, Black Cohosh, “Chinese herbs” |
| Multi-ingredient Nutritional Supplements | Products with mixtures of ingredients such as vitamins, minerals, amino acids, proteins and botanical extracts having proprietary names such as Hydroxycut and Airborne |
| Anabolic steroids | Body building products containing Anabolic Steroids |
Frequency of Liver Injury from HDS: U.S. and Worldwide
Although there are no population-based estimates for the frequency of liver injury from HDS in the United States, the incidence appears to be increasing. In the prospective study of drug-induced liver injury from the NIH-funded Drug Induced Liver Injury Network (DILIN), HDS accounted for 16% of cases overall. (8) Importantly, however, the proportion increased during the 8 years of the study from 7% in 2004–2005 to 19% by 2010–2012. Since then, the proportion of cases of liver injury attributable to HDS in the DILIN Prospective Study has remained high and is currently (2013–2014) 20% (Figure 1).
Figure 1.
Proportion of DILIN Cases due to HDS
Studies in Europe also show increases in HDS use over the last ten years. In the Spanish Drug-Induced Liver Injury registry, the proportion of cases attributed to HDS was 2% in 2006, representing a trend over a 10 year period (9) and increased to 13% for the period 2010–2013. (33) Interestingly, the proportion of liver injury cases attributed to HDS varies greatly in Asia, reported to be 70% in Singapore (10), 73% in Korea (11) but 18.6% in China (12) and only 2.5% in India (13) despite similar wide-scale use of alternative or traditional medicines in all four countries.
Perhaps the best estimate for the incidence of HDS related liver injury comes from a population-based survey in Iceland where the overall incidence of DILI in 2011–2012 was estimated to be 19 cases per 100,000 persons. In that study, 16% of cases were attributed to HDS, suggesting that the incidence of HDS-related acute liver injury was 3 per 100,000 persons. (14)
HDS Commonly Associated with Liver Injury
One hundred and thirty cases of HDS-related liver injury were reported in the course of the first 8 years of enrollment into the U.S. DILIN Prospective Study. (8) At least 45 were attributed to body building agents, presenting with a phenotype suggestive of injury due to anabolic steroids. Because prescription-strength anabolic steroids used in performance enhancing and bodybuilding supplements are largely synthetic derivatives of testosterone added illegally to products and without prescription, they are perhaps better designated as “agents of abuse” or hormonal compounds rather than HDS.
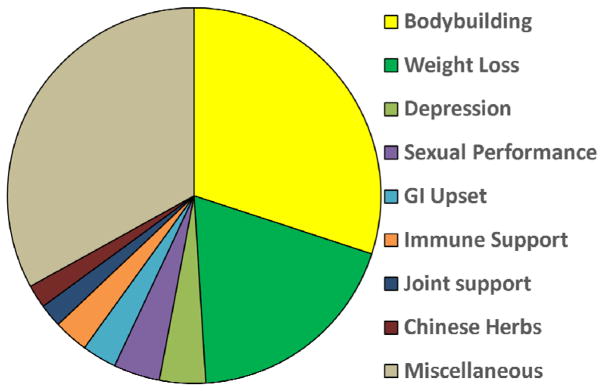
The remaining 85 HDS-related cases enrolled in the DILIN study were attributed to 116 products with labels specifying their ingredients. Importantly, these implicated products rarely contained only one ingredient. (8) The majority of agents implicated were complex mixtures sold under commercial names. Among the 85 non-anabolic steroid associated cases of liver injury, 14 (16%) were attributed to a single or multiple named herbal products (eg. green tea, kratom, black cohosh), 7 (8%) to traditional botanical mixtures (eg. “Chinese herbs”, “Korean herbs”, “Ayurvedic medications”), 6 (7%) to simple vitamins or minerals or dietary supplements (eg. niacin, multivitamins, levocarnitine), and the remaining 58 (68%), to multi-ingredient nutritional supplement(s) (MINS). Among these were products marketed under various companies’ labels, including “Slimquick” (n=6), “Herbalife” (n=4), “Hydroxycut” (n=4), “Move Free” (n=2) and “Airborne” (n=2). Assigning causality to these and other named agents proved daunting because they typically contained multiple ingredients (3 to 20), with only rare descriptions of their concentration and source. In 24 instances, including 15 attributed to a MINS, green tea was listed as a component and was believed to be the causative agent. The types of HDS implicated in the DILIN, organized by their marketed purpose for use, are displayed in Figure 2. (15)
Figure 2.
Distribution of HDS implicated in liver injury in the DILIN
Thus, HDS-induced liver injury encompasses a spectrum of presentations. One entity is anabolic steroid-related jaundice, resulting from the use of illicit synthetic derivatives of androgens that presents as a highly characteristic phenotype. The second is acute liver injury that follows the use of an identifiable specific, single botanical product or traditional herbal medicine, which generally proves no more difficult in assigning causality than is the case for pharmaceutical drug; the most common single agent in this category is green tea (Camellia sinensis). The third is the occurrence of acute liver injury associated with a MINS that usually challenges and often defies identification of the responsible component. A complicating factor is that the implicated product may also be contaminated with a synthetic chemical or with an unknown and toxic botanical.
Green Tea Extract Hepatotoxicity
Green tea is one of the most frequently consumed beverages in the world and is used daily by hundreds of millions of people. Green tea extract (GTE), derived from leaves of the Camellia sinensis plant, is considered to have beneficial medicinal properties. Recently, there are claims that GTEs have weight loss properties, enhancing fat metabolism (“fat burning). The bases for these claims are in vitro studies using concentrated GTEs that demonstrate antioxidant activity, inhibition of lipogenesis, and increase in several metabolic pathways.(16) Studies of green tea in humans have not demonstrated an effect on weight loss, although small studies have suggested a trend. (17) Nevertheless, a large number of commercial products have been developed containing GTE which are advertised as weight loss agents. While prospective clinical trials have not shown clear effects on weight, they also had not shown appreciable adverse events.
With this background, it came as a surprise when GTE was first linked to rare instances of acute hepatitis. (18) Since 2006, there have been more than 50 reports in the medical literature of clinically apparent acute liver injury with jaundice attributed to green tea extracts. (19, 20) In 2008, the United States Pharmacopeia (USP) assessed 34 reports of liver injury linked to GTE. (21) It concluded that a warning label in the quality monograph for GTE was not warranted at the time for several reasons, including a lack of additional adverse reports over time, missing epidemiological data, and paucity of information on the quality of the preparations specifically linked to liver injury. Among cases of HDS-related liver injury in the DILIN Prospective Trial, 97 implicated products were available for testing; 49 contained catechins indicative of GTE, 29 of which were from products that were not labelled as containing GTE. (22) The patients with liver injury attributed to GTE presented with a characteristic acute-hepatitis like illness occurring within 1 to 3 months of starting use of the product. The illness was generally self-limited, but fatal instances have been reported in up to 10% of cases, typically those who presented with acute hepatocellular injury and jaundice.
The cause of the liver injury due to GTE is not known. High doses of the types of catechins present in GTE are hepatotoxic in mice, particularly epigallocatechin gallate [EGCG] which represents 30–50% of the dry weight of GTE. (23) In most reports of GTE hepatotoxicity, however, the human dose of EGCG (generally less than 12 mg/kg daily) did not appear to be excessive or in the range that might have direct toxicity (estimated for humans to be 30–90 mg/kg). These findings suggest that the liver injury from GTE is an idiosyncratic reaction, typical of conventional DILI.
Other popular herbal products have been linked to cases of clinically apparent liver injury, but in many instances, there have been alternative explanations for the liver injury. One important potential contributor is contamination of the HDS product, not just with toxic elements but also with other unknown herbs or the illicit addition of actual conventional Western medications (such as 5-phosphodiesterase inhibitors [sildenafil], nonsteroidal anti-inflammatory agents, statins and corticosteroids). Other implicated herbal products in the DILIN database included black cohosh (Actaea racemosa), kratom (Mitragyna speciosa), valerian (Valeriana officinalis), Eurycoma longfolia, wormwood (Artemisia herba-alba), cat’s claw (Uncaria tomentosa), Ganoderma applantum (artist’s conk), Fo-Ti (Fallopia multiflora), Red Yeast rice (Monascus purpureus), and Garcinia cambogia. However, the role of these specific herbal products in causing the liver injury was often difficult to assign with any assurance, because of the lack of documentation of their chemical presence and purity, the possibility of contamination with other herbal products or mislabeling of the ingredients.
Acute Liver Injury from an OxyElitePro® weight loss product
A dramatic outbreak of severe acute hepatitis was reported to the Hawaii Department of Health in September 2013 in which 7 previously health young men and women developed jaundice with marked serum aminotransferase elevations, all of whom reported taking a product known as OxyELITE Pro® as a weight loss and muscle building agent. (24) Subsequently, Hawaiian investigators reported a total of 36 cases of acute liver injury with jaundice in persons taking this supplement (25,26), and the product was withdrawn by the manufacturer later in the year. The clinical features of the illness consisted of an acute-hepatitis-like illness with initial symptoms of fatigue and anorexia together with dark urine and jaundice. Fever and rash were uncommon. Laboratory tests showed peak mean serum bilirubin values of 9.4 (range: 2.6 to 41.6) mg/dL, mean ALT values of 1740 (428 to 3285) U/L and mean alkaline phosphatase values of 141 (72–277) U/L. Liver biopsies showed an acute hepatitis suggestive of a toxic injury. One patient died and 2 others underwent emergency liver transplantation, a fatality rate of 8%, which is typical of drug-induced acute hepatocellular injury. Other patients recovered, but many had a protracted course and some developed autoimmune hepatitis-like features and thus received corticosteroid therapy.
Additional cases have since been reported from the continental United States, particularly in military personnel, possibly due to the availability of OxyELITE Pro® in military post-exchanges. (27) While initial reports suggested a strong association with Asian-Pacific race, cases from the continental United States have included all racial groups. In the DILIN database covering this same period (May to December 2013), there were 6 cases of liver injury attributed to OxyELITE Pro®, 5 in women, 2 non-Hispanic whites, 1 Hispanic, and 3 Asians, most having taken the specific product for 1 to 5 months. All presented with a hepatocellular pattern of injury, requiring emergency liver transplantation in 2 patients. (28)
The cause of liver injury in consumers of OxyELITE Pro® was suspected to be the addition of aegeline to the commercial product in March 2013. Chemical analyses of implicated lots of OxyELITE Pro® showed the presence of aegeline but no evidence of other toxins or contaminants. Aegeline is the major alkaloid found in the fruit of the bael tree, Aegle marmelos, which has been used for centuries in Ayurvedic medicine to treat digestive complaints. Why aegeline might cause severe liver injury is uncertain, but the added product may have been synthetic and thus contain intermediates of its synthesis or racemic mixtures of the main components.
Anabolic Androgenic Steroid Jaundice
Testosterone and the anabolic androgenic steroids include many FDA-approved drugs that are used for male sex hormone replacement among other medical indications. Because anabolic steroids increase muscle growth and can improve athletic performance, they have attracted illicit use for body building and performance enhancement. (29)
Use of the 17- α-alkylated androgenic steroids has long been associated with a distinctive form of liver injury marked by intense and prolonged jaundice. (30, 31) Many of these products continue to be widely available on the internet. As shown through several case reports, injury typically presents in young or middle aged men interested in body building or performance enhancement who develop jaundice and pruritus 1 to 6 months after starting a supplement regimen that includes an anabolic steroid. (30–32) Laboratory tests demonstrate hyperbilirubinemia typically with minimal elevations in serum enzymes. Serum aminotransferase levels may be high initially, but soon fall into the range of 1 to 3 times the upper limit of normal (ULN) while alkaline phosphatase levels are normal or minimally elevated on presentation but slowly rise during the course of injury. Liver histology shows marked canalicular cholestasis with minimal inflammation and necrosis, a pattern often referred to as “bland cholestasis,” similar to that seen with estrogenic steroids.
Although jaundice can be severe and prolonged, with bilirubin levels reaching 40 to 50 mg/dL and jaundice persisting for 2 to 4 months, death from liver failure is uncommon. Severe cholestasis can be accompanied by renal dysfunction and need for temporary dialysis, but both the renal and liver injury ultimately resolve. (30–33) Chronic liver injury, cirrhosis and the vanishing bile duct syndrome as a result of anabolic steroid liver injury are exceedingly rare if they occur at all. Management of anabolic steroid jaundice involves watchful waiting. Symptomatic therapies for the pruritus (cholestyramine, ursodiol, antihistamines) are usually given but have only modest efficacy. Corticosteroids do not seem to ameliorate the liver injury or speed recovery.
The mechanism of cholestasis caused by anabolic steroids remains unknown. The pattern of canalicular jaundice with scant hepatocytic necrosis suggests selective impairment of canalicular function rather than hepatocellular and cholangiocytic damage or loss. The pattern is similar to that seen in benign recurrent intrahepatic cholestasis (BRIC), caused by mutations in the ATP8B1 gene (formerly FIC1), whose dysfunction leads to impaired bilirubin and bile acid secretion, or in ATPB11 (formerly FIC2), which encodes for the bile salt canalicular transporter. Sequencing of coding exons and intron-exon junctions of these two genes in two patients with anabolic steroid induced jaundice revealed no variants in the ATP8B1 gene and a non-synonymous coding variant in ABCB11 of unknown significance in one patient. (34)
Diagnosis of HDS Induced Liver Injury
The diagnosis of drug-induced liver injury (DILI) relies largely on a compatible history, a drug with a known record of causing liver injury and exclusion of other causes. There are no specific diagnostic tests for DILI and the pattern of injury can mimic virtually any acute or chronic liver disease. In many cases, the possibility of contamination or mis-identification of the botanical constituent remains a concern. The typical clinical presentation of HDS-associated liver injury is an acute hepatitis with marked elevations in serum aminotransferase levels but no or only modest increases in alkaline phosphatase values. Immunoallergic and autoimmune features are not common. The latency to onset is usually 1 to 4 months and the time to resolution typically within 1–2 months. Fatalities can occur but the true case fatality rate is unclear, given that the frequency of supplement use in the population is unknown.
Liver biopsy can rule out chronic liver injury and provide evidence for or against a contribution of other liver diseases. The liver histology of anabolic associated liver injury is quite distinctive in showing a bland cholestasis with canalicular cholestasis and scant hepatocyte necrosis and inflammation, a pattern rarely seen with other forms of liver injury, except for estrogen-associated cholestasis.
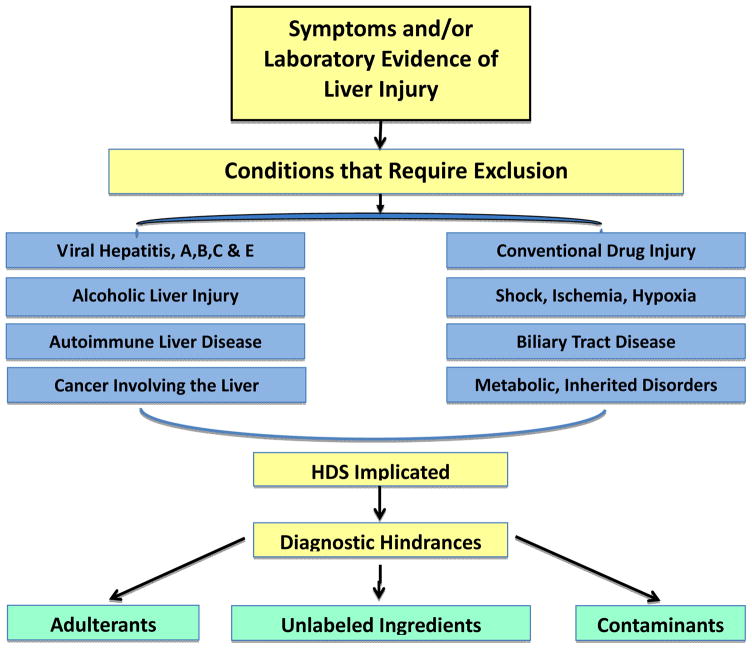
Further information on the hepatotoxicity of HDS products and clinical examples of liver injury from these products are available on the LiverTox website (http:/www.LiverTox.nih.gov) a resource developed by the Liver Disease Research Branch of NIDDK in collaboration with the National Library of Medicine. A proposed diagnostic approach to liver injury due to HDS is shown in Figure 3.
Figure 3.
The approach to diagnosing liver injury from Herbal and Dietary Supplements
Chemical analytical approaches for HDS
Current FDA regulations on good manufacturing practices (GMP) stipulate that manufacturers must provide full verification that “specifications are met for the identity, purity, strength, and composition of the dietary supplements”. (35) However, regulations do not specify which analytical methods are required for verification. The “identity” criterion states that each dietary ingredient must be authenticated by a specific, scientifically valid method, but no guidance is given regarding the sensitivity, specificity or accuracy of the method used. As discussed in this section, scientists have looked to various newer approaches to authenticate products and identify ingredients.
Botanical authentication consists of macroscopic and microscopic examination of the entire plant or plant parts as well as dried and/or processed plants. While usually reliable, these micro/macroscopic techniques are not always ideal, such as when trying to separate closely related genera, or when the sample has been extensively processed and particularly when working with a complex multi-component powdered sample. Thus, the authentication relies upon comparison to verified authentic material and expert opinion. In addition, the GMP require that manufacturers set specifications and test for reasonably anticipated contaminants, such as natural toxins, toxic elements, mycotoxins, pesticides, and pathogenic microorganisms.
Several approaches have been pursued in attempt to “standardize” botanical materials. The customary pharmacognostic approaches typically entail chemical standardization based on the quantification and manipulation of one or more selected marker compound(s) to assure batch to batch consistency of raw material extracts and finshed products. The most commonly used and dependable methods for chemical characterization of DS products are modern analytical separation techniques such as high-performance liquid chromatography (HPLC) or gas chromatography followed by a suitable detection mode such as UV or mass spectrometry (MS). In addition to information about the quantity of selected compounds of interest (marker compounds), these methods can provide an analytical “fingerprint” of botanical components. (36) A limiting factor to the widespread adoption of these types of quality control approaches for botanicals is expense or the lack of relevant pure marker compounds to be used as calibrants. To address this issue, many researchers have turned to utilizing non-targeted spectroscopic based analysis techniques in combination with chemometric analysis for identity testing. (37)
Genetic fingerprinting or profiling using DNA technologies is a fast growing research field in botanical authentication. (38) These methodologies begin with DNA extraction procedures that must be effective in yielding sufficient quantities of high quality DNA as well as being reproducible, economical and flexible enough to be compatible with high-throughput analysis. DNA authentication is most frequently confounded by poor quality plant material. DNA extracted from most commercially available, dehydrated powdered plant parts is often degraded and rarely suitable for analysis.
Statistical techniques for the interpretation of phytochemical fingerprinting data include Hierarchical Cluster Analysis (HCA) or Principal Component Analysis (PCA). These techniques can be used to compare the total mass spectrum, ultraviolet spectrum, or infrared spectrum of an extract of the authentic target botanical with that of an unknown material. HCA and PCA can also be used to compare chromatographic “fingerprints” of authentic materials with those of unknown materials. (39) One of the advantages of this type of statistical approach is that it utilizes pattern recognition parameters to evaluate peaks/components within the data clusters in order to see if the “test” sample correlates to the population of authenticated samples.
Toxicological analysis for HDS
Accurate identification of the chemical composition of HDS is a key step in determining ingredients responsible for injury, but it is only the beginning; a process must follow to determine which ingredient(s) has hepatotoxic potential and is responsible for injury. Conventional toxicological analysis requires in vitro and in vivo testing of a component for cellular and organ toxicity. Single compounds are well suited for this approach, as occurs in the conventional paradigm of pharmaceutical drug development. For HDS, however, the complexity of components and mixtures challenges the interrogation of a toxicological effect. That is to say, if a product induced cellular or organ toxicity, it would be incumbent upon the evaluator to determine which ingredient(s) was responsible and at what concentration. Thus, toxicological analysis of HDS must be predicated upon valid methods to tease apart the products into their component parts, so they can be interrogated, either alone or in a biologically relevant approach.
Liver Injury from HDS: Agenda for the Future
Research issues
The issues raised by HDS related liver injury pose several important research challenges that, when addressed, would help considerably in the understanding, control and prevention of this evolving problem (Table 2). The mechanisms by which HDS cause liver injury are unclear and require exploration. Identifying the chemical composition of HDS is a key step in determining the responsible ingredients, but this needs to be followed by studies of the toxic properties of the specific compounds using cell biology, cell cultures, and animal models. Prediction of toxicity from animal models is not always reliable, particularly for idiosyncratic liver injury, which is often immunologic but may have metabolic idiosyncrasy as well. HDS-associated liver injuries demonstrate some features of idiosyncrasy although marked immunoallergic and autoimmune features are uncommon.
Table 2.
Future challenges with research and regulation of HDS
| 1 | Analysis to verify ingredients and to identify pharmaceutical adulterants and chemical/botanical contaminants |
| 2 | Toxicological evaluation for suspected injurious ingredients and interactions with prescription drugs |
| 3 | Standardization of nomenclature and classification across countries, and causality assessment process for HDS associated injury |
| 4 | Enhance product monitoring and safety, as well as public awareness to the risks of injury from HDS |
| 5 | Improve spontaneous reporting of adverse events |
One example of a promising tool for testing HDS and purported active constituents is high throughput screening using in vitro assays, such as those included in the Tox21 Program. Tox21 is an initiative involving a partnership of the National Center for Advancing Translational Sciences (NCATS, NIH), National Institute of Environmental Health Sciences (NIEHS, NIH), the Environmental Protection Agency (EPA) and the FDA. (40) The goals of Tox21 are to identify mechanisms of compound-induced biological activity and toxicity and develop predictive models for both. The Tox21 Program has screened thousands of single chemicals, as well as some mixtures. In addition to the Tox21 high throughput screening platform, in vitro liver models for prediction of human drug metabolism and cell injury are being developed. The National Toxicology Program is currently applying cell-based models to the evaluation of biological and toxic responses of botanical products and multi-ingredient herbal supplement mixtures. Clinical research progress in HDS hepatotoxicity is also an important part of a future agenda. Most clinical research has been limited to accrual of case reports and analysis of commonality of the course and outcome of injury from related and unrelated HDS products. A major need is a standardized method of causality assessment in cases of liver injury in which HDS products are implicated that is reliable, reproducible and can be used by both the research and clinical community. The clinical instruments developed for assessing hepatotoxicity of medications do not perform well for HDS products and have inherent shortcomings that prevent their reliable use. (4) Compilation of well described cases of HDS liver injury is also important for collection of serum, tissue and DNA samples to allow assessment of immunologic, cell biologic and genetic factors that predict or contribute to the injury. Finally, since almost 20% of prescription drug users consume HDS concurrently (41), the concern for herb-drug interactions is one that must concern clinicians. This topic has been reviewed extensively elsewhere. (42)
Regulatory Issues
Current regulations of dietary supplements in the U.S. have been criticized as being inadequate and not completely rational. (43) The FDA has special and varied challenges when dealing with supplements. For instance, the FDA does not require manufacturers to register their products with the agency, so it has limited information on the number, types, and ingredients of products in the marketplace. Product labels may not provide a full disclosure of the ingredients, their concentrations, purity and source. There are also products that change in composition without appropriate notification of the FDA. When DSHEA was first published in 1994, there were an estimated 4000 supplements available in the United States. (43) Currently, there are more than 80,000. (44)
The FDA is also limited in its ability to monitor and identify adverse events from dietary supplements. The usual means of capturing adverse event information is through the FDAs Adverse Event Reporting System (AERS) which has accumulated a large database of spontaneous reports of adverse events from prescription drugs and products. (45,46) The difficulty is that consumers and others may be under reporting adverse events to the FDA. The “Dietary Supplement and Nonprescription Drug Consumer Protection Act” was passed in 2007 and requires DS and OTC drug manufacturers or distributors to report serious adverse events (SAE) to the FDA.
Most consumers of HDS are taking the supplement on their own, without medical direction or monitoring. Adverse events may go undetected and, if detected, are often underreported and, even if reported, are often inadequately documented for reliable assessment of causality, severity and outcome.
Even with adequate reporting or detection of adverse events due to HDS, the FDA is limited in its ability to act on the safety concerns. The FDA employs warning letters to manufacturers and alerts to consumers. Regulatory actions include recalls, which are largely voluntary, refusal of importation of suspicious products at U.S. borders, legal action against dietary supplement firms including product seizures and, most aggressively, banning of the production and distribution of the agent and removal of products from shelves. These more intrusive actions often occur after the fact, once clear toxic reactions have been identified. Examples of FDA actions include banning of ephedrine alkaloids because of multiple cases of sudden death in patients taking products (47); the voluntary withdrawal of OxyELITE Pro because of multiple cases of acute liver failure with its use (24, 26); and warning letters concerning kava kava and black cohosh containing products after reports of severe liver injury. (48) Despite actions affecting some products of a particular brand, other products from the same manufacturer remain available in stores and on the internet.
A major obstacle to better understanding and improving the safety of HDS is the difficulty in determining what is actually in a supplement. Although there are Current Good Manufacturing Practice regulations for supplements, which include some controls over the quality of products, the enigmatic term “proprietary blend” is commonly used. The disclaimer concerning the lack of medical indication is obviated by claims about structure and function, such as benefit for general health, joint health, liver wellness or weight control. Such claims do not require proof in prospective controlled trials, yet lead the consumer to believe that the product has demonstrated efficacy. Finally, of great concern is the presence of undeclared ingredients in HDS, not infrequently with pharmaceutical action or toxic potential. The authors’ proposal for changes to regulation of HDS are listed in Table 3.
Table 3.
Proposed changes to U.S.regulation for HDS
| 1 | Differentiate regulatory requirements for foods, vitamin- and mineral-containing dietary supplements and dietary supplements containing multiple ingredients that are not nutrients |
| 2 | Require FDA registration of HDS with a complete list of ingredients on the label. Proprietary blends, if listed, should list the actual amount of each ingredient in the blend. |
| 3 | List reported adverse events on the label/consumer information factsheet |
| 4 | Prohibit structure function claims without proof of effectiveness |
Liver injury from HDS is a growing problem that poses special challenges in clinical care, clinical and basic research and in regulatory oversight. A heightened awareness of the problem, stimulation of clinical and basic research and new approaches for the monitoring and regulation of supplements to insure their safety to the consumer are important priorities.
Conclusions
Bringing together experts of varied backgrounds through this workshop has made clear that to reduce the apparent rising burden of liver injury due to HDS, clinical, basic, and translational scientists must collaborate on a common agenda for the future. The most important priorities for this collaboration should be to better understand the epidemiological impact of HDS related liver injury, for clinicians to accurately identify those products that cause injury, and for chemists and toxicologists to isolate and test the products’ ingredients for their toxic potential. Ultimately, the findings from the collaborations must be used to inform regulation, thus providing regulatory authorities with information necessary to guide the development of safer products, and the removal of injurious products from the market.
Acknowledgments
The following were presenters at the May 2015 conference from this the content of this review was prepared: Joseph M. Betz, Ph.D., Director, Analytical Methods and Reference Materials Program, Office of Dietary Supplements, NIH; Einar Björnsson, M.D., Ph.D., The National University Hospital of Iceland; Mark Blumenthal, Founder and Executive Director, American Botanical Council, Editor, HerbalGram and HerbClip; Herbert Bonkovsky, M.D., Professor of Medicine and Molecular Medicine and Translational Science, Chief of Hepatology, Wake Forest University School of Medicine; Paul Coates, Ph.D., Director, Office of Dietary Supplements, NIH; Pieter Cohen, M.D., Cambridge Health Alliance, Harvard Medical School; Harshad Devarbhavi, M.D., D.M., Head, Department of Gastroenterology and Hepatology, St. John’s Medical College Hospital, Bangalore; Jamie Dunn, Center for Drug Evaluation and Research, Division of Pharmaceutical Analysis, FDA; Stephen Ferguson, National Toxicology Program, National Institute of Environmental Health Sciences, NIH; James Harnly, Research Leader, Food Composition and Methods Development Lab, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture; D. Craig Hopp, Ph.D., Program Director, National Center for Complementary and Integrative Health, NIH; Neil Kaplowitz, M.D., Division of Gastroenterology and Liver, University of Southern California Keck School of Medicine; David Kleiner, M.D., Ph.D., Laboratory of Pathology, National Cancer Institute, NIH; Ikhlas Khan, Ph.D., D. Litt. (Hon. Causa), Assistant Director, National Center for Natural Products Research (NCNPR), Director, FDA Center of Excellence, Research Professor, Department of Pharmacognosy, School of Pharmacy, University of Mississippi; Chenghai Liu, M.D., Ph.D., Institute of Liver Diseases, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine; M. Isabel Lucena, M.D., Ph.D., Professor of Pharmacology, Clinical Pharmacology Service, Biomedical Research Institute of Málaga, Hospital Universitario Virgen de la Victoria, Malaga University, Spain; Victor Navarro, M.D., Chairman, Division of Hepatology, Einstein Healthcare Network; Stephen Ostroff, M.D., Acting Chief Scientist, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration (FDA); Cynthia Rider, Ph.D., DABT, National Toxicology Program, National Institute of Environmental Health Sciences National Institute of Environmental Health Sciences, NIH; Nandakumara Sarma, R.Ph., Ph.D., Director, Dietary Supplements, United States Pharmacopeia; Leonard Seeff, M.D., Consultant in Hepatology, Einstein Healthcare Network; Andrew Stolz, M.D., Associate Professor of Medicine, Chief Gastrointestinal Service LAC+USC General Hospital; Ethel V. Taylor, D.V.M., M.P.H., Health Studies Branch, National Center for Environmental Health, Centers for Disease Control and Prevention; Lisa Van Arsdale, Senior Analyst, U.S. General Accountability Office; Larry Walker, Ph.D., Director, NCNPR, Professor of Pharmacology, School of Pharmacy, Associate Director for Basic Sciences, University of Mississippi Cancer Institute; Cara Welch, Ph.D., Senior Advisor, Office of Dietary Supplement Programs, Center for Food Safety and Applied Nutrition, FDA.
Abbreviations
- HDS
Herbal and Dietary Supplements
- NIH
National Institutes of Health
- AASLD
American Association for the Study of Liver Disease
- FDA
Food and Drug Administration
- DSHEA
Dietary Supplement Health and Education Act
- NDI
New dietary ingredient
- ODS
Office of Dietary Supplements
- NCCIH
National Center for Complementary and Integrative Health
- DILIN
Drug Induced Liver Injury Network
- GTE
Green tea extract
- ULN
Upper limit of normal
- BRIC
Benign recurrent intrahepatic cholestasis
- DILI
Drug induced liver injury
- GMP
Good manufacturing practices
- HPLC
High performance liquid chromatography
- HCA
Hierarchical cluster analysis
- NCATS
National Center for Advancing Translational Sciences
- EPA
Environmental Protection Agency
- AERS
Adverse Event Reporting System
- CDC
Centers for Disease Control and Prevention
- MINS
Multi-ingredient nutritional supplements
Footnotes
Conflicts of Interest: The authors report no commercial conflicts of interest.
Disclaimer: The opinions expressed are those of the authors; an opportunity for input on the content was given to presenters.
Financial Disclosures: This summary is based upon a research symposium entitled “Liver Injury from Herbal and Dietary Supplements” held May 4–5, 2015 in Bethesda, Maryland which was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) in collaboration with the American Association for the Study of Liver Diseases (AASLD) with support from: the Office of Dietary Supplements (ODS), Office of the Director, NIH; the National Center for Complementary and Integrative Health (NCCIH), NIH; the National Institute of Environmental Health Services (NIEHS), NIH; the U.S. Department of Agriculture (USDA); the Centers for Disease Control and Prevention (CDC) and with a special contribution by The National Center for Natural Products Research, School of Pharmacy, University of Mississippi, MS.
References
- 1.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. National Health Statistics Reports: no 79. Hyattsville, MD: National Center for Health Statistics; 2015. Trends in the use of complementary health approaches among adults: United States, 2002–2012. [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulgoni VL, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: where do Americans get their nutrients? J Nutr. 2011;141:1847–1854. doi: 10.3945/jn.111.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis. 2014;34:172–193. doi: 10.1055/s-0034-1375958. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. [Accessed February 20, 2016];Dietary Supplements. 2016 Jan 21; Available at http://www.fda.gov/Food/DietarySupplements/default.htm.
- 6.Lindstrom A, Ooyen C, Lynch ME, Blumenthal M, Kawa A. Sales of herbal dietary supplements increase by 7.9% in 2013, marking a decade of rising sales. HerbalGram. 2014;103:52–56. [Google Scholar]
- 7.Nutrition Business Journal. Supplement Business Report 2015. New York, NY: Penton; 2015. p. 67. [Google Scholar]
- 8.Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60:1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade RJ, Lucena MI, Fernández MC, Palaez G, Pachkoria K, Garcia-Ruiz E, et al. Spanish Group for the Study of Drug-Induced liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Wai CT, Tan BH, Chan CL, Sutedja DS, Lee YM, Khor C, et al. Drug-induced liver injury at an Asian center: a prospective study. Liver Int. 2007;27:465–474. doi: 10.1111/j.1478-3231.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 11.Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107:1380–1387. doi: 10.1038/ajg.2012.138. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Yang L, Liao Z, He X, Zhou Y, Guo H. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21,789 patients. Eur J Gastroenterol Hepatol. 2013;25:825–829. doi: 10.1097/MEG.0b013e32835f6889. [DOI] [PubMed] [Google Scholar]
- 13.Devarbhavi H, Dierkhising R, Kremers WK, Kremers WK, Sandeep MS, Karanth D, et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–2404. doi: 10.1038/ajg.2010.287. [DOI] [PubMed] [Google Scholar]
- 14.Björnsson E, Bergman OM, Björnsson HK, Kyaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Seeff LB, Bonkovsky HL, Navarro VJ, Wang G. Herbal products and the liver: A review of adverse reactions and mechanisms. Gastroenterology. 2015;148:517–532. doi: 10.1053/j.gastro.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. 2008;27:363–370. doi: 10.1016/j.clnu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Bonkovsky HL. Hepatotoxicity associated with supplements containing Chinese green tea (Camella sinensis) Ann Intern Med. 2006;144:68–71. doi: 10.7326/0003-4819-144-1-200601030-00020. [DOI] [PubMed] [Google Scholar]
- 19.Mazzanti G, Menniti-Ippolito F, Moro P, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–341. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 20.Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol. 2015;89:1175–1191. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 21.Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, et al. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf. 2008;31:469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- 22.Navarro VJ, Bonkovsky HL, Hwang SI, Vega M, Barnhart H, Serrano J. Catechins in dietary supplements and hepatotoxicity. Dig Dis Sci. 2013;58:2682–2690. doi: 10.1007/s10620-013-2687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Notes from the field: acute hepatitis and liver failure following the use of a dietary supplement intended for weight loss or muscle building--May–October 2013. MMWR Morb Mortal Wkly Rep. 2013;62:817–819. [PMC free article] [PubMed] [Google Scholar]
- 25.Roytman MM, Pörzgen P, Lee CL, Huddleston L, Kuo TT, Bryant-Greenwood P, et al. Outbreak of severe hepatitis linked to weight-loss supplement OxyELITE Pro. Am J Gastroenterol. 2014;109:1296–1298. doi: 10.1038/ajg.2014.159. [DOI] [PubMed] [Google Scholar]
- 26.Johnston DI, Chang A, Viray M, Chatham-Stephens K, He H, Taylor E, et al. Hepatotoxicity associated with the dietary supplement OxyELITE Pro™ - Hawaii, 2013. Drug Test Anal. 2015 Nov 2; doi: 10.1002/dta.1894. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley S, Butlin E, Shields W, Lacey B. Experience with OxyELITE pro and acute liver injury in active duty service members. Dig Dis Sci. 2014;59:3117–3121. doi: 10.1007/s10620-014-3221-4. [DOI] [PubMed] [Google Scholar]
- 28.Heidemann LA, Navarro VJ, Ahmad J, Hayashi PH, Stolz A, Kleiner DE, Fontana RJ. Severe acute hepatocellular injury attributed to OxyELITE Pro: A case series. Dig Dis Sci. 2016 May 3; doi: 10.1007/s10620-016-4181-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by US college students: results from four national surveys. Drug Alcohol Depen. 2007;90:243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan PV, Feng ZZ, Gordon SC. Prolonged intrahepatic cholestasis and renal failure secondary to anabolic androgenic steroid-enriched dietary supplements. J Clin Gastroenterol. 2009;43:672–675. doi: 10.1097/MCG.0b013e318188be6d. [DOI] [PubMed] [Google Scholar]
- 31.Kafrouni MI, Anders RA, Verma S. Hepatotoxicity associated with dietary supplements containing anabolic steroids. Clin Gastroenterol Hepatol. 2007;5:809–812. doi: 10.1016/j.cgh.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 32.Shah NL, Zacharias I, Khettry U, Afdhal N, Gordon FD. Methasteron-associated cholestatic liver injury: clinicopathological findings in 5 cases. Clin Gastroenterol Hepatol. 2008;6:255–158. doi: 10.1016/j.cgh.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, Stephens C, Garcia-Cortes M, Garcia-Munoz B, et al. Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther. 2015;41:116–135. doi: 10.1111/apt.13023. [DOI] [PubMed] [Google Scholar]
- 34.El Sherrif Y, Potts JR, Howard MR, Barnardo A, Cairns S, Knisely AS, et al. Hepatotoxicity from anabolic androgenic steroids marketed as dietary supplements: contribution from ATP8B1/ABCB11 mutations? Liver Int. 2013;33:1266–1270. doi: 10.1111/liv.12216. [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration. [Accessed 20FEB2016];Guidance for Industry: Current Good Manufacturing Practice in Manufacturing, Packaging, Labeling, or Holding Operations for Dietary Supplements; Small Entity Compliance Guide. 2014 Dec 30; Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm238182.htm.
- 36.Betz JM, Brown PN, Roman MC. Accuracy, precision, and reliability of chemical measurements in natural products research. Fitoterapia. 2011;82:44–52. doi: 10.1016/j.fitote.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares PK, Scarminio IS. Multivariate chromatographic fingerprint preparation and authentication of plant material from the genus Bauhinia. Phytochem Anal. 2008;19:78–85. doi: 10.1002/pca.1020. [DOI] [PubMed] [Google Scholar]
- 38.Techen N, Parveen I, Pan Z, Khan IA. DNA barcoding of medicinal plant material for identification. Current Opinion in Biotechnology. 2014;25:103–110. doi: 10.1016/j.copbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Zhao YY, Zhang Y, Lin RC, Sun WJ. An expeditious HPLC method to distinguish Aconitum kusnezoffii from related species. Fitoterapia. 2009;80:333–338. doi: 10.1016/j.fitote.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 40.United States Environmental Protection Agency. [Accessed on February 21, 2016];Toxicology testing in the 21st century (Tox21) 2015 Dec 15; Available at www.epa.gov/chemical-research/toxicology-testing-21st-century-tox21.
- 41.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–97. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 42.Alissa EM. Medicinal herbs and therapeutic drugs interactions. Ther Drug Monit. 2014;36:413–22. doi: 10.1097/FTD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 43.Cohen PA. Assessing supplement safety – the FDA’s controversial proposal. N Engl J Med. 2012;366:389–391. doi: 10.1056/NEJMp1113325. [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration. [Accessed February 22, 2016];FDA Initiates New Online Reporting Method for Dietary Supplement Adverse Events to Facilitate Reporting. 2014 Jan 13; Available at http://www.fda.gov/food/newsevents/constituentupdates/ucm381317.htm.
- 45.FDAble.com. [Accessed February 22, 2016]; Available at http://www.fdable.com/basic_query/aers.
- 46.Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci. 2016 Mar 3;17(3) doi: 10.3390/ijms17030331. Pii: E33a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Food and Drug Administration. [Accessed on February 22, 2016];FDA Issues Regulation Prohibiting Sale of Dietary Supplements Containing Ephedrine Alkaloids and Reiterates Its Advice That Consumers Stop Using These Products. 2004 Feb 06; Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108242.htm.
- 48.US Food and Drug Administration. [Accessed on February 22, 2016];Inspections, Compliance, Enforcement, and Criminal Investigations. Available at http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/