Abstract
The causes of excess cardiovascular mortality associated with chronic kidney disease (CKD) have been attributed in part to the CKD-mineral bone disorder syndrome (CKD-MBD), wherein, novel cardiovascular risk factors have been identified. New advances in the causes of the CKD-MBD are discussed in this review. They demonstrate that repair and disease processes in the kidneys release factors to the circulation that cause the systemic complications of CKD. The discovery of WNT inhibitors, especially Dickkopf 1 (Dkk1), produced during renal repair as participating in the pathogenesis of the vascular and skeletal components of the CKD-MBD implied that additional pathogenic factors are critical. This lead to the discovery that activin A is a second renal repair factor circulating in increased levels during CKD. Activin A derives from peritubular myofibroblasts of diseased kidneys, wherein it stimulates fibrosis, and decreases tubular klotho expression. Activin A binds to the type 2 activin A receptor, ActRIIA, which is variably affected by CKD in the vasculature. In diabetic/atherosclerotic aortas, specifically in vascular smooth muscle cells (VSMC), ActRIIA signaling is inhibited and contributes to CKD induced VSMC dedifferentiation, osteogenic transition and neointimal atherosclerotic calcification. In nondiabetic/nonatherosclerotic aortas, CKD increases VSMC ActRIIA signaling, and vascular fibroblast signaling causing the latter to undergo osteogenic transition and stimulate vascular calcification. In both vascular situations, a ligand trap for ActRIIA prevented vascular calcification. In the skeleton, activin A is responsible for CKD stimulation of osteoclastogenesis and bone remodeling increasing bone turnover. These studies demonstrate that circulating renal repair and injury factors are causal of the CKD-MBD and CKD associated cardiovascular disease.
Keywords: CKD-MBD, activin, dickkhopf 1, klotho, FGF23, parathyroid hormone, renal osteodystrophy, vascular calcification
Introduction and Epidemiology of the CKD-MBD
The kidney disease pandemic1 is associated with high mortality rates, in part due to cardiovascular complications 2–5. The kidney disease produced increase in cardiovascular risk extends to type 2 diabetes6, where the presence of mild to moderate kidney disease increases atherosclerotic cardiovascular disease risk by 87% 7. The causes of the increased cardiovascular risk associated with kidney diseases partly reside in the chronic kidney disease – mineral bone disorder (CKD-MBD) syndrome 8. Three novel cardiovascular risk factors (hyperphosphatemia, vascular calcification, and elevated fibroblast growth factor 23 (FGF23) levels) have been discovered within the CKD-MBD 9–11, and their risk factor status confirmed in the general population 12–14. The CKD-MBD begins early in CKD (stage 2) 15–18 consisting of vascular osteoblastic transition/calcification, an osteodystrophy, loss of klotho and increased FGF23 secretion 15, and progress into its causes have been made 18–21. Recent studies demonstrating that factors participating in renal repair and injury and released into the circulation contribute to the pathogenesis of the CKD-MBD will be reviewed here.
Recent Advances in the Pathogenesis of the CKD-MBD
Multiple investigators and we have shown that kidney diseases reactivate developmental programs involved in nephrogenesis during disease stimulated renal repair 22–26. Among the nephrogenic factors reactivated in renal repair, the Wnt (portmanteau of Wingless and Integrated) family (stimulated family members include Wnt4, Wnt7b, and Wnt10) is critical for tubular epithelial reconstitution 25–28. In the control of Wnt function, canonical signaling transcriptionally induces the expression of a family of Wnt inhibitory proteins which are secreted proteins that serve to restrict the distances of Wnt stimulation as autocrine or paracrine factors 29–33. The Wnt inhibitors are circulating factors, and the family includes the Dickkopfs (Dkk). We and others have shown that various forms of kidney disease increase renal expression of Wnt inhibitors including Dkk1, and increase their levels in the circulation 19,23.
Developmentally, Dkk1 is the only critical Wnt inhibitor in the kidney, the other family members have overlapping and redundant functions. Dkk1 functions in limiting Wnt7b stimulation of loop of Henle and collecting duct epithelial cell proliferation driving renal papillary length. Dkk1 deficiency produces excessively long renal papillae 34. In kidney diseases, Dkk1 expression in the kidney and circulating levels are increased early in disease associated with tubular epithelial proliferation and repair 19, then decrease but remain elevated as a transcriptional target of canonical Wnts stimulating fibrosis 19,35–39.
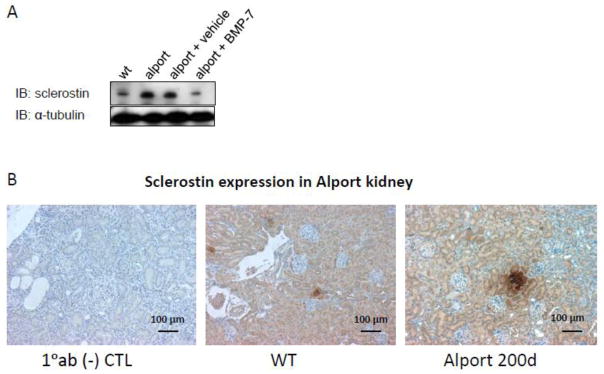
Another Wnt inhibitor whose circulating levels are increased in CKD and considered an important CKD-MBD factor is sclerostin 40–42, a critical regulator of bone mass 43–46. Sclerostin is considered an osteocyte specific protein 47–52. Although message levels are known to be high in the kidney, protein is not thought to be normally expressed 52. Sclerostin protein is expressed in the developing kidney (see IHC at the sclerostin antibody ab194940, abcam website), and in discreet pockets of medullary tubules in normal mouse kidneys (Figure 1). Development of CKD increases kidney sclerostin expression as shown in the Alport kidneys with reduced kidney function (Figure 1), and this may contribute to its increased urinary excretion in CKD 53. It is unclear whether renal sclerostin contributes to the increased circulating levels in CKD, but osteocyte expression is increased early in CKD 20,21, and the skeleton is thought to be the source of increased circulating sclerostin in CKD 41,54–57.
Figure 1.
Renal Expression of Sclerostin. A, Westerns of kidney homogenates. Sclerostin is expressed in 200 day old normal mouse kidneys. Sclerostin expression is increased in 200 day old Col4a5 deficient mice with severe kidney failure (GFR 15% of normal). Treatment of Col4a5 deficient Alport syndrome mice with BMP-7 decreased sclerostin expression. B, Immunohistochemistry of renal sclerostin. Renal cortical sections from kidneys of normal 200 day old C57BL6J mice show patches of tubular sclerostin expression. Tubular sclerostin expression was increased in 200 day old Col4a5 Alport mice.
Neutralization of sclerostin elevated in the circulation of Cy/+ polycystic kidney disease (PKD) rats with CKD failed to inhibit vascular calcification and did not affect cardiac hypertrophy 58. The anti-sclerostin monoclonal antibody (mab) treatment increased trabecular bone volume of CKD rats with low PTH levels but not high PTH levels, but did not improve cortical bone porosity or biomechanical properties of long bones 58.
Neutralization of Dkk1 elevated in the circulation of atherosclerotic diabetic mice with CKD, inhibited CKD induced vascular dedifferentiation, vascular calcification, and renal osteodystrophy 19. The osteodystrophy effect of Dkk1 mab treatment involved stimulation of bone formation and remodeling leading to an increase in trabecular bone volume, and decreased cortical porosity 19. The different results associated with neutralization Dkk1 versus neutralization of sclerostin may be due to differing effects of the two molecules on the frizzled/LRP5/5 receptor complex such that sclerostin has a tissue and context dependent effect not necessarily Wnt inhibitory whereas Dkk1 is always inhibitory, or due to the differing experimental models. Dkk1 is known to be stimulated in diabetes 59, and this could have influenced our results with Dkk1 inhibition.
The vascular effects of Dkk1 inhibition were surprising since Wnt signaling in the vascular smooth muscle is implicated in stimulating osteoblastic transition and vascular calcification 60,61. However, recent studies demonstrate that Dkk1 mediated inhibition of aortic Wnt7b stimulates smad mediated aortic endothelial-mesenchymal transition (EndMT) and vascular calcification 62. EndMT is a developmental physiologic process involved in the development of the cardiac valves, the cardiac septum and the aortic root 63,64, and it may 65 or may not 66 contribute to cardiac fibrosis in various adult disease states. Since EndMT is a process driven by smad transcription factors activated by factors in the transforming growth factor beta (TGFβ) superfamily 67, we investigated whether other factors involved in attempted renal repair during kidney disease derive from the TGFβ superfamily and are increased in the circulation during CKD. Of the TGFβ superfamily members, activin A, a known renal developmental factor and circulating hormone, was the primary candidate 24,68.
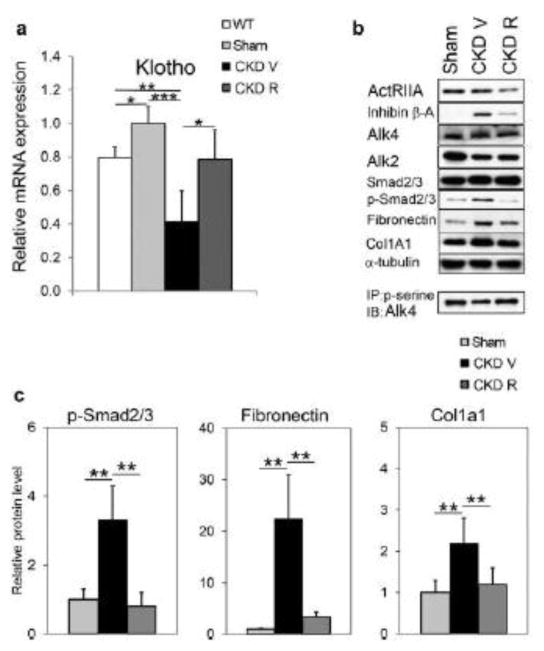
Activin is increased in the circulation by CKD associated with increased expression of activin in the kidney 69. Surprisingly, the activin type 2A receptor (ActRIIA) was reduced by CKD in the aortic vascular smooth muscle and not the endothelium of diabetic/atherosclerotic mice, and a ligand trap for the receptor increased rather than decreased aortic ActRIIA signaling in this model. The ActRIIA ligand trap blocked the stimulation of vascular smooth muscle osteoblastic transition, vascular calcification, and renal fibrosis by CKD 69. In the kidney, the ligand trap inhibited activin signaling, decreased renal Wnt activation and circulating Dkk1 and increased renal klotho expression (Figure 2). As a result a composite vascular effect of indirectly increasing endothelial Wnt signaling through loss of Dkk1 in the circulation, and vascular smooth muscle differentiation through increased p-Smad 2/3 produced loss of osteoblastic transition and decreased atherosclerotic calcification.
Figure 2.
Renal αklotho mRNA and activin A (inhibin β-A homodimer) signaling in renal homogenates. A, Compared to sham operated ldlr−/− high fat fed mice, ldlr−/− high fat fed CKD mice (CKD V) had reduced αklotho expression that was restored by treatment with a ligand trap for the activin receptor type IIA (ActRIIA) (CKD R). B, Inhibin β-A (activin A) expression was increased in homogenates of ldlr−/− high fat fed CKD kidneys and reduced by treatment with the ActRIIA ligand trap. B and C, Homogenates of ldlr−/− high fat fed CKD kidneys had increased levels of p-Samd2/3, the transcription factor activated by ActRIIA signaling. C, Smad2/3 transcriptional targets, fibronectin and Col1a1, were increased by CKD and decreased by treatment with the ActRIIA ligand trap. (Reproduced with permission from Agapova et al., Kid Int 89: 1231–1243, 2016).
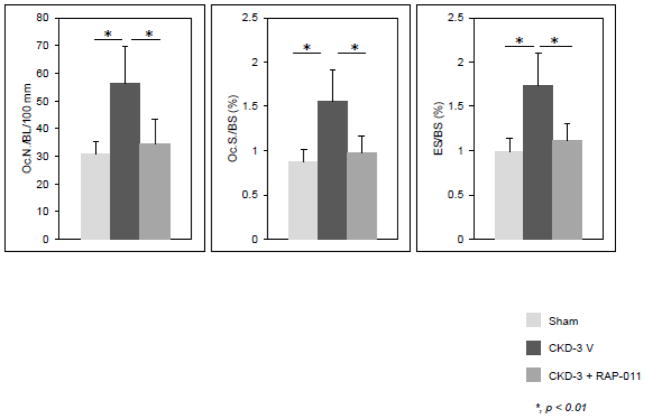
In the skeleton, the ActRIIA ligand trap blocked CKD stimulation of osteoclastogenesis, bone resorption and remodeling (Figure 3) despite not affecting the high PTH levels 70. This suggests that Activin A stimulation of osteoclast p-Smad 2 is required for the effects of CKD. How the role of activin A interacts with the effects of PTH and sclerostin in CKD remains to be determined.
Figure 3.
Osteoclast number, surfaces, and eroded surfaces in trabecular bones of sham operated ldlr−/− high fat fed mice, ldlr−/− high fat fed CKD mice (CKD V), and ldlr−/− high fat fed CKD mice treated with RAP-011, an ActRIIA ligand trap, (CKD R). CKD increased and RAP-011 treatment reversed the increase in osteoclast numbers, surfaces and eroded surfaces. (Reproduced with permission from Sugatani et al., Kid Int 91: 86–95, 2107).
Pathogenic Mechanisms in the Components of the CKD-MBD
vascular dedifferentiation/calcification
There are two forms of vascular calcification stimulated by CKD – intimal and medial calcification. CKD stimulated intimal calcification takes the form of atherosclerotic plaque neointimal calcification produced by osteoblastic transition of cells in the neointima whose origin have been linked to smooth muscle cells and circulating mesenchymal cells 71,72. Likewise, medial calcification has been linked to vascular smooth muscle cells undergoing chondroosseous transition 72–74. Although elevations in plasma DKK1, sclerostin, bone morphogenetic protein-9 (BMP-9)75 and activin have been found in human kidney diseases 69,76 (Williams M and Hruska K, unpublished observations), and linked in preclinical studies to CKD stimulated vascular calcification and vascular osteoblastic transition 19,69,75, these studies are preliminary and need confirmation and characterization.
Loss of Renal Klotho
FGF23 signaling through FGF receptors typically requires the co-receptor function of membrane-bound αklotho. High levels of αklotho expression are restricted to a few tissues and define the primary targets of FGF23 action as the proximal and distal renal tubules, the parathyroid glands and the brain 77,78. Klotho also circulates as a physiologically active hormone after either being cleaved at the cell surface by ADAM-10 and -17 in the renal tubules. Alternative splicing of the klotho gene transcript produces a secreted protein with only one klotho domain of unknown function. Insulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17 79. Cleaved klotho directly regulates calcium and phosphorus excretion in the kidney and participates in systemic mineral homeostasis by regulating 1-alpha hydroxylase activity, PTH and FGF23 secretion 80,81. Klotho expression is significantly reduced by kidney injuries such as acute kidney injury, glomerulonephritis, calcineurin inhibitor use and chronic allograft injury 82. We have shown that the reduction of klotho is in part related to activin and ActRIIA signaling 69. The resulting klotho deficiency limits its regulation of FGF23 production and leaves hyperphosphatemia as the principal regulator of FGF23 secretion in CKD. Furthermore, the loss of membrane-bound klotho expression limits FGF23-stimulated signal transduction through FGF receptor/klotho complexes. One result is the loss of negative feedback to FGF23 secretion and the continual production of FGF23 and secretion by the osteocyte. In late CKD, the very high levels of FGF23 permit anomalous FGF receptor activation independent of Klotho and result in unique FGF23-stimulated pathologies such as cardiac myocyte hypertrophy 83,84. In addition, recent mechanistic studies have directly linked klotho deficiency with cardiovascular disease including vascular calcification, vascular stiffness, and uremic vasculopathy 18,85.
Hyperphosphatemia
As renal injury decreases the number of functioning nephrons, phosphate excretion is maintained by reducing the tubular reabsorption of filtered phosphate in the remaining nephrons under the influence of FGF23 and PTH 86. The effects of FGF23 on phosphate excretion are limited by proximal tubular klotho deficiency in CKD, and PTH becomes a major adaptive mechanism maintaining phosphate homeostasis. In stage 4–5 CKD (GFR < 30% of normal), this adaptation is no longer adequate and hyperphosphatemia develops despite high PTH and FGF23 levels 86.
CKD contributes to hyperphosphatemia and vascular calcification through inhibition of skeletal function. Bone resorption increases phosphate release to the plasma and decreases phosphate deposition resulting in increased serum phosphorus levels 87. Hyperphosphatemia stimulates osteoblastic transition in the vasculature and directly contributes to extraskeletal mineralization through an elevated calcium-phosphorus product 88.
Hyperphosphatemia exerts other important effects in the CKD-MBD axis. In the kidney, hyperphosphatemia suppresses 1-alpha-hydroxylase activity that further contributes to calcitriol deficiency 89. In the parathyroid gland, hyperphosphatemia directly stimulates parathyroid cells independent of calcium and calcitriol levels, producing nodular hyperplasia and increasing PTH secretion 90. In the skeleton, phosphorus stimulates FGF23 secretion from osteocytes 91,92.
Osteodystrophy
With progressive loss of renal function, cancellous bone volume may be increased along with a loss of cortical bone, but this is in part due to deposition of woven immature collagen fibrils instead of lamellar mature fibrils. Thus, bone strength suffers despite an apparent increase in mass detected by dual energy x-ray absorptiometry (DXA) 93. Patients with advanced CKD could have a loss or gain in bone volume depending on overall bone balance. When the bone balance is positive, osteosclerosis may be observed when osteoblasts are active in depositing new bone composed primarily of immature woven collagen. However, this scenario is rare in the 21st century due to improved therapy of secondary hyperparathyroidism. When the bone balance is negative both cortical and cancellous bone loss occurs, resulting in osteopenia or osteoporosis detected by DXA. The prevalence of osteoporosis in CKD patients exceeds that of the general population and is a major public health concern in CKD 94. With high-turnover renal osteodystrophy, and osteitis fibrosa, bone resorption rates are in excess of bone formation and osteopenia progressing to osteoporosis may result 95. With low-turnover renal osteodystrophy, both bone formation and resorption rates may be reduced although resorption is still in relative excess and loss of bone mass occurs 96. Therefore in CKD, osteoporosis may be observed with either high-turnover or low-turnover renal osteodystrophy. The impact of this phenomenon extends far beyond bone health in CKD, as excessive bone resorption rates contribute to hyperphosphatemia with stimulation of heterotopic mineralization including vascular calcification 88. This disrupted systems biology links kidney, skeletal, and parathyroid dysfunction to cardiovascular risk and mortality through the CKD-MBD.
FGF23
FGF23 is the original phosphatonin (hormone regulating phosphate excretion) discovered in studies of autosomal dominant hypophosphatemic rickets and oncogenic osteomalacia 97. FGF23 is produced by osteocytes and osteoblasts, and it represents direct bone-kidney and bone–parathyroid connections in the multiorgan systems biology involved in the CKD-MBD 98. Circulating FGF23 levels rise after mild renal injury and progressively increase several fold during the course of CKD due to increased osteocyte secretion as well as decreased catabolism by the injured kidney. FGF23 levels rise prior to changes in calcium, phosphorus, or PTH levels and are now recognized as one of the earliest detectable biomarkers of the CKD-MBD 16,99.
FGF23 levels have been associated with cardiovascular risk in CKD, and kidney transplant loss and mortality 100,101. In humans and animal models, Faul et al demonstrated that FGF23 is not only a biomarker associated with cardiovascular risk in CKD, but is also a direct pathogenic factor causing left ventricular hypertrophy (LVH) through activation of the calcineurin-NFAT pathway in cardiac myocytes 83,84.
Recently, the pathogenic nature of circulating FGF23 has become more intriguing. Andrukhova et al showed that FGF23 directly regulates the abundance of the thiazide-sensitive sodium-chloride transporter (NCC) in the distal convoluted tubule, leading to increased distal sodium reabsorption, effective circulating volume expansion, hypertension, and cardiac hypertrophy, effects that were abrogated by a thiazide diuretic 102. Interestingly, these FGF23-mediated effects on cardiovascular pathophysiology were augmented in animal models ingesting a low sodium diet (which stimulates aldosterone secretion), raising the possibility of an interaction between FGF23 and the renin-angiotensin-aldosterone axis in CKD-stimulated cardiovascular disease. Furthermore, Humalda et al demonstrated that humans with higher baseline FGF23 levels had a reduced antiproteinuric response to dietary sodium restriction and ACE inhibitor therapy, which has been associated with heighted cardiovascular and end-stage renal disease (ESRD) risk in CKD 103. Andrukhova et al also demonstrated that FGF23 promotes calcium reabsorption through stimulation of the apical calcium entry channel, TRPV5, in the distal tubule 104. Because the calcium entry channel is also regulated by klotho 105, the Andrukhova et al findings 102,104 raise the issue of the mechanism of klotho’s actions. Are they direct through glucuronidase activity and FGF23 independent, or as the FGF23 co-receptor and FGF23 dependent?
Vitamin D deficiency
In early CKD, the physiologic actions of FGF23 secretion from the osteocyte include inhibition of 1-alpha-hydroxylase and stimulation of 24-hydroxylase in proximal renal tubules, thereby decreasing calcitriol production and producing 25-hydroxyvitamin D deficiency 106. As CKD advances, the decrease in functioning nephron mass combined with hyperphosphatemia and increased FGF23 levels results in calcitriol (1,25-hydroxyvitamin D) deficiency as well 107. Calcitriol deficiency decreases intestinal calcium absorption leading to hypocalcemia and diminishes tissue levels of vitamin D receptors, which in the parathyroid gland results in resistance to calcitriol-mediated regulation and stimulation of PTH secretion leading to secondary hyperparathyroidism 108.
Hyperparathyroidism
PTH regulates secretion of FGF23 and is required for the early stimulation of FGF23 secretion 109, which is the earliest detected abnormality of the CKD-MBD 99. Thus, there is a regulation of PTH secretion early in CKD that remains to be clarified. As CKD progresses, components of the CKD-MBD result in increased production of PTH and nodular hyperplasia of the parathyroid glands. Sustained elevation in PTH levels, while adaptive to maintain osteoblast surfaces, are associated with an abnormal phenotype of osteoblast function and osteocyte stimulation with relatively less type 1 collagen and more RANKL ligand production than anabolic osteoblasts 110. New studies discussed above indicate that other factors such as FGF23 and activin may impact osteoblast function besides PTH, and produce the mineralization disorder of CKD changing the material properties of bone. The outcome is a high turnover renal osteodystrophy, excess bone resorption, skeletal frailty and elevated fracture risk 111.
Cardiovascular disease
The CKD-MBD is a contributing factor to vascular stiffness and calcification that increases the systolic blood pressure, pulse wave velocity, and left ventricular mass, all of which are surrogates for cardiovascular risk in the general population and in those with CKD 112. Structural and functional abnormalities of the vasculature are seen in early CKD, including vascular stiffness and endothelial dysfunction that progress to vascular calcification, a common phenomenon in the aging general population that is accelerated in CKD to the highest level seen in clinical medicine. Vascular calcification further intensifies vascular stiffness and promotes the development of LVH, all processes that contribute to cardiovascular risk and excess cardiac mortality in native and transplant CKD.
In animal models with mild renal insufficiency (equivalent to human stage 2 CKD), we have demonstrated that expression of proteins involved in the contractile apparatus of aortic smooth muscle cells are decreased, reflecting a dedifferentiated state of the vasculature in early CKD 19. Within the developmental program of mesenchymal stem cells and early vascular progenitors, dedifferentiated vascular smooth muscle cells are susceptible to osteoblastic transition, which contributes to vascular calcification in CKD. Osteoblastic transition of vascular smooth muscle cells produces CKD-stimulated calcification of atherosclerotic plaques as well as the tunica media, resulting in either neointimal or medial vascular calcification 113.
Emerging Concepts in the Systems Biology of the CKD-MBD
The Wnt pathway and reactivation developmental pathways during renal repair mechanisms in kidney diseases
Kidney injuries produce circulating signals that directly affect the vasculature, the myocardium and the skeleton. These signals are derived from reactivation of developmental programs of nephrogenesis in an attempt at kidney repair, which are typically silent in the normal adult kidney 19. The classic example is the reactivation of the Wnt pathway that controls tubular epithelial proliferation and polarity during nephrogenesis and is a driving force in renal fibrosis 26. Activation of the canonical Wnt pathway increases expression of downstream transcriptional targets, including Wnt inhibitors that function in a negative feedback loop to autoregulate Wnt activation. These Wnt inhibitors include Dickkopf-1 (Dkk1), soluble frizzled related proteins, Wnt-modulator in surface ectoderm (Wise), and sclerostin among others. While Wnts are strictly autocrine/paracrine factors, the Wnt inhibitors also function as circulating systemic factors 114. The role of Wnt in renal development largely precedes the invasion of the microcirculation forming the glomerulus and the peritubular capillaries. Therefore, while the Wnt inhibitors did not evolve as circulating factors produced by the normal kidney, during kidney injury and repair they are released into the systemic circulation and may inhibit the physiologic roles of Wnt in extrarenal tissues 19.
We and others have recently shown this “unintended” systemic inhibition of Wnt activity and production of activin A stimulated by kidney disease to have major consequences in the skeleton and vasculature. In animal models of early CKD, incomplete recovery from acute kidney injury led to increased expression of Wnt inhibitors (e.g., Dkk1, sclerostin) and activin in the injured kidney and increased levels in the systemic circulation 19,69. The skeleton was affected through both changes in remodeling (decreased bone formation rates) and increased bone resorption and in secretory properties of the osteocytes (increased FGF23 secretion). The cardiovascular system was affected through loss of vascular contractile machinery and dedifferentiation of vascular smooth muscle cells, stimulation of osteoblastic transition and vascular calcification, and promotion of cardiac hypertrophy 19,69. Neutralization of circulating Dkk1 using a monoclonal antibody or a ligand trap for ActRIIA resulted in increased bone formation rates and bone volume, improved vascular function, and decreased osteoblastic transition and vascular calcification 19,69.
Conclusion and Future Directions
The CKD-MBD defines a disruption in the systems biology between the injured kidney, skeleton, and cardiovascular system that has a profoundly negative impact on survival in CKD. Recent translational discoveries have introduced a new paradigm where kidney injury directly leads to skeletal and cardiovascular injury through the production of pathogenic circulating factors during attempted renal repair, including molecules that inhibit the canonical Wnt pathway and activin, both processes that have been implicated in chronic allograft injury as well as cardiovascular disease. Future studies must clarify whether incomplete recovery from acute kidney injury is sufficient to stimulate these disturbances in the kidney-skeletal-cardiovascular axis that contribute to decreased patient and allograft survival. This would identify the early CKD-MBD as an important therapeutic target for improving long-term outcomes in CKD.
Highlights.
This review discusses novel aspects of CKD-MBD pathogenesis
Acknowledgments
Financial support and sponsorship:
This work was funded by NIH grants, RO1DK070790 (KAH) and RO1DK089137 (KAH), an investigator stimulated grant from Celgene, and by NIH grants UL1 TR000448, KL2 TR000450, and L40 DK099748-01 (MS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. The Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu Cy. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 5.Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrology Dialysis Transplantation. 2005;20:1048–1056. doi: 10.1093/ndt/gfh813. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic Kidney Disease: A Report From an ADA ConsensusConference. American Journal of Kidney Diseases. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87(3):649–659. doi: 10.1038/ki.2014.296. [DOI] [PubMed] [Google Scholar]
- 8.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium X phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 10.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. New Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhingra R, Sullivan LM, Fox CS, et al. Relations of Serum Phosphorus and Calcium Levels to the Incidence of Cardiovascular Disease in the Community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Sang Y, Ballew SH, et al. Subclinical Atherosclerosis Measures for Cardiovascular Prediction in CKD. J Am Soc Nephrol. 2014;26(2):439–447. doi: 10.1681/ASN.2014020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalal M, Sun K, Cappola AR, et al. Relationship of serum fibroblast growth factor 23 with cardiovascular disease in older community-dwelling women. Eur J Endocrinol. 2011;165(5):797–803. doi: 10.1530/EJE-11-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Y, Ginsberg C, Sugatani T, Faugere MC, Malluche H, Hruska KA. Early chronic kidney disease-mineral bone disorder stimulates vascular calcification. Kidney Int. 2014 Jul 24;85:142–150. doi: 10.1038/ki.2013.271. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1 and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161–1168. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang Y, Zhang Y, Mathew S, Futhey J, Lund RJ, Hruska KA. Early chronic kidney disease (CKD) stimulates vascular calcification (VC) and decreased bone formation rates prior to positive phosphate balance. J Am Soc Nephrol. 2009;20:36A. [Google Scholar]
- 18.Hu MC, Shi M, Zhang J, et al. Klotho Deficiency Causes Vascular Calcification in Chronic Kidney Disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Y, Ginsberg C, Seifert M, et al. CKD-induced Wingless/Integration1 Inhibitors and Phosphorus Cause the CKD-Mineral and Bone Disorder. J Am Soc Nephrol. 2014;25(8):1760–1763. doi: 10.1681/ASN.2013080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira RB, Graciolli FG, dos Reis LM, et al. Disturbances of Wnt/β-catenin pathway and energy metabolism in early CKD: effect of phosphate binders. Nephrol Dial Transplant. 2013;28(10):2510–2517. doi: 10.1093/ndt/gft234. [DOI] [PubMed] [Google Scholar]
- 21.Sabbagh Y, Graciolli FG, O’Brien S, et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res. 2012;27(8):1757–1772. doi: 10.1002/jbmr.1630. [DOI] [PubMed] [Google Scholar]
- 22.Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;282:F431–F441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- 23.Surendran K, Schiavi S, Hruska KA. Wnt-dependent- β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 24.Maeshima A, Nojima Y, Kojima I. The role of the activin-follistatin system in the developmental and regeneration processes of the kidney. Cytokine & Growth Factor Reviews. 2001;12(4):289–298. doi: 10.1016/s1359-6101(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 25.Terada Y, Tanaka H, Okado T, et al. Expression and Function of the Developmental Gene Wnt-4 during Experimental Acute Renal Failure in Rats. J Am Soc Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229(2):221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 27.Rinkevich Y, Montoro Daniel T, Contreras-Trujillo H, et al. In Vivo Clonal Analysis Reveals Lineage-Restricted Progenitor Characteristics in Mammalian Kidney Development, Maintenance, and Regeneration. Cell Reports. 2014;7(4):1270–1283. doi: 10.1016/j.celrep.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin S-L, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proceedings of the National Academy of Sciences. 2010;107(9):4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niida A, Hiroko T, Kasai M, et al. DKK1, a negative regulator of Wnt signaling, is a target of the á-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 30.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 31.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 32.Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2004;24(1):73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Sancho JM, Aguilera O, Garcia JM, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of [beta]-catenin//TCF and is downregulated in human colon cancer. Oncogene. 2004;24(6):1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 34.Pietilä I, Ellwanger K, Railo A, et al. Secreted Wnt antagonist Dickkopf-1 controls kidney papilla development coordinated by Wnt-7b signalling. Developmental Biology. 2011;353(1):50–60. doi: 10.1016/j.ydbio.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Surendran K, Schiavi SC, Hruska K. A putative Wnt antagonist secreted frizzled-related protein 4 (sFRP4) suppresses the progression of renal fibrosis follwoing unilateral ureteral obstruction. J Am Soc Nephrol. 2004;15:245A. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 36.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 37.DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β Catenin Signaling in Medullary Kidney Myofibroblasts. Journal of the American Society of Nephrology. 2013 doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren S, Johnson BG, Kida Y, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proceedings of the National Academy of Sciences. 2013;110(4):1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/{beta}-Catenin Signaling Promotes Renal Interstitial Fibrosis. Journal of the American Society of Nephrology. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evenepoel P, D’Haese P, Brandenburg V. Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney International. 88(2):235–240. doi: 10.1038/ki.2015.156. [DOI] [PubMed] [Google Scholar]
- 41.Thambiah S, Roplekar R, Manghat P, et al. Circulating Sclerostin and Dickkopf-1 (DKK1) in Predialysis Chronic Kidney Disease (CKD): Relationship with Bone Density and Arterial Stiffness. Calcified Tissue International. 2012;90(6):473–480. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- 42.Asamiya Y, Tsuchiya K, Nitta K. Role of sclerostin in the pathogenesis of chronic kidney disease-mineral bone disorder. Renal Replacement Therapy. 2016;2(1):1–8. [Google Scholar]
- 43.Loots GG. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15:928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 2001. 2001 Mar;:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. American Journal of Medical Genetics. 2002;110(2):144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 46.Balemans W. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wein MN, Spatz J, Nishimori S, et al. HDAC5 Controls MEF2C-Driven Sclerostin Expression in Osteocytes. Journal of Bone and Mineral Research. 2015;30(3):400–411. doi: 10.1002/jbmr.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler DG, Sutherland MK, Geoghegan JC, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. The EMBO Journal. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poole KE. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 51.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moester MJC, Papapoulos SE, Löwik CWGM, van Bezooijen RL. Sclerostin: Current knowledge and future perspectives. Calcif Tissue Int. 2011;87:99–107. doi: 10.1007/s00223-010-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cejka D, Marculescu R, Kozakowski N, et al. Renal Elimination of Sclerostin Increases With Declining Kidney Function. The Journal of Clinical Endocrinology & Metabolism. 2014;99(1):248–255. doi: 10.1210/jc.2013-2786. [DOI] [PubMed] [Google Scholar]
- 54.Vervloet MG, Massy ZA, Brandenburg VM, et al. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. The Lancet Diabetes & Endocrinology. 2(5):427–436. doi: 10.1016/S2213-8587(14)70059-2. [DOI] [PubMed] [Google Scholar]
- 55.Pelletier S, Dubourg L, Carlier M-C, Hadj-Aissa A, Fouque D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clinical Journal of the American Society of Nephrology. 2013;8(5):819–823. doi: 10.2215/CJN.07670712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in Renal Osteodystrophy. Clin J Am Soc Nephrol. 2011;6(4):877–882. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanbay M, Siriopol D, Saglam M, et al. Serum Sclerostin and Adverse Outcomes in Nondialyzed Chronic Kidney Disease Patients. The Journal of Clinical Endocrinology & Metabolism. 2014;99(10):E1854–E1861. doi: 10.1210/jc.2014-2042. [DOI] [PubMed] [Google Scholar]
- 58.Moe SM, Chen NX, Newman CL, et al. Anti-Sclerostin Antibody Treatment in a Rat Model of Progressive Renal Osteodystrophy. Journal of Bone and Mineral Research. 2015;30(3):499–509. doi: 10.1002/jbmr.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee P-H, Hsu Y-C, Lin C-L, Wang F-S. New Paradigm for Progression of Diabetic Nephropathy. Acta Nephrologica. 2013;27(2):70–75. [Google Scholar]
- 60.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt Calcification Cascade Is Regulated by TNF-{alpha} Dependent Signals in Diabetic Ldlr/Mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 61.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng S-L, Shao J-S, Behrmann A, Krchma K, Towler DA. Dkk1 and Msx2–Wnt7b Signaling Reciprocally Regulate the Endothelial–Mesenchymal Transition in Aortic Endothelial Cells. Arterio Thromb Vasc Biol. 2013;33(7):1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eisenberg LM, Markwald RR. Molecular Regulation of Atrioventricular Valvuloseptal Morphogenesis. Circ Res. 1995;77(1):1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Camenisch TD, Molin DGM, Person A, et al. Temporal and Distinct TGFβ Ligand Requirements during Mouse and Avian Endocardial Cushion Morphogenesis. Develop Biol. 2002;248(1):170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 65.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 66.Moore-Morris T, Guimarães-Camboa N, et al. Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooley BC, Nevado J, Mellad J, et al. TGF-β Signaling Mediates Endothelial-to-Mesenchymal Transition (EndMT) During Vein Graft Remodeling. Sci Transl Med. 2014;6(227):227ra234. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ying S-Y. Inhibins, Activins, and Follistatins: Gonadal Proteins Modulating the Secretion of Follicle-Stimulating Hormone. Endocrine Reviews. 1988;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- 69.Agapova OA, Fang Y, Sugatani T, Seifert ME, Hruska KA. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney International. 2016;89(6):1231–1243. doi: 10.1016/j.kint.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toshifumi Sugatani OAA, Fang Yifu, Berman Alycia G, Wallace Joseph M, Malluche Hartmut H, Smith William, Sung Victoria, Hruska Keith A. A Ligand of the Activin Receptor Type IIA Mediates Osteoclast Stimulation of Bone Remodeling in Diabetic Mice with CKD. Kidney International. 2016 doi: 10.1016/j.kint.2016.07.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naik V, Leaf EM, Hu JH, et al. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovascular Research. 2012;94(3):545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kokubo T, Ishikawa N, Uchida H, et al. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol. 2009;20:1236–1245. doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shanahan CM, Cary NRB, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 74.Speer MY, Yang HY, Brabb T, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu D, Mackenzie NCW, Shanahan CM, Shroff RC, Farquharson C, MacRae VE. BMP-9 regulates the osteoblastic differentiation and calcification of vascular smooth muscle cells through an ALK1 mediated pathway. Journal of Cellular and Molecular Medicine. 2015;19(1):165–174. doi: 10.1111/jcmm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seifert ME, De las Fuentes L, Rothstein M, et al. Effects of phosphate binder therapy on vascular stiffness in the early chronic kidney disease. Amer J Nephrol. 2013;38(2):158–167. doi: 10.1159/000353569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by Klotho. J Biol Chem. 2006;281(10):6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ide N, Olauson H, Sato T, et al. In vivo evidence for a limited role of proximal tubular Klotho in renal phosphate handling. Kidney International. 2016:90. doi: 10.1016/j.kint.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Chen C-D, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proceedings of the National Academy of Sciences. 2007;104(50):19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakan HNK, Asai O, Imura A, Tanaka T, et al. Reduced Renal α-Klotho Expression in CKD Patients and Its Effect on Renal Phosphate Handling and Vitamin D Metabolism. PLoS ONE. 2014;9(1):e86301. doi: 10.1371/journal.pone.0086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith RC, O’Bryan LM, Farrow EG, et al. Circulating αKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122(12):4710–4715. doi: 10.1172/JCI64986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu M-C, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010;78(12):1240–1251. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grabner A, Amaral Ansel P, Schramm K, et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metabolism. 2015;22(6):1020–1032. doi: 10.1016/j.cmet.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu MC, Shi M, Cho HJ, et al. Klotho and Phosphate Are Modulators of Pathologic Uremic Cardiac Remodeling. J Am Soc Nephrol. 2015;26(6):1290–1302. doi: 10.1681/ASN.2014050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silver J, Rodriguez M, Slatopolsky E. FGF23 and PTH—double agents at the heart of CKD. Nephrology Dialysis Transplantation. 2012;27(5):1715–1720. doi: 10.1093/ndt/gfs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16(4):917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 88.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in chronic kidney disease. J Am Soc Nephrol. 2008;19(6):1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-Protein Binding and Post-transcriptional Regulation of Parathyroid Hormone Gene Expression by Calcium and Phosphate. Journal of Biological Chemistry. 1998;273(9):5253–5259. doi: 10.1074/jbc.273.9.5253. [DOI] [PubMed] [Google Scholar]
- 91.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 92.Isakova T, Barchi-Chung A, Enfield G, et al. Effects of dietary phosphate restriction and lanthanum carbonate on FGF23 in CKD. J Am Soc Nephrol. 2012;23:P45A. doi: 10.2215/CJN.09250912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9(11):671–680. doi: 10.1038/nrneph.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cunningham J, Sprague S, Cannata-Andia J, et al. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43(3):566–571. doi: 10.1053/j.ajkd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56(3):1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 96.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 97.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 98.Liu S, Tang W, Zhou J, Vierthaler L, Quarles LD. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. AJP - Endocrinology and Metabolism. 2007;293:E1636–E1644. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- 99.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf M, Molnar MZ, Amaral AP, et al. Elevated Fibroblast Growth Factor 23 is a Risk Factor for Kidney Transplant Loss and Mortality. Journal of the American Society of Nephrology. 2011;22:956–966. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fliser D, Kollerits B, Neyer U, et al. Fibroblast Growth Factor 23 (FGF23) Predicts Progression of Chronic Kidney Disease: The Mild to Moderate Kidney Disease (MMKD) Study. Journal of the American Society of Nephrology. 2007;18(9):2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 102.Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Molecular Medicine. 2014;6(6):744–759. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Humalda JK, Lambers Heerspink HJ, Kwakernaak AJ, et al. Fibroblast Growth Factor 23 and the Antiproteinuric Response to Dietary Sodium Restriction During Renin-Angiotensin-Aldosterone System Blockade. American Journal of Kidney Diseases. 2015;65(2):259–266. doi: 10.1053/j.ajkd.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 104.Andrukhova O, Smorodchenko A, Egerbacher M, et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. Embo Journal. 2014;33 doi: 10.1002/embj.201284188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The á-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 106.Prié D, Friedlander G. Reciprocal Control of 1,25-Dihydroxyvitamin D and FGF23 Formation Involving the FGF23/Klotho System. Clinical Journal of the American Society of Nephrology. 2010;5(9):1717–1722. doi: 10.2215/CJN.02680310. [DOI] [PubMed] [Google Scholar]
- 107.KDIGO Clinical Practice Guideline for the Diagnosis E, Prevention, and Treatment of the CKD-MBD. Chapter 4.1: Treatment of CKD-MBD targeted at lowering high serum phosphorus and maintaining serum calcium. Kidney Int. 2009;76(S113):S50–S99. doi: 10.1038/ki.2009.192. [DOI] [PubMed] [Google Scholar]
- 108.Naveh-Many T, Marx R, Keshet E, Pike JW, Silver J. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin D3 in the parathyroid in vivo. J Clin Invest. 1990;86(6):1968–1975. doi: 10.1172/JCI114931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meir T, Durlacher K, Pan Z, et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86(6):1106–1115. doi: 10.1038/ki.2014.215. [DOI] [PubMed] [Google Scholar]
- 110.Wesseling-Perry K, Salusky IB. Chronic Kidney Disease: Mineral and Bone Disorder in Children. Seminars in Nephrology. 2013;33(2):169–179. doi: 10.1016/j.semnephrol.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moe SM, Abdalla S, Chertow GM, et al. Effects of Cinacalcet on Fracture Events in Patients Receiving Hemodialysis: The EVOLVE Trial. Journal of the American Society of Nephrology. 2014 doi: 10.1681/ASN.2014040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ix JH, Katz R, De Boer IH, et al. Association of Chronic Kidney Disease with the Spectrum of Ankle Brachial Index: The Cardiovascular Health Study. Journal of the American College of Cardiology. 2009;54(13):1176–1184. doi: 10.1016/j.jacc.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moe SM, Chen NX. Pathophysiology of Vascular Calcification in Chronic Kidney Disease. Circulation Research. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 114.Diarra D. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]