Abstract
The excitatory amino acid transporters 1 and 2 (EAAT1 and EAAT2), mostly located on astrocytes, are the main mediators for glutamate clearance in humans. Malfunctions of these transporters may lead to excessive glutamate accumulation and subsequent excitotoxicity to neurons, which has been implicated in many kinds of neurodegenerative disorders including Alzheimer’s disease (AD). Yet, the specific mechanism of the glutamate system dysregulation remains vague. To explore whether the insulin/protein kinase B (Akt)/EAAT signaling in human astrocytes could be disturbed by beta-amyloid protein (Aβ) and be protected by insulin, we incubated HA-1800 cells with varying concentrations of Aβ1–42 oligomers and insulin. Then the alterations of several key substrates in this signal transduction pathway were determined. Our results showed that expressions of insulin receptor, phospho-insulin receptor, phospho-protein kinase B, phospho-mammalian target of rapamycin, and EAAT1 and EAAT2 were decreased by the Aβ1–42 oligomers in a dose-dependent manner (p < 0.05) and this trend could be recovered by insulin treatment (p < 0.05). However, the expressions of total Akt and mTOR were invariant (p > 0.05), and the mRNA levels of EAAT1 and EAAT2 were also unchanged (p > 0.05). Taken together, this study indicates that Aβ1–42 oligomers could cause disturbances in insulin/Akt/EAAT signaling in astrocytes, which might be responsible for AD onset and progression. Additionally, insulin can exert protective functions to the brain by modulating protein modifications or expressions.
Keywords: Alzheimer’s disease, Aβ1–42 oligomers, Insulin signaling, Excitatory amino acid transporter, Human astrocytes
Introduction
Alzheimer’s disease (AD), which is an age-related neurodegenerative disorder, is the most common form of dementia among old people. It is a heterogeneous disease that affected 1.93 million people in China in 1990 and 5.69 million in 2010 (Chan et al. 2013). Clinically, AD is characterized by the insidious onset and gradual progression of cognitive decline. The well-known histopathological changes namely extracellular senile plaques (SPs) depositing of beta-amyloid protein (Aβ), neurofibrillary tangles (NFTs) consisting of hyperphosphorylated tau protein, as well as damage to neurons represents the effects of brain insulin resistance and deficiency (de la Monte 2014). Although the pathogenesis of AD is still elusive, there is ample consensus that the abnormal Aβ deposition in the central nervous system (CNS) plays a crucial role, especially the Aβ1–42 in the form of soluble oligomers (Tam and Pasternak 2012). Additionally, disturbances in insulin signaling and abnormalities of the glutamate neurotransmitter system are also involved in AD (On 2013a; Kulijewicz-Nawrot et al. 2013).
Brain insulin signaling has been widely researched in the last decade. It is critical for memory processing, synaptic genesis and remodeling. Furthermore, insulin is a kind of neuroprotective hormone that contributes not only to metabolism but also to cognition (Chen et al. 2014). Several recent reports have indicated that both brain IR density and insulin level decrease with age (Cole and Frautschy 2007; Laron 2009) and many key substrates in the insulin signal transduction pathway are significantly reduced in AD (Liu et al. 2011; Pearson-Leary and McNay 2012). Furthermore, mammalian target of rapamycin (mTOR), one of the most important downstream targets of protein kinase B (Akt), can regulate glutamate transporter expression in astrocytes (Lopez-Colome et al. 2012). A number of studies have shown that direct exposure to Aβ could result in animal cognitive decline and downregulation of Akt and mTOR (Caccamo et al. 2010; Pearson-Leary and McNay 2012).
Astrocytes are the major glial cells in the CNS providing a nurturing environment for neurons. To be specific, they contribute to synaptic functions, transfer and storage information, participate in cognition, produce trophic factors, remove toxins and debris, maintain redox potential and regulate concentrations of neurotransmitters and ions (Sidoryk-Wegrzynowicz et al. 2011). In addition, astrocytes express abundant insulin receptors (IRs) and can be influenced by insulin (Henneberger et al. 2010). Furthermore, it has been reported that astrocyte dysfunction is associated with AD (Birch 2014). Glutamate, the main excitatory neurotransmitter in the brain, plays a key role in learning and memory. It is released into the synaptic cleft during neuron activity and rapidly taken up by the excitatory amino acid transporters (EAATs), among which EAAT1 and EAAT2 (known in rodents as glutamate/aspartate transporter, GLAST, and glutamate transporter-1, GLT-1) preferentially located in astrocytes are responsible for clearance of the majority of glutamate (Danbolt 2001). Consequently, malfunctions of EAAT1 and EAAT2 may lead to aberrant glutamate accumulation and neuron injury known as excitotoxicity.
Although a great deal of evidence has revealed that insulin signaling disturbances and glutamate neurotoxicity are implicated in AD pathophysiology, it remains to be determined how Aβ oligomers are related to glutamate excitotoxicity. The aim of this study was to explore whether the insulin/Akt/EAAT signaling in human astrocytes could be disturbed by Aβ and if insulin has protective effects on the cells. Hence, we treated human astrocytes with Aβ1–42 oligomers and insulin, and then investigated the changes of downstream targets in this signaling pathway both in protein expressions and mRNA levels.
Materials and Methods
Preparation of Aβ1–42 Oligomers
The method employed to generate Aβ1–42 oligomers was described by Ryan et al. (Ryan et al. 2010). Briefly, 1 mg human synthetic Aβ1–42 lyophilized powder (American Peptide Sunnyvale, CA, USA) was suspended in 1,1,1,3,3,3-hexa-fluoro-2-propanol (HFIP, Sigma, Saint Louis, MO, USA) to 1 mM concentration, followed by vibration mixing to produce a homogenous suspension. Subsequently, the suspension was aliquoted into five microfuge tubes and the HFIP was removed in a ventilation cabinet overnight, leaving a hyaloid peptide film. The peptide film was resuspended in anhydrous dimethyl sulfoxide (DMSO, Sigma, Saint Louis, MO, USA) to 5 mM and sonicated for 10 min. Then the dimethyl sulfoxide-Aβ1–42 solution was diluted with cold PBS containing 0.05 % SDS to 100 μM followed by a 24-h incubation at 4 °C. For higher aggregation, the peptide solution was further diluted with PBS to 11.1 μM and incubated for another 2 weeks at 4 °C. For experiments, these Aβ1–42 oligomer preparations were centrifuged at 13,000 rpm for 10 min to remove the insoluble aggregates and diluted to final concentrations with serum-free Dulbecco’s modified Eagle’s medium (DMEM, low-glucose, Carlsbad, CA, USA).
Identification of Aβ1–42 Oligomers
Aβ1–42 oligomeric preparations were identified by transmission electron microscopy (H-800, Japan’s Hitachi). Briefly, 20 μL Aβ1–42 oligomeric preparation was spotted onto a copper mesh and dried for 10 min followed by washing twice with distilled water. Then, it was stained with 2 % phosphotungstic acid (pH 6.8) for 2 min, washed once, dried, and examined. The acceleration voltage was 100 kV and the magnification was ×100,000.
Astrocyte Culture
Human astrocyte cell line (HA-1800) was obtained from ScienCell Research Laboratories (ScienCell, Carlsbad, CA, USA). Cells were grown in low-glucose DMEM containing 10 % fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and maintained in a humidified 5 % CO2 atmosphere at 37°C.
Pharmacological Treatments
The astrocytes were plated in 25 cm2 culture flasks at 2.0 × 105 cells/mL for Western blot and real-time PCR analyses. Cells were plated onto poly-D-lysine-coated 24-well chamber slides at 1.0 × 105 cells/well for immunofluorescence staining analysis, and plated in 96-well plates at 9.0 × 103 cells/well for MTT analysis. There were six groups in our experiments: control group and groups I to V. The control group, group I, and group II were successively treated with equivalent volumes of FBS-free DMEM, 100 nM Aβ1–42 oligomers and 1 μM Aβ1–42 oligomers for 24 h (Ferreira et al. 2012; Ito et al. 2012; Zhou et al. 2012); groups III, IV, and V were treated with 100 nM human recombinant insulin (Novo Nordisk, Bagsvard, Denmark) for 30 min after the treatments with DMEM vehicle, 100 nM Aβ1–42 oligomers and 1 μM Aβ1–42 oligomers, respectively (Bomfim et al. 2012; De Felice et al. 2009; Ji et al. 2011).
RNA Isolation and Real-Time RT-PCR Analysis
Total RNA was isolated from astrocytes according to the manufacturer’s protocol for RNAiso Plus (TakaRa, Dalian, China). RNA concentration was measured by spectrophotometric absorbance at 260 nm and 1 μg total RNA was reverse transcribed using a Prime Script RT reagent kit (TakaRa, Dalian, China). Quantitative real-time PCR analysis was performed by the LightCycler Carousel-Based System (Roche Applied Science, Mannheim, Germany) with a SYBR Premix Ex Taq (Tli RNaseH Plus) kit (TakaRa, Dalian, China). The shuttle PCR conditions were as follows: initial denaturation at 95 °C for 30 s followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. After shuttle PCR, a melting curve was constructed by increasing the temperature to 95 °C. β-actin was used as an endogenous control for normalization of RNA quantity. Relative changes of gene expression were calculated using the comparative ΔΔCt method. This procedure was repeated three times for each gene. All primers purchased from Sangong Biotech (Shanghai, China) were as follows:
GFAP forward primer: 5′-CTGGAGGTTGAGAGGGA CAAT-3′; reverse primer: 5′-GTGGCTTCATCTGCTTCC TGT-3′; IR forward primer: 5′-TTTCGTCCCCAGAAAA ACCTCT-3′; reverse primer: 5′-CCACCGTCATCCCAA C-3′; Akt forward primer: 5′-GGTATTTTGATGAGGAG TTCACG-3′; reverse primer: 5′-GAGTAGGAGAACTGG GGGAAGT-3′; mTOR forward primer: 5′-CTTGTTTGTG GCTCTGAATGAC-3′; reverse primer: 5′-GGCACTCTG CTCTTTGATTCTT-3′; EAAT1 forward primer: 5′-GCTT ACTCATTCACGCAGTCAT-3′; reverse primer: 5′-AAC CCTCCAATAAAAACCCAAG-3′; EAAT2 forward primer: 5′-GGTCATTCTGGTCTTGGCTATC-3′; reverse primer: 5′-AGGCTGGACACTTCATCATTCT-3′; β-actin forward primer: 5′-TGACGTGGACATCCGCAAAG-3′; reverse primer: 5′-CTGGAAGGTGGACAGCGAGG-3′.
Western Blot Analysis
After the treatment, the cells were lysed for 30 min in ice-cold RIPA lysis buffer (Beyotime, Haimen, China) containing phenylmethanesulfonyl fluoride (PMSF, Beyotime, Haimen, China) and protein phosphatase inhibitor complex I (Aidlab Biotechnologies, Beijing, China) to extract total proteins. The protein content was measured using a BCA protein assay kit (Beyotime, Haimen, China), adjusted to 30 μg, and then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE with 10 % acrylamide gel). Subsequently, the separated proteins were electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA) and blocked with 5 % bovine serum albumin (BSA, Sigma, Saint Louis, MO, USA) in Tris-buffered saline Tween-20 (TBST, 50 mM Tris–HCl, 150 mM NaCl, pH 7.4, 0.1 % Tween-20) for 1 h at room temperature. Then the membranes were incubated with primary antibodies at 4 °C overnight as follows: mouse monoclonal anti-GFAP (1:200, Abcam, Cambridge, UK); rabbit polyclonal anti-insulin receptor alpha (1:200, Abcam, Hong Kong, China); rabbit polyclonal anti-insulin receptor beta (1:500, ImmunoWay, Newark, DE, USA); rabbit polyclonal anti-phospho-insulin receptor (Y1361, 1:500, ImmunoWay, Newark, DE, USA); rabbit polyclonal anti-Akt1 (1:1000, ImmunoWay, Newark, DE, USA); rabbit polyclonal anti-phospho-Akt (Ser473, 1:1000, ImmunoWay, Newark, DE, USA); rabbit polyclonal anti-mTOR (1:5000, ImmunoWay, Newark, DE, USA); rabbit polyclonal phospho-mTOR (Ser2448, 1:5000, Immuno-Way, Newark, DE, USA); rabbit polyclonal anti-EAAT1 (1:500, ImmunoWay, Newark, DE, USA), and mouse monoclonal anti-EAAT2 (1:500, Abcam, HongKong, China). After extensive washing with TBST, the blots were probed with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5000, ZSGB-BIO, Beijing, China) or anti-rabbit IgG (1:10000, ZSGB-BIO, Beijing, China) diluted in blocking buffer for 1 h at room temperature. The immunoreactive bands were visualized using enhanced chemiluminescence (ECL, Millipore, Bedford, MA, USA) and (Kodak). Band density values of individual proteins were normalized by β-actin (1:5000, Santa Cruz Biotechnology, CA, USA) and measured using ImageJ software (NIH, Bethesda, MD, USA). Results were means of three replicates of experiments.
Immunofluorescence Staining
After treatment with Aβ, the slides were fixed with 4 % paraformaldehyde (Solarbio, China, Beijing) for 30 min. After three rinses with PBS, cells were incubated with 0.5 % Triton X-100 (DingGuo, Beijing, China) for 15 min and blocked with 10 % goat serum (ZSGB-BIO, Beijing, China) for 30 min at 37 °C. Subsequently, the astrocytes were incubated with primary antibodies at 4 °C overnight (mouse monoclonal anti-GFAP, 1:100, Abcam, Cambridge, UK). After adequate washing, rhodamine (TRITC)-conjugated goat anti-mouse IgG (1:200, ZSGB-BIO, Beijing, China) was added to the samples and incubated at 37 °C for 1 h in the dark. Finally, the slides were washed extensively and stained with 4′,6′-diamidino-2-phenylindole (DAPI, Boisynthesis Biotechnology, Beijing, China) for 2 min at room temperature. Images were obtained at ×200 magnification under a fluorescent microscope (DP72, Olympus, Tokyo, Japan). Densitometry quantification was performed using ImageJ (NIH, Bethesda, MD, USA) software after converting the image to gray scale. All experiments were performed in triplicates.
MTT Reduction Test
Cell viability was examined through conversion of methyl thiazolyl tetrazolium (MTT, Sigma, Saint Louis, MO, USA) to colored formazan crystals. Briefly, medium in each well was replaced with 180 μL FBS-free DMEM containing 20 μL of 5 mg/mL MTT, and the astrocytes were incubated for 4 h. At termination, MTT was removed by aspiration and the formazan crystals were solubilized by 200 μL DMSO, and then the cultures were incubated for an additional 20 min. The optical density was measured at a test wavelength of 570 nm and a reference wavelength of 630 nm using a spectrophotometer. Results are expressed as percent of control values.
Data Analysis
Results are expressed as mean ± SD. To determine the significance of difference among various groups, the statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Tukey’s test using SPSS 17.0. p < 0.05 was considered statistically significant in all tests.
Results
Identification of Aβ1–42 Oligomers
Transmission electron microscope analysis showed that the oligomers were regular and uniform spheres with a diameter of 12–25 nm, and no fibrillar aggregates were present in this preparation (Fig. S1). The magnification of the images is ×100,000 and the scale bar is 250 nm. Our results suggested that the Aβ1–42 oligomers used in this experiment were acceptable for experiments.
Aβ1–42 Oligomers-Induced Reactive Astrogliosis
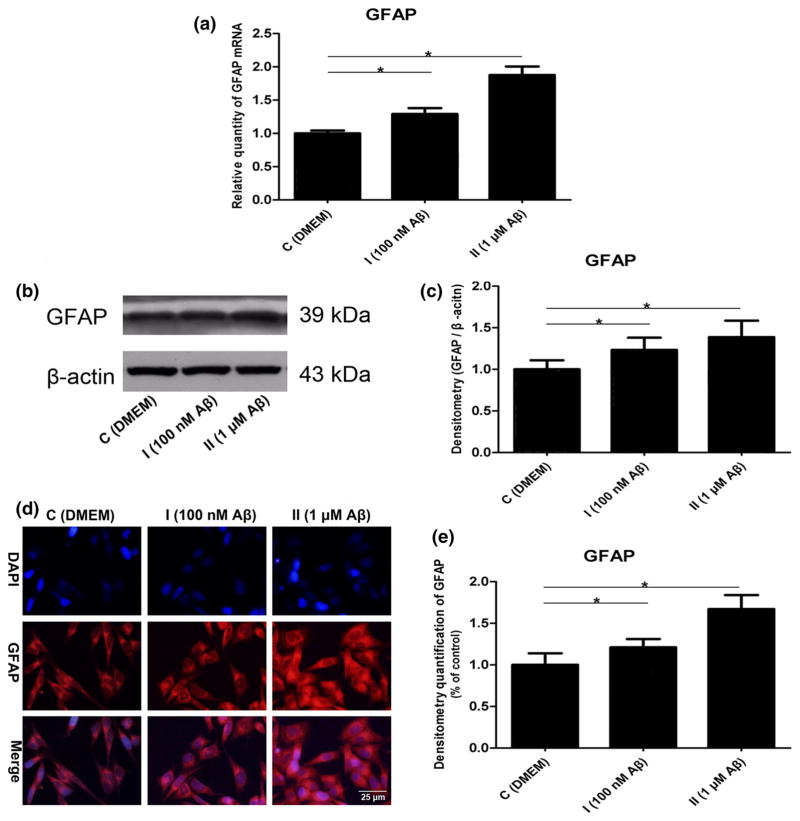
Glial fibrillary acidic protein (GFAP), widely used as an indicator of astrocyte activation, is progressively increased in AD. Furthermore, it is correlated with severity of AD pathologic changes (Wharton et al. 2009). Therefore, we treated the astrocytes with different concentrations of Aβ1–42 oligomers to simulate the different stages of AD, and then detected the changes of GFAP at the gene and protein levels. First, our real-time RT-PCR and Western blot results showed that expression of GFAP was increased by the Aβ1–42 oligomers in a dose-dependent manner (Fig. 1a–c, p < 0.05). Subsequently, we found that Aβ could cause morphological changes of the astrocytes, namely enlarged cell bodies and nuclei, hypertrophic processes, and increased GFAP expression. Furthermore, there was an absence of cell proliferation (Fig. 1d, e, p < 0.05).
Fig. 1.
Reactive astrogliosis was induced by the Aβ1–42 oligomers: a mRNA levels of GFAP (F = 67.304, *p < 0.05); b representative western blot bands of GFAP; c densitometric measurements of GFAP normalized by β-actin (F = 9.443, *p < 0.05); d representative figures of GFAP immunofluorescence stain; DAPI, nuclei; TRITC, GFAP; e densitometry quantification of GFAP immunofluorescence stain (F = 67.399, *p <0.05)
Aβ1–42 Oligomers and Insulin Changed the Expression of Insulin Receptor in Astrocytes
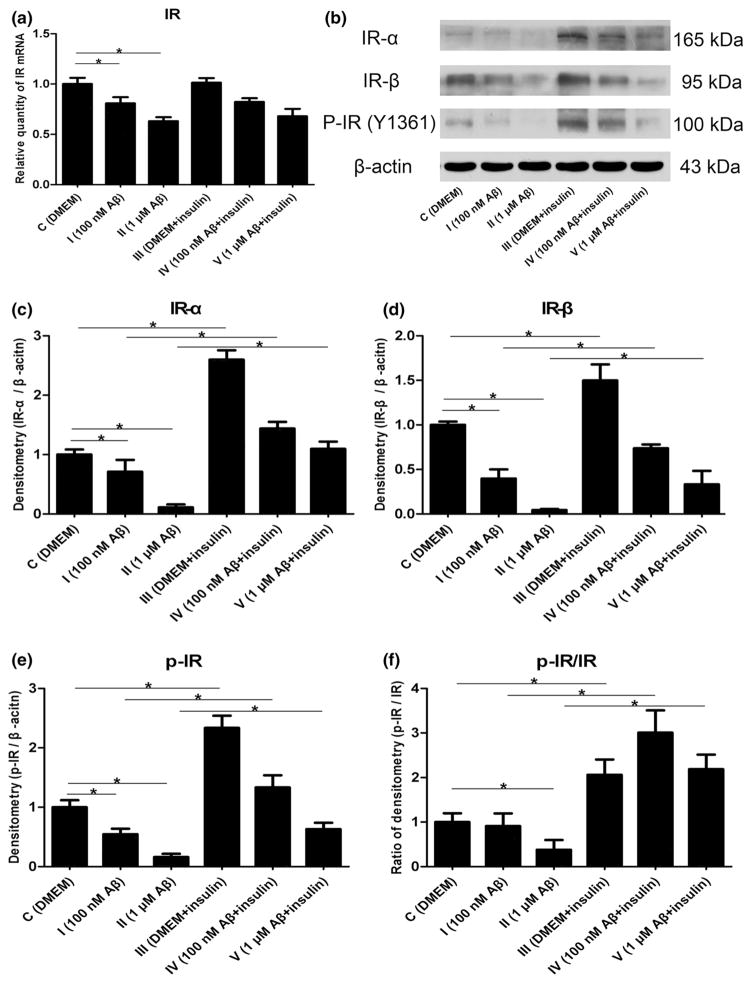
Brain IRs that are densely localized on the hippocampus and entorhinal cortex contribute to learning and memory, and are activated by tyrosine autophosphorylation of β subunits when insulin binds to α subunits. This receptor and its tyrosine kinase activity are substantially lost in AD brain, which could be markedly prevented by insulin (Talbot et al. 2012). To explore the impact of Aβ1–42 oligomers and insulin on IRs in astrocytes, we employed real-time RT-PCR analysis demonstrating that mRNA encoding IR was decreased by Aβ in a dose-dependent manner (Fig. 2a, p < 0.05). In addition, the protein levels of IR-α, IR-β, and p-IR at Tyr1361 were evaluated by Western blot analysis. We found that lower expressions of IR-α and IR-β, as well as a reduction in p-IR at Tyr1361, were potentiated with concentrations of the Aβ1–42 oligomers (Fig. 2b–e, p < 0.05). Although insulin treatment for 30 min had no effects on IR mRNA (Fig. 2a, p > 0.05), we found a significant effect on IR-α, IR-β and phosphoryla-tion of IR after exposure to insulin compared with their respective control groups (Fig. 2b–e, p < 0.05). In addition, the ratio of p-IR/IR-β was also raised by insulin (Fig. 2f, p < 0.05), meaning that the increment of p-IR was more than IR-β after insulin treatment.
Fig. 2.
Changes of IR mRNA and protein expression induced by Aβ1–42 oligomers and insulin: a mRNA levels of IR (F = 24.952, *p < 0.05); b representative western blot bands of IR-α, IR-β and p-IR at Tyr1361; c densitometric measurements of IR-α normalized by β-actin (F = 242.107, *p < 0.05); d densitometric measurements of IR-β normalized by β-actin (F = 70.964, *p < 0.05); e densitometric measurements of p-IR normalized by β-actin (F = 176.129, *p < 0.05); f the ratio of p-IR/IR-β (F = 27.365, *p < 0.05)
Aβ1–42 Oligomers and Insulin Altered the Expression of Protein Kinase B in Astrocytes
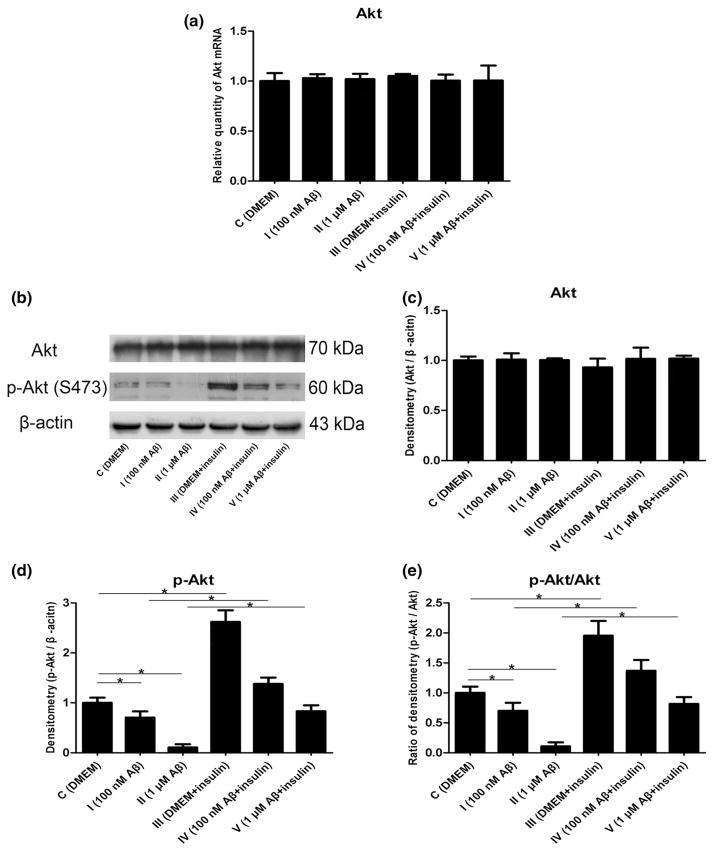
Akt, a component of the insulin signal pathway, has been shown to have beneficial functions in the brain, such as improving cell survival and promoting protein synthesis. It is detectable in astrocytes and can respond to growth factors including insulin. Additionally, it has been shown that the reduced phosphorylation of Akt is closely associated with increased intracellular Aβ levels (Lee et al. 2009), and insulin completely reverses this tendency (Yang et al. 2013). To determine whether Akt was affected by Aβ and insulin in astrocytes, we initially measured mRNA expression by real-time RT-PCR analysis and found that Akt mRNA was unchanged after incubation with Aβ1–42 oligomers or insulin (Fig. 3a, p >0.05). To determine the total protein and phosphorylation of Akt, we employed Western blot analysis and found that Akt total protein was unaltered (Fig. 3b, c, p >0.05), whereas Akt phosphorylation at Ser473 was significantly decreased by the soluble Aβ1–42 oligomers (Fig. 3b, d, p <0.05). Moreover, we also observed that insulin enhanced the phosphorylation of Akt compared with the control groups (Fig. 3b, d, p <0.05) but had no influ-ence on total protein of Akt (Fig. 3b, c, p >0.05). Furthermore, the ratio of phospho-protein kinase B (p-Akt)/Akt was also increased by insulin (Fig. 3e, p <0.05).
Fig. 3.
Changes in Akt mRNA and protein expression induced by Aβ1–42 oligomers and insulin: a mRNA levels of Akt (F = 0.193, p > 0.05); b representative Western blot bands of Akt; c densitometric measurements of Akt normalized by β-actin (F = 1.415, p > 0.05); d densitometric measurements of p-Akt normalized by β-actin (F = 172.558, *p < 0.05); e The ratio of p-Akt/Akt (F = 102.084, *p < 0.05)
Aβ1–42 Oligomers and Insulin Changed the Expression of Mammalian Target of Rapamycin in Astrocytes
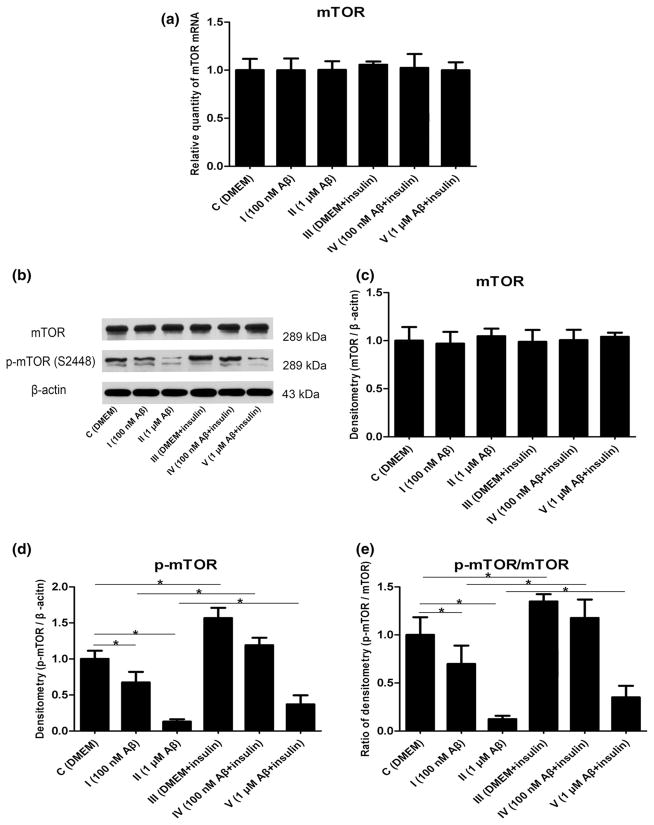
mTOR, a downstream target of Akt, is a key regulator of protein translation. It has been reported that its activity is directly linked to cell survival, axon guidance, protein synthesis, synaptic plasticity, and memory formation (Hulmi et al. 2012; Machado-Neto et al. 2011). To assess whether the mRNA of mTOR was modulated by Aβ and insulin, our real-time RT-PCR analysis demonstrated no significant differences among each group (Fig. 4a, p >0.05). To evaluate whether the protein levels of mTOR were affected by Aβ and insulin, we conducted further Western blot analysis. In our experiments, total protein of mTOR remained constant among all groups (Fig. 4b, c, p >0.05). However, decreased levels of phospho-mammalian target of rapamycin (p-mTOR) at Ser2448 were observed in the astrocytes by treatment with Aβ1–42 oligomers in a dose-dependent manner, which were reversed by insulin treatment for 30 min (Fig. 4b, d, p < 0.05). The ratio of p-mTOR/mTOR was also reduced by Aβ and increased by insulin in group III, group IV, and group V (Fig. 4e, p <0.05).
Fig. 4.
Changes in mTOR mRNA and protein expression induced by Aβ1–42 oligomers and insulin: a mRNA levels of mTOR (F = 0.149, p > 0.05); b representative Western blot bands of mTOR; c densitometric measurements of mTOR normalized by β-actin (F = 0.470, p > 0.05); d densitometric measurements of p-mTOR normalized by β-actin (F = 124.794, *p < 0.05); e The ratio of p-mTOR/mTOR (F = 64.520, *p <0.05)
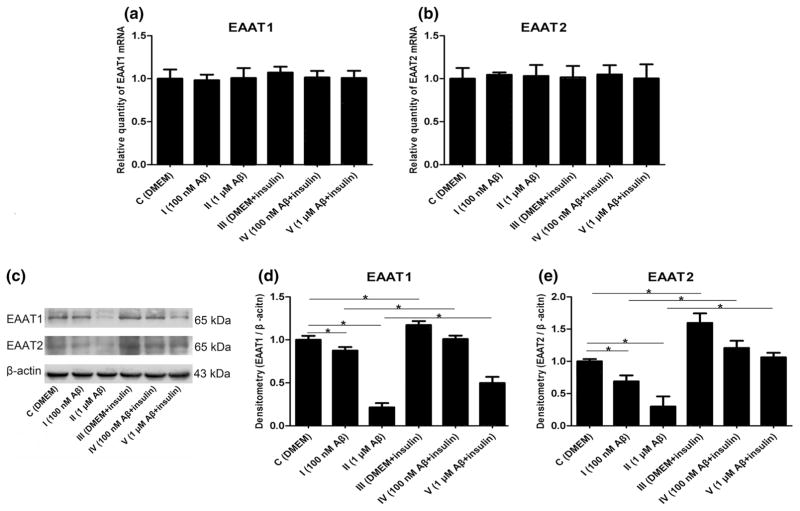
Aβ1–42 Oligomers and Insulin Changed the Expression of Excitatory Amino Acid Transporters in Astrocytes
EAAT1 and EAAT2 are expressed exclusively in astrocytes and are responsible for the bulk of glutamate uptake, which is a major mechanism that prevents excessive glutamate accumulation in the synaptic space and thus protects neurons from excitotoxicity. In the normal brain, both EAAT1 and EAAT2 are detectable in the hippocampus, a region of the brain that participates in learning and memory (Jacob et al. 2007). To determine whether the expressions of EAAT1 and EAAT2 were regulated by Aβ and insulin, we employed RT-PCR and found that the mRNA levels were not affected by these two drugs (Fig. 5a, b, p > 0.05). Subsequently, our Western blot results showed that the protein expressions of EAAT1 and EAAT2 were both lowered by the Aβ1–42 oligomers, whereas they were increased by insulin (Fig. 5c–e, p < 0.05).
Fig. 5.
Changes in EAAT1 and EAAT2 induced by Aβ1–42 oligomers and insulin: a mRNA levels of EAAT1 (F = 0.362, p > 0.05); b mRNA levels of EAAT2 (F = 0.092, p > 0.05); c representative western blot bands of EAAT1 and EAAT2; d densitometric measurements of EAAT1 normalized by β-actin (F = 312.936, *p < 0.05); e densitometric measurements of EAAT2 normalized by β-actin (F = 95.475, *p < 0.05)
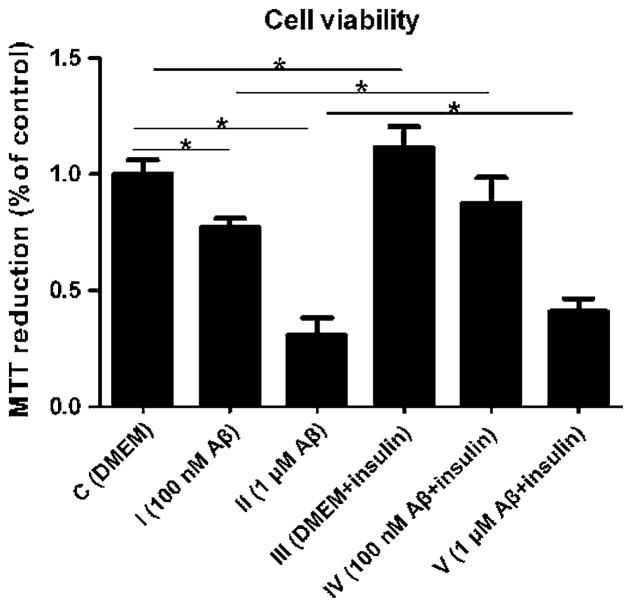
Cell Viability of the Astrocytes was Affected by Aβ1–42 Oligomers and Insulin
To further evaluate whether alteration of the insulin/Akt/ EAAT signaling pathway was associated with changes in cell viability, we incubated the astrocytes with Aβ1–42 oligomers and insulin and performed MTT analysis. Incubation with Aβ1–42 oligomers at 100 nM and 1 μM for 24 h caused a significant decrease of MTT reduction (23 and 70 %, respectively), whereas insulin exposure for 30 min induced an approximate 10 % increase of MTT reduction in both group IV and group V compared with the control groups (Fig. 6, p <0.05), suggesting that Aβ1–42 oligomers at 100 nM and 1 μM were toxic to the astrocytes and insulin at 100 nM had protective functions to the cells in the presence of Aβ1–42 oligomers at 100 nM and 1 μM (p < 0.05).
Fig. 6.
Cell viability was measured by MTT reduction: Aβ1–42 oligomers at 100 nM and 1 μM induced a significant decrease in MTT reduction compared with the control group and insulin exposure increased the MTT reduction in the presence of Aβ1–42 oligomers at 100 nM and 1 μM in both group IV and group V (F = 185.142, *p < 0.05)
Discussion
Aβ deposition in the brain is a crucial step in the onset and progress of AD through interference with a number of signaling cascades. However, the precise molecular pathway remains to be elucidated. It has been proposed as an initiating event in AD, and other neuropathologic features might be the consequence of aberrant accumulation of Aβ. Notably, it could disturb neuronal insulin signaling by desensitizing IRs, thus contributing to insulin resistance (de la Monte 2012). Therefore, we chose Aβ treatment of astrocytes as a cell culture AD model and adopted a modified method to prepare Aβ1–42 oligomers that could be kept in a stable state at −80 °C for an extended period of time so that it did not have to be used immediately after its preparation. Four weeks later, we detected Aβ1–42 oligomers with only traces of fibrillary aggregates (data not shown). This study aimed to investigate whether the disturbance in insulin/Akt/EAAT signaling was involved in AD pathogenesis and if insulin was a protective factor.
Astrocytes, which are basic components of the brain support system, play a critical role in controlling ion and transmitter concentration, regulating brain-blood barrier, resisting noxious substances, preserving connections in brain networks, maintaining synaptic birth and maturation, coordinating neuronal activity, and aiding in cognition (Giaume et al. 2007; Gibbs et al. 2008). Aβ appears to induce reactive astrogliosis, especially at higher concentrations (Fig. 1). When transforming into a reactive state, astrocytes lose their normal physiologic functions. Consequently, astrocyte malfunction might cause lesions to the CNS and play a central role in triggering a cascade, ultimately leading to neurodegenerative disease. It has been shown recently that astrocytes are actively involved in the inflammatory events associated with Alzheimer-type pathology (Avila-Munoz and Arias 2014) and astrocyte activation is discovered in the early stages of AD (Carter et al. 2012; Olabarria et al. 2010). Therefore, it is of great value to investigate changes in astrocytes in AD. Because there is much distinction between human astrocytes and rodent astrocytes in terms of morphology, physiology, and function (Sukhorukova et al. 2012), human cells are more suitable to study AD pathogenesis. However, primary cultured astrocytes from the human cerebral cortex are always difficult to obtain, thus we chose the cell line HA-1800 (Wang et al. 2013) for our research.
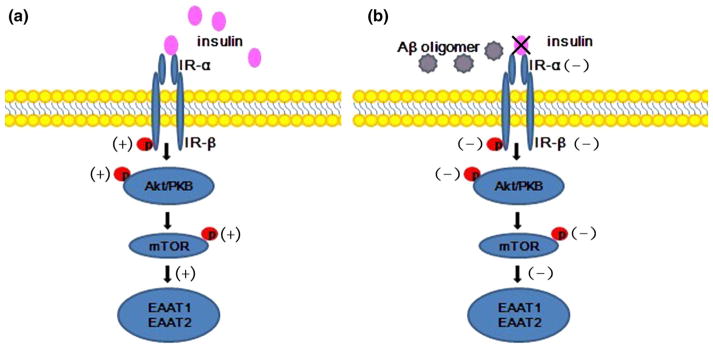
Brain insulin signaling is responsible for neuroendocrine function and cognition. An overview of this signal transduction pathway is shown in Fig. 7 (Franke 2008; Hers et al. 2011; Laplante and Sabatini 2009; Wu et al. 2010). Briefly, insulin binding to IR-α induces dimerization and autophosphorylation of IR-β, which subsequently triggers Akt phosphorylation at Ser473; mTOR is a downstream target of Akt. Hence Akt activation can result in increased phosphorylation of mTOR at Ser2448 and then induce EAAT protein synthesis. Moreover, all substrates have been detected in neurons and glial cells (On 2013a), and our previous study indicated that aberrant insulin signaling was present in AD (Han et al. 2012; Zhang et al. 2015). In this paper, we present evidence from cell cultures that alteration of the insulin/Akt/EAAT signaling cascade might be one of the potential pathogenesis of AD and insulin is capable to protect human astrocytes when oli-gomeric beta amyloid is added. First, we showed that Aβ1–42 oligomers decreased the levels of IR mRNA and IR protein and blocked IR activation in the astrocytes (Fig. 2). Persistent suppression of IR can cause brain insulin resistance. Moreover, the ratio of p-IR to IR was lessened by Aβ1–42 oligomers in a dose-dependent manner (Fig. 2f), meaning that the reduction of IR phosphorylation at Tyr1361 was more obvious than total IR expression when Aβ1–42 oligomers was added. Furthermore, it was revealed that the decrease in p-Akt occurred in brains from rats with type 2 diabetes; a disease that disrupts the common cellular and molecular pathways associated with AD (Correia et al. 2012; Yang et al. 2013). In addition, Aβ could cause reductions in mTOR phosphorylation at the Ser2448 site (Lafay-Chebassier et al. 2005, 2006). The findings are consistent with our observations that the astrocytes exhibited a striking loss of p-Akt at Ser473 and p-mTOR at Ser2448 (Figs. 3b, d, 4b, d). mRNA and total protein levels of both these kinases were similar to that of the control groups (Figs. 3a–c, 4a–c). We speculate that there might be three explanations for this phenomenon. First, Aβ can remove IR via Aβ-triggered endocytosis or by disturbing gene transcription and protein translation or by destroying the IR mRNA and protein directly. Second, Aβ may interfere with the modifications of Akt and mTOR proteins, rather than affect the gene transcription and protein translation directly. Third, the impairment of IR or p-IR might interfere with the phosphorylation of Akt and mTOR through the insulin signaling pathway. However, the specific mechanism is still unknown and further research will be needed. On the contrary, increased phospho-Ser473 Akt was reported in AD patients (Griffin et al. 2005).This divergence may be due to the different degrees of the disease and the diverse regions of the pathological changes. In addition, several studies showed neuronal PI3 K/Akt/ mTOR signaling was activated aberrantly and sustainedly in AD brain (On 2013b; Gupta and Dey 2012), this is mechanistically linked to progressive desensitization of insulin responses. More detailed studies will be necessary to clarify this point.
Fig. 7.
Schematic representation of insulin/Akt/EAAT signaling pathway. a Insulin binding to IR-α induces dimerization and autophosphorylation of IR-β, which subsequently triggers Akt phosphorylation. mTOR is an important downstream target of Akt, and Akt activation can result in increased phosphorylation of mTOR, which then induces EAAT protein synthesis. b Aβ oligomers can destroy the IRs, disrupting expression of the downstream substrates and disturbing glutamate metabolism
Glutamate is the main excitatory neurotransmitter in the brain. Excessive glutamate accumulation in the extracellular spaces triggers excitotoxic neuronal damage, which has been implicated in many neurodegenerative disorders including AD. There are no enzymes for metabolizing this neurotransmitter in the synaptic space and the main pathway to maintain glutamate homeostasis is its reuptake via EAAT1 and EAAT2 (Danbolt 2001). It was reported that the loss of EAAT can impair glucose utilization and promote CNS dysfunction over time (Cassano et al. 2012). In our experiment, although the mRNA levels of EAAT1 and EAAT2 were unchanged, the protein expressions were reduced by the Aβ1–42 oligomers (Fig. 5). We concluded that the capacity of glutamate clearance was attenuated by Aβ. There are conflicting results demonstrating that Aβ could upregulate glutamate transporters in primary cultured cortical astrocytes or in mice brains (Peters et al. 2009). It may be a compensatory reaction a low dose of Aβ can increase the expressions of EAAT1 and EAAT2 at the early stage of AD, whereas a high burden of Aβ may cause injury to these transporters, which results in the disturbance of glutamate recycling.
Insulin contributes to cognition, cerebral glucose metabolism, potentiation of synaptic responses, and regulation of neuronal survival through the insulin signaling pathway. In addition, insulin can attenuate pathogenic binding of Aβ and synaptic deficits (Chen et al. 2014). Although insulin mRNA and insulin protein synthesis is not measurable in astrocytes, these glial cells could be influenced by insulin (Henneberger et al. 2010). It has been reported that IR and p-IR expressions are markedly raised by insulin in neurons (Jolivalt et al. 2008). In our experiment, insulin induced a significant increase in IR protein and its phosphorylation at Tyr1361 without altering IR mRNA in astrocytes (Fig. 2). Furthermore, we found that insulin enhanced the levels of p-Akt at Ser473 and p-mTOR at Ser2448 (Figs. 3b, d, 4b, d), and we also showed that protein expressions of EAAT1 and EAAT2 were both improved by insulin compared with the control groups (Fig. 5). As a consequence, we deduce that insulin can compete with Aβ and reduce the affinity of Aβ binding to the remaining IRs, thereby triggering the activation of insulin signaling. Moreover, we uncovered an adverse effect of Aβ1–42 oligomers on astrocyte survival and a considerably beneficial role of insulin in improving cell viability (Fig. 6). Randomized and double-blind trials have proved that intranasal insulin therapy improves cognitive function in patients with mild cognitive impairment or with AD (Craft et al. 2012). However, others have shown that chronically high insulin levels might be deleterious to the brain (Cole and Frautschy 2007). One possible explanation for this inconsistency might be the different drug doses or diverse administration routes of insulin. Together, large-scale investigations are essential to explore the status and efficacy of insulin therapy.
In conclusion, the present study demonstrated that soluble Aβ oligomers could disrupt the glutamate metabolic system in astrocytes by perturbing insulin signaling and impairing EAATs. Subsequently, astrocytes lose their neuroprotective ability and induce neuronal injury. In addition, our data also indicate that insulin activated protective functions for the astrocytes, even though chronically high levels in the brain might be deleterious.
The limitations of this study are that experiments in vivo were not done, and the precise interaction mechanism among these signaling proteins has not been elucidated. However, this study does suggest that strategies aimed at restoring astrocytic function might be a potential treatment for AD, and insulin or drugs designed to specifically enhance CNS insulin signaling may provide therapeutic benefits for the treatment of AD.
Supplementary Material
Acknowledgments
This study was supported by the Natural Science Foundation of Shandong Province (Y2008C116) and the National Natural Science Foundation of China (Grant No. 81100802). We gratefully acknowledge the excellent assistance of the Education Key Laboratory of Experimental Teratology and Institute of Molecular Medicine and Genetics, Shandong University Medicine School.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10571-015-0268-5) contains supplementary material, which is available to authorized users.
References
- Avila-Munoz E, Arias C. When astrocytes become harmful: functional and inflammatory responses that contribute to Alzheimer’s disease. Ageing Res Rev. 2014;18:29–40. doi: 10.1016/j.arr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Birch AM. The contribution of astrocytes to Alzheimer’s disease. Biochem Soc Trans. 2014;42(5):1316–1320. doi: 10.1042/bst20140171. [DOI] [PubMed] [Google Scholar]
- Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Investig. 2012;122(4):1339–1353. doi: 10.1172/jci57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, Nordberg A. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53(1):37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- Cassano T, Serviddio G, Gaetani S, Romano A, Dipasquale P, Cianci S, Bellanti F, Laconca L, Romano AD, Padalino I, LaFerla FM, Nicoletti F, Cuomo V, Vendemiale G. Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging. 2012;33(6):1121.e1. doi: 10.1016/j.neurobiolaging.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Rudan I. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381(9882):2016–2023. doi: 10.1016/s0140-6736(13)60221-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Deng Y, Zhang B, Gong CX. Deregulation of brain insulin signaling in Alzheimer’s disease. Neurosci Bull. 2014;30(2):282–294. doi: 10.1007/s12264-013-1408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer’s Disease. Exp Gerontol. 2007;42(1–2):10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106(6):1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr Alzheimer Res. 2012;9(1):35–66. doi: 10.2174/156720512799015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Type 3 diabetes is sporadic Alzheimers disease: mini-review. European Neuropsychopharmacol. 2014;24(12):1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, Rego AC. Amyloid beta peptide 1–42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium. 2012;51(2):95–106. doi: 10.1016/j.ceca.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27(50):6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14(7):1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32(5):927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93(1):105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dey CS. PTEN, a widely known negative regulator of insulin/PI3 K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell. 2012;23(19):3882–3898. doi: 10.1091/mbc.E12-05-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Ma Y, Liu X, Wang L, Qi S, Zhang Q, Du Y. Changes in insulin-signaling transduction pathway underlie learning/ memory deficits in an Alzheimer’s disease rat model. J Neural Transm. 2012;119(11):1407–1416. doi: 10.1007/s00702-012-0803-1. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463(7278):232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Hulmi JJ, Silvennoinen M, Lehti M, Kivela R, Kainulainen H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am J Physiol Endocrinol Metab. 2012;302(3):E307–315. doi: 10.1152/ajpendo.00398.2011. [DOI] [PubMed] [Google Scholar]
- Ito S, Menard M, Atkinson T, Gaudet C, Brown L, Whitfield J, Chakravarthy B. Involvement of insulin-like growth factor 1 receptor signaling in the amyloid-beta peptide oligomers-induced p75 neurotrophin receptor protein expression in mouse hippocampus. J Alzheimer’s Dis. 2012;31(3):493–506. doi: 10.3233/jad-2012-120046. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimer’s Dis. 2007;11(1):97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- Ji YF, Xu SM, Zhu J, Wang XX, Shen Y. Insulin increases glutamate transporter GLT1 in cultured astrocytes. Biochem Biophys Res Commun. 2011;405(4):691–696. doi: 10.1016/j.bbrc.2011.01.105. [DOI] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86(15):3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulijewicz-Nawrot M, Sykova E, Chvatal A, Verkhratsky A, Rodriguez JJ. Astrocytes and glutamate homoeostasis in Alzheimer’s disease: a decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. ASN Neuro. 2013;5(4):273–282. doi: 10.1042/an20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer’s disease. J Neurochem. 2005;94(1):215–225. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- Lafay-Chebassier C, Perault-Pochat MC, Page G, Rioux Bilan A, Damjanac M, Pain S, Houeto JL, Gil R, Hugon J. The immunosuppressant rapamycin exacerbates neurotoxicity of Abeta peptide. J Neurosci Res. 2006;84(6):1323–1334. doi: 10.1002/jnr.21039. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(Pt 20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115(2):112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kumar P, Fu Q, Rosen KM, Querfurth HW. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20(5):1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225(1):54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Colome AM, Martinez-Lozada Z, Guillem AM, Lopez E, Ortega A. Glutamate transporter-dependent mTOR phosphorylation in Muller glia cells. ASN Neuro. 2012 doi: 10.1042/an20120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Neto JA, Favaro P, Lazarini M, Costa FF, Saad STO, Traina F. Knockdown of insulin receptor substrate 1 reduces proliferation and downregulates Akt/mTOR and MAPK pathways in K562 cells. Biochim Biophys Acta. 2011;8:1404–1411. doi: 10.1016/j.bbamcr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58(7):831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- On C. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013a doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- On C. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013b;48(7):647–653. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Pearson-Leary J, McNay EC. Intrahippocampal administration of amyloid-beta(1–42) oligomers acutely impairs spatial working memory, insulin signaling, and hippocampal metabolism. J Alzheimer’s Dis. 2012;30(2):413–422. doi: 10.3233/jad-2012-112192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters O, Schipke CG, Philipps A, Haas B, Pannasch U, Wang LP, Benedetti B, Kingston AE, Kettenmann H. Astrocyte function is modified by Alzheimer’s disease-like pathology in aged mice. J Alzheimer’s Dis. 2009;18(1):177–189. doi: 10.3233/jad-2009-1140. [DOI] [PubMed] [Google Scholar]
- Ryan DA, Narrow WC, Federoff HJ, Bowers WJ. An improved method for generating consistent soluble amyloid-beta oligomer preparations for in vitro neurotoxicity studies. J Neurosci Methods. 2010;190(2):171–179. doi: 10.1016/j.jneumeth.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39(1):115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhorukova EG, Alekseeva OS, Kirik OV, Grudinina NA, Korzhevskii DE. Comparative aspects of structural organization of astrocytes of the first layer of the human and rat cerebral cortex. Zh Evol Biokhim Fiziol. 2012;48(3):280–286. [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Investig. 2012;122(4):1316–1338. doi: 10.1172/jci59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JH, Pasternak SH. Amyloid and Alzheimer’s disease: inside and out. Can J Neurol Sci. 2012;39(3):286–298. doi: 10.1017/s0317167100013408. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang K, Zhao J, Liu X, Bu J, Yan X, Huang R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J Am Chem Soc. 2013;135(12):4799–4804. doi: 10.1021/ja312221g. [DOI] [PubMed] [Google Scholar]
- Wharton SB, O’Callaghan JP, Savva GM, Nicoll JA, Matthews F, Simpson JE, Forster G, Shaw PJ, Brayne C, Ince PG. Population variation in glial fibrillary acidic protein levels in brain ageing: relationship to Alzheimer-type pathology and dementia. Dement Geriatr Cogn Disord. 2009;27(5):465–473. doi: 10.1159/000217729. [DOI] [PubMed] [Google Scholar]
- Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H. PI3K/ Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393(3):514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma D, Wang Y, Jiang T, Hu S, Zhang M, Yu X, Gong CX. Intranasal insulin ameliorates tau hyperphosphorylation in a rat model of type 2 diabetes. J Alzheimer’s Dis. 2013;33(2):329–338. doi: 10.3233/jad-2012-121294. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Guo S, Zhang X, Tang S, Wang L, Han X, Shao W, Cong L, Du Y. Amyloid beta oligomer-induced ERK1/2-dependent serine 636/639 phosphorylation of insulin receptor substrate-1 impairs insulin signaling and glycogen storage in human astrocytes. Gene. 2015;561(1):76–81. doi: 10.1016/j.gene.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chan KH, Chu LW, Kwan JS, Song YQ, Chen LH, Ho PW, Cheng OY, Ho JW, Lam KS. Plasma amyloid-beta oligomers level is a biomarker for Alzheimer’s disease diagnosis. Biochem Biophys Res Commun. 2012;423(4):697–702. doi: 10.1016/j.bbrc.2012.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.