Abstract
Objective
Surgery is the most important curative treatment modality for patients with early stage non-small cell lung cancer (NSCLC). We examined the pattern of surgical resection for NSCLC in a high incidence and mortality region of the US over a 10-year period (2004–2013) in the context of a regional surgical quality improvement initiative.
Methods
We abstracted patient-level data on all resections at 11 hospitals in 4 contiguous Dartmouth Hospital Referral Regions in North Mississippi, East Arkansas and West Tennessee. Surgical quality measures focused on intraoperative practice, with emphasis on pathologic nodal staging. We used descriptive statistics and trend analyses to assess changes in practice over time. To measure the impact of an ongoing regional quality improvement intervention with a lymph node specimen collection kit, we used period effect analysis to compare trends between the pre- and post-intervention periods.
Results
Of 2,566 patients, 18% had no preoperative biopsy, only 15% had a preoperative invasive staging test, and 11% underwent mediastinoscopy. The rate of resections with no mediastinal lymph nodes examined decreased from 48% to 32% (p<0.0001) while the rate of resections examining 3 or more mediastinal stations increased from 5% to 49% (p<0.0001). There was a significant period effect in the increase in the number of N1, mediastinal and total lymph nodes examined (all p<0.0001).
Conclusion
A quality improvement intervention including a lymph node specimen collection kit shows early signs of having a significant positive impact on pathologic nodal examination in this population-based cohort. However, gaps in surgical quality remain.
INTRODUCTION
The predicted aggregate 5-year survival of the 230,000 individuals diagnosed annually with lung cancer in the US is approximately 18%.1 Most of these 5-year survivors are patients with early stage non-small cell lung cancer (NSCLC) who had curative-intent surgical resection.2 However, fewer than 50% of patients who have surgery for lung cancer survive 5 years.3, 4 Gaps in the quality of resection and pathologic nodal staging are associated with lower-than-expected long term survival rates in populations.5–8
In the surgical resection population, the pathologic nodal stage is a major determinant of prognosis.9 Variation in the quality of pathologic nodal staging drives long-term survival disparities.5–8,10,11 Theoretically, improving access to high-quality surgical care for early-stage NSCLC is a major opportunity to improve population-level lung cancer survival. Such efforts must focus on improving surgical and pathologic processes critical for thorough and accurate pathologic nodal staging in order to provide the intrinsic benefit of oncologically sound resection, ensure identification of residually high-risk patients who may benefit from existing adjuvant therapy and, importantly, correctly categorize patients enrolled into clinical trials of novel adjuvant treatments.
The Mid-South region of the US, which includes North Mississippi, East Arkansas and West Tennessee, has some of the highest lung cancer incidence and mortality rates in the US. Major problems of healthcare access, quality, and outcome disparities exist in this region.12–14 Using a comparative observational population-based cohort study design, we examined the pattern of surgical resection for NSCLC in a heterogeneous group of institutions in this region to test the early, population-level impact of specific regional quality improvement efforts.
METHODS
Patient cohort
The study was approved, with a waiver of informed consent, by the Institutional Review Boards at all participating institutions. We retrospectively studied all patients receiving primary lung cancer surgery from January 1, 2009, to December 31, 2013, at 11 hospitals from 4 pre-specified, geographically contiguous Dartmouth Referral Regions in East Arkansas, North Mississippi and West Tennessee. Six of the 11 institutions had participated in a previous, more limited retrospective review of lung cancer resections from January 1, 2004 to December 31, 2008 (Appendix 1).14 Institutions within these Hospital Referral Regions were eligible to participate in this Mid-South Quality of Surgical Resection (MS-QSR) database if they averaged 5 or more curative-intent resections per year. The degree of detail about preoperative tests and disease characteristics in the earlier database was more limited, but operative, post-operative, and pathology details were consistent across all 10 years. The MS-QSR database is now prospectively updated, but the current analysis is based on retrospectively collected data up to the point of prospective collection.
Patients at each institution were identified and screened for eligibility by cross-referencing three separate lists provided by each institution’s Health Information Management (using ICD-9 procedure codes 32.20 – 32.90 [wedge to pneumonectomy, plus “other excision”]), Tumor Registry, and Pathology Departments (using self-selected search algorithms). Resections for benign lung disease, small cell lung cancer, and non-lung primary cancers were excluded. Only the first resection was included for analysis for patients receiving multiple lung resections. At the time of data censoring, the MS-QSR database contained records of 2,566 eligible patients (Appendix 2). Except where noted, analysis was limited to variables available over the full 10 years.
Data abstraction
Trained, dedicated research staff reviewed all available inpatient and outpatient clinical records, starting with the final post-resection pathology report and working back to the initial radiology report that showed an abnormal lung lesion, to abstract all relevant data from operation reports, admission notes, discharge summaries, nurses’ notes, progress notes, pre-surgical physiologic clearance tests, clinic notes, radiologic studies, invasive diagnostic and staging tests, and their associated pathology reports. The number of lymph nodes examined was strictly abstracted from the pathology reports. Whole lymph nodes were counted individually, all reference to lymph node fragments or non-quantitative descriptions such as ‘multiple’ were counted as a single lymph node to avoid over-counting bias.
Definition of quality measures for surgical care
We grouped surgical quality care measures into three categories: preoperative, intraoperative, and postoperative. We measured the pattern of use of certain preoperative diagnostic and staging tests as a surrogate for the quality of pre-surgical care. Diagnostic tests were defined as any attempted pre-resection biopsy to confirm a suspected lung cancer. Staging tests were classified as either non-invasive or invasive. Non-invasive staging tests included PET/CT scans and non-thoracic radiographic scans performed after lesion identification, but before surgical resection. Invasive staging tests were defined as any attempted biopsy of an anatomic site other than the site of the primary lung lesion. These included mediastinoscopy and bronchoscopic lymph node biopsies.
Intraoperative measures of surgical quality included the rates of pneumonectomy, margin-positivity, and use of minimally-invasive surgical techniques. Intraoperative lymph node examination quality was defined by two broad parameters: median lymph node counts and rates of lymph node examination. Median lymph counts included lymph nodes sampled from station 10 (hilar), stations 11–14 (intrapulmonary), all N1, all mediastinal, and all lymph node stations. Lymph node examination rates included resections with zero lymph nodes (pNX rate), resections with no mediastinal lymph nodes, resections with sampling of hilar, and subcarinal (station 7) lymph nodes, and resections with 3 or more mediastinal stations (a quality standard recommended by the National Comprehensive Cancer Network).15 Intraoperative lymph node counts were restricted to the surgical resection only, and do not include lymph node sampling done pre-operatively with invasive staging biopsies.
Postoperative quality measures were 30- and 60-day readmission rates, mortality rates at 30-, 60-, and 90-days, and 1-year overall survival rates.
Quality improvement initiatives
From 2008 to 2010, our research group analyzed the pattern of surgical resection in Metropolitan Memphis Hospitals from 2004 to 2007 and shared summary details on the prevalence and survival implications of the pathologic nodal staging quality gap with administrators, surgeons and pathologists at participating hospitals.7,8,12,14 In 2009, we began a coordinated program to improve mediastinal lymph node examination. This initiative had two parallel tracks: a regional educational effort to inform clinicians about the quality gap in surgical lymph node examination (problem definition)14, 16 and the introduction, in 2010, of a lymph node specimen collection kit for use in the operating room (solution implementation).12,17,18 Details on the characteristics of this kit have been provided previously (Appendix 3).17,18 The ongoing educational effort includes multiple individual and group meetings with hospital administrators, surgeons, pathologists and cardiovascular surgery operating room staff across the region. In 2010, we began pilot studies with the lymph node specimen collection kit at 2 institutions. This has since expanded to a regional intervention project using a multiple baseline, staggered implementation strategy.19 Using a conservative approach, we chose 2010, the year the kit was introduced, as the fulcrum date between the pre- and post-implementation periods.
By comparing the difference between trends in the pre-intervention (2004 – 2009) and post-intervention (2010 – 2013) periods, we sought early signals of the impact of this quality improvement program on process measures of the quality of lung cancer surgical practice at the broad population level.
Statistical methodology
We used descriptive statistics to summarize patients’ socio-demographic, clinical and management characteristics, including procedures performed pre-, intra- and post-operatively. All trend plots are unadjusted; T-test was used for comparing continuous variables, and chi-squared test for categorical variables.
To test linear trends and period effects of surgical quality indicators, we employed linear regression models for continuous outcomes, and logistic regression for binary outcomes including pneumonectomy, resection margin, and open thoracotomy over time. We used Poisson regression to assess the trend of the total number of lymph nodes, mediastinal lymph nodes, and intrapulmonary lymph nodes examined over time. Period effects were assessed by including the period indicator, 2010 (when the lymph node collection kit was introduced), in the various models. The difference in trends between the pre-intervention and post-intervention periods was assessed with the interaction between time and period in the same models. If the difference was statistically significant, this constituted a period effect. If the difference was not statistically significant, then the interaction term was dropped from the model. Multi-variable analyses were also performed, adjusting for stage, margin status, age, sex, race, insurance, histology, number of comorbidities, institution, and use of the lymph node kit (not included in the period effect model), and are reported where notably different from univariate analyses. We explicitly included the surgical kit use indicator in the various models to assess the impact of surgical kit use on the quality indicators related to lymph nodes. Finally, we performed a sensitivity analysis restricted to the 6 institutions (representing 79% of the cohort) with data available over the full timespan of the study.
In this way, we used a trend analysis to illustrate significant changes in practice over the full 10-year timespan, and the period effect analysis to examine for significant change in trends between the pre-intervention and post-intervention periods that were associated with our quality improvement initiatives. All statistical analyses were performed using SAS 9.4. (2013, SAS Institute Inc., Cary, NC). All tests were two-sided, and p<0.05 was considered statistically significant. We made no adjustments for multiple testing.
RESULTS
Overall cohort characteristics
For this analysis, the MS-QSR cohort includes 2,566 patients with NSCLC, of which 1,215 (47%) were female and 1,351 (53%) male. The majority of patients were Caucasian (78%), 21% were African-American and 1% were of another race. The median age of the cohort was 67 years, 49% had Medicare as their primary insurance, 35% had primary commercial insurance, 12% had Medicaid and 4% were uninsured.
Fifty-two percent of patients had adenocarcinoma, 35% had squamous cell carcinoma and 13% had other histologic variants. In this surgical resection population, 84% had either pT1 or pT2 tumors. Nodal metastasis was noted in the final pathology result in 21% of cases: 13% were pN1 and 8% were pN2. The majority of patients (83%) were Stage IA – IIB; 15% were Stage IIIA – IV; 17 patients (1%) were pathologic Stage 0; and the records were insufficient to report pathologic stage for 15 patients (Table 1).
Table 1.
Patient Demographic and Clinical Characteristics
| All Stages (n = 2,566) |
Stage IA – IIB (n = 2,143) |
Early Era (2004–2009) (n = 1,296) |
Late Era (2010–2013) (n=1,270) |
|||||
|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics |
||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Age | 67 | (61,74) | 68 | (61,74) | 67 | (61,74) | 68 | (61,74) |
| No. | % | No. | % | No. | % | No. | % | |
| Age Group | ||||||||
| <65 | 950 | 37 | 766 | 36 | 484 | 37 | 466 | 37 |
| 65–74 | 1049 | 41 | 880 | 41 | 512 | 40 | 537 | 42 |
| 75–84 | 534 | 21 | 471 | 22 | 282 | 22 | 252 | 20 |
| >85 | 33 | 1 | 26 | 11.22 | 18 | 1 | 15 | 1 |
| Sex | ||||||||
| Male | 1351 | 53 | 1125 | 52 | 685 | 53 | 666 | 52 |
| Female | 1215 | 47 | 1018 | 48 | 611 | 47 | 604 | 48 |
| Race | ||||||||
| White | 2013 | 78 | 1693 | 79 | 999 | 77 | 1014 | 80 |
| Black | 532 | 21 | 431 | 20 | 291 | 22 | 241 | 19 |
| Other | 21 | 1 | 19 | 1 | 6 | 1 | 15 | 1 |
| Insurance | ||||||||
| Medicare | 1257 | 49 | 1081 | 50 | 673 | 52 | 584 | 46 |
| Medicaid | 313 | 12 | 256 | 12 | 152 | 12 | 161 | 13 |
| Commercial | 901 | 35 | 738 | 34 | 427 | 33 | 474 | 37 |
| None | 95 | 4 | 68 | 3 | 44 | 3 | 51 | 4 |
| Histology | ||||||||
| Adenocarcinoma | 1348 | 52 | 1108 | 52 | 664 | 51 | 684 | 54 |
| Squamous | 889 | 35 | 772 | 36 | 461 | 36 | 428 | 34 |
| Adenosquamous | 72 | 3 | 61 | 3 | 45 | 3 | 27 | 2 |
| Large Cell | 115 | 4 | 91 | 4 | 63 | 5 | 52 | 4 |
| Others | 142 | 6 | 111 | 5 | 63 | 5 | 79 | 6 |
| Histologic Grade | ||||||||
| Well differentiated | 271 | 11 | 249 | 12 | 117 | 9 | 154 | 12 |
| Moderately differentiated |
1076 | 42 | 922 | 43 | 556 | 43 | 520 | 41 |
| Poorly differentiated | 774 | 30 | 627 | 29 | 392 | 30 | 382 | 30 |
| Undifferentiated | 55 | 2 | 42 | 2 | 24 | 2 | 31 | 2 |
| Not Reported | 390 | 15 | 303 | 14 | 207 | 16 | 183 | 14 |
| Median | Std. Dev. | Median | Std. Dev. | Median | Std. Dev. | |||
| Tumor Size (in cm) | 2.50 | 4.68 | 2.5 | 5.98 | 2.6 | 2.8 | ||
| No. | % | No. | % | No. | % | No. | % | |
| Pathologic T classification | ||||||||
| T1 | 1172 | 46 | 1097 | 51 | 614 | 47 | 558 | 44 |
| T2 | 987 | 38 | 864 | 40 | 502 | 39 | 485 | 38 |
| T3 | 266 | 10 | 177 | 8 | 88 | 7 | 178 | 14 |
| T4 | 101 | 4 | -- | -- | 72 | 6 | 29 | 2 |
| Others | 40 | 2 | 5 | 0 | 20 | 1 | 20 | 2 |
| Pathologic N classification |
||||||||
| N0 | 1785 | 70 | 1671 | 78 | 902 | 70 | 883 | 70 |
| N1 | 333 | 13 | 256 | 12 | 171 | 13 | 162 | 13 |
| N2 | 212 | 8 | -- | -- | 95 | 7 | 117 | 9 |
| NX | 236 | 9 | 216 | 10 | 128 | 10 | 108 | 8 |
| Pathologic M classification |
||||||||
| M0 | 2051 | 80 | 1727 | 81 | 804 | 62 | 1247 | 98 |
| M1 | 49 | 2 | -- | -- | 27 | 2 | 22 | 1 |
| MX | 466 | 18 | 416 | 19 | 465 | 36 | 1 | 1 |
| Pathologic stage | ||||||||
| IA | 1011 | 39 | 1011 | 47 | 522 | 40 | 489 | 38 |
| IB | 629 | 24 | 629 | 29 | 335 | 26 | 294 | 23 |
| IIA | 232 | 9 | 232 | 11 | 92 | 7 | 140 | 11 |
| IIB | 271 | 11 | 271 | 13 | 136 | 10 | 135 | 10 |
| IIIA | 284 | 11 | -- | -- | 112 | 9 | 172 | 13 |
| IIIB | 57 | 2 | -- | -- | 54 | 4 | 3 | 1 |
| IV | 50 | 2 | -- | -- | 28 | 2 | 22 | 2 |
| 0 | 17 | 1 | -- | -- | 4 | 1 | 13 | 1 |
| Not Reported | 15 | 1 | -- | -- | 13 | 1 | 2 | 1 |
Pattern of pre-operative care
Because of missing data in the earlier (2004–2008) dataset, the pre-operative quality of care analysis over the full 10 years was limited to use of PET/CT scan and mediastinoscopy as staging tests. Over that timespan, 1,631 patients (64%) had a pre-operative PET/CT, but only 283 (11%) had a pre-operative mediastinoscopy. Pre-operative chemotherapy and/or radiation therapy was used in 136 patients (5%).
Pattern of operative care
Seventy-three percent of patients underwent lobectomy, 13% had sublobar resections and 14% received bi-lobectomy or pneumonectomy. Seventy-seven percent of resections were completed as open thoracotomy, 14% were video-assisted thoracoscopic resections and 9% were robotically-assisted. Final resection margins were positive in 6% of cases (Table 2a).
Table 2.
| a - Surgical Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| All Stages (N=2566) |
Stage IA – IIB (N=2143) |
Early Era (2004–2009) (N=1296) |
Late Era (2010–2013) (N=1270) |
|||||
| Surgical Characteristics | No. | % | No. | % | No. | % | No. | % |
| Extent of Resection | ||||||||
| Pneumonectomy | 209 | 8 | 141 | 7 | 121 | 9 | 88 | 7 |
| Bilobectomy | 159 | 6 | 127 | 6 | 84 | 6 | 75 | 6 |
| Lobectomy | 1880 | 73 | 1608 | 75 | 936 | 72 | 944 | 74 |
| Segmentectomy | 76 | 3 | 66 | 3 | 34 | 3 | 42 | 3 |
| Wedge | 242 | 10 | 201 | 9 | 121 | 9 | 121 | 10 |
| Surgical Technique | ||||||||
| Open | 1976 | 77 | 1619 | 76 | 1049 | 81 | 927 | 73 |
| RATS | 238 | 9 | 209 | 10 | 50 | 4 | 188 | 15 |
| VATS | 352 | 14 | 315 | 15 | 197 | 15 | 155 | 12 |
| Gross Margins | ||||||||
| Positive | 147 | 6 | 92 | 4 | 80 | 6 | 67 | 5 |
| Negative | 2326 | 91 | 1984 | 93 | 1173 | 91 | 1153 | 91 |
| Not reported | 93 | 3 | 67 | 3 | 43 | 3 | 50 | 4 |
| 30 Day Readmission* (2009–2013) | ||||||||
| No | 1505 | 94 | 1267 | 94 | N/A | 1190 | 94 | |
| Yes | 110 | 7 | 83 | 6 | N/A | 80 | 6 | |
| 60 Day Readmission*(2009–2013) | ||||||||
| No | 1404 | 87 | 1188 | 88 | N/A | 1118 | 88 | |
| Yes | 211 | 13 | 162 | 12 | N/A | 152 | 12 | |
| Mortality (number of deaths, %) | ||||||||
| In-hospital mortality | 118 | 5 | 98 | 5 | 69 | 5 | 49 | 4 |
| 30-day mortality | 114 | 4 | 93 | 4 | 62 | 5 | 52 | 4 |
| 60-day mortality | 172 | 7 | 136 | 6 | 99 | 8 | 73 | 6 |
| 90-day mortality | 218 | 8 | 167 | 8 | 120 | 9 | 98 | 8 |
| 1 year survival (number initially at risk, %) |
||||||||
| All Stages | 2118 | 83 | ||||||
| Stage IA | 1011 | 90 | ||||||
| Stage IB | 629 | 82 | ||||||
| Stage IIA | 232 | 81 | ||||||
| Stage IIB | 271 | 74 | ||||||
| Stage IIIA | 284 | 73 | ||||||
| Stage IIIB | 57 | 72 | ||||||
| Stage IV | 50 | 68 | ||||||
| b – Surgical Lymph Node Examination | ||||||||
|---|---|---|---|---|---|---|---|---|
| All Stages (N=2566) |
Stage IA – IIB (N=2143) |
Early Era (2004–2009) (N=1296) |
Late Era (2010–2013) (N=1270) |
|||||
| Surgical Lymph Node (LN) | ||||||||
| Examination | No. | % | No. | % | No. | % | No. | % |
| pNx | ||||||||
| No | 2330 | 91 | 1927 | 90 | 1168 | 90 | 1162 | 91 |
| Yes | 236 | 9 | 216 | 10 | 128 | 10 | 108 | 9 |
| Any LNs with metastatic disease? | ||||||||
| No | 2017 | 79 | 1883 | 88 | 1026 | 79 | 991 | 78 |
| Yes | 549 | 21 | 260 | 12 | 270 | 21 | 279 | 22 |
| Any N1 LNs examined? | ||||||||
| No | 399 | 16 | 351 | 16 | 239 | 18 | 160 | 12 |
| Yes | 2167 | 84 | 1792 | 84 | 1057 | 82 | 1110 | 86 |
| Any N1 LNs with metastasis? | ||||||||
| No | 2110 | 72 | 1883 | 88 | 1065 | 82 | 1045 | 82 |
| Yes | 456 | 18 | 260 | 12 | 231 | 18 | 225 | 18 |
| Any mediastinal LN examination? | ||||||||
| No | 794 | 31 | 700 | 33 | 489 | 38 | 305 | 24 |
| Yes | 1772 | 69 | 1443 | 67 | 807 | 62 | 965 | 76 |
| Any N2 LN with metastasis? | ||||||||
| No | 2354 | 92 | 2143 | 100 | 1201 | 93 | 1153 | 91 |
| Yes | 212 | 8 | 0 | 0 | 95 | 7 | 117 | 9 |
| Station 10 examination? | ||||||||
| No | 1262 | 49 | 1069 | 50 | 741 | 57 | 521 | 41 |
| Yes | 1304 | 51 | 1074 | 50 | 555 | 43 | 749 | 59 |
| No. of mediastinal LN stations sampled |
||||||||
| 0 | 794 | 31 | 721 | 34 | 489 | 38 | 305 | 24 |
| 1–2 | 1170 | 46 | 954 | 45 | 661 | 51 | 509 | 40 |
| >=3 | 602 | 23 | 468 | 22 | 146 | 11 | 456 | 36 |
| LN counts | Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Total LNs | 6 | (3,10) | 6 | (3,10) | 5 | (2,8) | 8 | (4,12) |
| Total N2 LNs | 2 | (0,5) | 2 | (0,4) | 1 | (0,3) | 3 | (1,6) |
| Total mediastinal stations sampled |
1 | (0,2) | 1 | (0,2) | 1 | (0,2) | 2 | (1,3) |
| Total N1 LNs | 3 | (1,6) | 3 | (1,6) | 3 | (1,5) | 4 | (2,7) |
| Total station 10 LNs | 1 | (0,2) | 1 | (0,2) | 0 | (0,1) | 1 | (0,2) |
| Total intrapulmonary LNs (stations 11–14) |
2 | (0,5) | 2 | (0,5) | 2 | (0,4) | 2 | (0,5) |
| Total LN count by extent of resection |
Median | IQR | ||||||
| Pneumonectomy | 6 | (7,17) | ||||||
| Lobectomy/Bilobectomy | 10 | (3,11) | ||||||
| Segmentectomy | 3 | (1,7) | ||||||
| Wedge | 0 | (0,2) | ||||||
| Mediastinal LN count by extent of resection |
Median | IQR | ||||||
| Pneumonectomy | 3 | (1,6) | ||||||
| Lobectomy/Bilobectomy | 2 | (0,5) | ||||||
| Segmentectomy | 2 | (0,4.5) | ||||||
| Wedge | 0 | (0,2) | ||||||
Overall quality of pathologic nodal staging
Nine percent of resections had no lymph nodes (pNX), 16% had no N1 lymph nodes, and 31% had no mediastinal lymph nodes. The median number of lymph nodes examined per resection was 6, with an interquartile range (IQR) of 3–10. The median (IQR) number of mediastinal lymph nodes, and mediastinal lymph node stations were 2 (0–5), and 1 (0–2), respectively. The median number of N1 lymph nodes was 3 (1–6), which consisted of a median of 2 (0–5) intrapulmonary lymph nodes (stations 11–14), and 1 (0–2) hilar lymph node (Table 2b).
Post-operative events
The 30-day and 60-day readmission rates for the 2009–2013 era were 7% and 13%, respectively. Postoperative mortality rates were 4% at 30-, 7% at 60-, and 8% at 90-days. 1-year overall survival was 83% (95% confidence interval: 81–84%). One-year survival was 90% for Stage IA patients; 82% for Stage IB; 81% for Stage IIA; 74% for Stage IIB; 73% for Stage IIIA; 72% for Stage IIIB; and 68% for Stage IV. Median (IQR) follow up for the cohort was 2.9 years (1.5,5.4) (Table 2a).
Overall trend analysis
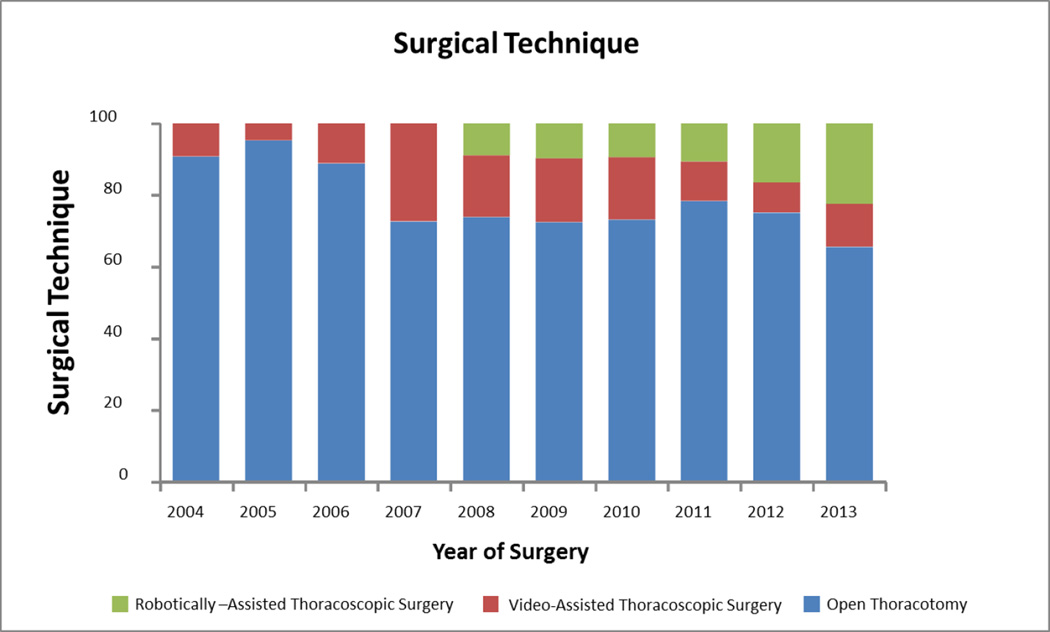
There was a significant overall trend (p<0.0001) in the use of PET/CT as a non-invasive staging test, increasing from an average usage rate of 36% from 2004–2008, to 80% from 2009–2013. The use of pre-operative mediastinoscopy was stable over the 10-year period. The pneumonectomy rate decreased from a high of 14% in 2005 down to 5% in 2013 (unadjusted p=0.0007; adjusted p=0.22). The use of open thoracotomy decreased significantly (p<0.0001) from a high of 95% in 2005 to a low of 66% in 2013, as the rates of minimally-invasive resection techniques increased (p=0.73) (Figure 1).
Figure 1.
significant decrease in use of open thoracotomy (p<0.0001) mirrored in rise of minimally-invasive surgical techniques.
The evolution of major adverse post-operative outcomes varied. In-hospital mortality decreased over time (p=0.0940; adjusted p=0.0332) but 30-, 60-, and 90-day mortality rates were not significantly different. A similar pattern of fluctuation with no significant trend was observed with the rate of resections with positive margins.
Period effect analysis
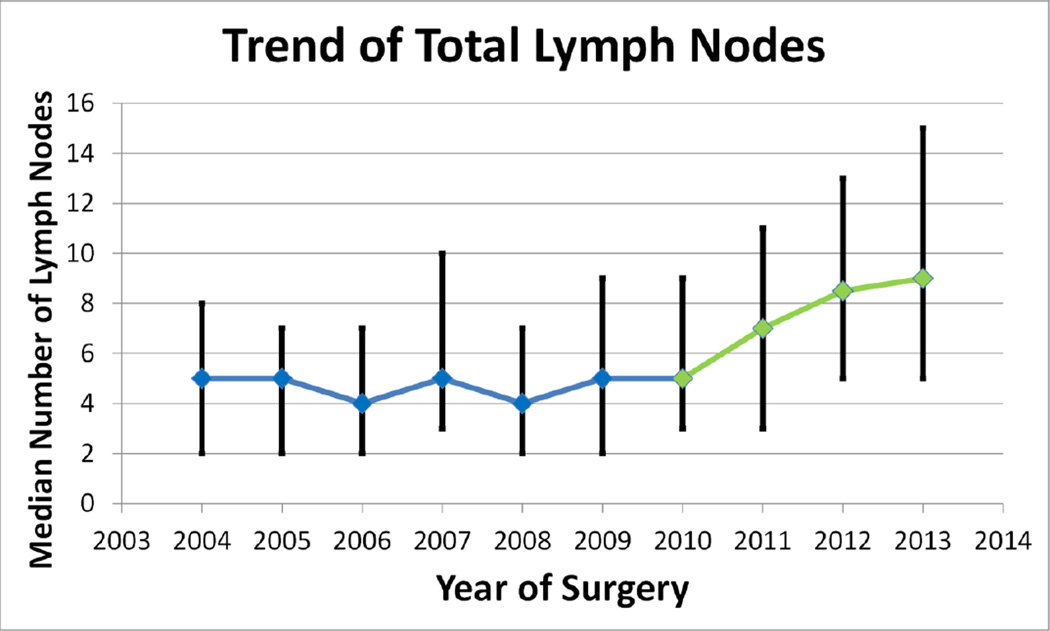
Having looked at significant trends over the full 10-year span, we next looked at differences between trends in the periods before (2004–2009) and after (2010–2013) the implementation of our quality improvement initiatives (period effect analysis), in order to test the impact of our quality improvement efforts. The pNX rate showed no significant overall (p=0.24) or period effect (p=0.072; adjusted p=0.31) trend (Figure 2a). However, there was a significant period effect for total lymph node count, as the median number of lymph nodes examined increased from 5 in the pre-intervention period (2004–2009) to 8 in the post-intervention period (2010–2013) (p<0.0001) (Figure 2b).
Figure 2.
a) no significant trend (p=0.24) or period effect (p=0.072; adjusted p=0.99) in rate of resections without lymph node examination;
b) significant period effect increase in median total number of lymph nodes per resection (p<0.0001).
We observed a period effect increase in station 10 sampling, with a median of 0 lymph nodes in the pre-intervention era and a median of 1 in the post-intervention era (p<0.0001). There was also an increase in the total number of N1 (stations 10–14) lymph nodes examined, and the rate of resections with examination of station 10 (both p<0.0001) (Figures 3a, b).
Figure 3.
a) significant period effect increase in number of station 10 (p<0.0001) and total N1 lymph nodes (p<0.0001);
b) significant period effect increase in rate of station 10 examination (p<0.0001)
Similarly, a period effect increase (p<0.0001) was observed with the median number of mediastinal lymph nodes examined. However, although we observed a significant overall decrease in resections without mediastinal lymph nodes examined (p<0.0001; adjusted p=0.0021) and increase in resections with 3 or more mediastinal lymph node stations examined (p<0.0001), no significant period effect was observed for either of these quality measures (Figures 4a, b, c). Despite these changes in the pattern of lymph node examination, pathologic stage distribution remained stable over time.
Figure 4.
a) period effect increase in median total number of mediastinal lymph nodes examined (p<0.0001; adjusted p=0.87);
b) significant overall decrease in rate of resections with no mediastinal lymph nodes examined (p<0.0001), period effect (p=0.93; adjusted p=0.88);
c) significant overall increase in rate of resections with 3 or more mediastinal lymph node stations examined (p<0.0001), period effect (p=0.6; adjusted p=0.16).
Subset analysis of an early intervention-adopting institution
In order to isolate the effect of our primary quality improvement intervention – a lymph node specimen collection kit – we also performed a descriptive sub-analysis of the most recent 5 years (2009–2013) of surgical practice at an early adopting institution. At this institution, use of the surgical lymph node specimen collection kit rose from 0% in 2010, to 4% in 2011, 57% in 2012, and 62% in 2013.
During this period, 488 resections were performed without, and 159 resections were performed with, the lymph node collection kit at this institution. Resections performed without the kit had a pNX rate of 9%, whereas those performed with the kit had a pNX rate of 0% (p<0.0001). The rate of resections with no mediastinal lymph node examination dropped from 30% with non-kit cases to 0% for kit cases (p<0.0001). With use of the kit, the rate of resections without examination of stations 10 and 7 dropped from 44% to 4% and from 64% to 3%, respectively. Use of the kit was associated with an increase in the median N1 nodal count from 4 to 6 (p<0.0001; adjusted p=0.0213), N2 nodal count from 2 to 8 (p<0.0001), and total nodal count from 7 to 15 (p<0.0001) (Table 3).
Table 3.
Effect of Kit in an Early Adopting Institution
| NON-KIT CASES n=488 |
KIT CASES n=159 |
p-value | Adjusted p-value |
|
|---|---|---|---|---|
| pNx Rate | 9% | 0% | <.0001 | <0.0001* |
| pN0 | 69% | 75% | 0.1381 | 0.0081 |
| pN1 | 13% | 14% | 0.6646 | 0.73 |
| pN2 | 9% | 11% | 0.5843 | 0.97 |
| Rate of non-examination of mediastinal lymph nodes | 30% | 0% | <.0001 | <0.0001* |
| Rate of non-examination of station 10 | 44% | 4% | <.0001 | <0.0001 |
| Rate of non-examination of station 7 | 64% | 3% | <.0001 | <0.0001 |
| N1 lymph node count: median (IQR) | 4 (1–7) | 6 (3–11) | <.0001 | 0.0213 |
| N2 lymph node count: median (IQR) | 2 (0–5) | 8 (5–12) | <.0001 | <0.0001 |
| Total lymph node count: median (IQR) | 7 (3–11) | 15 (10–23) | <.0001 | <0.0001 |
model only controlled for stage due to zero cell frequency
DISCUSSION
The pattern of surgical resection for NSCLC in this population-based cohort showed trends towards higher quality. Most measures of the quality of lymph node examination, except for the pNX rate, showed significant improvement in the whole cohort, with a period effect correlated to the timing of our quality improvement initiative. The pNX rate in this community-based series compares favorably to analyses of large US databases, from which the detrimental prognostic implications of this major quality of care deficit have been demonstrated.8 From the early results in the early-adopting institution, we expect that the pNX rate will diminish with wider adoption of the specimen collection kit.
Missing data, such as the absence of early-era data for 5 institutions, absence of data on performance status and recurrence-free survival, limit this retrospective study. The study design precludes rigorous control for unrelated secular changes and a causal analysis. The evolution towards higher quality surgical practice is probably attributable to multiple factors. Quantifying local practice patterns and providing direct feedback on the quality gap probably had a beneficial effect, exemplified by the declining rate of resections without mediastinal nodal examination. There may also have been a Hawthorne effect from awareness of the effort to monitor surgical quality. This effect cannot be separated from the effect of our direct stakeholder education campaign. We also observed statistically significant trends in practice within the pre-intervention period that suggests movement, which accelerated in the post-intervention period, had begun before formal introduction of our intervention.
The 5 institutions contributing data for only 2009–2013 were also not yet directly involved in our quality improvement work. They represent a smaller percentage of cases (21% of total cohort) than the other 6 institutions. A sensitivity analysis performed with only the 6 institutions present over the full timespan of this study showed results consistent with the results of the analysis of the whole cohort.
Both the period effect analysis and the early results from an institution with early adoption of the surgical kit intervention highlight the potential significance of a solution specifically designed to eliminate the pathologic nodal staging quality gap. Simply educating surgeons and their operating room teams about the quality gap may be insufficient to sustain practice improvement. In our pilot studies, we showed how post-intervention surgical practice falls back to pre-intervention levels when the kit is not used.18 The problem of suboptimal nodal staging is multifaceted and extends beyond the operation and the surgical team, to include aspects of specimen labeling and transfer, and the pathology gross examination.12,17,18 Heightened awareness is insufficient to eliminate the problem. Simple, practical, logically designed and carefully tested corrective interventions are needed.
More broadly, we see secular changes in lung cancer surgical practice. The use of pneumonectomy is decreasing across the region, minimally-invasive resection techniques are supplanting open thoracotomy, and adjusted in-hospital mortality has improved. However, other measures of short-term post-operative mortality have not changed; the rate of margin-positive resections remains at 6%, slightly higher than the National Cancer Database average of 4.6%.5 The 30-day mortality rate in this cohort, although slightly higher than recent reports from analysis of the Society of Thoracic Surgeons General Thoracic Surgery database (STS-GTS), is competitive with other reports.20,21 Unlike the STS-GTS, and reports from Japan, the MS-QSR cohort is population-based, relatively unselected (all data from all institutions with 5 or more resections within pre-specified Dartmouth Hospital Referral Regions are included), rigorously audited, and not based on self-reporting.20,22,23
Other problems remain. The ultimate goal of eliminating the nodal staging quality gap is to change the distribution of pathologic nodal stage, by correctly identifying previously under-staged patients who may be offered beneficial adjuvant therapy, or enrollment into clinical trials of novel therapies. Unrelated changes in preoperative evaluation, such as greater use of PET/CT scanning in the more recent era, may have blunted the expected migration to higher pathologic nodal stage. Despite this bias to the null, we see early signals of potential stage-migration. Our staggered implementation design, which allows each institution to be used in the future as its own control while adjusting for secular changes in the aggregate population, will enable rigorous future analyses of pathologic nodal stage distribution and survival, which are not yet possible at this early phase.
The infrequent use of invasive pre-operative nodal staging procedures is probably worthy of specific intervention, given the likely additional impact on patient selection, use of neo-adjuvant therapeutic modalities, and long-term survival.24,25 Such intervention studies will potentially confound the analysis of stage migration, as more patients with mediastinal nodal metastasis are triaged away from primary surgical resection.
The ongoing surgical specimen collection kit deployment only addresses two of the three points of origin of the nodal staging quality gap: improved surgical hilar and mediastinal lymph node harvest, and communication between the operating room and pathology teams including the secure transfer of resected nodal specimens. It does not address the problem of incomplete intrapulmonary lymph node retrieval which is usually the responsibility of the specimen gross dissector in the pathology laboratory.26,27 We are currently testing a novel gross dissection method to address this problem.28
The nodal staging quality gap is widest in community-level institutions, where 80% of NSCLC patients receive their care in the US. Although slightly narrower, the quality gap also exists in academic centers.29 Therefore, large pragmatic dissemination and implementation studies are needed to evaluate the impact on care provided to a diversity of patients, by different types of surgery and pathology teams, in different types of institutions. The ultimate aim must be to understand the best approaches to raise the overall standards of surgical care for patients with early stage NSCLC in the places where they choose to seek care, with the goal of improving population-level lung cancer survival.
Supplementary Material
Perspective.
Over 80% of lung cancer patients receive their care at community-level hospitals. Pathologic nodal stage is the key prognostic factor for survival of lung cancer patients. In a high lung cancer incidence and mortality region of the US, a quality improvement initiative in community hospitals shows significant trends in improving the quality of pathologic nodal staging.
Central Message.
A regional quality improvement project has a positive impact on the quality of lung cancer resections as measured by pathologic nodal assessment at community-level hospitals.
Acknowledgments
1 R01 CA172253-01
Dr. Osarogiagbon has a patent application for the surgical specimen collection kit under review.
We thank all past and present members of Dr. Osarogiagbon’s Thoracic Oncology Research Group for their direct and indirect contributions to this work.
Glossary of Abbreviations
- NSCLC
Non-small cell lung cancer
- MS-QSR
Mid-South Quality of Surgical Resection
- PET/CT
Positron-emission tomography/computerized axial tomography
- IQR
Interquartile range
- STS-GTS
Society of Thoracic Surgeons General Thoracic Surgery database
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1. [Accessed May 28, 2015]; http://seer.cancer.gov/statfacts/html/lungb.html.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Pfannschmidt J, Muley T, Bulzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer. 2007;55:371–377. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey CR, Marks LB, Hollis D, Hubbs JL, Ready NE, D’Amico TA, et al. Local recurrence after surgery for early stage lung cancer. Cancer. 2009;115:5218–5227. doi: 10.1002/cncr.24625. [DOI] [PubMed] [Google Scholar]
- 5.Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal AJ. Prevalence, prognostic implications, and survival modulators of incompletely resected non-small cell lung cancer in the U.S. National Cancer Data Base. J Thorac Oncol. 2016 Jan;11(1):e5–e16. doi: 10.1016/j.jtho.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2014 Feb;97(2):385–393. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012 Dec;7(12):1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 8.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013 Oct;96(4):1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF, et al. International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015 Dec;10(12):1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 10.Ou SI, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3:880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig MS, Goodman M, Miller DL, Johnstone PAS. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. CHEST. 2005;128:1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 12.Osarogiagbon RU, Allen JW, Farooq A, Wu JT. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7:390–396. doi: 10.1097/JTO.0b013e31823e5e2d. [DOI] [PubMed] [Google Scholar]
- 13.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 14.Allen JW, Farooq A, O’Brien TF, Osarogiagbon RU. The quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer. 2011;117:134–142. doi: 10.1002/cncr.25334. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. [Accessed May 18, 2016];Non-small cell lung cancer (Version 4.2016) doi: 10.6004/jnccn.2016.0031. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooq A, Osarogiagbon RU, Allen JW, O’Brien TF, Spencer D, Berry A. Accuracy and comprehensiveness of pathology reportage after lung cancer resection. J Clin Oncol. 2009;27:15s. (suppl; abstr 6523) [Google Scholar]
- 17.Osarogiagbon RU, Sareen S, Eke R, Yu X, McHugh LM, Kernstine KH, et al. Audit of lymphadenectomy in lung cancer resections using a specimen collection kit and checklist. Ann Thorac Surg. 2015 Feb;99(2):421–427. doi: 10.1016/j.athoracsur.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osarogiagbon RU, Miller LE, Ramirez RA, Wang CG, O’Brien TF, Yu X, et al. Use of a surgical specimen-collection kit to improve mediastinal lymph-node examination of resectable lung cancer. J Thorac Oncol. 2012;7:1276–1282. doi: 10.1097/JTO.0b013e318257fbe5. [DOI] [PubMed] [Google Scholar]
- 19.Barlow DH, Hersen M. Multiple baseline designs. In: Goldstein AP, Krasner L, editors. Single case experimental designs: strategies for studying behavior change. New York: Pergamon Press; 1984. pp. 210–251. [Google Scholar]
- 20.Boffa DJ, Allen MS, Grab JD, Gaissert HA, harpole DH, Wright CD. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: The surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 21.Little AG, Rusch VW, Bonner JA, Gaspar LE, Green MR, Webb WR, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005 Dec;80(6):2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 22.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: A Japanese lung cancer registry study. Lung Cancer. 2005;50:227–234. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry Study: Prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi: 10.1097/JTO.0b013e31815e8577. [DOI] [PubMed] [Google Scholar]
- 24.Farjah F, Flum DR, Ramsey SD, Heagerty PJ, Symons RG, Wood DE. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol. 2009 Mar;4(3):355–363. doi: 10.1097/JTO.0b013e318197f4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faris N, Yu X, Sareen S, Signore RS, McHugh LM, Roark K, et al. Preoperative evaluation of lung cancer in a community health care setting. Ann Thorac Surg. 2015 Aug;100(2):394–400. doi: 10.1016/j.athoracsur.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Osarogiagbon RU, Hilsenbeck HL, Sales EW, Berry A, Jarrett RW, Jr, Giampapa CS, et al. Improving the pathologic evaluation of lung cancer resection specimens. Transl Lung Cancer Res. 2015 Aug;4(4):432–437. doi: 10.3978/j.issn.2218-6751.2015.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramirez RA, Wang CG, Miller LE, Adair CA, Berry A, Yu X, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30:2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 28.Osarogiagbon RU, Eke R, Sareen S, Leary C, Coleman L, Faris N, et al. The impact of a novel lung gross dissection protocol on intrapulmonary lymph node retrieval from lung cancer resection specimens. Ann Diagn Pathol. 2014 Aug;18(4):220–226. doi: 10.1016/j.anndiagpath.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osarogiagbon RU, Lin CC, Smeltzer M, Jemal A. Overall survival (OS) implications of institutional disparities in non-small cell lung cancer (NSCLC) pathologic nodal (pN) staging practice in the National Cancer Data Base (NCDB) J Clin Oncol. 2016;34 (suppl; abstr 6554) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.