Abstract
Recent findings indicate that emotional arousal can enhance memory consolidation of goal-relevant stimuli while impairing it for irrelevant stimuli. According to one recent model, these goal-dependent memory tradeoffs are driven by arousal-induced release of norepinephrine (NE), which amplifies neural gain in target sensory and memory processing brain regions. Past work also shows that ovarian hormones modulate activity in the same regions thought to support NE’s effects on memory, such as the amygdala, suggesting that men and women may be differentially susceptible to arousal’s dual effects on episodic memory. Here, we aimed to determine the neurohormonal mechanisms that mediate arousal-biased competition processes in memory. In a competitive visuo-attention task, participants viewed images of a transparent object overlaid on a background scene and explicitly memorized one of these stimuli while ignoring the other. Participants then heard emotional or neutral audio-clips and provided a subjective arousal rating. Hierarchical generalized linear modeling (HGLM) analyses revealed that greater pre-to-post task increases in salivary alpha-amylase (sAA), a biomarker of noradrenergic activity, was associated with significantly greater arousal-enhanced memory tradeoffs in women than in men. These sex-dependent effects appeared to result from phasic and background noradrenergic activity interacting to suppress task-irrelevant representations in women but enhancing them in men. Additionally, in naturally cycling women, low ovarian hormone levels interacted with increased noradrenergic activity to amplify memory selectivity independently of emotion-induced arousal. Together these findings suggest that increased noradrenergic transmission enhances preferential consolidation of goal-relevant memory traces according to phasic arousal and ovarian hormone levels in women.
Keywords: locus coeruleus, norepinephrine, arousal, memory, emotion, sex hormones
1. Introduction
Emotionally arousing events form some of our most vivid and enduring memories (LaBar & Cabeza, 2006; Sharot et al., 2004). Yet arousal does more than simply enhance memory of emotional stimuli; it modulates processing of all information perceived during, shortly before, or shortly after an arousing event. Across experimental conditions, emotional stimuli can either enhance or impair memory for nearby neutral stimuli (Anderson et al., 2006; Bocanegra & Zeelenberg, 2009; Kensinger et al., 2007; Knight & Mather, 2009; Mather, 2007; Mather & Sutherland, 2011).
A reliable finding in the emotion-cognition literature is that foreground emotionally arousing objects often elicit a tradeoff by yielding enhanced object memory but impaired memory for neutral background scenes (Kensinger et al., 2007; Mather, 2007; Waring & Kensinger, 2011). Relatedly, when eyewitnesses to a crime recall their experience, they often remember the weapon itself – the source of arousal - at the cost of memory for the perpetrator’s face, a phenomenon known as the “weapon focus effect” (Steblay, 1992). Memory tradeoffs are also commonly observed when emotional and neutral memoranda compete nearby in time. For instance, several studies demonstrate that emotional images or words induce retrograde amnesia for preceding neutral stimuli (Hurlemann et al., 2005; Hurlemann et al., 2007; Knight & Mather, 2009; Strange et al., 2003). The adaptive significance of such arousal-related tradeoffs is that processing of seemingly inconsequential information is suppressed to allocate limited mental resources to processing the emotional object, which is likely to have greater motivational significance than nearby neutral information.
Recent studies, however, indicate that arousal can enhance rather than impair memory for neutral information when those stimuli have priority, such as goal relevance (Lee et al., 2015; Sakaki et al., 2014). The arousal-biased competition (ABC) model proposes that a momentary surge of arousal amplifies the effects of top-down attention, such that processing goal-relevant information is enhanced at the expense of processing distracting information (Mather & Sutherland, 2011). This framework builds upon the idea of biased competition in the brain, whereby - by virtue of their motivational relevance or perceptual salience - prioritized stimuli outcompete less salient information for limited mental resources to gain representation and reach awareness (Desimone & Duncan, 1995).
Increasing evidence supports ABC’s predictions that emotional arousal enhances the effects of goal relevance in memory. For example, seeing an emotional image can enhance memory for a preceding goal-relevant neutral object, while impairing memory for a subsequent less-attended neutral object (Sakaki et al., 2014). Likewise, hearing an emotional versus neutral sound suppresses memory consolidation for a neutral scene paired with a salient foreground object that was seen just beforehand (Ponzio & Mather, 2014). Based on these findings, it seems that emotional arousal amplifies competitive processes more generally, leading to “winner-take-more” and “loser-take-less” effects in memory regardless of whether priority is determined by emotional factors or not.
Presently, most brain-based models fail to account for the arousal’s dual effects on cognition. To address how arousal interacts with priority in the brain, the glutamate amplifies noradrenergic effects (GANE) model posits that activity-dependent increases in norepinephrine (NE) enable arousal to enhance already highly activated, prioritized mental representations even further (Mather et al., in press). According to GANE, the brain’s primary excitatory neurotransmitter, glutamate, serves as a flexible marker of priority. When arousal is induced, elevated glutamate levels corresponding with high priority (e.g., goal-relevant) stimuli spill over from the synaptic gap to interact with nearby NE axons and stimulate additional local NE release; in turn, local elevations of NE at these synapses engage low-binding-affinity β-adrenoreceptors that up-regulate glutamate release even further. In addition to enhancing the activation strength of important representations, the engagement of β-adrenoreceptors on nearby glutamate terminals triggers synaptic plasticity processes, thereby promoting the storage of “winning” mental representations into memory. Elsewhere, the predominantly inhibitory effects of NE release prevail where lower priority, “losing” representations fail to engage this positive glutamate-NE feedback loop. Together these dichotomous influences of NE on local regional activity amplify the gain on competition between high and lower priority representations, providing a neuromechanism by which arousal optimizes selectivity in perception and memory.
The GANE model helps reconcile and extend previous influential theories of how NE modulates episodic memory by highlighting the role of priority in regulating different memory outcomes. For instance, GANE aligns with models positing that NE biases attention (Markovic et al., 2014) and memory consolidation (McGaugh & Roozendaal, 2002; McGaugh, 2013) to favor emotional or affectively salient information over neutral information (Markovic et al., 2014). More recently, it was demonstrated that increasing NE levels with isometric handgrip in young women enhances selective memory for negative emotional stimuli (Nielsen et al., 2015), suggesting that NE release enhances memory selectivity even when sympathetic arousal is manipulated externally. Thus, the dominance of emotional stimuli in perception and memory may be due both to the prioritization they acquire through their motivational relevance (e.g., reward or punishment) as well as the presence of elevated NE.
Building upon these ideas, the GANE model proposes that that the beneficial mnemonic effects of NE released under arousal are not unique to emotional stimuli but rather apply to any prioritized stimulus, including goal-relevant neutral stimuli. Consistent with this, one recent study showed that elevated levels of noradrenergic activity, indexed by salivary alpha-amylase (sAA), are positively associated with retrograde memory enhancements for goal-relevant neutral stimuli encountered prior to an emotional event (Clewett et al., 2017). Arousal-related NE release may also amplify competition in memory consolidation (e.g. Ponzio & Mather, 2014).
Another important consideration is how NE might affect memory consolidation differently in men and women given ample evidence of sex-dependent effects of emotional arousal on episodic memory. At the behavioral level, women show even stronger memory enhancements for emotional versus neutral stimuli and tend to recall emotional material more quickly and more vividly than men (Hamann, 2005; Hamann & Canli, 2004). Young women who show greater increases in salivary biomarkers of noradrenergic activity exhibit retrograde memory enhancement for high priority neutral images preceding something emotional (Clewett et al., 2017). Women also exhibit greater emotion-induced retrograde amnesia for inconspicuous neutral words preceding an emotional versus neutral oddball word, an effect that is β-adrenoreceptor-dependent (Strange et al., 2003).
Other work suggests that such sex differences in emotion-related memory consolidation may arise due interactions between NE released under arousal and background levels of sex steroid hormones. For instance, sex steroid hormone levels have been shown to modulate emotional memory enhancements both in the presence and absence of stress, such that higher levels of sex hormones lead to stronger emotional memory enhancements (Andreano et al., 2008; Ertman et al., 2011). There are also indications that estradiol potentiates NE release (Bangasser et al., 2015) and, along with progesterone, alters the responsivity of the amygdala and hippocampus – key regions that are regulated by NE and that support emotional memory consolidation (McIntyre et al., 2012) - to emotional images (Andreano & Cahill, 2010). However, there is also evidence of contradictory mnemonic effects of sex steroid hormones under arousal. For example, Nielsen et al. (2015) found that the memory-biasing effects of handgrip-induced increases in NE only occurred in women with lower estradiol and progesterone levels at encoding. Wegerer et al. (2014) also revealed that low levels of estradiol are associated with stronger intrusive memories for emotional materials (Wegerer et al., 2014). Together these data suggest that GANE effects may differ between young women and men due to fluctuating sex steroid hormone levels influencing the consolidation of prioritized information either independently or via interactions with NE release.
The goal of the present study was to determine whether noradrenergic activity, as indexed by salivary alpha-amylase (Ditzen et al., 2014), amplifies the effects of top-down priority in cognition, such that memory for goal-relevant neutral images is enhanced at the expense of memory for competing neutral distracters in men and women as predicted by GANE. Alternatively, other models would predict that emotional events disrupt ongoing cognitive selection processes due to NE re-allocating limited attention and mental resources towards processing emotionally salient stimuli (Bouret & Sara, 2005; Markovic et al., 2014). From this alternative perspective, encountering something emotional should always result in prioritization of the emotionally salient stimuli and thus attenuate processing of neutral stimuli irrespective of their goal relevance. In addition, given the mixed findings on the effects of sex steroid hormones, we examined whether there were significant sex-dependent effects of tonic noradrenergic activity and phasic arousal on the selective consolidation of neutral episodic memories. Based on prior emotion-cognition research, we were particularly interested in whether estradiol and progesterone altered the strength of NE’s effects on memory in young women.
2. Methods
2.1 Participants
One hundred and two healthy young adults were recruited from the University of Southern California Psychology Subject Pool to participate in this experiment. All participants provided written informed consent approved by the University of Southern California Institutional Review Board and were awarded course credits for their participation. All eligible individuals had normal or normal-to-corrected vision and hearing. Female participants were either using hormonal contraception or naturally cycling.
To avoid contamination of saliva samples, participants were instructed to: 1) Refrain from eating, gum chewing, or teeth brushing for at least one hour prior to the experiment, 2) Refrain from cardiovascular exercise and alcohol 24 hours prior to the start of the experiment, and 3) Refrain from caffeine for 4 hours prior to the experiment.
Prior to analysis, we applied several exclusion criteria: 1) failure to comply with saliva collection criteria (n = 23; most violations involved failure to comply with the 4-hr caffeine and aerobic exercise criteria); 2) categorization performance on the task was below chance (n = 7); 3) memory for target stimuli was below chance (n = 14); 4) experimenter error (e.g., script crashing) or participant failed to follow instructions and indicated that they tried to memorize both the target and distracter stimulus on every trial (n = 5), and 5) missing distraction ratings (n = 3). Note that some of these overlap since some participants violated multiple criteria. After exclusions, a total of 57 participants remained and were analyzed in this study (30 females: Mage = 21 years, SD = 2.61; 27 males: Mage = 21 years, SD = 1.88).
2.2 Stimuli
Visual stimuli were composed of 48 neutral object and 48 neutral scene grayscale images selected from previous datasets (Gabrieli et al., 1997; Kensinger et al., 2006) and the Internet. Half of the object images depicted kitchen utensils, while the other half depicted animals; half of the scene images depicted indoor scenes, while the other half depicted outdoor scenes. With these images, 48 “overlap” stimuli were created by overlaying each object image on top of a scene image using Adobe Photoshop 5.0. The object image was then rendered transparent such that the foreground object and background scene images were equally discernable. Each of the un-merged object and scene stimuli were yoked with a semantically related image that served as a foil during the recognition memory test.
Emotional sound stimuli consisted of 16 neutral (Mvalence = 5.74, SDvalence = 0.26; Marousal = 4.27, SDarousal = 0.097), 16 positive (Mvalence = 1.96, SDvalence = 0.066; Marousal = 7.59, SDarousal = 0.094), and 16 negative (Mvalence = 7.38, SDvalence = 0.069; Marousal = 6.24, SDarousal = 0.17) audio-clips from the International Affective Digital Sounds (Bradley & Lang, 2007). The most salient 2s portion of each original 6s audio-clip was selected for the task using Audacity. For neutral sounds, we clipped the original audio files to exclude any brief periods of silence.
2.3 Procedure
Upon arrival participants were consented and then completed a variety of behavioral questionnaires. Participants then drank an 8-oz bottle of water and answered the saliva screening questions. A few minutes later, participants provided a 1-mL saliva sample via passive drool and then performed a competitive visuo-attention task.
2.3.1 Competitive visuo-attention task
To examine how emotional arousal influences memory selectivity, participants performed a competitive visuo-attention task consisting of an encoding phase and a memory test (Figure 1). The overall structure of the experiment was a 2 (Prioritized: object vs. scene) x 3 (Valence: neutral vs. negative vs. positive) within-subjects design. The task consisted of four blocks of 12 trials (i.e., overlap images), with three trials of each valence in each block. The order of the overlap stimuli was randomized within each block. Prior to the start of each block, participants were cued to which visual stimulus category (object or scene) to focus on and memorize. Object-focused and scene-focused blocks always alternated, with the first block type counterbalanced across participants. The prioritized visual category and valence associated with each overlap stimulus was also counterbalanced across participants. To familiarize participants with the experiment, they were given six practice trials and one example memory trial prior to the main task.
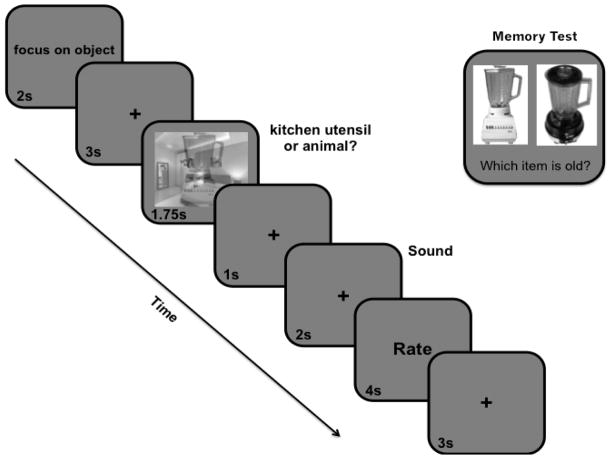
Figure 1.
An example trial from the competitive visuo-attention task (left side) and subsequent recognition memory test (right side). Each encoding trial consisted of an “overlap” image of a transparent object overlaid on a background scene. Participants were cued to categorize and memorize either the scene or object stimulus while ignoring the other. Following the overlap image, participants heard an emotional or neutral sound and were prompted to rate how arousing they found each sound via button press. Approximately 5 minutes after encoding, participants performed a two-alternative forced-choice recognition memory test that probed memory for all target and distracter items from the task.
2.3.2 Encoding Phase
During the encoding phase, participants were presented with a series of grayscale “overlap” images of a transparent, centered object overlaid on a background scene (Figure 1). Each trial commenced with a 2s verbal reminder of which visual category to prioritize (“focus on object” or “focus on scene”) followed by a 3s inter-stimulus-interval (ISI) consisting of a fixation cross in 24-point black font. Following this fixation period, an overlap image appeared in the middle of the screen for 1.75 seconds.
To manipulate stimulus priority, or goal relevance, participants were instructed to focus on and memorize the exact image of either the object or background scene while ignoring the other visual category. To facilitate encoding and ensure participants were prioritizing the correct stimulus, they were also instructed to categorize the target stimulus (object: animal or kitchen utensil; scene: indoor or outdoor) as quickly and accurately as possible via button press when the overlap image appeared. In addition, participants were told that their memory would only be tested for the scenes and objects they categorized (i.e., specifically focused on) during the task.
The overlap image was followed by a 1s fixation period. To manipulate arousal, a negative, positive, or neutral emotional audio-clip was then played for 2s. After hearing the sound, participants were prompted to rate how arousing they found each sound on a scale from 1 = not intense at all to 8 = extremely intense via button press. Participants were instructed to provide their rating within the 4 seconds that the prompt was displayed on the screen. These subjective ratings were used as the primary trial-by-trial estimates of phasic arousal in the hierarchical generalized linear modeling (HGLM) analyses rather than the pre-defined valence categories or normative IADS arousal ratings. Each trial concluded with a 3s fixation cross inter-trial-interval.
2.3.3 Post-task delay period
Immediately following the visuo-attention task, participants provided a second 1-mL saliva sample via passive drool. Participants then worked on a number search puzzle for 5 minutes to introduce a delay between encoding and retrieval.
2.3.4 Recognition memory test
To examine the differential effects of arousal on memory for high versus lower priority images, we tested memory for all target and distracter images. In a two-alternative forced choice memory test, the scenes and objects from each overlap stimulus were displayed individually. Each of these items was shown alongside a similar but perceptually different new image of the same semantic category (e.g., two different images of kitchens, but with similar cabinetry). Participants were tasked with identifying which image they had seen during the encoding phase. After making each memory choice, participants were prompted to rate their confidence in their memory accuracy on a scale ranging from 1 = not confident at all to 6 = extremely confident. There were no time limits for either the memory choice or confidence rating.
2.3.5 Emotional sound distraction ratings
After the memory test, we also acquired a rating of how distracting the participants found the emotional sounds on a scale ranging from 1 = not distracting to 8 = very distracting. These distraction ratings provided an estimate of how effectively participants prioritized goal-relevant stimuli relative to the task-irrelevant emotional sound stimuli, with lower distraction scores being associated with better goal-directed attention for the neutral target stimulus. We anticipated that lower distraction scores would therefore moderate a greater positive influence of arousal on memory selectivity.
2.4 Saliva assay and analysis
Saliva samples were immediately frozen, and kept frozen for a minimum of 24 hours to allow mucins to precipitate. Prior to the assays, they were thawed and centrifuged at 3,000 x g for 15 min to extract particulates from saliva. Clear supernatant was decanted into microtubes. Alpha-amylase levels were estimated using Salimetrics, LLC (State College, PA) enzyme kinetic assay kits and measured optically using Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). To estimate task-evoked noradrenergic activity, we calculated a sAA change score by subtracting sAA concentration values at baseline (sample 1) from values immediately post-task (sample 2). The results of a 2 (Time: baseline vs. post-task) x 2 (Sex: male vs. female) mixed ANOVA indicated that sAA levels significantly increased from baseline (M = 83.86, SD = 67.68) to post- task (M = 121.73, SD = 83.48), F(1,55) = 16.09, p < .001, ηp2 = .23. However, there were no main or interaction effects of Sex on sAA levels (ps > .5).
The saliva samples from female participants were then analyzed for 17β-estradiol and progesterone levels using Salimetrics, LLC (State College, PA) ELISA kits and optical measurements acquired from a Molecular Devices, LLC SpectraMax M3 Multi-mode Microplate Reader (Sunnyvale, CA). We assayed the first and the last saliva samples for 17β-estradiol and progesterone; from these samples, we determined the average levels of these hormones. The observed ranges from the assay of 17β-estradiol (M = 1.91, SD = 0.78) and progesterone (M = 118.95, SD = 105.84) were also similar to the expected ranges for women (Salimetrics, LLC, State College, PA).
2.5 Mean item recognition performance
First, we performed a 2 (Sex: male vs. female) x 2 (Priority: target vs. distracter) x 3 (Valence: positive vs. negative vs. neutral) mixed ANOVA on mean recognition performance, with Valence and Priority as within-subjects factors, to verify that participants properly prioritized the goal-relevant target images over distracters. Prior work indicates that positive emotional stimuli appear to amplify priority effects in the same manner as negative emotional stimuli, suggesting that GANE effects are invariant to the valence of the arousal-eliciting emotional stimulus (Sakaki et al., 2014). Nonetheless, we explored the possibility that valence also influenced mean distracter/target item recognition in supplementary analyses (Section 6.1 of Supplementary Materials; Figure S1). Because Priority was modeled as a predictor in this mixed ANOVA, we also report the results of our priority manipulation-check in supplementary materials. Of note, additional linear modeling analyses verified that the semantic properties of the target stimulus (i.e., object-focused vs. scene-focused) did not influence item or tradeoff memory outcomes (Section 6.2 of Supplementary Materials).
2.6 Memory codependency analysis
To examine how arousal influenced competitive mnemonic processes at the trial level, we performed a memory codependency analysis. Specifically, we examined how memory accuracy for the distracting stimulus (lower priority) in a given overlap image differed as a function of memory accuracy for its corresponding target stimulus (high priority). Each of the 48 trials in the encoding phase were sorted by one of four possible memory codependency outcomes: 1) Remembered Target and Forgot Distracter, 2) Forgot Target and Remembered Distracter, 3) Remembered both, or 4) Forgot both.
According to the ABC model, emotional arousal biases mental resource allocation towards prioritized representations, increasing memory for goal-relevant stimuli at the cost of memory for less salient distracters (Mather & Sutherland, 2011). Thus, an “optimal memory tradeoff” was operationalized as trials in which participants remembered the target stimulus while forgetting its corresponding distracter. To increase statistical power, memory performance was not differentiated by the specific visual category of the target (object-target vs. scene-target trials).
2.7 Hierarchical generalized linear modeling analyses
2.7.1 Trial-level effects of emotional arousal on selective memory tradeoffs and target/distracter item recognition
To test our main prediction that NE facilitates arousal-biased competition memory effects during encoding, we performed a hierarchical generalized linear modeling (HGLM) analysis with the glmer function in the lme4 library (Baayen et al., 2008). The parameters were estimated with the maximum likelihood method in R (R Core Team, 2012). Each trial was used as a Level 1 unit and each participant was used as a Level 2 unit. Optimal memory tradeoff scores were modeled as the dependent variable with a binomial (logit) distribution (1 = RememberedtargetForgotdistracter, 0 = other memory outcome). Subjective arousal ratings for the sounds were group-centered and modeled as the level-1 trial-by-trial predictors, which were nested within three grand-mean-centered level-2 predictors: Sex (1 = female, −1 = male), Sound Distraction Score, and Task-Induced sAA Change. Trials with missing arousal ratings were excluded. All main effects, two-way, three-way, and four-way interaction terms were included in the model (see Table 1).
Table 1.
Hierarchical generalized linear model (HGLM) examining interaction effects between trial-level arousal ratings, distraction ratings, and salivary alpha-amylase on memory selectivity (i.e., remembered target but forgot its distracter) across all participants.
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Memory Tradeoffs Across All Participants | ||||
| Intercept | −0.6189 | 0.0529 | −11.70 | <2e-16 |
| Emotional Sound Arousal Rating | 0.0139 | 0.0185 | 0.75 | 0.45 |
| Sex | −0.0295 | 0.0528 | −0.56 | 0.58 |
| Task-Induced sAA Change | 0.0975 | 0.0688 | 1.42 | 0.16 |
| Emotional Sound Distraction Rating | −0.0236 | 0.0409 | −0.58 | 0.56 |
| Arousal Rating x Sex | 0.0127 | 0.0185 | 0.69 | 0.49 |
| Arousal Rating x sAA Change | 0.0198 | 0.0245 | 0.81 | 0.42 |
| Arousal Rating x Distraction | −0.0037 | 0.0143 | −0.26 | 0.80 |
| sAA Change x Sex | 0.0218 | 0.0688 | 0.32 | 0.75 |
| sAA Change x Distraction | −0.0386 | 0.0512 | −0.75 | 0.45 |
| Distraction x Sex | 0.0215 | 0.0408 | 0.53 | 0.60 |
| Arousal Rating x Sex x sAA | 0.0582 | 0.0245 | 2.37 | 0.018* |
| Arousal Rating x Distraction x sAA | 0.00929 | 0.018067 | 0.51 | 0.61 |
| Distraction x Sex x sAA Change | 0.004538 | 0.05119 | 0.089 | 0.93 |
| Arousal Rating x Sex x Distraction | −0.019399 | 0.014334 | −1.35 | 0.18 |
| Arousal x Sex x Distraction x sAA | −0.043818 | 0.018067 | −2.43 | 0.015* |
Key: sAA Change: Change score in salivary alpha-amylase (sAA) concentration (U/mL) levels from baseline to immediately post-task. Sex (female = 1, male = −1). Distraction: A single post-task rating of how distracting participants found the emotional sounds to be; Arousal Rating: Subjective arousal rating for each emotional sound. Significant results are bolded.
p < .05.
First, to determine the optimal HGLM model, we tested whether there were random effects in the slope across participants. A comparison (ANOVA) between models with and without a random effects term was not significant (p > .05), suggesting that the effects of arousal on memory tradeoffs did not differ across participants. Thus, we did not include a random slope effects term in the final model.
To determine which aspects of memory consolidation led to altered memory tradeoffs under arousal, we also examined how arousal/hormones impacted mean recognition for target and distracter items. Two additional HGLMs were carried out using the same structure as the previous memory tradeoffs model. In separate analyses, target memory accuracy and distracter memory accuracy were modeled as dependent variables with a binomial (logit) distribution (1 = Remembered, 0 = Forgotten).
2.7.2 Sex-dependent effects of trial-level emotional arousal and noradrenergic activity on target/distracter memory and their tradeoffs
To interpret the directionality of any sex-related interaction on memory tradeoff effects in the first HGLM, we performed the same HGLM analysis in men and women, separately. These models were identical to the whole-group HGLM, except any predictors including the Sex predictor were removed (see Table 4). We again conducted separate follow-up HGLMs focused on memory for individual target and distracter stimuli to examine whether arousal influenced item recognition differently in men and women.
Table 4.
Hierarchical generalized linear models (HGLMs) examining Interaction effects between trial-level arousal ratings, distraction ratings, and salivary alpha-amylase on memory selectivity in men and women, separately.
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Memory Tradeoffs in Men Only | ||||
| Intercept | −0.5773 | 0.0731 | −7.90 | 2.8E−15 |
| Emotional Sound Arousal Rating | −0.0038 | 0.0265 | −0.14 | 0.89 |
| Task-Induced sAA Change | 0.0727 | 0.0992 | 0.73 | 0.46 |
| Emotional Sound Distraction Rating | −0.0495 | 0.0658 | −0.75 | 0.45 |
| Arousal Rating x sAA Change | −0.0390 | 0.0363 | −1.07 | 0.28 |
| Arousal Rating x Distraction | 0.0205 | 0.0240 | 0.86 | 0.39 |
| sAA Change x Distraction | −0.0396 | 0.0798 | −0.50 | 0.62 |
| Arousal Rating x sAA x Distraction | 0.0489 | 0.0293 | 1.67 | 0.09 |
| Effects on Memory Tradeoffs in Women Only | ||||
| Intercept | −0.6597 | 0.0755 | −8.73 | <2e-16 |
| Emotional Sound Arousal Rating | 0.0188 | 0.0249 | 0.76 | 0.45 |
| Task-Induced sAA Change | 0.1242 | 0.0908 | 1.37 | 0.17 |
| Emotional Sound Distraction Rating | 0.0008 | 0.0470 | 0.02 | 0.99 |
| Arousal Rating x sAA Change | 0.0803 | 0.0323 | 2.49 | 0.013* |
| Arousal Rating x Distraction | −0.0204 | 0.0162 | −1.26 | 0.21 |
| sAA Change x Distraction | −0.0363 | 0.0512 | −0.71 | 0.48 |
| Arousal Rating x sAA x Distraction | −0.0366 | 0.0182 | −2.01 | 0.044* |
Key: sAA Change: Change score in salivary alpha-amylase concentration (U/mL) levels from baseline to immediately post-task. Distraction: a single post-task rating of how distracting participants found the emotional sounds to be; Arousal Rating: subjective arousal rating for each emotional sound. Significant results are bolded.
p < .05.
2.7.3 Interaction between ovarian hormones, noradrenergic activity and trial-level emotional arousal on target/distracter memory and their tradeoffs in women only
Next, we performed a female-only HGLM analysis to test whether sex steroid hormone levels altered the strength of arousal’s influence on mean recognition performance and selective memory tradeoffs favoring goal-relevant target items. To simplify the model, continuous estrogen and progesterone values were z-scored and multiplied to produce a single ovarian hormone predictor.
As in the previous HGLMs, optimal tradeoffs vs. other trial-level memory outcomes were coded as the binomial dependent variable. Subjective arousal ratings were group-centered and modeled as the level-1 trial-by-trial predictor, which was nested within three grand-mean-centered level-2 predictors: ovarian hormone levels, sound distraction scores, and task-induced sAA change scores. The HGLM included the main effects of each predictor and all of their interaction effects (see Table 2). As before, we also performed exploratory HGLM analyses to examine whether ovarian hormones, noradrenergic activity, and subjective arousal rating were associated with successful item recognition for target and distracter stimuli.
Table 2.
Hierarchical generalized linear model (HGLM) examining interaction effects between trial-level arousal ratings, distraction ratings, and salivary alpha-amylase on target item recognition across all participants.
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Target Item Memory Across All Participants | ||||
| Intercept | 1.26205 | 0.06404 | 19.71 | <2e-16 |
| Emotional Sound Arousal Rating | 0.00782 | 0.02135 | 0.37 | 0.71 |
| Sex | 0.04393 | 0.06324 | 0.70 | 0.49 |
| Task-Induced sAA Change | 0.11394 | 0.08378 | 1.36 | 0.17 |
| Emotional Sound Distraction Rating | −0.02975 | 0.04916 | −0.61 | 0.55 |
| Arousal Rating x Sex | 0.00261 | 0.02135 | 0.12 | 0.90 |
| Arousal Rating x sAA Change | 0.00559 | 0.02892 | 0.19 | 0.85 |
| Arousal Rating x Distraction | 0.01046 | 0.01670 | 0.63 | 0.53 |
| sAA Change x Sex | 0.13363 | 0.08378 | 1.60 | 0.11 |
| sAA Change x Distraction | 0.00453 | 0.06326 | 0.07 | 0.94 |
| Distraction x Sex | −0.04859 | 0.04915 | −0.99 | 0.32 |
| Arousal Rating x Sex x sAA | −0.01383 | 0.02892 | −0.48 | 0.63 |
| Arousal Rating x Distraction x sAA | 0.01367 | 0.02183 | 0.63 | 0.53 |
| Distraction x Sex x sAA Change | −0.07417 | 0.06326 | −1.17 | 0.24 |
| Arousal Rating x Sex x Distraction | −0.00073 | 0.01670 | −0.04 | 0.97 |
| Arousal x Sex x Distraction x sAA | 0.00488 | 0.02183 | 0.22 | 0.82 |
Key: sAA Change: Change score in salivary alpha-amylase (sAA) concentration (U/mL) levels from baseline to immediately post-task. Sex (female = 1, male = -1). Distraction: A single post-task rating of how distracting participants found the emotional sounds to be; Arousal Rating: Subjective arousal rating for each emotional sound. Significant results are bolded.
In this study, we did not control for menstrual cycle phase or whether or not women taking hormonal contraception were in the active or inactive days of their pill pack. To reduce complications regarding alternative hormone-related mechanisms engaged by endogenous vs. exogenous hormones, we conducted an additional HGLM in the naturally cycling women only with regular menstrual cycles (n = 18).
3. Results
3.1 Sound arousal and distraction ratings
Mean distraction ratings for the sounds did not significantly differ between men (M = 3.26, SD = 1.16) and women (M = 3.40, SD = 1.67; p > .05). Mean subjective arousal ratings for the sounds also did not significantly differ between men and women in any of the three pre-defined valence categories. However, there was a robust main effect of Valence on subjective arousal ratings, F(2,56) = 773.89, p < .001, ηp2 = .97, such that participants perceived negative sounds (M = 7.11, SD = .66) as being significantly more arousing than positive sounds (M = 5.11, SD = 1.19) and neutral sounds (M = 2.62, SD = .68). Positive emotional sounds were also rated as being more arousing than neutral sounds (all pairwise comparisons: ps > .001).
3.2 Effects of phasic emotional arousal, noradrenergic activity, and sound-induced distraction on memory selectivity
Statistical results from the whole-group HGLM analysis on memory tradeoffs are displayed in Table 1. Across all participants, there was a significant three-way interaction between Arousal Rating, Sex and sAA Change, z = 2.37, p = .018. We also found a significant four-way interaction between all predictors, z = −2.43, p = .015.
3.3 Effects of phasic emotional arousal, noradrenergic activity, and sound-induced distraction on target/distracter recognition
When examining target item recognition, there were no significant main or interaction effects of any of the four predictors on target memory accuracy (all ps > .05; Table 2). However, another follow-up HGLM revealed that the previously observed effects were likely driven by the influence of these predictors on distracter item recognition (Arousal Rating x Sex x sAA Change: z = −3.29, p = .001; four-way interaction: z = 2.76, p = .0058; Table 3).
Table 3.
Hierarchical generalized linear model (HGLM) examining interaction effects between trial-level arousal ratings, distraction ratings, and salivary alpha-amylase on distracter item recognition across all participants.
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Distracter Item Memory Across All Participants | ||||
| Intercept | 0.18520 | 0.04081 | 4.54 | 5.7E-06 |
| Emotional Sound Arousal Rating | −0.01466 | 0.01765 | −0.83 | 0.41 |
| Sex | 0.03973 | 0.04081 | 0.97 | 0.33 |
| Task-Induced sAA Change | −0.08924 | 0.05375 | −1.66 | 0.10 |
| Emotional Sound Distraction Rating | 0.03205 | 0.03166 | 1.01 | 0.31 |
| Arousal Rating x Sex | −0.00134 | 0.01765 | −0.08 | 0.94 |
| Arousal Rating x sAA Change | −0.00008 | 0.02400 | 0.00 | 1.00 |
| Arousal Rating x Distraction | 0.00492 | 0.01375 | 0.36 | 0.72 |
| sAA Change x Sex | 0.04107 | 0.05375 | 0.76 | 0.44 |
| sAA Change x Distraction | 0.04851 | 0.03990 | 1.22 | 0.22 |
| Distraction x Sex | −0.03748 | 0.03166 | −1.18 | 0.24 |
| Arousal Rating x Sex x sAA | −0.07898 | 0.02401 | −3.29 | 0.001** |
| Arousal Rating x Distraction x sAA | −0.01509 | 0.01760 | −0.86 | 0.39 |
| Distraction x Sex x sAA Change | −0.02861 | 0.03990 | −0.72 | 0.47 |
| Arousal Rating x Sex x Distraction | 0.02342 | 0.01375 | 1.70 | 0.09 |
| Arousal x Sex x Distraction x sAA | 0.04853 | 0.01760 | 2.76 | 0.0058** |
Key: sAA Change: Change score in salivary alpha-amylase (sAA) concentration (U/mL) levels from baseline to immediately post-task. Sex (female = 1, male = −1). Distraction: A single post-task rating of how distracting participants found the emotional sounds to be; Arousal Rating: Subjective arousal rating for each emotional sound. Significant results are bolded.
p < .05;
p < .01.
3.4 Sex-dependent effects of phasic emotional arousal, noradrenergic activity, and sound induced distraction on optimal memory selectivity
To better understand the nature of these complex interactions on memory tradeoffs favoring the goal-relevant target item found across all participants, we performed several follow-up HGLMs in men-only and women-only groups.
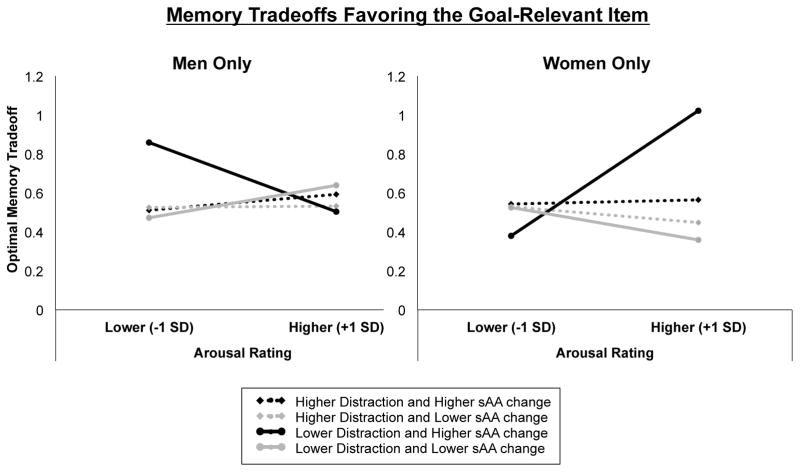
In men, there were no significant main or interaction effects of the predictors on optimal memory tradeoffs in which participants remembered the target item but forgot its corresponding distracter (ps > .05; Table 4, Figure 2, left panel). In women, larger task-induced increases in sAA were associated with greater trial-by-trial positive modulatory effects of emotional arousal on memory selectivity, z = 2.49, p = .013 (Table 4). To interpret the pattern of this interaction, we calculated -1 and +1 standard deviation values for these two predictors and estimated the corresponding optimal memory tradeoff values using the HGLM regression equation. Plotting these results revealed that, in women who showed greater increases in sAA levels across the task, higher subjective emotional arousal was associated with greater memory selectivity for the high priority target of attention (Figure 2, right panel).
Figure 2.
Estimation of the trial-by-trial influence of emotional arousal, sound distraction, and task-induced sAA change on optimal memory selectivity from the HGLM analyses in men only (left panel) and women only (right panel). Optimal memory tradeoff score was operationalized as trials in which participants correctly remembered the target visual stimulus (scene or object) while forgetting its corresponding distracter. Optimal memory tradeoff scores were estimated for sound arousal rating, sound distraction scores and task-induced salivary alpha-amylase (sAA) change values that were relatively higher or lower (+/− 1 SD, respectively) than the mean. Black lines represent relatively higher sAA change, whereas gray lines represent relatively lower sAA change. Broken lines represent relatively higher subjective distraction ratings for the emotional sounds, whereas intact lines represent relatively smaller distraction ratings.
The results also revealed a significant three-way interaction between Arousal Ratings, sAA Change, and Sound Distraction Ratings, z = −2.01, p = .044, such that arousal-enhanced memory tradeoffs were even greater in women who rated the task-irrelevant emotional sounds as being less distracting (Figure 2, right panel). These findings suggest that, under conditions of elevated background NE release, women were significantly more likely to show arousal-enhanced memory selectivity than men, particularly in those who were better able to direct their attention towards the neutral target stimulus.
3.5 Sex-dependent effects of phasic emotional arousal, noradrenergic activity, and sound-induced distraction on target/distracter item recognition
Because there was no significant four-way interaction on target item recognition in the initial analyses, we only list the statistical results of the HGLM on distracter item recognition (Table 5). Although we did not find any significant effects of the three predictors on memory selectivity in men, we did find that, in men showing a greater increase in sAA across the task, higher arousal ratings tended to improve distracter memory accuracy, z = 2.13, p = .033, particularly in those who rated the sounds as less distracting, z = −2.04, p = .041 (Figure 3, left panel; Table 5). There were no significant effects of predictors on memory for target items in men.
Table 5.
Hierarchical generalized linear models (HGLMs) examining Interaction effects between trial-level arousal ratings, distraction ratings, and salivary alpha-amylase on distracter item recognition in men and women, separately.
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Distracter Item Memory in Men Only | ||||
| Intercept | 0.127834 | 0.059036 | 2.17 | 0.030 |
| Emotional Sound Arousal Rating | −0.004322 | 0.025641 | −0.17 | 0.87 |
| Task-Induced sAA Change | −0.125244 | 0.08121 | −1.54 | 0.12 |
| Emotional Sound Distraction Rating | 0.076548 | 0.053346 | 1.44 | 0.15 |
| Arousal Rating x sAA Change | 0.076988 | 0.036133 | 2.13 | 0.033* |
| Arousal Rating x Distraction | −0.024278 | 0.023198 | −1.046 | 0.30 |
| sAA Change x Distraction | 0.070961 | 0.065025 | 1.091 | 0.28 |
| Arousal Rating x sAA x Distraction | −0.058562 | 0.028694 | −2.041 | 0.041* |
| Effects on Distracter Item Memory in Women Only | ||||
| Intercept | 0.228624 | 0.058051 | 3.94 | 8.21E-05 |
| Emotional Sound Arousal Rating | −0.007802 | 0.023625 | −0.33 | 0.74 |
| Task-Induced sAA Change | −0.049643 | 0.070534 | −0.70 | 0.48 |
| Emotional Sound Distraction Rating | −0.007197 | 0.036033 | −0.2 | 0.84 |
| Arousal Rating x sAA Change | −0.081531 | 0.030885 | −2.64 | 0.0083** |
| Arousal Rating x Distraction | 0.025661 | 0.015213 | 1.69 | 0.09 |
| sAA Change x Distraction | 0.021114 | 0.039594 | 0.53 | 0.59 |
| Arousal Rating x sAA x Distraction | 0.0355 | 0.017352 | 2.05 | 0.041* |
Key: sAA Change: Change score in salivary alpha-amylase (sAA) concentration (U/mL) levels from baseline to immediately post-task. Distraction: a single post-task rating of how distracting participants found the emotional sounds to be; Arousal Rating: subjective arousal rating for each emotional sound. Significant results are bolded.
p < .05;
p < .01.
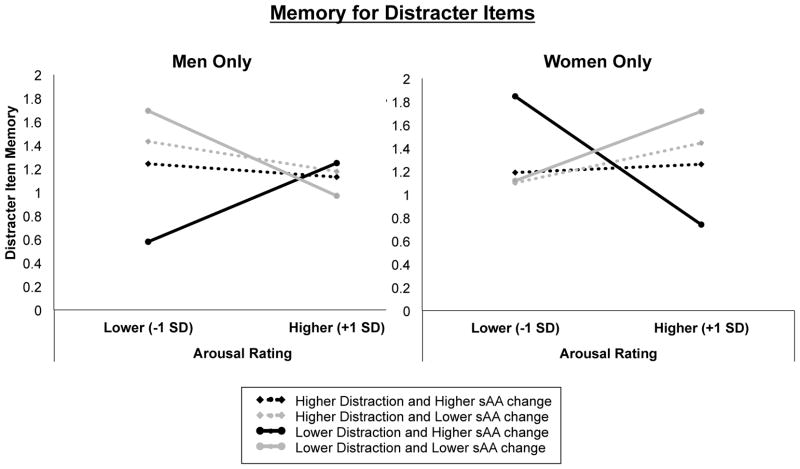
Figure 3.
Estimation of the trial-level influence of emotional arousal, sAA change, and sound distraction on distracter item memory from the hierarchical generalized linear modeling (HGLM) analyses in men (left) and women (right). Distracter memory performance was estimated for sAA change, distraction scores and subjective arousal ratings that were relatively higher or lower (+/− 1 SD, respectively) than the mean. Black lines represent relatively higher sAA change, whereas gray lines represent relatively lower sAA change. Broken lines represent relatively higher subjective distraction ratings for the emotional sounds, whereas intact lines represent relatively smaller distraction ratings.
In contrast with men, phasic arousal interacted with elevated tonic noradrenergic activity in women to impair memory for distracters (Arousal x sAA Change: z = −2.64, p = .0083), particularly in those who rated the sounds as being less distracting (Arousal x sAA Change x Distraction: z = 2.05, p = .041; Figure 3, right panel; Table 5). Together these results suggest that when phasic arousal is in sync with elevated tonic noradrenergic activity, women are significantly better than men at suppressing task-irrelevant information in attention and memory (see Table 3 for statistics). Additionally, unlike men, overall levels of noradrenergic activity across the task were also associated with better target memory accuracy in women, z = 2.26, p = .024 (not pictured).
3.6 The effects of ovarian hormone levels, phasic emotional arousal, noradrenergic activity, and sound-induced distraction on memory selectivity in women
Contrary to our second main prediction, adding a ovarian hormone (estradiol/progesterone interaction) predictor to the HGLM did not change the significance of the two-way interaction effect between Arousal Rating and sAA Change nor the three-way interaction effect between Arousal Rating, Distraction Rating, and sAA Change on memory selectivity (Table 6, top panels).
Table 6.
Hierarchical generalized linear models (HGLMs) examining interaction effects between trial-level arousal ratings, distraction ratings, salivary alpha-amylase and ovarian hormones (estradiol/progesterone interaction) on memory selectivity in all women and naturally cycling women (n = 18).
| Predictors | Estimate | SE | z | p |
|---|---|---|---|---|
| Effects on Memory Tradeoffs in All Women | ||||
| Intercept | −0.637805 | 0.074848 | -8.52 | <2e-16 |
| Emotional Sound Arousal Rating | 0.027282 | 0.026806 | 1.02 | 0.31 |
| Task-Induced sAA Change | 0.108572 | 0.086882 | 1.25 | 0.21 |
| Emotional Sound Distraction Rating | −0.002499 | 0.043519 | −0.06 | 0.95 |
| Ovarian Hormone Level | 0.195296 | 0.102899 | 1.90 | 0.06 |
| Arousal Rating x Ovarian Hormone | 0.055174 | 0.035765 | 1.54 | 0.12 |
| Ovarian Hormone x sAA Change | −0.118256 | 0.085726 | −1.38 | 0.17 |
| Arousal Rating x sAA Change | 0.072917 | 0.032839 | 2.22 | 0.026* |
| Arousal Rating x Distraction | −0.025967 | 0.016333 | −1.59 | 0.11 |
| sAA Change x Distraction | −0.046194 | 0.048577 | −0.95 | 0.34 |
| Ovarian Hormone x Distraction | 0.024431 | 0.065102 | 0.38 | 0.71 |
| Ovarian H. x sAA x Distraction | 0.012787 | 0.113595 | 0.11 | 0.91 |
| Arousal Rating x sAA x Ovarian H. | 0.035977 | 0.033429 | 1.08 | 0.28 |
| Arousal Rating x sAA x Distraction | −0.042072 | 0.018609 | −2.26 | 0.024* |
| Arousal Rating x Ovarian H. x Dist. | 0.001923 | 0.023753 | 0.08 | 0.94 |
| Arousal x sAA x Ovarian H. x Dist. | −0.064006 | 0.042296 | −1.51 | 0.13 |
| Effects on Memory Tradeoffs in Naturally Cycling Women Only | ||||
| Intercept | −0.4058 | 0.0995 | −4.08 | |
| Emotional Sound Arousal Rating | 0.0380 | 0.0424 | 0.90 | 0.37 |
| Task-Induced sAA Change | 0.3500 | 0.1684 | 2.08 | 0.038* |
| Emotional Sound Distraction Rating | −0.1535 | 0.1260 | −1.22 | 0.22 |
| Ovarian Hormone Level | 0.2691 | 0.1739 | 1.55 | 0.12 |
| Arousal Rating x Ovarian Hormone | 0.0824 | 0.0723 | 1.14 | 0.25 |
| Ovarian Hormone x sAA Change | −0.2621 | 0.1240 | −2.11 | 0.035* |
| Arousal Rating x sAA Change | 0.0657 | 0.0776 | 0.85 | 0.40 |
| Arousal Rating x Distraction | −0.0412 | 0.0536 | −0.77 | 0.44 |
| sAA Change x Distraction | −0.1934 | 0.1312 | −1.47 | 0.14 |
| Ovarian Hormone x Distraction | 0.0864 | 0.1702 | 0.51 | 0.61 |
| Ovarian H. x sAA x Distraction | 0.0400 | 0.2803 | 0.14 | 0.89 |
| Arousal Rating x sAA x Ovarian H. | 0.0072 | 0.0576 | 0.13 | 0.90 |
| Arousal Rating x sAA x Distraction | −0.0394 | 0.0595 | −0.66 | 0.51 |
| Arousal Rating x Ovarian H. x Dist. | −0.0256 | 0.0710 | −0.36 | 0.72 |
| Arousal x sAA x Ovarian H. x Dist. | −0.1112 | 0.1176 | −0.95 | 0.34 |
Key: sAA Change: Change score in salivary alpha-amylase concentration (U/mL) levels from baseline to immediately post-task; Distraction: a single post-task rating of how distracting participants found the emotional sounds to be; Ovarian Hormone: an interaction term between progesterone and estradiol levels; Arousal Rating: subjective arousal rating for each emotional sound. Significant results are bolded.
p < .05.
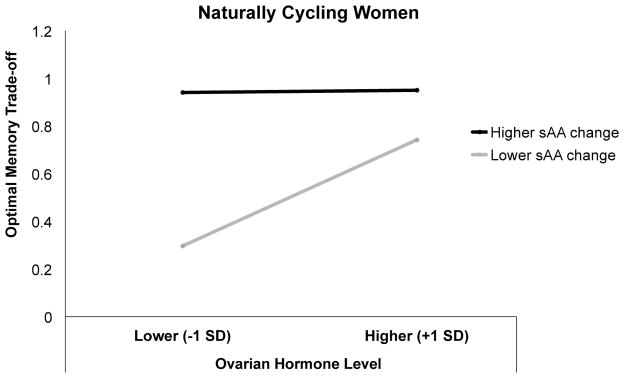
However, when examining the influence of ovarian hormone levels on memory in naturally cycling women only (n = 18), we found that women showing greater increases in sAA across the task exhibited greater top-down memory selectivity favoring the goal-relevant target item, z = 2.08, p = .038. The results also revealed a significant two-way interaction between Ovarian Hormone Levels and sAA Change, such that relatively lower sex steroid hormone levels were associated with enhanced memory tradeoffs in women who also showed a greater increase in noradrenergic activity across the task, z = −2.11, p = .035 (Table 6, bottom panels; Figure 4). These results indicated that, in naturally cycling women, ovarian hormones interacted with noradrenergic activity to influence mnemonic processing independently of phasic arousal.
Figure 4.
Estimation of the trial-level influence of emotional arousal and ovarian hormone levels (progesterone and estradiol) on optimal memory selectivity from the hierarchical generalized linear modeling (HGLM) analysis in natural cycling women only (n = 18). Optimal memory tradeoff score was operationalized as trials in which participants correctly remembered the target visual stimulus (scene or object) while forgetting its corresponding distracter. Optimal memory tradeoff scores were estimated for sAA change and ovarian hormone values that were relatively higher or lower (+/− 1 SD, respectively) than the mean. The black line represents higher sAA change across the task, whereas the gray line represents lower sAA change across the task.
3.7 The effects of ovarian hormone levels, phasic emotional arousal, noradrenergic activity, and sound-induced distraction on target/distracter item recognition
As in the memory tradeoffs analysis, adding an ovarian hormone predictor to the models did not alter the significance of the main effect of sAA Change on target memory accuracy, the arousal-by-sAA interaction effect on distracter memory accuracy, nor the arousal-by-sAA-by-distraction interaction effect on distracter memory accuracy found in women (ps < .05). When the same model was run with the subset of naturally cycling women, overall levels of background NE activity were still significantly positively associated with target recognition, z = 2.37, p = .018, whereas the just-mentioned two- and three-way interaction effects on distracter memory accuracy were no longer significant (ps > .05).
4. Discussion
Given the broad and diffuse release of NE under arousal, the noradrenergic system is ideally positioned to modulate any on-going cognitive selection process when emotional events occur. Yet, although it is well established that NE released under arousal helps strengthen emotional memories (McGaugh & Roozendaal, 2002), there has been less examination of whether NE can also bias memory in favor of prioritized, goal-relevant neutral information. Our results supported this prediction, revealing that, in women with greater task-increased NE levels, emotional arousal enhanced memory of goal-relevant stimuli at the cost of memory for competing distracters. These NE effects were sex-dependent, such that young women showed significantly greater modulatory effects of NE on arousal-related memory tradeoffs than young men. In women who found emotional sounds less distracting, we also found a stronger NE-arousal interaction on memory selectivity. This finding suggests that individuals who were better able to prioritize the preceding goal-relevant stimuli under greater background noradrenergic activity also showed greater arousal biases in memory consolidation.
Overall, our finding with women supports the recent GANE model of emotion-cognition interactions, which proposes that NE yields different memory outcomes as a function of stimulus priority (Mather et al., in press). According to GANE, strong goal-relevant representational activity triggers a positive feedback loop with nearby NE axons that garners even higher levels of NE and brain activity. In contrast, more modest NE release elsewhere suppresses task-irrelevant activity. Although GANE focuses primarily on local synaptic interactions between brain activity and NE release, its core prediction that NE amplifies cognitive selectivity is testable at the behavioral level using behavioral (top-down attention) and hormone (sAA) proxies of glutamate transmission and NE activity, respectively. Specifically, global increases in NE, as indexed by task-induced increases in sAA, should enhance NE’s local effects by fueling both priority-related activity (i.e., glutamate) and broad-scale inhibition of weaker lower priority-related activity.
Supporting this view, human genotyping studies demonstrate that ADRA2B deletion carriers, who presumably have greater extracellular NE availability, show greater activity in the insula and amygdala when viewing or encoding emotional versus neutral images (Cousijn et al., 2010; Rasch et al., 2009). This NE modulation of limbic activity is believed to promote the superiority of emotionally salient stimuli in perception (Todd et al., 2013) and memory (de Quervain et al., 2007) in these individuals. The present results extend these findings by showing that higher NE levels also bias cognitive processing in favor of goal-relevant neutral stimuli and not just emotional stimuli.
Akin to genotyping studies, a recent study showed that isometric handgrip-induced increases in NE bias memory towards highly arousing negative information, suggesting that incidentally elevating noradrenergic activity selectively amplifies the effects of phasic arousal on memory (Nielsen et al., 2015). Critically, our results suggest that greater background NE levels were also associated with competitive tradeoffs in memory consolidation: women with greater noradrenergic activity showed greater arousal-biased competition memory outcomes at the trial level. Examining item recognition performance revealed that these mnemonic tradeoffs were driven by memory costs for distracters rather than selective memory enhancements for prioritized target items. In fact, we also found significant sex differences in how effectively men and women suppressed task-irrelevant memory traces: under conditions of elevated noradrenergic activity and high subjective arousal, women were significantly better than men at filtering out distracter representations. These findings are highly consistent with reports in animals that NE enhances signal-to-noise processing in target brain regions primarily by suppressing weak or spontaneous inputs, while also sparing or inducing a less robust suppression of strong sensory inputs (Foote et al., 1975; Hasselmo et al., 1997). Importantly, our data offer a neuromechanistic account for findings that arousing sounds only impair memory consolidation for scenes paired with foreground objects (i.e., making scenes less salient) but not for scenes that are presented individually (Ponzio & Mather, 2014). That is, emotional arousal serves to amplify competition in memory consolidation between high and lower priority inputs primarily via the suppression of less salient information. In accordance with the current findings, 74% of the participants in that study were young women.
At least in women, the observed memory tradeoff effects conform to the widely recognized role of the noradrenergic system in regulating neural gain in the brain (Aston-Jones & Cohen, 2005; Eldar et al., 2013; Eldar et al., 2016; Servan-Schreiber et al., 1990; Usher et al., 1999); that is, NE enhances the excitability of active neurons, while further suppressing the excitability of inhibited neurons. LC recordings in monkeys (Aston-Jones et al., 1999; Aston-Jones et al., 1994) demonstrate that target discrimination performance is optimal under conditions of moderate tonic (i.e., baseline/background) LC activity, which is most permissive to LC phasic (i.e., rapid) responses that enhance goal-directed attention (Aston-Jones & Cohen, 2005). Consistent with this, our linear modeling analyses in all of the women indicated that greater sAA change is associated with better target item recognition, whereas greater sAA change was associated with enhanced memory selectivity in naturally cycling women only. It is possible that these differences relate to discrepancies in sample size. One interpretation of these results is that relatively larger sAA increases across the task corresponded to the peak of the inverted-U function between arousal/NE and cognitive function (Aston-Jones & Cohen, 2005). Given the significant NE-by-arousal interaction effect on memory tradeoffs, it is possible that GANE effects are particularly strong when phasic emotional arousal influences ongoing mental activity under conditions of higher background NE release.
One of our key findings was a significant sex-dependent effect of arousal and hormonal activity on memory, such that noradrenergic activity, or tone, and emotional arousal biased memory in favor of preceding top-down prioritized stimuli significantly more in women than in men. A large body of work shows that men and women exhibit different responses to emotional stimuli, which may alter arousal’s spillover effects on processing nearby neutral information. For example, emotionally arousing words elicit larger suppressive effect on preceding inconspicuous words in women than in men (Strange et al., 2003). It has been speculated that this may relate, at least in part, to sex differences in the ability to recall emotional events (Seidlitz & Diener, 1998). Past research shows that noradrenergic activation of the amygdala mediates emotional arousal’s suppressive effects on preceding information, while also enhancing memory of emotionally salient items (Strange & Dolan, 2004; Strange et al., 2003). Women exhibit larger startle reflex amplitude – a putative index of amygdala modulation - to emotional images than men (Bianchin & Angrilli, 2012). Moreover, a large meta-analysis found that women show greater amygdala responses to negatively valenced emotional stimuli, which, in the current study, were rated as being significantly more arousing than positive and neutral sounds (Stevens & Hamann, 2012). Together these findings suggest that noradrenergic modulation of amygdala activity under arousal is a critical locus of these sex-dependent memory effects. We also cannot rule out the possibility that the null arousal-by-priority effects in males were related to varying testosterone levels modulating the impact of arousal on memory, particularly through regulating amygdala activation (Ackermann et al., 2012).
When we limited our modeling analyses to naturally cycling women only, we found that those with a combination of lower levels of ovarian hormones and higher levels of noradrenergic activity showed greater gain effects in memory; however, such neurohormonal modulation of memory consolidation did not interact with phasic arousal induced by emotional sounds but rather enhanced memory selectivity in favor of top-down prioritized memoranda more generally. Thus, the ability of naturally cycling women to prioritize motivationally relevant information may differ according to their sex hormone status and overall arousal state. Indeed, many studies show that sex hormone effects on emotional memory differ by menstrual cycle phase (Andreano & Cahill, 2009; Nielsen et al., 2013; Pompili et al., 2016; Sakaki & Mather, 2012) and that the mnemonic effects of arousal differ between women who are on hormonal contraception and who are naturally cycling (Nielsen et al., 2011).
This key hormone-related finding expands upon recent evidence that handgrip-enhanced NE release at encoding, as indexed by pupil dilation and sAA increase, amplifies an emotional negativity bias in memory in women with lower levels of sex steroid hormones (Nielsen et al., 2015). In a similar finding, women on hormonal contraception (i.e., lower sex hormone state) with greater post-encoding sAA increases have been shown to exhibit greater memory enhancements for arousing negative images (Nielsen et al., 2013). Because negative emotional stimuli tend to garner greater attention in young adults and are usually more arousing than positively valenced images, it is somewhat difficult to isolate the effects of arousal from biases in priority for emotional stimuli. Yet, by manipulating top-down attention separately from arousal, our results suggest that - in women with lower levels of ovarian hormones - noradrenergic activity amplifies the effects of goal relevance in memory consolidation. Thus, one alternative explanation for Nielsen et al.’s (2015) findings is that sex hormone-dependent effects of noradrenergic activity on memory relate more closely to the priority of emotional stimuli rather than to the phasic arousal they induce (Markovic et al., 2014). It is difficult to verify this interpretation, however, due to important design differences between their study and the current one, such as differences in the memory outcome variable. Nonetheless, our results point to an important role of ovarian hormones and NE in regulating the ability of women to prioritize memory contents in a top-down manner.
One limitation of the current study was that we could not disentangle valence effects, because subjective emotional arousal ratings were highly correlated with pre-defined valence categories. Previous research indicates that positive emotional stimuli also amplify the effects of priority in memory, suggesting that GANE effects are invariant to the valence of the arousal-eliciting emotional stimulus (Sakaki et al., 2014). Yet other evidence shows that the spillover effects of emotional arousal may differ by the valence of to-be-remembered information when background NE levels are manipulated independently via isometric exercise (Nielsen et al., 2015) or pharmacology (Hurlemann et al., 2005). A key distinction between these studies is whether they manipulated the priority of the neutral stimuli appearing near something emotional. Since we manipulated and focused on arousal’s effects on competition between goal-relevant and distracting neutral stimuli during consolidation, we would also expect our results to be invariant to the valence of the emotional stimuli (Sakaki et al., 2014). Nonetheless, future studies should focus on examining whether valence also modulates arousal-biased competition effects.
By highlighting the important role of priority in neuronal processing, the GANE model provides predictions about how neutral information will fare under arousing conditions. However, since priority is relative, it is difficult to determine whether the goal-relevance or perceptual salience of a neutral stimulus is high enough to outcompete emotional stimuli, which tend to be preferentially processed and attended to (LaBar & Cabeza, 2006). We attempted to limit such competition using three strategies. First, we presented the goal-relevant stimulus (image) and arousing stimulus (sound) in different sensory modalities. Second, we introduced a 1s lag between the presentation of the neutral overlap and emotional stimuli, which is associated with emotion-enhanced processing of standalone neutral information (Bocanegra & Zeelenberg, 2009). Third, we acquired ratings of how distracted participants were by the sounds to approximate the competitive weight of the emotional sounds. As predicted, we found that arousal-enhanced memory tradeoffs were greater in women who not only showed increased NE levels but were also better able to prioritize the goal relevant neutral images over the emotional sounds. Thus, an important consideration in future emotion-cognition research is to measure how effectively participants are able to voluntarily focus their attention on a goal amidst task-irrelevant emotional distraction.
It is important to note that there has been some controversy concerning the use of sAA as a specific biomarker of central noradrenergic activity (Bosch et al., 2011; Nater et al., 2006). Past evidence indicates that there is a positive association between sAA secretion and changes in plasma NE levels induced by exercise (Chatterton et al., 1996) or acute stress (Thoma et al., 2012). Using multilevel modeling, Ditzen et al. (2014) demonstrated that plasma levels of NE and epinephrine statistically mediated changes in sAA secretion in response to pharmacological challenge (Ditzen et al., 2014). Because peripheral epinephrine can stimulate central release of NE (Gold and van Buskirk, 1978), particularly in the amygdala (Chen and Williams, 2012), it is possible that such increases in plasma NE, epinephrine, and perhaps sAA are also associated with increased activation of memory consolidation mechanisms in the brain. However, this link is only speculative. Earlier work shows that pharmacologically increasing (Ehlert et al., 2006) or decreasing (van Stegeren et al., 2006) noradrenergic system activity leads to increased or decreased sAA levels, respectively. Ehlert et al. (2006) demonstrated that administering the alpha2-adrenoreceptor antagonist yohimbine increases sAA secretion and plasma levels of NE and epinephrine; however, they did not find correlations between sAA and these hormones, suggesting that central noradrenergic system activity triggered sAA secretion. Further, one recent study identified a potential link between isometric exercise-induced changes in sAA levels and pupil dilation (Nielsen & Mather, 2015), another putative biomarker of noradrenergic activity (Joshi et al., 2015; Murphy et al., 2014; Reimer et al., 2014; Reimer et al., 2016). Taken together, while current evidence suggests an association between sAA levels and catecholamine release, our results should be interpreted with caution given that more work is needed to validate this relationship.
5. Conclusion
Years of research demonstrate that emotional arousal can enhance or impair cognitive processing. But, until recently, we lacked a unifying brain-based model to account for such dual effects. Our results support the recent hypothesis that NE released under arousal enhances the gain on competition between goal-relevant and distracting neutral information in memory (Mather et al., in press). Thus, beyond simply enhancing emotional memories, NE appears to amplify selectivity in memory consolidation to ensure that any behaviorally relevant stimulus is encoded under arousing situations that demand our attention or threaten our wellbeing.
Intensified competition in memory consolidation, however, was only observed in young women and not in young men, which appeared to be driven by phasic arousal and background noradrenergic activity interacting to suppress weaker, task-irrelevant representations. Fundamentally, this result highlights the importance of accounting for sex-dependent effects of arousal on mnemonic selection processes. In addition, we found that naturally cycling women with a combination of lower ovarian hormone levels and greater task-induced increases in noradrenergic activity showed greater goal-relevant biases in episodic memory. Together these findings suggest that, in women, increased noradrenergic transmission enhances the preferential retention of prioritized stimuli according to overall arousal state and ovarian hormone status. That the scope of memory under arousal is not only narrowed towards emotional stimuli, as demonstrated in prior research, but also towards nearby salient information has important implications for understanding and treating certain disorders of emotion, including Post-Traumatic Stress Disorder (PTSD) and anxiety, which are more prevalent in women than in men.
Highlights.
Arousal enhanced competition effects in memory in women with high noradrenergic activity
Men did not show such arousal-biased competition effects in memory
Under high noradrenergic activity, arousal impaired distracter recognition in women
Under high noradrenergic activity, arousal enhanced distracter recognition in men
Memory selectivity was greater in naturally cycling women with high NE/low sex hormones
Acknowledgments
This project was funded by federal NIH grants R01AG038043 (M.M.), R01AG025340 (M.M.), a grant from the European Commission (FP7-PEOPLE-2013-CIG; M.S.), a University of Southern California Endowed Fellowship (D.C.), and federal NIA grant F32AG047840 (S.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann S, Spalek K, Rasch B, Gschwind L, Coynel D, Fastenrath M, … Dominique J-F. Testosterone levels in healthy men are related to amygdala reactivity and memory performance. Psychoneuroendocrinology. 2012;37(9):1417–1424. doi: 10.1016/j.psyneuen.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Wais PE, Gabrieli JD. Emotion enhances remembrance of neutral events past. Proc Natl Acad Sci U S A. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33(6):874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learning and Memory. 2009;16(4):248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493(1):99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46(9):1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. The Journal of neuroscience. 1994;14(7):4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of memory and language. 2008;59(4):390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Research. 2015 doi: 10.1016/j.brainres.2015.11.021. [DOI] [PMC free article] [PubMed]
- Bianchin M, Angrilli A. Gender differences in emotional responses: A psychophysiological study. Physiol Behav. 2012;105(4):925–932. doi: 10.1016/j.physbeh.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion improves and impairs early vision. Psychol Sci. 2009;20(6):707–713. doi: 10.1111/j.1467-9280.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Veerman EC, de Geus EJ, Proctor GB. α-Amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–453. doi: 10.1016/j.psyneuen.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends in Neurosciences. 2005;28(11):574–582. doi: 10.1016/j.tins.2005.09.002. doi: http://dx.doi.org/10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Tech Rep B-3. University of Florida; Gainesville, FL: 2007. The International Affective Digitized Sounds (; IADS-2): Affective ratings of sounds and instruction manual. [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clinical Physiology. 1996;16(4):433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Clewett D, Sakaki M, Nielsen S, Petzinger G, Mather M. Noradrenergic mechanisms of arousal’s bidirectional effects on episodic memory. Neurobiology of Learning and Memory. 2017;137:1–14. doi: 10.1016/j.nlm.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, … Fernández G. Acute stress modulates genotype effects on amygdala processing in humans. Proceedings of the National Academy of Sciences. 2010;107(21):9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nature Neuroscience. 2007;10(9):1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Ehlert U, Nater UM. Associations between salivary alpha-amylase and catecholamines – A multilevel modeling approach. Biological Psychology. 2014;103:15–18. doi: 10.1016/j.biopsycho.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Salivary α-amylase levels after yohimbine challenge in healthy men. The Journal of Clinical Endocrinology & Metabolism. 2006;91(12):5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- Eldar E, Cohen JD, Niv Y. The effects of neural gain on attention and learning. Nat Neurosci. 2013;16(8):1146–1153. doi: 10.1038/nn.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Niv Y, Cohen JD. Do you see the forest or the tree? Neural gain and breadth versus focus in perceptual processing. Psychological Science. 2016;27(12):1632–1643. doi: 10.1177/0956797616665578. [DOI] [PubMed] [Google Scholar]
- Ertman N, Andreano JM, Cahill L. Progesterone at encoding predicts subsequent emotional memory. Learn Mem. 2011;18(12):759–763. doi: 10.1101/lm.023267.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Research. 1975;86(2):229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Seperate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11(4):288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14(2):233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. Journal of Neurophysiology. 1997;77(6):3326–3339. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, … Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. J Neurosci. 2005;25(27):6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, … Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25(27):6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Wagner M, Hawellek B, Reich H, Pieperhoff P, Amunts K, … Dolan RJ. Amygdala control of emotion-induced forgetting and remembering: Evidence from Urbach-Wiethe disease. Neuropsychologia. 2007;45(5):877–884. doi: 10.1016/j.neuropsychologia.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani Rishi M, Gold Joshua I. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron. 2015 doi: 10.1016/j.neuron.2015.11.028. http://dx.doi.org/10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005. [DOI] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of memory and language. 2007;56(4):575–591. [Google Scholar]
- Knight M, Mather M. Reconciling findings of emotion-induced memory enhancement and impairment of preceding items. Emotion. 2009;9(6):763–781. doi: 10.1037/a0017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lee T-H, Greening SG, Mather M. Encoding of goal-relevant stimuli is strengthened by emotional arousal in memory. Frontiers in psychology. 2015:6. doi: 10.3389/fpsyg.2015.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, Todd RM. Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research. 2014;259:229–241. doi: 10.1016/j.bbr.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2(1):33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hot spots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences. doi: 10.1017/S0140525X15000667. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12(2):205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Making lasting memories: Remembering the significant. Proceedings of the National Academy of Sciences. 2013;110(Supplement 2):10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neuroscience and Biobehavioral Reviews. 2012;36:1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O'Connell RG, O'Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping. 2014;35(8):4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. Stress-induced changes in human salivary alpha-amylase activity-associations with adrenergic activity. Psychoneuroendocrinology. 2006;31(1):49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ahmed I, Cahill L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiology of Learning and Memory. 2013;106:56–65. doi: 10.1016/j.nlm.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Barber SJ, Chai A, Clewett DV, Mather M. Sympathetic arousal increases a negative memory bias in young women with low sex hormone levels. Psychoneuroendocrinology. 2015;62:96–106. doi: 10.1016/j.psyneuen.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory. 2011;96(2):378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Mather M. Comparison of two isometric handgrip protocols on sympathetic arousal in women. Physiology and Behavior. 2015;142:5–13. doi: 10.1016/j.physbeh.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biological Psychology. 2013;92(2):257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili A, Arnone B, D'Amico M, Federico P, Gasbarri A. Evidence of estrogen modulation on memory processes for emotional content in healthy young women. Psychoneuroendocrinology. 2016;65:94–101. doi: 10.1016/j.psyneuen.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Ponzio A, Mather M. Hearing something emotional affects memory for what was just seen: How arousal amplifies trade-off effects in memory consolidation. Emotion. 2014;14:1137–1142. doi: 10.1037/a0037983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio A, Mather M. Hearing something emotional influences memory for what was just seen: How arousal amplifies effects of competition in memory consolidation. Emotion. 2014;14(6):1137. doi: 10.1037/a0037983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106(45):19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron. 2014;84(2):355–362. doi: 10.1016/j.neuron.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nature communications. 2016;7 doi: 10.1038/ncomms13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, Mather M. Emotion strengthens high priority memory traces but weakens low priority memory traces. Psychological Science. 2014;25:387–395. doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Fryer K, Mather M. Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychol Sci. 2014;25(2):387–395. doi: 10.1177/0956797613504784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Mather M. How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and Personality Psychology Compass. 2012;6(1):1–17. doi: 10.1111/j.1751-9004.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlitz L, Diener E. Sex differences in the recall of affective experiences. Journal of Personality and Social Psychology. 1998;74(1):262–271. doi: 10.1037//0022-3514.74.1.262. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Printz H, COHEN J. A network model of catecholamine effects- Gain, signal-to-noise ratio, and behavior. Science. 1990;249(4971):892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nat Neurosci. 2004;7(12):1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Steblay NM. A meta-analytic review of the weapon focus effect. Law and Human Behavior. 1992;16(4):413. [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. beta-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(31):11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(23):13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biological Psychology. 2012;91(3):342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Todd RM, Muller DJ, Lee DH, Robertson A, Eaton T, Freeman N, … Anderson AK. Genes for emotion-enhanced remembering are linked to enhanced perceiving. Psychol Sci. 2013;24(11):2244–2253. doi: 10.1177/0956797613492423. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283(5401):549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31(1):137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. How emotion leads to selective memory: Neuroimaging evidence. Neuropsychologia. 2011;49(7):1831–1842. doi: 10.1016/j.neuropsychologia.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiology of Learning and Memory. 2014;116:145–154. doi: 10.1016/j.nlm.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]