Abstract
BACKGROUND
Recent clinical trials highlight the need for better models to identify patients at higher risk for death from ventricular arrhythmia (VA).
OBJECTIVE
We hypothesized that the Seattle Heart Failure Model (SHFM) for overall survival and the Seattle Proportional Risk Model (SPRM) for proportional risk of sudden death from VA would predict the survival benefit with an implantable cardioverter defibrillator (ICD).
METHODS
Patients with primary prevention ICDs from the National Cardiovascular Data Registry (NCDR) were compared with control patients with heart failure (HF) without ICDs with respect to 5-year survival using multivariable Cox proportional hazards regression.
RESULTS
Among 98,846 patients with HF (87,914 with ICDs and 10,932 without ICDs), the SHFM was strongly associated with all-cause mortality (p <0.0001). The ICD-SPRM interaction was significant (p <0.0001), such that SPRM quintile 5 patients had approximately twice the reduction in mortality with the ICD versus SPRM quintile 1 patients (adjusted HR 0.602 [95% CI 0.537–0.675] vs. 0.793 [95% CI 0.736–0.855] for patients with and without ICD, respectively). Among patients with SHFM-predicted annual mortality ≤5.7%, those with a SPRM-predicted risk of VA below the median had no reduction in mortality with the ICD (adjusted ICD HR 0.921; 95% CI 0.787–1.08; p =0.31), while those with SPRM above the median derived the greatest benefit (adjusted HR 0.599; 95% CI 0.530–0.677; p <0.0001).
CONCLUSIONS
The SHFM predicts all-cause mortality in a large cohort with and without ICDs, and the SPRM discriminates and calibrates the potential ICD benefit. Together, the models identified patients less likely to derive a mortality benefit from primary prevention ICDs.
Keywords: heart failure, implantable cardioverter defibrillator, risk models
INTRODUCTION
At least 5 million people in the United States have heart failure (HF) (1); more than 500,000 are diagnosed each year (2), and 2.5 million are hospitalized for this disease (3). Although randomized trials have shown an overall mortality benefit of prophylactic implantable cardioverter defibrillators (ICDs) in those with severe systolic HF (4–6), studies have questioned the effectiveness of ICDs in certain subgroups of patients. Moreover, the majority of patients with ICDs implanted for primary prevention do not receive therapeutic shocks, and only 21% of patients in the Sudden Cardiac Death in Heart Failure Trial received appropriate shocks during the trial (4). The DANISH-ICD trial recently demonstrated that patients with nonischemic heart failure had approximately a 50% reduction in sudden death with ICD implantation, but this did not translate into an improvement in survival, possibly due to the low rate of sudden death during follow-up (approximately 1.5%/year in the control group) and the low proportion of sudden death relative to all-cause mortality (35%) (10). For patients with HF associated with a prior myocardial infarction in the Multicenter Automatic Defibrillator Implantation Trial II, for which a risk model has been validated (11), ICD-associated reduction in mortality was almost entirely due to a reduction in arrhythmic death (12), highlighting the importance of identifying subgroups of patients with a greater proportional risk of arrhythmic death. Considering health resources used for ICDs implanted for primary prevention and the potential increase in such implants if all patients meeting guideline-based criteria were implanted, the public health impact improving risk stratification and prognostication in ICD patients could be considerable.
The Seattle Heart Failure Model (SHFM) (9) and the Seattle Proportional Risk Model (SPRM) (13) are innovative models for prediction of mortality and sudden death. The SHFM is a well validated scoring system (9) that predicts the risk of all-cause mortality, and the SPRM is designed to predict the mode of death (sudden versus non-sudden) by identifying the proportional risk of death from ventricular arrhythmia (VA) (14). Application of these models in a large cohort of potential ICD patients could improve our ability to target ICDs to the right patients using precision medicine (15) and demonstrate the external validity of these models in an important cohort of patients. We hypothesized that the SHFM and SPRM scores would identify HF patients more and less likely to derive survival benefit from an ICD intended for primary prevention of sudden cardiac death. Our analysis applies both of these models to a large real-world population of National Cardiovascular Data Registry (NCDR) patients and a large control group of patients with HF without ICDs for comparison.
METHODS
GENERAL DESIGN
The analysis was approved by the University of Virginia Human Subjects Institutional Review Board, Yale University’s Human Investigation Committee, and a Swedish multi-site ethics committee. Data from the NCDR ICD Registry Version 1 was linked to Social Security Death Index (SSDI) records to determine long-term mortality up to 5 years following device implantation. SPRM and SHFM risk scores were determined for all patients in the ICD Registry. In addition, SPRM and SHFM risk scores were also determined for a control cohort of 10,932 subjects (left ventricular ejection fraction [LVEF] ≤35%) without ICDs from 3 HF registries and 3 clinical trials: the University of Washington Registry (16), the Italian Network on Congestive Heart Failure (17), the Swedish Heart Failure Registry (18), Carvedilol or Metoprolol European Trial (19), Valsartan Heart Failure Trial (20), and the Prospective Randomized Amlodipine Survival Evaluation 1 trial (21). These studies were chosen as a novel control group because they provided high-quality and generalizable data on patients who would otherwise be candidates for primary prevention ICDs but did not receive them, mainly because they enrolled patients prior to widespread use of ICDs. Survival data were collected for patients in these studies and registries throughout follow-up and used for the present analysis. We performed overall survival analysis by calculating the SHFM and SPRM scores in both the ICD Registry and control patients in order to assess the association of a primary prevention ICD with survival. We further utilized these scores to identify key subgroups that would potentially derive more and less survival benefit from ICD implantation.
COHORT SELECTION
The cohort included: 1) patients in the ICD Registry undergoing ICD implantation between 2006 and 2009 for primary prevention of sudden cardiac death with follow-up through 2011, linkage to the SSDI for determination of dates of death, and complete essential data in the registry, and 2) the control cohort with HF but without ICDs (approximate enrollment period 2000 to 2010), as described above, with the same data fields as those available in the ICD Registry and similar follow-up for death events. The control patients were not part of the ICD Registry, and data were collected separately for these patients. Based on current guidelines for ICD implantation (22), patients in both the ICD and control cohorts were required to have LVEF ≤35%. The following exclusion criteria were also applied to both ICD and control patients (Figure 1): systolic blood pressure <80 mm Hg or >220 mm Hg, NYHA class IV, NYHA class I with nonischemic cardiomyopathy, advanced chronic kidney disease with creatinine >4.0 mg/dL or requiring dialysis, age <21 years or >90 years, serum sodium <120 mEq/L or >155 mEq/L or missing, and prior pacemaker implantation. ICD Registry patients were excluded if the ICD implantation was performed during an inpatient hospitalization, or if a cardiac resynchronization therapy defibrillator (CRT-D) was implanted, as CRT-D devices are distinct because they modify the HF substrate through biventricular pacing.
Figure 1. CONSORT Diagram.
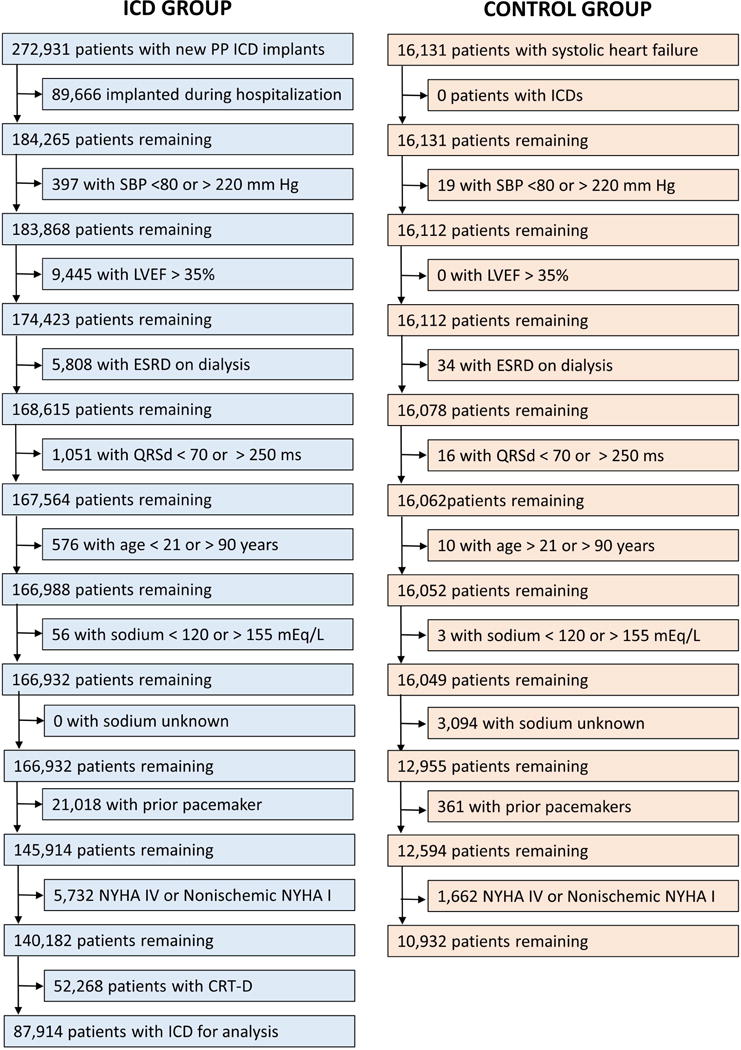
A flow diagram showing the derivation of the ICD and Control cohorts is shown. ICD = Implantable Cardioverter Defibrillator.
LINKAGE AND DETERMINATION OF OUTCOMES
Linkage between the ICD Registry and the SSDI was performed based on social security number by the NCDR Analytic Center to determine vital status through October 2011. In the control cohort, survival data were obtained directly from the associated registries and clinical trials. Of note, time to death was available for all patients. While the cause of death was not available for this analysis, the magnitude of the ICD-associated improvement in survival relative to control patients was easily determined and used as the primary outcomes measure to address the study hypotheses. The rationale for this approach is that the mechanism by which ICDs improve survival is through treatment of VA to prevent arrhythmic death (12).
DETERMINATION OF SHFM AND SPRM SCORES FROM ICD REGISTRY
As some variables for the SHFM model were missing in the NCDR registry (weight, carvedilol use, and diuretic daily dose) and could not be used in the SHFM model, the following variables were used to create a slightly revised version of the SHFM with comparable statistical power that could be prospectively applied to both NCDR registry patients and controls: age, gender, NYHA class, ischemic etiology, LVEF, systolic blood pressure, sodium, creatinine, angiotensin converting enzyme inhibitor use or angiotensin receptor blocker use, beta blocker use, digoxin use, diuretic use (daily dose not available), statin use, and the new variables of diabetes mellitus, lung disease, and QRS width. The SPRM in this cohort was calculated as previously described using age, gender, NYHA class, LVEF, systolic blood pressure, sodium, creatinine, digoxin use, diabetes mellitus, and the substitution of ischemic etiology of cardiomyopathy for BMI (not available in NCDR cohort), as both ischemic etiology of cardiomyopathy and BMI had similar statistical power. Both models were derived in the control group and prospectively applied in the NCDR registry. For simplicity, we refer to these slightly revised models as SHFM and SPRM in the present analysis.
STATISTICAL ANALYSIS
Analyses were performed for the primary statistical analysis using SAS version 9.4 (Cary, NC). Baseline continuous variables in the control and ICD cohorts were described using the mean and standard deviation if normality criteria were satisfied with the Shapiro-Wilk test or with the median and interquartile range, while categorical variables were described based on their frequency and percentage. Differences between continuous variables between groups were assessed using t-tests if normality criteria were satisfied or the Wilcoxon rank sum test if not, while differences between categorical variables were assessed using chi square tests.
A Cox proportional hazards (CPH) analysis was performed based on the combined cohort with and without ICDs with adjustment by SHFM, which has been previously shown to account for the contribution of baseline variables to overall mortality in the setting of heart failure (9). We used CPH regression to determine how overall survival varied depending on the SHFM score and tested the interaction term of SHFM*ICD in SHFM-adjusted CPH models. We evaluated the extent to which increased survival associated with ICD implantation varied as a function of the SPRM score by testing an interaction term of SPRM*ICD in the adjusted model. We performed a multivariable CPH model to assess the effect of the ICD on survival based on SPRM after adjustment for SHFM and SPRM. We determined the association of the ICD with survival in key subgroups by grouping patients relative to the median SHFM value, and then further subdividing each SHFM group based on the SPRM median. Associations between ICD use and survival were also assessed in quintiles of SHFM and quintiles of SPRM. Multivariable logistic regression and receiver operating characteristic analysis were also performed to determine the association of the SHFM with 3-year survival in the NCDR cohort.
The number of life years gained and years needed to treat (YNT) to save a life were determined as previously described (23). The additional years of life gained for each year of therapy was calculated as: [total survival with ICD − total survival without ICD]/total survival with ICD. The inverse of this value was defined as the YNT value. YNT was calculated based on the scenario that all patients would continue in their treatment assignment until death.
RESULTS
CHARACTERISTICS OF COHORT
The final analysis (n = 98,946) was based on 87,914 NCDR patients with primary prevention non-CRT ICD implants between 2006 and 2009, and 10,932 patients with systolic HF derived from registries and clinical trials enrolled during the period between approximately 2000 to 2010 (Figure 1). Characteristics of these 2 groups are described in Table 1, which includes the standardized differences for all parameters. We accounted for parameter differences between groups through adjustment in the subsequent Cox proportional hazards models described below.
Table 1.
Baseline Characteristics
| ALL (n = 98,846) | ICD (n = 87,914) | Control (n = 10,932) | Stand. Diff. | |
|---|---|---|---|---|
|
| ||||
| Age (mean[SD]) | 65.4 (12.1) | 65.6 (12.1) | 63.8 (11.7) | 0.112 |
| Female (N[%]) | 23,626 (23.9) | 21,276 (24.2) | 2350 (21.5) | 0.064 |
|
| ||||
| NYHA Class | ||||
| Class I (N[%]) | 8,332 (8.4) | 8,231 (9.4) | 101 (0.9) | 0.392 |
|
| ||||
| Class II (N[%]) | 58,410 (59.1) | 52,557 (59.8) | 5,853 (53.5) | 0.127 |
|
| ||||
| Class III (N[%]) | 32,104 (32.5) | 27,126 (30.9) | 4,978 (45.5) | −0.304 |
| Ischemic CM (N[%]) | 69,221 (70.0) | 63,059 (72.2) | 5712 (52.3) | −0.419 |
|
| ||||
| Lung Disease (N[%]) | 18,702 (18.9) | 17,792 (20.2) | 910 (8.3) | 0.345 |
| Diabetes Mellitus (N[%]) | 34,298 (34.7) | 31,587 (35.9) | 2,711 (24.8) | 0.243 |
|
| ||||
| LVEF (mean[SD]) | 25.8 (6.1) | 25.8 (6.1) | 25.2 (6.1) | 0.098 |
| Creatinine (mean[SD]) | 1.19 (0.40) | 1.18 (0.40) | 1.25 (0.38) | −0.179 |
|
| ||||
| Sodium (mean[SD]) | 139.1 (3.1) | 139.0 (3.1) | 139.5 (3.3) | −0.156 |
| Systolic BP (mm Hg, mean[SD]) | 130.6 (21.7) | 131.4 (21.8) | 123.5 (19.1) | 0.385 |
|
| ||||
| QRS (ms, mean[SD]) | 110.6 (23.5) | 110.3 (24.6) | 112.9 (11.7) | −0.135 |
| Med: ACEI or ARB (N[%]) | 82,453 (83.4) | 71,936 (81.8) | 10,517 (96.2) | −0.472 |
| Med: Beta Blocker (N[%]) | 84,584 (85.6) | 78,162 (88.9) | 6,422 (58.7) | 0.731 |
| Med: Digoxin (N[%]) | 25,971 (26.3) | 19,589 (22.3) | 6,382 (58.4) | −0.791 |
| Med: Diuretic (N[%]) | 64,029 (64.8) | 54,473 (62.0) | 9,556 (87.4) | −0.611 |
Limited to 5 years
OVERALL EFFECT OF THE ICD ON SURVIVAL
Among the 98,946 patients in the cohort, patients who underwent ICD implantation had a 25% lower risk of death after adjustment for the SHFM (hazard ratio [HR] 0.751; 95% confidence interval [CI] 0.721 to 0.782) during follow-up over 5 years (mean 3.2 ± 1.4 years) compared with control patients. Kaplan-Meier curves for patients with ICDs and control patients are shown in Figure 2A.
Figure 2. Overall Survival by SHFM Score in Control and ICD Cohorts.
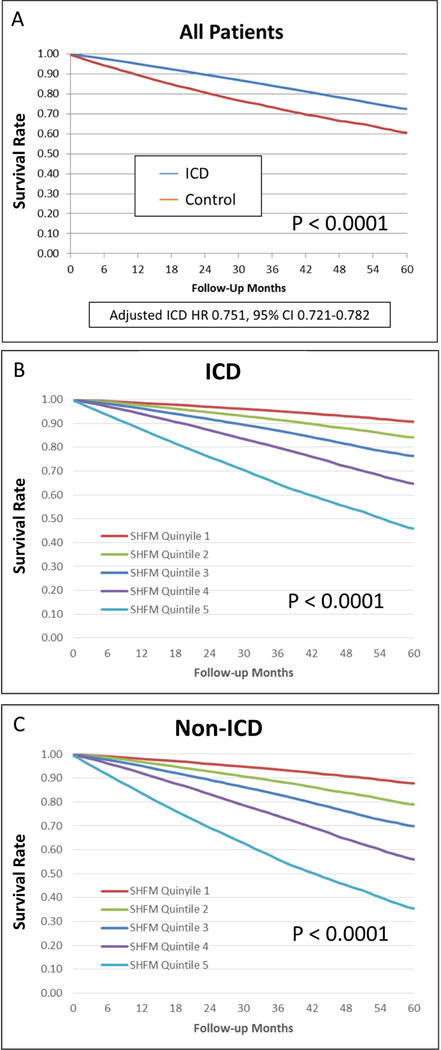
Kaplan-Meier curves demonstrate survival by SHFM in ICD (A) and control (B) cohorts. In both groups, an increase in the SHFM quintile is associated with decreased survival over 5 years. ICD = Implantable Cardioverter Defibrillator; SHFM = Seattle Heart Failure Model; SPRM = Seattle Proportional Risk Model.
SEATTLE HEART FAILURE MODEL SCORE AND OVERALL MORTALITY
An increase in SHFM quintile was associated with increased mortality in both the ICD and non-ICD cohorts (panels B and C of Figure 2). Table 2 demonstrates robust prediction of overall survival with the ICD based on SHFM quintiles. In a multivariable logistic regression models, SHFM was essentially as good as all the covariates combined for prediction of 3-year mortality (area under the curve [AUC] for SHFM and all other covariates = 0.730, p <0.0001; AUC with SHFM only = 0.723, p <0.0001). The ICD HRs are shown in specific subgroups of interest based on the model covariates in Figure 3. The findings in Figure 3 with respect to subgroups based on ischemic versus nonischemic etiology of cardiomyopathy stratified by age groups of <59 years old, 59 to 67 years old, and ≥68 years old show decreased benefit in patients with nonischemic cardiomyopathy and age ≥68 years old, consistent with the results of the recent DANISH ICD trial (10). Although both women and men had statistically significant hazard ratios favoring the ICD, the ICD was favored even more strongly in men than in women (HR 0.73 vs. 0.85 respectively; interaction p =0.004).
Table 2.
SHFM-Adjusted Hazard Ratios and 95% Confidence Intervals for the Effect of the ICD on Mortality versus Controls by Quintile of SHFM and SPRM
| SPRM Q1: PPAD 6.97–40.15% |
SPRM Q2: PPAD 40.16–47.55% |
SPRM Q3: PPAD 47.56–53.96% |
SPRM Q4: PPAD 53.97–61.16% |
SPRM Q5: PPAD 61.17–89.41% |
|
|---|---|---|---|---|---|
| SHFM Q1: P1YM 0.58–3.18% |
–* | 1.00 (0.25–4.09) |
0.73 (0.37–1.42) |
0.53† (0.36–0.79) |
0.81 (0.61–1.07) |
| SHFM Q2: P1YM 3.19–4.75% |
–* | 1.00 (0.58–1.70) |
1.11 (0.74–1.67) |
0.90 (0.67–1.22) |
0.52† (0.43–0.64) |
| SHFM Q3: P1YM 4.76–6.78% |
0.87 (0.59–1.27) |
1.16 (0.85–1.57) |
0.80† (0.63–1.00) |
0.58‡ (0.48–0.71) |
0.58‡ (0.46–0.73) |
| SHFM Q4: P1YM 6.79–10.39% |
0.89 (0.72–1.10) |
0.82† (0.69–0.98) |
0.71‡ (0.61–0.84) |
0.62‡ (0.52–0.74) |
0.63‡ (0.48–0.82) |
| SHFM Q5: P1YM ≥ 10.40% |
0.78‡ (0.72–0.85) |
0.75‡ (0.67–0.85) |
0.65‡ (0.56–0.76) |
0.59‡ (0.48–0.73) |
0.59† (0.37–0.93) |
Note: P-values correspond to HRs for ICD versus no ICD in SHFM-SPRM subgroups.
Not enough data to determine HR
<0.05
<0.001
P1YM = Predicted 1-Year Mortality
PPAD = Predicted Proportional Arrhythmic Death
SHFM = Seattle Heart Failure Model
SPRM = Seattle Proportional Risk Model
Figure 3. Forest Plot for the Effect of the ICD on Survival in Subgroups of Interest.
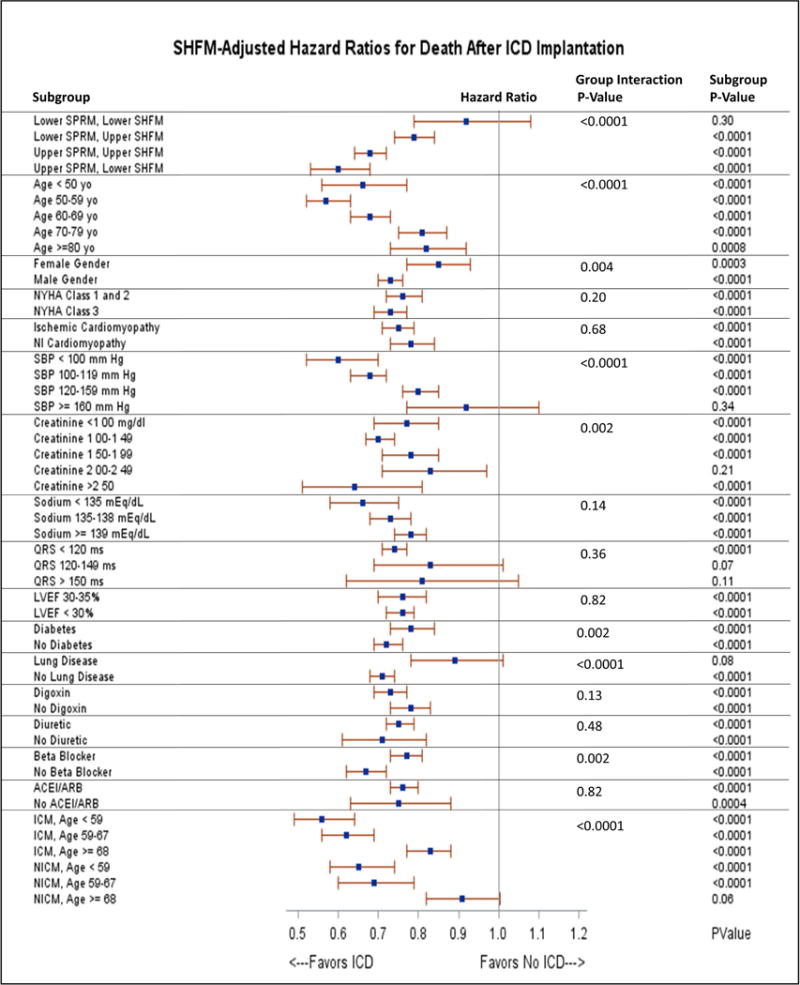
The hazard ratios for death with ICD implantation in subgroups of interests based on covariates of interest are plotted in the figure. Group interaction p-values are shown in addition to the p-value for ICD benefit in each subgroup of interest. ACE = Angiotensin Converting Enzyme; ARB = Angiotensin Receptor Blocker. ICD = Implantable Cardioverter Defibrillator; ICM = Ischemic Cardiomyopathy; LVEF = Left Ventricular Ejection Fraction; NICM = Nonischemic Cardiomyopathy; NYHA = New York Heart Association; SBP = Systolic Blood Pressure.
SEATTLE PROPORTIONAL RISK MODEL AND OVERALL MORTALITY
Whereas the SHFM score was strongly associated with all-cause mortality even with the ICD, the SPRM score was strongly associated with the magnitude of benefit from the ICD even after adjustment for SHFM. Figure 4 demonstrates how increasing SPRM score was associated with the effect of the ICD on survival based on the fitted line of the SPRM ICD interaction in the Cox proportional hazards model. For example, patients in SPRM quintiles 1 and 2 had a 19 to 21% reduction in mortality with the ICD, while patients in SPRM quintiles 4 and 5 had a 38 to 40% adjusted reduction in mortality with the ICD (p <0.0001 for improvement in survival with the ICD in these groups). In a multivariable model for survival, the covariates of ICD (p <0.0001), SPRM (p =0.15), SHFM (p <0.0001), and the interaction terms SPRM*ICD (p <0.0001) and SHFM*ICD (p =0.0006) were all significant. The high level of significance for the SPRM*ICD interaction term (HR 0.811; 95% CI 0.751 to 0.875; chi-square 29.2) demonstrated that the benefit of ICD implantation clearly increased as the SPRM estimated proportion of sudden death increased. Of note, the SPRM*ICD interaction term favored the ICD more strongly when the cohort was limited to patients with ischemic cardiomyopathy (HR 0.776; 95% CI 0.703 to 0.856) versus those with nonischemic cardiomyopathy (HR 0.871; 95% CI 0.770 to 0.985).
Figure 4. SPRM and the Improvement in Survival with ICDs.
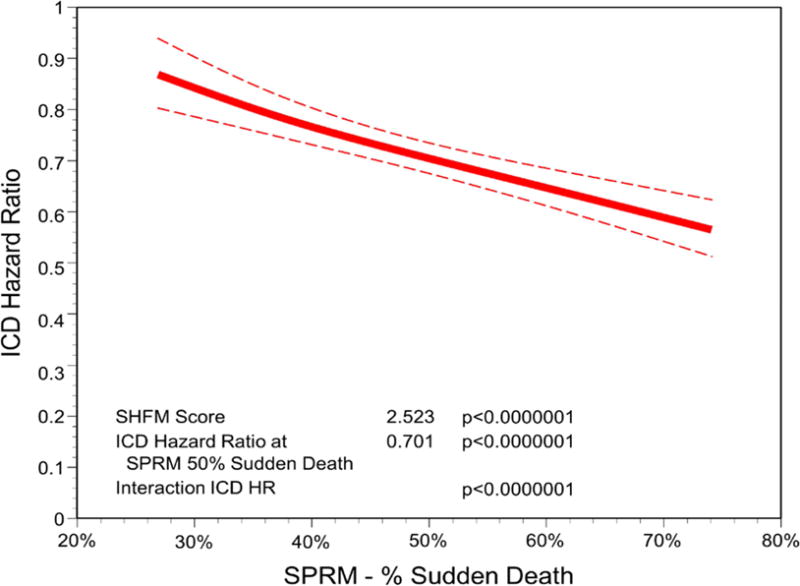
A fitted interaction from the Cox proportional hazards model of the ICD with the SPRM score as a continuous variable is shown with the ICD hazard ratio (solid line) and 95% upper and lower confidence bounds (dashed lines) plotted against the predicted proportional risk of sudden death based on the SPRM. As the SPRM-predicted proportional risk of sudden death increases, the ICD hazard ratio for death becomes more favorable. ICD = Implantable Cardioverter Defibrillator; SHFM = Seattle Heart Failure Model; SPRM = Seattle Proportional Risk Model.
COMBINED ASSESSMENT OF OVERALL SURVIVAL AND EFFECT OF ICD ON SURVIVAL WITH SHFM AND SPRM
Combined use of the SHFM score and SPRM score for comprehensive risk stratification in ICD candidates is of particular interest. In order to create 4 groups of equal size based on SHFM and SPRM (lower SHFM/lower SPRM; lower SHFM/higher SPRM; higher SHFM/lower SPRM; and higher SHFM, higher SPRM), we used threshold values based on the median SPRM-predicted proportional rate of arrhythmic death and the median SHFM-predicted 1-year mortality rate (5.7%). Characteristics of the patients in these 4 groups can be found in the Online Table. Kaplan-Meier survival curves for patients in these 4 groups with and without an ICD are shown in Figure 5A–D. The ICD had a clear benefit (adjusted ICD HR 0.599; 95% CI 0.530 to 0.677; p <0.0001) in patients with both SHFM-predicted 1-year mortality ≤5.7% and a SPRM-predicted proportional risk of sudden death above the median, but not in patients with an SHFM-predicted one year mortality ≤5.7% and a SPRM-predicted proportional risk of sudden death below the median (adjusted HR 0.921; 95% CI 0.787 to 1.08; p =0.31). Among patients with an SHFM-predicted 1-year mortality rate >5.7%, patients with a higher SPRM-predicted proportional risk of arrhythmic death above the median (>44.3%) had a greater adjusted improvement in survival over 5 years with the ICD (adjusted ICD HR 0.683; 95% CI 0.641 to 0.727; p <0.0001) compared with patients having a SPRM-predicted proportional risk of arrhythmic death ≤44.3% (adjusted ICD HR 0.791; 95% CI 0.742 to 0.843; p <0.0001). HRs for the ICD versus control patients are shown in more granular fashion in Table 2 in a 5×5 matrix form for specific quintiles of SPRM and SHFM. The SHFM-adjusted mortality rates at 1 and 5 years by quintile of SPRM score and quintile of SHFM score is shown in Figure 6.
Figure 5. Survival in ICD and Control Cohorts Stratified by SHFM and SPRM.
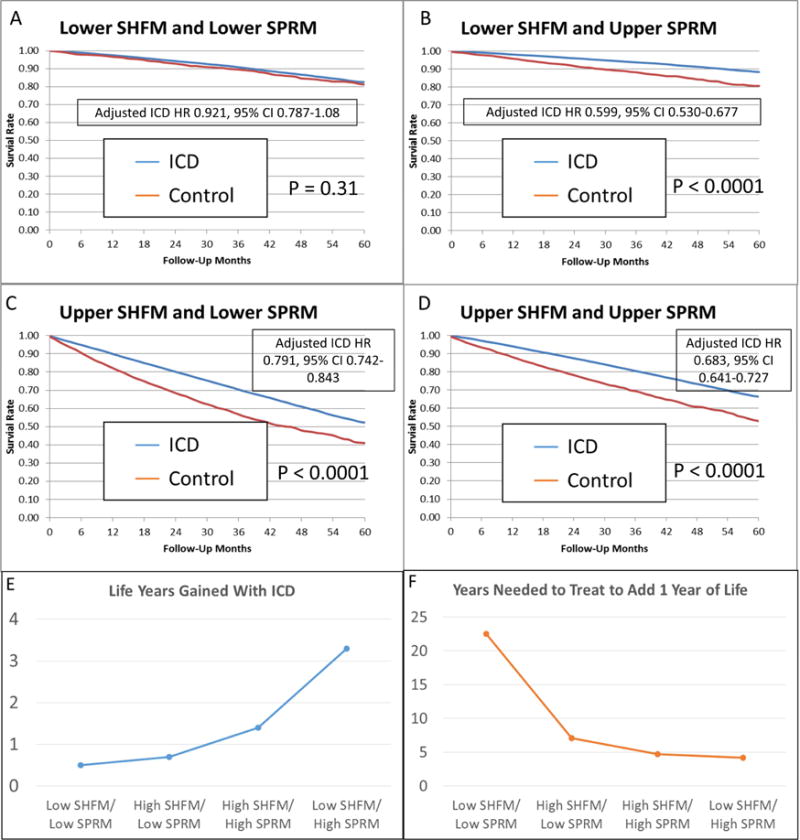
Adjusted survival is shown in 4 groups based on whether SHFM and SPRM are above and below the median (A–D). Differences in life years gained with the ICD in these 4 groups (E) and the years needed to treat to add 1 year of life in the 4 groups (F) are also shown. ICD = Implantable Cardioverter Defibrillator; SHFM = Seattle Heart Failure Model; SPRM = Seattle Proportional Risk Model.
Figure 6. Adjusted Survival at 1 Year and 5 Years by Quintile of SPRM and SHFM.
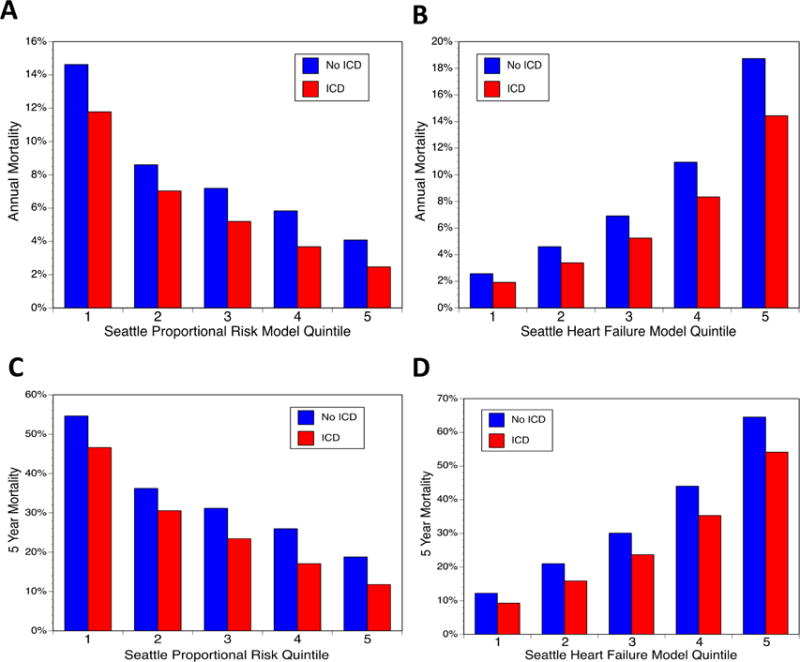
Adjusted 1-year survival with and without ICD implantation is shown for SPRM (A) and SHFM (B). Adjusted survival after 5 years of follow-up with and without ICD implantation is also provided for SPRM (C) and SHFM (D). ICD = Implantable Cardioverter Defibrillator; SHFM = Seattle Heart Failure Model; SPRM = Seattle Proportional Risk Model.
As shown in Table 3 and in Figure 5E–F, patients with lower predicted overall mortality (lower SHFM) and lower SPRM-predicted proportional risk of arrhythmic death (the group without a statistically significant improvement in survival during follow-up with ICDs) would be projected to have minimal improvement in life expectancy with the ICD (0.5 years) during their lifetime, such that these patients would need to be treated 22.5 years with an ICD to add 1 year of life. In contrast, patients with lower predicted overall mortality (lower SHFM) and a higher SPRM-predicted proportional risk of arrhythmic death (the group with the greatest survival improvement with the ICD) would be projected to have a life expectancy improvement of 3.3 years resulting from the ICD, and these patients would only need to be treated for 4.2 years to add one year of life.
Table 3.
Hazard Ratios for the Effect of the ICD on Mortality by SPRM/SHFM Grouping*
| SHFM | SPRM | 5-Year Mortality ICD | 5-Year Mortality No ICD | 1-Year Mortality ICD | 1-Year Mortality No ICD | Life Span ICD* | Life Span No ICD* | Life Years Gained | Years Needed to Treat |
|---|---|---|---|---|---|---|---|---|---|
| Low (P1YM ≤5.7%) |
Low (PPAD ≤57.2%) |
16.7% | 18.0% | 3.6% | 3.9% | 10.7 | 10.2 | 0.5 | 22.5 |
| High (P1YM >5.7%) |
Low (PPAD ≤44.3%) |
47.1% | 55.3% | 12.0% | 14.9% | 5.1 | 4.4 | 0.7 | 7.1 |
| High (P1YM >5.7%) |
High (PPAD> 44.3%) |
33.3% | 44.8% | 7.8% | 11.2% | 6.7 | 5.3 | 1.4 | 4.7 |
| Low (P1YM ≤5.7%) |
High (PPAD> 57.2%) |
10.8% | 17.3% | 2.3% | 3.7% | 13.7 | 10.5 | 3.3 | 4.2 |
The cohort was first divided into lower and upper SHFM groups based on the median predicted one-year mortality [P1YM] of 5.7% based on the SHFM score. In order to create 4 groups of equal size, the median predicted proportional arrhythmic death (PPAD) based on the SPRM score was determined for both the lower SHFM group (57.2%) and the upper SHFM group (44.3%). As a result, the SPRM cutoff is different for the upper and lower SHFM groups.
P1YM = Predicted 1-Year Mortality
PPAD = Predicted Proportional Arrhythmic Death
SHFM = Seattle Heart Failure Model
SPRM = Seattle Proportional Risk Model
DISCUSSION
We found that the SPRM and SHFM together provide a useful assessment of overall mortality and expected ICD effectiveness. The SHFM provides a powerful measure of overall survival, while the SPRM provides a very effective assessment of how overall survival with the SHFM is modified by the ICD implantation (Central Illustration). Overall, the ICD was associated with an approximately 25% improvement in adjusted survival in the cohort of approximately 100,000 patients, consistent with results from clinical trials. We also identified a subgroup composing a quarter of the NCDR cohort who did not receive significant survival benefit with an ICD. These patients would have minimal expected improvement in life expectancy, such that they would have to be treated for 22.5 years to add a year of life. In contrast, another group with a high proportion of arrhythmic death composing about one-quarter of the NCDR cohort had a 40% reduction in mortality with the ICD during follow-up. These patients would need to be treated for only 4.2 years to add a year of life. The remaining half of the NCDR cohort with increased heart failure severity received intermediate survival benefit with an ICD.
Central Illustration. Seattle Heart Failure Model, Seattle Proportional Risk Model, and ICD Benefit.
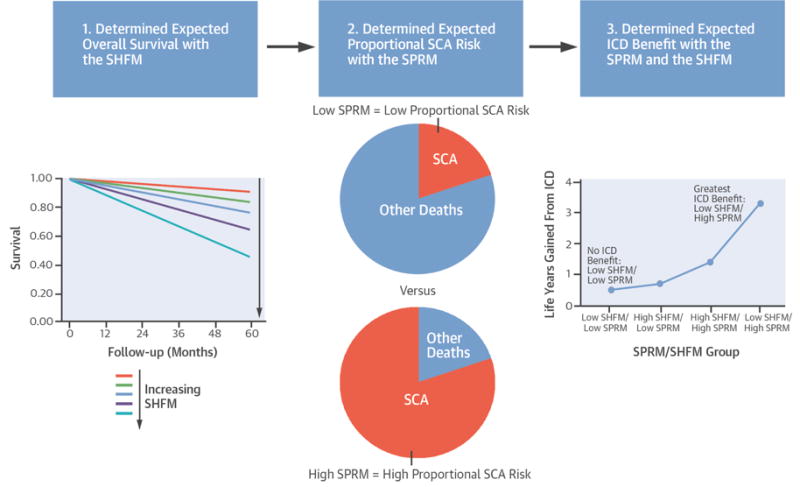
These models together can be used to predict the survival benefit from the ICD. ICD = Implantable Cardioverter Defibrillator; SCA = Sudden Cardiac Arrest; SHFM = Seattle Heart Failure Model; SPRM = Seattle Proportional Risk Model.
There were several strengths of this study. These include the use of high-quality registry data, linkage to a reliable death index, and the use of a high-quality and generalizable control group from multiple sources and with adjustment for many covariates. The use of a control group has been overlooked in some previous registry studies evaluating ICD prognosis (24), but it does provide important insights into the magnitude of the effect of the ICD on survival in addition to the overall expected survival with the ICD. Use of the contemporary control group in this case facilitated an analysis of the modulation of ICD effect on survival based on the SPRM after adjustment for overall expected survival based on heart failure parameters, as determined by the SHFM.
Both women and men derived a significant benefit from standard (non-CRT) ICDs in our study, but the more favorable HR for the ICD in men versus women is consistent with previous data from our group showing that women have a lower proportion of sudden death and a higher proportion of pump failure death at any given SHFM score (25), as well as a network clinical trial meta-analysis showing that men have a greater survival benefit than women with ICD therapy for the primary prevention indication (26). In contrast, the higher proportion of pump failure death in women may explain why women had a greater benefit with CRT-D in the MADIT-CRT trial (27).
This analysis provides much needed data to support the use of the SPRM and SHFM in the general population of patients with ICDs implanted in the community for primary prevention of sudden cardiac death from VA, while providing the tools to improve and individualize the clinical care of patients with heart failure according to the principles of precision medicine in several ways. First, these results provide evidence from the largest registry of ICD implants that the SHFM score provides an effective assessment of overall survival, which promises to be very useful for discussions between providers and patients regarding prognosis. Second, these results provide evidence for the use of the novel SPRM to characterize the proportional risk of sudden death, which has been shown to translate into the magnitude of survival improvement with the ICD in the present study. This promises to be useful in clinical decision making. Third, the combined use of the SPRM and SHFM appears to identify a low-risk cohort composing as many as one-quarter of patients with ICDs in the NCDR registry who had an overall survival during 5 years of follow-up of 83% that was not modified by the ICD. This is particularly relevant with the finding from the DANISH-ICD trial that ICDs reduce sudden death in patients with nonischemic heart failure, but not overall survival, because of the smaller proportion of patients with sudden death (approximately 35%) (10).
The combined use of the SPRM and SHFM in the present study could be particularly useful in identifying patients unlikely to need ICDs because they have a low proportional risk of sudden death. The potential impact on the health care system if further confirmatory studies showed that up to one-quarter of all patients receiving ICDs would do well without them would be enormous. Considering that current ICD indications are broad and include low risk patients unlikely to have improved survival as a result of the ICD (22), we believe a randomized clinical trial of ICDs in low-risk patients characterized by low SHFM and low SPRM risk is appropriate. Application of these models at the time of ICD generator replacement may be useful as many patients have ICDs replaced even if they have not received appropriate therapy and no longer meet criteria for primary prevention ICDs.
LIMITATIONS
One limitation of our analysis is that it is not a randomized clinical trial and uses patient data from datasets from prior clinical trials and registries as the control group. Although we accounted for differences between groups with statistical adjustment, there may be unmeasured factors that differ between groups. This is an inherent limitation of analyses of large databases and registries; however, this limitation is offset to a significant degree by the opportunity of study designs such as this to evaluate risk models and other interventions in real-world patients. This is particularly important considering the differences in patient selection in treatment between clinical trials and registries. In addition, although the time periods during which patients in the control group were enrolled in registries and clinical studies (2000 to 2010) was similar to that of the patients in the NCDR group (2006 to 2009), the patients in ICD and control cohorts were not enrolled at the same time in the same way, which could introduce bias. The reasons that control patients did not receive ICDs were likely related to differences among clinical practice settings and the lower prevalence of ICD use before 2005 in those patients enrolled before 2005 when ICDs were not widely available for primary prevention, especially in nonischemic patients. Even so, available medical therapy for HF was similar for patients in the control group, and analyses were adjusted for differences in the use of these medications, which are included in the SHFM, itself. Of note, we did use slightly adapted versions of the SHFM and SPRM based on available data fields, but the models used has similar statistical performance compared with the original models, and the same re-derived models were used in both ICD and control groups.
CONCLUSIONS
Our analysis shows how the SHFM and SPRM can be used to predict both overall survival and ICD benefit in a large cohort of approximately 100,000 patients. In particular, the SHFM provides a highly effective measure of HF outcomes, while the SPRM provides a powerful measure of the proportional risk of arrhythmic death. Considering the large amount of time and expense presently allocated to patients with ICDs for implantation, postoperative care, and subsequent generator changes, application of these models could more effectively allocate these health care resources and personalize device therapy for the benefit of patients and society.
Supplementary Material
CONDENSED ABSTRACT.
In a study of 98,846 heart failure patients with a primary prevention indication for an implantable cardioverter defibrillator (ICD), we found that the combination of the Seattle Heart Failure Model to assess overall survival and the Seattle Proportional Risk Model to determine the proportional risk of sudden death from ventricular arrhythmia effectively predicted the survival benefit from the ICD in the National Cardiovascular Data Registry (NCDR) ICD Registry. We not only identified patients with the greatest expected survival benefit from the ICD, but also a quarter of the cohort with good overall survival that was not altered by ICD implantation.
PERSPECTIVES.
Competency in Patient Care
Patients with ischemic or nonischemic cardiomyopathy face competing risks of heart failure and ventricular arrhythmias, limiting the benefit of implantable cardioverter-defibrillator (ICD) therapy to those at higher risk of sudden death.
Competency in Interpersonal and Communication Skills
Shared decision-making around ICD therapy should address both overall survival and the proportional risk of death from ventricular arrhythmias.
Translational Outlook
Further studies are needed to assess the value of ICD therapy for patients with cardiomyopathy at low proportional risk of ventricular arrhythmia.
Acknowledgments
Acknowledgements: The authors acknowledge the staff of the NCDR Analytic Center at Yale University and the American College of Cardiology for their assistance in facilitating the present analysis.
This work was supported by NIH grant R03 HL135463 (KCB) and a research grant from the National Cardiovascular Data Registry (KCB, WL, AC).
Sources of Funding: This research is supported by a grant to Drs. Bilchick, Cheng, and Levy from the NCDR and Dr. Bilchick’s NIH grant R03 HL135463.
ABBREVIATIONS
- AUC
Area under the curve
- CI
Confidence interval
- CPH
Cox proportional hazards
- CRT-D
Cardiac resynchronization therapy defibrillator
- HF
Heart failure
- HR
Hazard ratio
- ICD
Implantable cardioverter defibrillator
- LVEF
Left ventricular ejection fraction
- NYHA
New York Heart Association
- NCDR
National Cardiovascular Data Registry
- ROC
Receiver Operating Characteristic
- SHFM
Seattle Heart Failure Model
- SPRM
Seattle Proportional Risk Model
- YNT
Years needed to treat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Cheng is employed by Medtronic. Dr. Curtis receives salary support under contract with the NCDR and the Centers for Medicare & Medicaid Services. Dr. Dharmarajan is supported by grant K23AG048331 from the National Institute on Aging and the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. He is also supported by grant P30AG021342 via the Yale Claude D. Pepper Older Americans Independence Center. Dr. Dharmarajan also receives salary support under contract with the Centers for Medicare & Medicaid Services and serves as a consultant and scientific advisory board member for Clover Health. Dr. Levy has research grants from Amgen, Resmed, and Novartis, and he serves as a consultant for Novartis, St. Jude Medical, GE Healthcare, Abbott, and Biotronik. The University of Washington CoMotion holds the copyrights for the SHFM and SPRM and has received licensing fees from Thoratec (St Jude Medical), HeartWare (Medtronic), GE Healthcare, and Athena Health. Dr. Anand is a consultant for Amgen, ARCA, Cyberonics, Novartis and Zensun. Dr. Sartipy has nothing to disclose. Dr. Lund has research grants from Boston Scientific and speaker’s honoraria from St. Jude Medical. Dr. Dahlstrom has received speaker´s/consulting honoraria from Novartis and research grants from AstraZeneca. Dr. Swedberg has research grants from Servier and serves as a consultant for Amgen, AstraZeneca, Novartis, Pfizer, Servier, and Vifor Pharma.
References
- 1.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–60. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Theuns DA, Smith T, Hunink MG, Bardy GH, Jordaens L. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–70. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson LW. Implantable cardioverter-defibrillators for primary prevention of sudden death in heart failure: are there enough bangs for the bucks? Circulation. 2006;114:101–3. doi: 10.1161/CIRCULATIONAHA.106.637405. [DOI] [PubMed] [Google Scholar]
- 8.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–42. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 10.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER, Andrews ML. Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT-II) J Am Coll Cardiol. 2004;43:1459–65. doi: 10.1016/j.jacc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Shadman R, Poole JE, Mozaffarian D, et al. Predicting the Proportional Risk of Sudden Cardiac Death in a Multicenter Cohort (abstr) Circulation. 2011;124:A17819. [Google Scholar]
- 14.Levy WC, Poole JE, Hellkamp AS, et al. Benefits of ICD Therapy: Connecting Treatment Decisions to Individualized SCD Risk Estimates Within SCD-HEFT - Seattle Proportional Risk Model (abstr) ECAS-Heart Rhythm. 2015 [Google Scholar]
- 15.Levy WC, Li Y, Reed SD, et al. Does the Implantable Cardioverter-Defibrillator Benefit Vary With the Estimated Proportional Risk of Sudden Death in Heart Failure Patients? JACC Electrophysiology. 2016 doi: 10.1016/j.jacep.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan MD, Levy WC, Crane BA, Russo JE, Spertus JA. Usefulness of depression to predict time to combined end point of transplant or death for outpatients with advanced heart failure. Am J Cardiol. 2004;94:1577–80. doi: 10.1016/j.amjcard.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 17.Fabbri G, Gorini M, Maggioni AP, Cacciatore G, Di Lenarda A. [Italian Network on Congestive Heart Failure: ten-year experience] G Ital Cardiol. 2006;7:689–94. [PubMed] [Google Scholar]
- 18.Jonsson A, Edner M, Alehagen U, Dahlstrom U. Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12:25–31. doi: 10.1093/eurjhf/hfp175. [DOI] [PubMed] [Google Scholar]
- 19.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335:1107–14. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 22.Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:1297–313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Levy WC, Mozaffarian D, Linker DT, et al. Years-needed-to-treat to add 1 year of life: a new metric to estimate treatment effects in randomized trials. Eur J Heart Fail. 2009;11:256–63. doi: 10.1093/eurjhf/hfn048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Khatib SM, Hellkamp AS, Fonarow GC, et al. Association between prophylactic implantable cardioverter-defibrillators and survival in patients with left ventricular ejection fraction between 30% and 35% JAMA. 2014;311:2209–15. doi: 10.1001/jama.2014.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rho RW, Patton KK, Poole JE, et al. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–7. doi: 10.1161/CIRCULATIONAHA.111.069245. [DOI] [PubMed] [Google Scholar]
- 26.Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–6. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


