Abstract
Background & aims
Cholangiocarcinoma (CCA) is a biliary malignancy linked to genetic and epigenetic abnormalities, such as hypermethylation of SOX17 promoter. Here, the role of SOX17 in cholangiocyte differentiation and cholangiocarcinogenesis was studied.
Methods
SOX17 expression/function was evaluated along the differentiation of human induced pluripotent stem cells (iPSC) into cholangiocytes, in the dedifferentiation process of normal human cholangiocytes (NHC) in culture and in cholangiocarcinogenesis. Lentiviruses for SOX17 overexpression or knock-down were used. Gene expression and DNA methylation profiling were performed.
Results
SOX17 expression is induced in the last stage of cholangiocyte differentiation from iPSC and regulates the acquisition of biliary markers. SOX17 becomes downregulated in NHC undergoing dedifferentiation; experimental SOX17 knock-down in differentiated NHC downregulated biliary markers and promoted baseline and Wnt-dependent proliferation. SOX17 expression is lower in human CCA than in healthy tissue, which correlates with worse survival after tumor resection. In CCA cells, SOX17 overexpression decreased their tumorigenic capacity in murine xenograft models, which was related to increased oxidative stress and apoptosis. In contrast, SOX17 overexpression in NHC did not affect their survival but inhibited their baseline proliferation. In CCA cells, SOX17 inhibited migration, anchorage-independent growth and Wnt/β-catenin-dependent proliferation, and restored the expression of biliary markers and primary cilium length. In human CCA, SOX17 promoter was found hypermethylated and its expression inversely correlates with the methylation grade. In NHC, Wnt3a decreased SOX17 expression in a DNMT-dependent manner, whereas in CCA, DNMT1 inhibition or silencing upregulated SOX17.
Conclusions
SOX17 regulates the differentiation and maintenance of the biliary phenotype and functions as a tumor suppressor for CCA, being a potential prognostic marker and a promising therapeutic target.
Keywords: cholangiocyte differentiation, cholangiocarcinoma, SOX17, epigenetics, prognosis
Introduction
Cholangiocarcinoma (CCA) is a heterogeneous group of biliary malignancies with poor prognosis [1]. The etiopathogenesis remains largely unknown [1]. Incidence is rising worldwide and CCA already represents the second most common primary liver tumor and ~3% of all gastrointestinal cancers [1]. CCA is generally asymptomatic in early stages and is commonly diagnosed at advanced phases, when symptoms associated with biliary obstruction arise; this circumstance compromises the potential therapeutic options [1]. Recurrence after surgery and elevated chemoresistance of CCAs contribute to the limited prognosis [1]. Therefore, it is essential to investigate in detail the molecular mechanisms involved in the pathogenesis of CCA to find new potential targets for therapy.
CCA is characterized by genetic and epigenetic abnormalities [1]. Hypermethylation of certain gene promoters was previously reported in CCA tumors, including the promoter of SRY-related HMG-box 17 (SOX17) transcription factor [2]. The family of SOX genes plays key roles in tissue homeostasis, organogenesis and cell fate decision from embryonic to adult stages [3]. In particular, SOX17 appears essential in the development of the biliary epithelium during embryogenesis since Sox17-deficient (Sox17−/−) mice exhibit premature lethality due to alterations in the formation of the definitive gut endoderm [4] and Sox17 haploinsufficiency (Sox17+/−) in mice leads to congenital biliary atresia, acute embryonic hepatitis and high perinatal death [5]. Moreover, SOX17 is necessary for the development of the biliary primordium and loss of SOX17 leads to gallbladder agenesia and ectopic development of pancreatic tissue in the common bile duct [5, 6]. On the other hand, SOX genes are also involved in carcinogenesis, acting as oncogenes or tumor suppressor genes [7]. Little information is available about the role of SOX17 in carcinogenesis. Some reports suggest that it may act as a tumor suppressor in some types of cancer and that is able to antagonize the pro-tumorigenic Wnt/β-catenin signaling pathway [8–11].
In the present study, we have investigated the role of SOX17 in the differentiation and maintenance of the biliary phenotype and in cholangiocarcinogenesis. Moreover, the prognostic clinical value of SOX17 expression was evaluated.
Material and methods
Complete transparent accurate and timely account (CTAT) methods are included as Supplementary CTAT table.
Human samples
Whole transcriptome profiling was performed using humanRef-8v2 BeadChips (Illumina) on 104 CCA surgical specimens (68 intrahepatic and 36 perihilar CCAs) and 6 normal intrahepatic bile ducts, as previously described (GEO: GSE26566) (“Copenhagen cohort” of patients) [12]. Additionally, laser microdissection (LMD) was performed using LMD6000 system (Leica) to generate 23 pairs of matched tumor epithelia and tumor stroma [12]. DNA promoter methylation was analyzed in 48 CCAs using Infinium HumanMethylation27 BeadChips (Illumina Inc.). To evaluate the relationship between SOX17 expression and the outcome of patients, samples were grouped as high vs low SOX17 expression in the resected tumor, which was obtained according to an arbitrary average expression level (cut-off).
Moreover, 13 CCA human biopsies and 14 normal human gallbladder tissues were obtained from the Biobank of Donostia University Hospital for the analysis of SOX17 expression by qPCR (“San Sebastian cohort” of patients) (Supplementary Table 1). Research protocols were approved by the Ethical Committees for Clinical Research of supporting Institutions, and all patients signed written consents for the use of their samples for biomedical research.
CCA xenograft animal models
Immune-deficient CD-1 nude mice (Crl:CD1-Foxn1nu; strain 086, homozygous) were purchased from Charles River Company. As a first procedure, CCA (EGI1) cells (1×105 cells resuspended in 100 µL of PBS) infected either with Lent-control or Lent-SOX17 (MOI 3) were subcutaneously injected in the dorsal flanks of animals. In a second approach, 1×106 CCA (EGI1) cells were subcutaneously injected and once tumors were well-established (average size: ~175 mm3) Lent-control or Lent-SOX17 (1×107 lentiviral particles) were intratumorally injected. Tumor size was measured every 2–4 days and tumor volume (TV) was calculated as “TV = (D × d2)/2”, where “D” represents the largest diameter measured and “d” the shortest.
Isolation and reprogramming of human myofibroblasts into cholangiocytes
Human myofibroblasts were isolated from biopsy specimens and then cultured and reprogrammed as previously described (Supplementary data) [13].
Normal human cholangiocytes and CCA human cells
Normal human cholangiocytes (NHC) were isolated from normal liver tissue and characterized as we previously described [14–16]. The protocol of isolation and culture is summarized in Supplementary data. In addition, 3 CCA human cell lines (EGI1, TFK1 and Witt) were also used. Both EGI1 and Witt cell lines are KRAS mutated whereas TFK1 is wild-type. Cells were tested as mycoplasma negative along the experiments.
Quantitative polymerase chain reaction
RNA extraction, retrotranscription and quantitative polymerase chain reaction (qPCR) were performed as previously described [14–16]. Primer sequences are included in Supplementary Table 2.
Immunoblotting
Analysis of cell protein expression by immunoblotting was performed as described in Supplementary data. Antibodies are listed in Supplementary Table 3.
Immunofluorescence
Detection of protein expression by immunofluorescence (IMF) was performed as described in Supplementary data.
Cell transduction with lentiviral vectors
iPSC cells were differentiated to definitive endoderm (DE) and then cells were either left untreated (control) or infected with lentiviruses carrying small hairpin RNA (shRNA) against SOX17 (Lent-shRNA-SOX17; Santa Cruz) or control shRNA (Lent-shRNA-control: Santa Cruz) at the first day of each differentiation phase (HS, HP, iDC) and with a multiplicity of infection (MOI) of 3. NHC were infected with Lent-shRNA-SOX17 or Lent-shRNA-control at MOI 3. CCA cells (EGI1 or TFK1) and NHC were infected with lentiviruses carrying SOX17 (Lent-SOX17) or empty viruses (Lent-control) at MOI 3. Lent-SOX17 production and lentiviral infections are described in Supplementary data.
Cell Death Analysis
Cell death was determined by using three different flow cytometry-based assays: Annexin-V (Invitrogen), Propidium Iodide (Invitrogen) and Caspase-3 activity (Phiphilux-G2D2, OncoImmunin® Inc), as described in Supplementary data.
Cell redox stress
Levels of oxidative stress were determined in CCA (EGI1) cells infected with Lent-SOX17 or Lent-control by using the CellROX® “Deep Red” Flow Cytometry Assay Kit (Invitrogen). Non-infected cells were also used as controls.
Cell proliferation
Proliferation of NHC and CCA (EGI1) cells was evaluated under Lent-shRNA-SOX17 (or Lent-shRNA-control) and/or Lent-SOX17 (or Lent-control) infection. Non-infected cells were also used as controls. Protocol is described in Supplementary data.
Anchorage-independent growth in soft agar
CCA (EGI1) cell growth in soft agar was analyzed as described in Supplementary data.
Cell senescence
NHC were used to analyze cell senescence (β-galactosidase activity) over passages and under Lent-shRNA-SOX17 or Lent-shRNA-control infections as described in Supplementary data.
Cell migration
CCA cell migration was evaluated in cells (EGI1) infected with Lent-SOX17 or Lent-control, as well as in non-infected cells, as described in Supplementary data.
Illumina mRNA expression array
Illumina Human HT12 v4 BeadChips (GPL10558) were chosen to characterize gene expression in NHC and CCA (EGI1) cells under Lent-shRNA-SOX17/Lent-shRNA-control or Lent-SOX17/Lent-control infection, respectively (Supplementary data). Non-infected cells were also used as controls.
Knock-down of DNMT1 mRNA expression with siRNAs and pharmacological inhibition of DNMT activity
DNA methyltransferase 1 (DNMT1) mRNA expression was knocked-down in CCA cells by using specific small interference RNA (siRNA) oligos (Supplementary data). SOX17 expression was measured in CCA cells under DNMT1 knocked-down or under the presence or absence of 5-aza-2'-deoxycytidine (1 µM) (also known as decitabine) for 48 h. SOX17 mRNA expression was measured in NHC under the presence or absence of Wnt3a (100 ng/mL, R&D systems) in DMEM/F12+Glutamax/1%P/S culture media for 48 h and with or without decitabine (pre-incubated for 18 h).
SOX17 promoter methylation
The methylation status of SOX17 promoter was studied as described in Supplementary data. DNA methylation was quantified using beta (β)-value metric (range: 0–1; 0=0% methylation, 1=100% methylation).
To further investigate the extent of aberrant DNA methylation in SOX17, level 1 Infinium HumanMethylation450 BeadChip (Illumina Inc.) data were analyzed in specimens obtainable through the cancer genome atlas (TCGA) (Supplementary data).
Statistical analysis
Results were statistically analyzed using GraphPad program. For comparisons between two groups, parametric t-Student test or nonparametric Mann-Whitney test were used. For comparisons between more than two parametric groups, One-Way analysis of variance (ANOVA) with Tukey´s post-hoc was employed. In data correlation, nonparametric Spearman test was employed. Differences were considered significant when p<0.05.
Results
Role of SOX17 in cholangiocyte differentiation
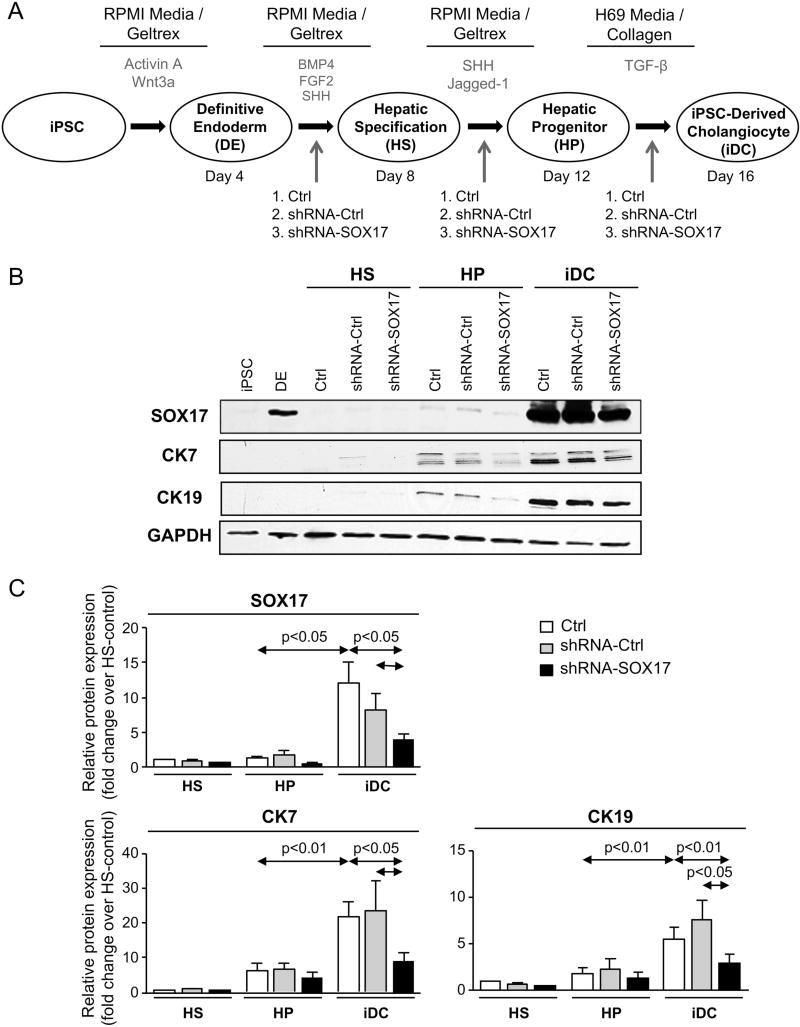
iPSC were differentiated into mature cholangiocytes (i.e iDCs) by using a multistep process with exposure to biliary morphogens (Figure 1A). Similarly to the biliary markers cytokeratin 7 and 19 (CK7 and CK19, respectively), SOX17 protein expression is also highly induced in the last step of the differentiation process (from HP into iDC) (Figure 1B,C) and its partial experimental knock-down with Lent-shRNA-SOX17 decreased CK7 and CK19 protein expression compared to cells infected with Lent-shRNA-control or non-infected cells (Figures 1B,C).
Figure 1. SOX17 regulates the differentiation of induced pluripotent stem cells into mature cholangiocytes.
A) Chart summarizing the multistep process of differentiation from iPSC into iDC. Time-points of lentiviral administration are shown. B) Representative immunoblots and C) quantitative protein levels of SOX17, CK7 and CK19 in the differentiation steps from HS into iDC. Pooled data from 5 independent experiments (mean±SEM; p-value vs controls, Student’s t-test).
Involvement of SOX17 in the maintenance of the biliary phenotype
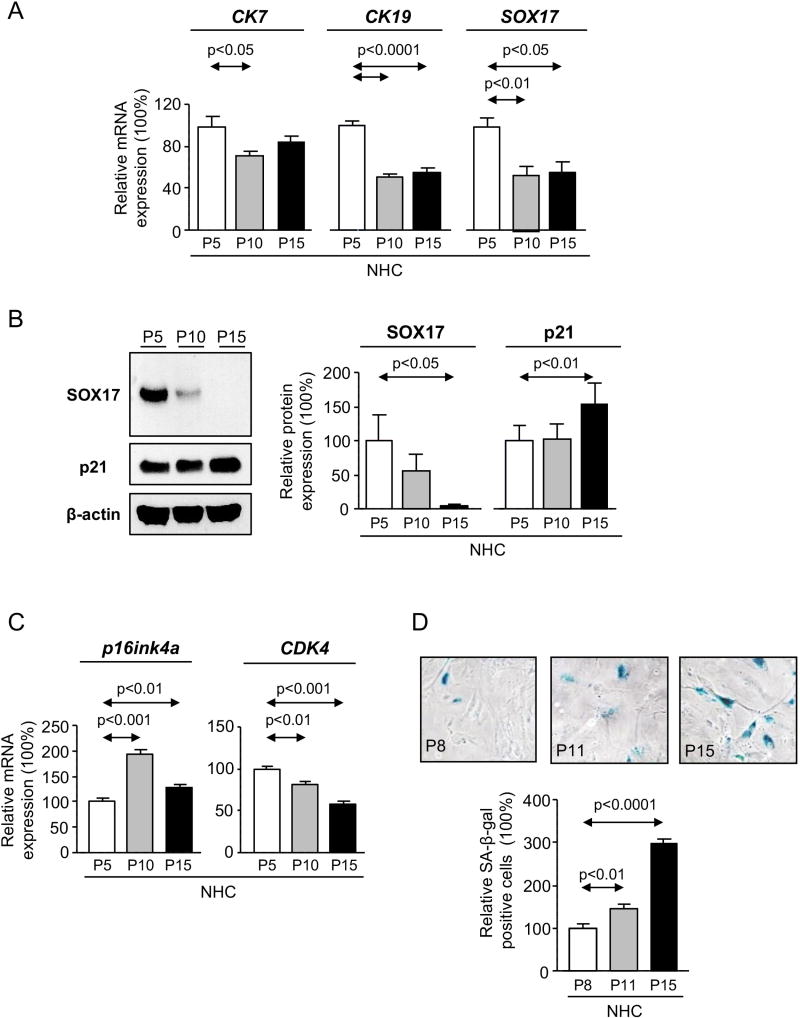
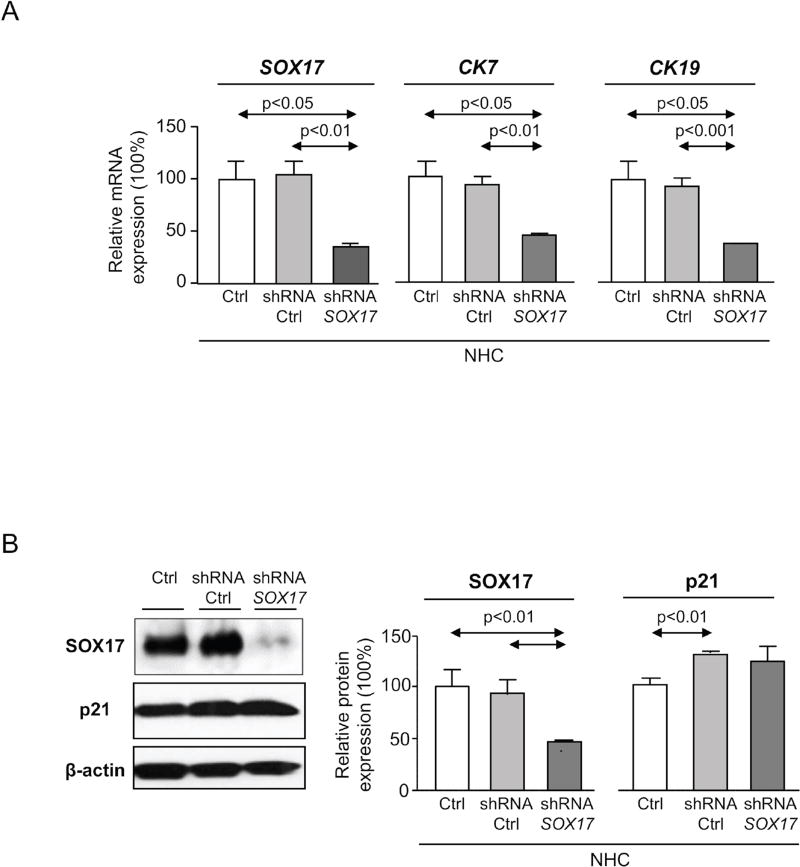
Normal primary epithelial cells progressively decrease the expression of differentiation markers along cell passages in vitro and gradually enter in senescence. Here, the expression of SOX17, similarly to CK7 and CK19, decreases with NHC passages (Figure 2A). In contrast, the expression (p21, p16ink4a) and activity (β-galactosidase) of senescence markers increased, whereas the cell-cycle kinases [cyclin-dependent kinase 4 (CDK4)] decreased (Figures 2B–D). To further study the role of SOX17 in cholangiocyte phenotype and senescence, SOX17 was experimentally downregulated in NHC at low passages with Lent-shRNA-SOX17 (Figure 3A). Experimental SOX17 knock-down prompted the downregulation of both CK7 and CK19 (Figure 3A), but did not affect NHC senescence (p21, p16ink4a, CDK4 expression and β-galactosidase activity) compared to control conditions (Figures 3B–D). In addition, mRNA microarrays were carried out to test the effect of SOX17 downregulation on the NHC mRNA profile. Heatmaps showed that most of the differentially-expressed genes were found overexpressed under SOX17 downregulation; many of them were oncogenes, cell-cycle promoters, invasion/migration inducers and genes involved in drug resistance and cell survival (Supplementary Figure 1).
Figure 2. NHC dedifferentiate and enter into senescence over cell passages in vitro.
A) Expression of the biliary markers CK7, CK19 and SOX17 (n=4–6). B) Immunoblots and quantification of SOX17 and p21 (senescence marker) levels (n=3). C) Expression of p16ink4a (senescence marker) and CDK4 (cell cycle promoter) (n=4–5). D) Representative light microscopy images and relative quantification of β-galactosidase staining (n=6–8 wells from two independent experiments). Panels A-D: mean±SEM; p-value vs lowest cell passage (P5 or P8, Student’s t-test).
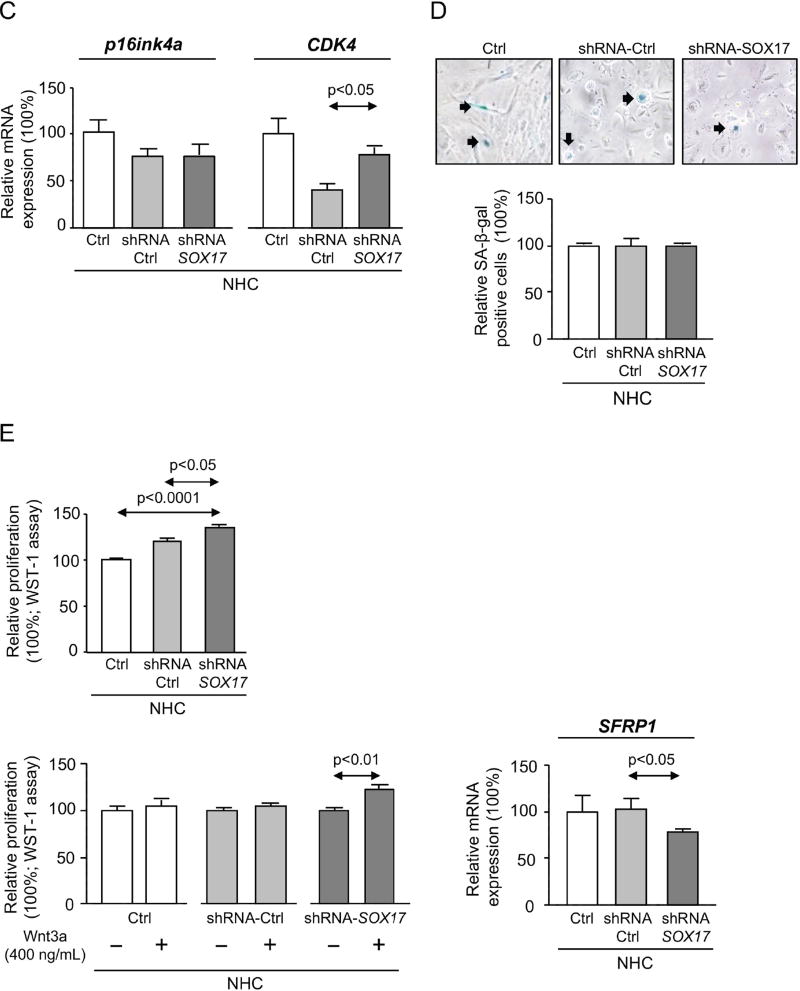
Figure 3. Experimental knock-down of SOX17 levels in NHC decreases the expression of biliary markers but does not affect senescence.
A) Expression of SOX17 and biliary markers (n=3–4). B) Representative immunoblots and quantification of SOX17 and p21 levels (n=3 independent experiments). C) Expression of p16ink4a and CDK4 (n=5). D) Representative light microscopy images of β-galactosidase staining (n=7 wells from two independent experiments; NHC of passage 8). E) Basal and Wnt3a–dependent proliferation (n=8–9) and levels of the Wnt3a inhibitor SFRP1 (n=4). Panels A-E: mean±SEM; p-value vs controls, Student’s t-test.
Then, we investigated the role of SOX17 and Wnt on NHC proliferation. Under baseline conditions, experimental SOX17 knock-down with Lent-shRNA-SOX17 increased NHC proliferation compared to controls (Figure 3E). The presence of Wnt3a ligand did not modify the baseline proliferation of NHC infected with Lent-shRNA-control or non-infected. However, experimental SOX17 knock-down rendered these cells susceptible to Wnt3a-induced proliferation (Figure 3E); this event was associated with decreased secreted frizzled-related protein 1 (SFRP1) mRNA levels (Figure 3E), which translate into a protein that is extracellularly secreted and may bind and inhibit Wnt ligands.
Expression of SOX17 in CCA human tissue and cells
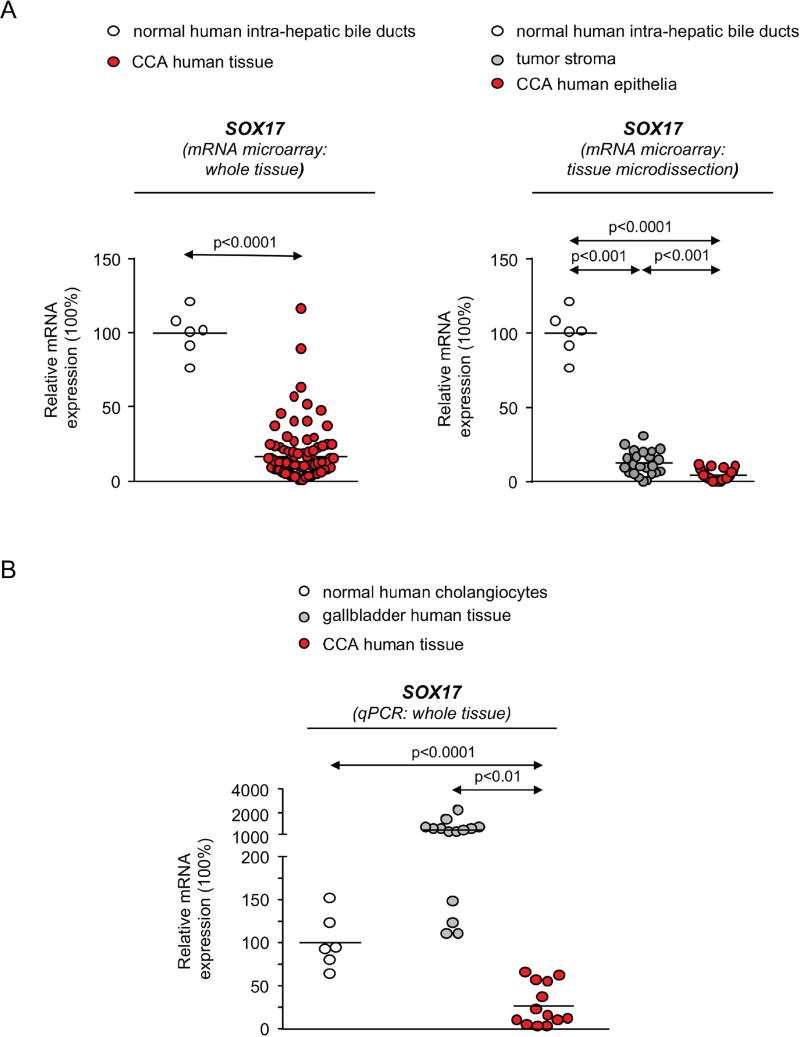
The analysis of SOX17 expression was performed in CCA human biopsies and controls by using 2 approaches (mRNA microarray and qPCR) and 2 different cohorts of patients (Copenhagen and San Sebastian), respectively. By using mRNA microarrays in samples from the Copenhagen cohort, SOX17 expression was found downregulated in 104 CCA biopsies compared to 6 normal intrahepatic bile ducts (Figure 4A). SOX17 expression was also found downregulated in CCA epithelia obtained by laser microdissection, compared with their matched tumor stroma [mainly composed by cancer associated fibroblasts (CAFs), immune cells such as tumor associated macrophages (TAMs) and endothelial cells] [1] (Figure 4A).
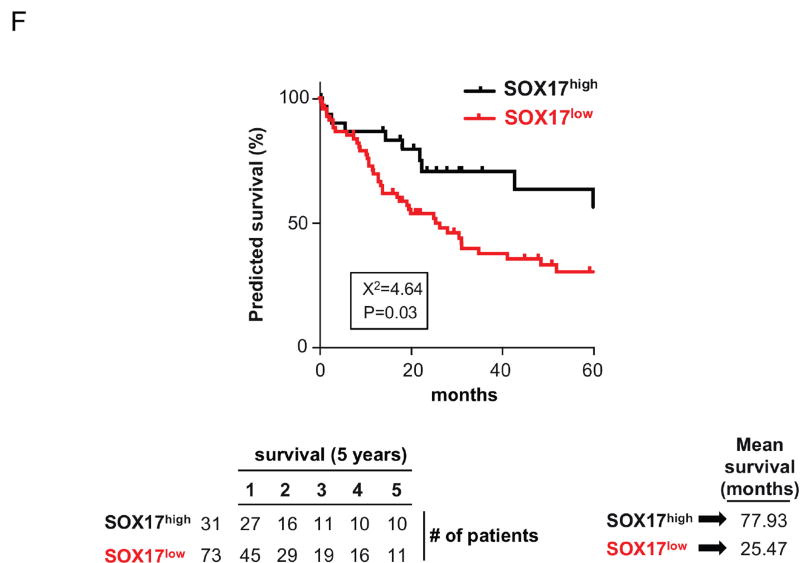
Figure 4. SOX17 expression is decreased in CCA tumors and cell lines and correlates with survival.
A) SOX17 expression (mRNA microarray) in CCA tumors (whole tissue) compared to normal intrahepatic bile ducts, and in CCA epithelia compared to stroma (mean±SEM; p-value vs controls, Mann-Whitney t-test and Wilcoxon matched-pairs signed rank test, respectively) (Copenhagen cohort). B) SOX17 expression (qPCR) in CCA tumors compared to NHC and normal gallbladder tissue (San Sebastian cohort). Dots=number of patients. C) Representative IMFs of SOX17 (green) and CK19 (red) in normal human liver and CCA human tissue (n=5). SOX17 D) mRNA (n=4–6) and E) protein (n=4) levels in CCA cell lines and NHC. F) Kaplan-Meier analysis (Gehan-Breslow-Wilcoxon test) of SOX17 mRNA expression in CCA tumors (Copenhagen cohort). Panels B,D,E: mean±SEM; p-value vs controls, Student’s t-test.
Next, the expression of SOX17 was evaluated by qPCR in the San Sebastian cohort. Data revealed that SOX17 levels are also lower in 13 CCA tissues compared to NHC and 14 normal gallbladder tissues (which is mainly composed by cholangiocytes but also by progenitor cells that express high SOX17 levels) [17] (Figure 4B). In addition, SOX17 protein expression was evaluated by IMF in normal human livers as well as in CCA human tissues. In normal human livers, CK19-positive cells (cholangiocytes) show high SOX17 expression (Figure 4C), which is mainly localized in the perinuclear and nuclear compartments. In contrast, SOX17 immunostaining was negligible in CK19-positive human iCCA tissue (Figure 4C). SOX17 expression (mRNA and protein) was also very poor in three human CCA cell lines (EGI1, TFK1 and Witt) compared to NHC (Figures 4D,E, respectively).
Relationship of SOX17 expression in CCA tumors and the outcome of patients
SOX17 expression was retrospectively evaluated in 104 CCA patients (Copenhagen cohort) who had undergone tumor resection and were monitored for 5 years after surgery. Data showed that SOX17 expression correlates with the outcome of patients. Thus, patients with lower SOX17 mRNA levels in CCA tumors showed shorter overall survival time (25.5 months) than those with higher levels of SOX17 (77.9 months) (Figure 4F).
Role of SOX17 in cholangiocarcinogenesis
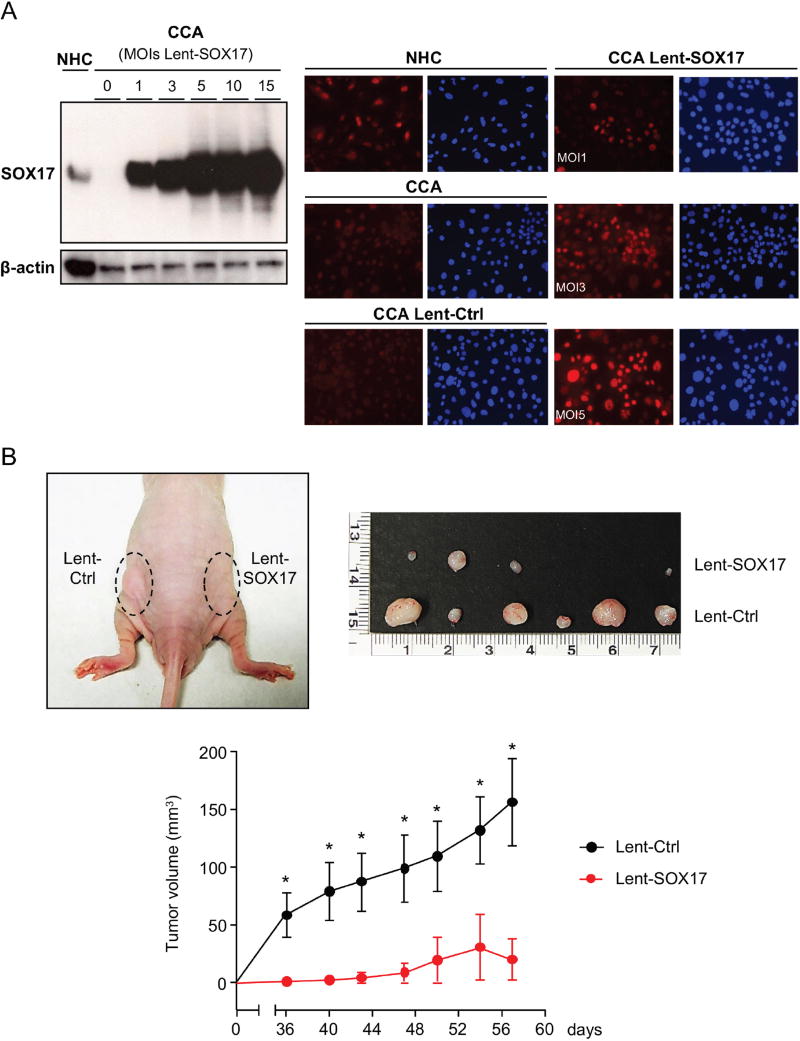
In order to evaluate the role of SOX17 on biliary tumorigenesis, this transcription factor was overexpressed in CCA (EGI1) cells by using Lent-SOX17. First, we evaluated the MOI needed to overexpress SOX17 in CCA cells in vitro. Immunoblots revealed a MOI-dependent SOX17 overexpression in CCA cells after Lent-SOX17 infection (Figure 5A). IMF analysis confirmed higher SOX17 baseline levels in NHC than in CCA cells. SOX17 immunostaining was mainly located at the nucleus, although some staining was also found in the cytoplasm (Figure 5A). Experimental SOX17 overexpression in CCA cells resulted in its protein accumulation in the nucleus. The percentage of transduced cells was dependent on the MOI employed (1, 3 or 5) (Figure 5A). MOI 3 was used for further experiments as this was the minimal value able to transduce all cells with non-saturating SOX17 overexpression.
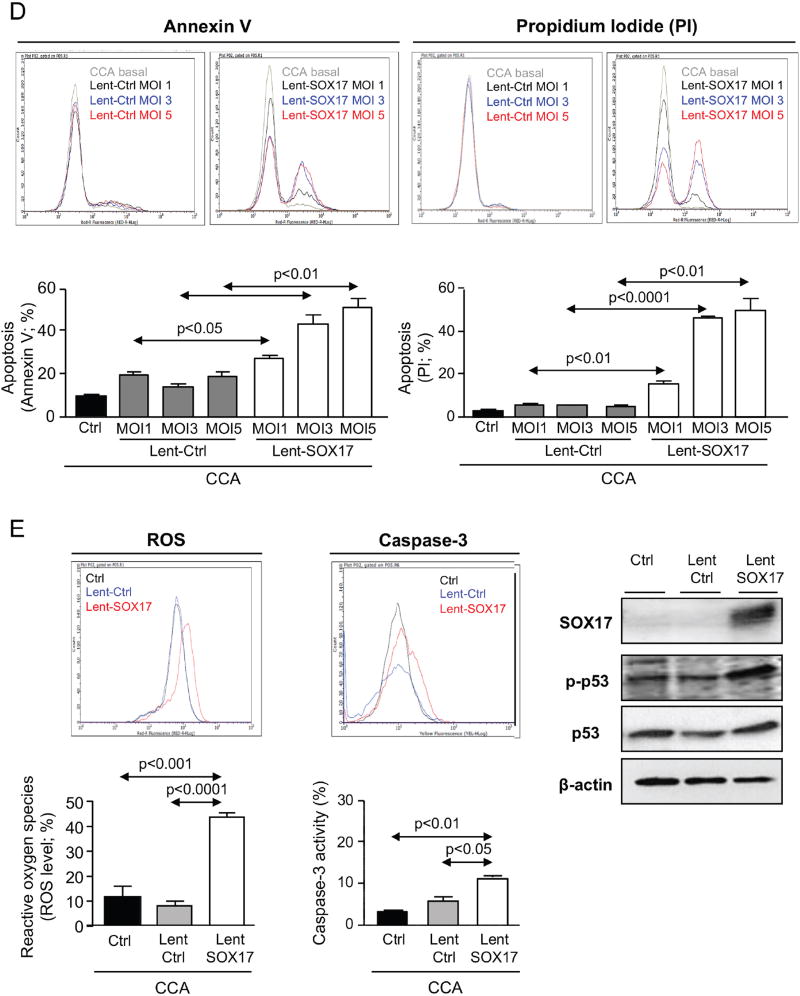
Figure 5. Experimental overexpression of SOX17 in CCA cells decreases their tumorigenic capacity by increasing apoptosis.
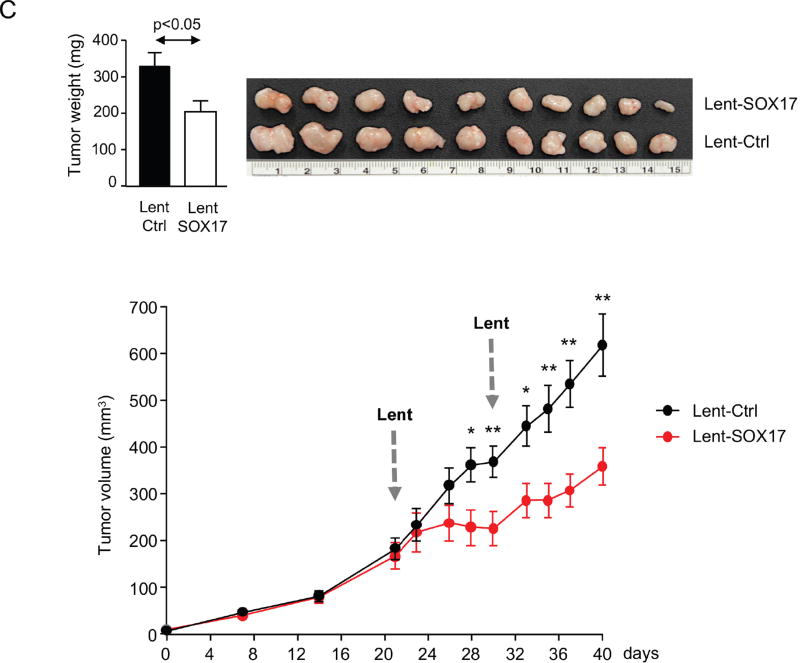
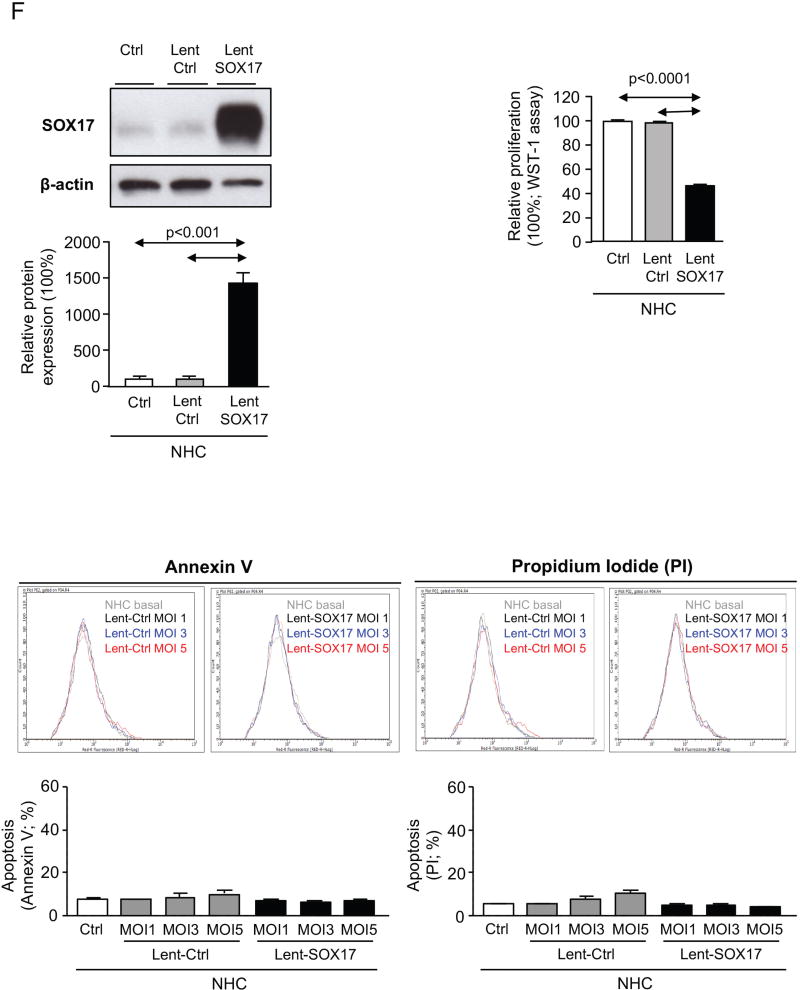
A) Representative immunoblots and IMFs of SOX17 in CCA cells and NHC under experimental modulation of SOX17 expression (red: SOX17; blue: nucleus). B) SOX17-dependent subcutaneous CCA implantation and growth in immunodeficient mice. C) SOX17 experimental overexpression inhibits the growth of already developed subcutaneous CCA tumors in immunodeficient mice. Arrows: time-points of intratumoral lentiviral injection. Quantification (n=3, MOI 3) of flow cytometry-based D) apoptosis (n=3), E) oxidative stress and caspase-3 activity in CCA cells under experimental modulation of SOX17 expression. Representative immunoblots of p-p53, p53, SOX17 and β-actin. F) Representative immunoblot and quantification of SOX17 expression in NHC under experimental modulation of SOX17 expression (n=3) and effects on proliferation (n=10) and apoptosis (n=3). Panels B-F: mean±SEM; p-value vs controls, Student’s t-test. Data in D-F are representative of at least two independent experiments. (* and **:p-value <0.05 and <0.01, respectively).
When implanted subcutaneously in immunodeficient nude mice, CCA (EGI1) cells pre-infected with Lent-control showed a continuous lineal growth overtime (Figure 5B), which was similar to that observed in non-infected CCA cells (data not shown). In contrast, overexpression of SOX17 in CCA human cells inhibited their tumorigenic capacity (implantation and growth) in vivo compared to cells infected with Lent-control (Figure 5B). Moreover, intratumoral injection of Lent-SOX17 in pre-existing CCA tumors also suppressed the tumor growth in vivo (Figure 5C). Elucidation of the mechanisms accounting for this anti-tumorigenic effect revealed that SOX17 overexpression promoted a MOI-dependent cell death in CCA cells compared to control conditions (Figure 5D). SOX17-induced cell death was associated with increased cellular levels of reactive oxygen species (ROS), increased pro-apoptotic caspase-3 activity, and phosphorylation of the pro-apoptotic p53 protein (Figure 5E). Notably, and in contrast to CCA cells, experimental overexpression of SOX17 in NHC did not display deleterious effects but reduced their baseline proliferation promoting a quiescent phenotype (Figure 5F).
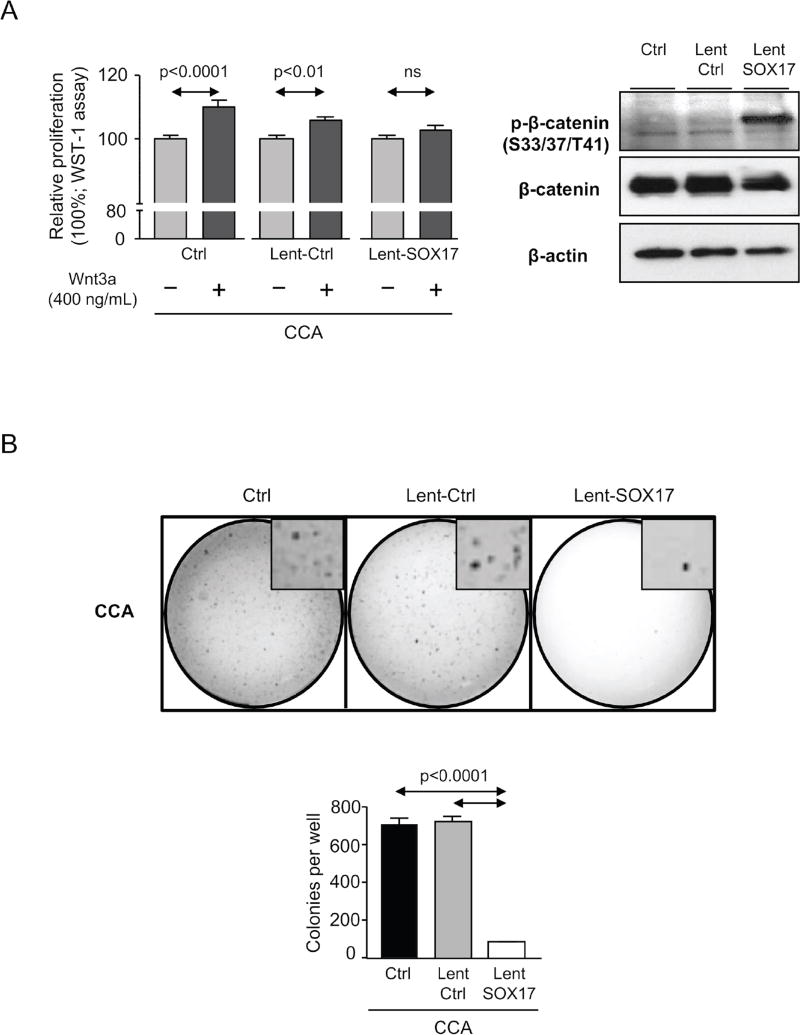
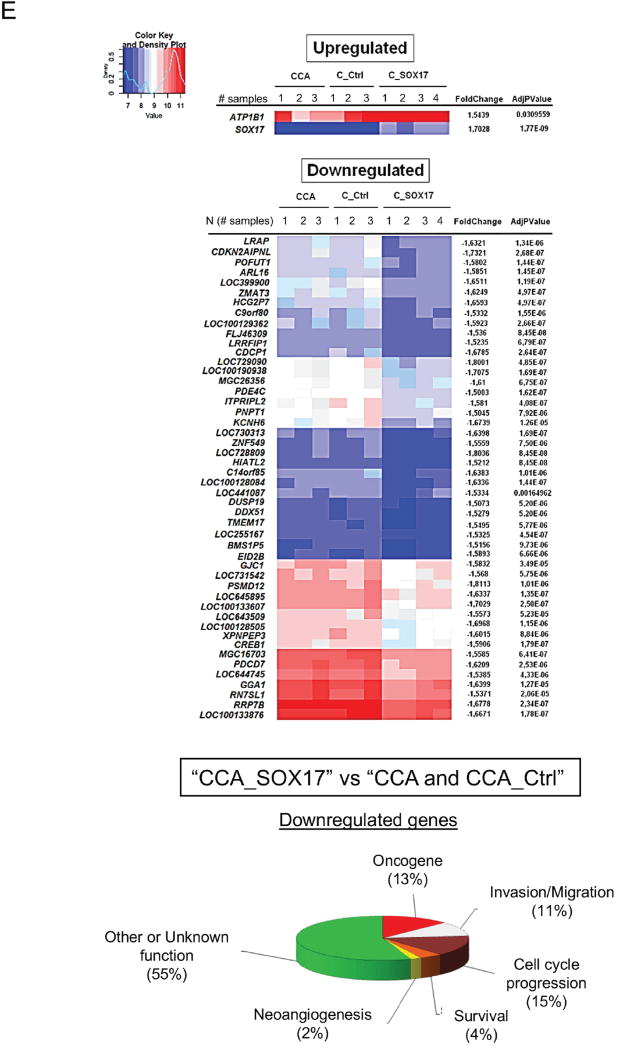
Since a fraction of CCA cells overexpressing SOX17 did not enter into apoptosis we used them to investigate several characteristics involved in carcinogenesis and malignancy, such as proliferation, invasion/migration and differentiation. The presence of Wnt3a promotes the proliferation of CCA (EGI1) cells in a dose-dependent manner (Supplementary Figure 2). Notably, overexpression of SOX17 in CCA cells (EGI1 and TFK1) inhibited the Wnt3a–dependent proliferation (Figure 6A and Supplementary Figure 3). This was associated with increased phosphorylation of β-catenin (key effector of the Wnt signaling pathway) in the ser33/37/thr41 residues that promotes its ubiquitin/proteasome-dependent protein degradation [18] (Figure 6A). Moreover, experimental overexpression of SOX17 impaired the anchorage-independent growth in soft agar and the invasion/migration properties of CCA cells (Figures 6B,C). SOX17-mediated effects were associated with increased mRNA levels of biliary/epithelial markers CK7, fibronectin (FN) and zonula occludens 1 (ZO-1), as well as with the normalization of the upregulated CK19 levels and decreased expression of the mesenchymal and pro-tumor/metastatic marker S100 calcium-binding protein A4 (S100A4) compared to controls (Figures 6C,D and Supplementary Figure 4). Heatmaps obtained from mRNA microarrays analysis showed that most of the differentially-expressed genes in CCA cells under SOX17 overexpression were found downregulated. These were mainly oncogenes, cell-cycle promoters, invasion/migration inducers and genes involved in angiogenesis and cell survival (Figure 6E).
Figure 6. Experimental overexpression of SOX17 in CCA cells decreases their proliferation and migration capacity and increases their biliary differentiation features.
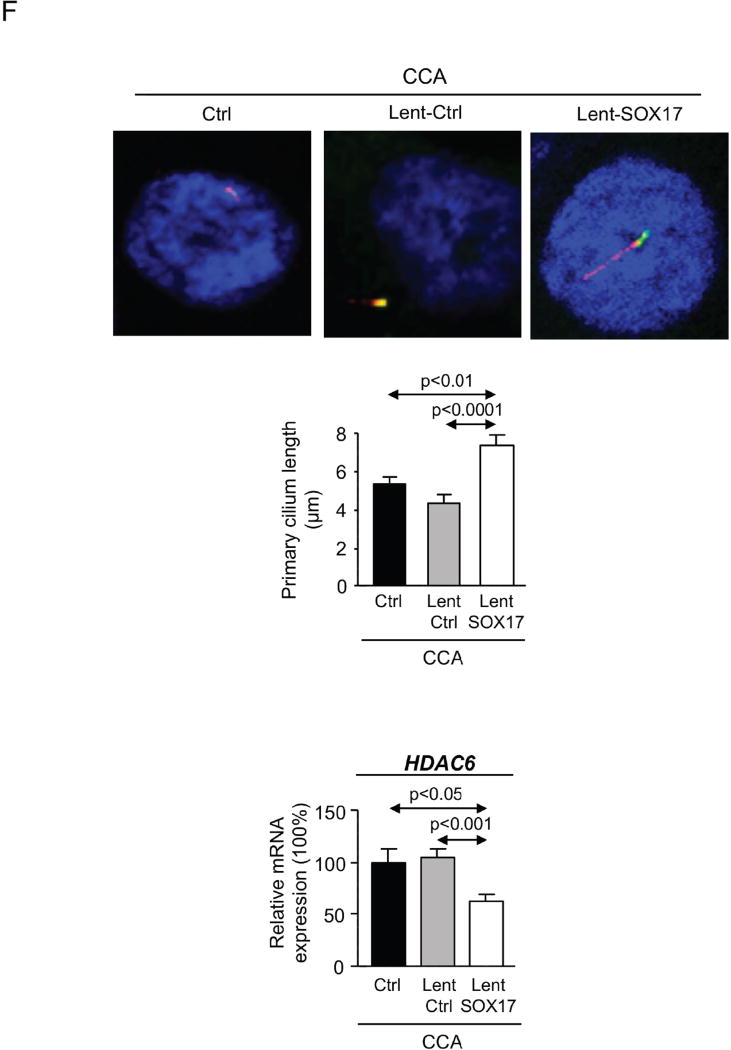
A) Wnt3a–dependent proliferation (n=24) and immunoblots of p-β-catenin (S33/37/T41), β-catenin and β-actin. B) Anchorage-independent CCA cell growth in soft agar (n=6 from two independent experiments). C) Representative light microscopy images and quantification of CCA scratch-migration assays (n=6–8 from three independent experiments) and S100A4 mRNA expression (n=4). D) Expression (mRNA) of biliary and epithelial markers (n=4). E) Heatmaps and clusters of dysregulated genes based on cellular function (n=3–4; Bayes moderated t-statistics). F) Representative IMF images and quantification of the primary cilium length [acetylated α-tubulin (axoneme): red; γ-tubulin (centrioles): green; nucleus: blue)] (n=40–60) and HDAC6 mRNA levels (n=4). Panels A–D,F: mean±SEM; p-value vs controls, Student’s t-test.
Role of SOX17 in CCA primary cilium length
CCA cells show shorter or even absent primary cilia due to histone deacetylase 6 (HDAC6) overexpression that de-acetylates the ciliary α-tubulin [19]. The decreased primary cilium length is in part responsible of the depolarization and hyperproliferation observed in CCA cells. Our data indicate that those CCA human cells overexpressing SOX17, but that did not enter apoptosis, show longer cilia than controls (Figure 6F). This event was associated with decreased HDAC6 mRNA levels (Figure 6F).
Regulatory mechanisms of SOX17 expression in normal human cholangiocytes and CCA human cells
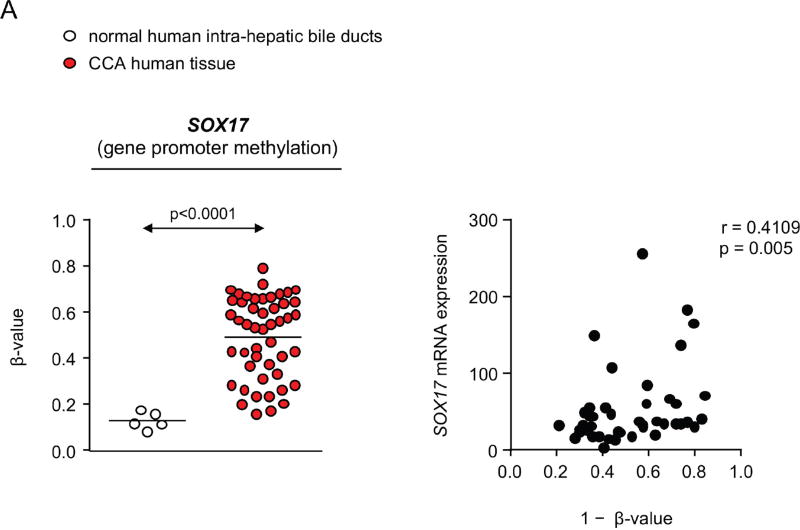
Epigenetic abnormalities have been described in CCA, including hypermethylation of some promoters of genes that inhibit Wnt/β-catenin signaling like SOX17 [2]. Here, we also confirmed in 48 CCAs from the Copenhagen cohort that the SOX17 promoter is hypermethylated at probe cg02919422 compared to normal human intrahepatic bile ducts (Figure 7A). Moreover, we observed an inverse correlation between SOX17 methylation and gene expression (Figure 7A). To further investigate the extent of such aberrant methylation, we also analyzed TCGA Infinium 450k data from 35 CCA patients and 6 surrounding normal samples. Remarkably, 2 large differentially methylated regions were uncovered – one localized at the SOX17 promoter (chr8: 55,368,995–55,370,994 corresponding to 11 significant probes, including cg02919422) and other localized at the SOX17 gene body (chr8: 55,370,495 – 55,373,448 corresponding to 10 significant probes).
Figure 7. SOX17 expression is epigenetically regulated by DNMTs in NHC and CCA cells.
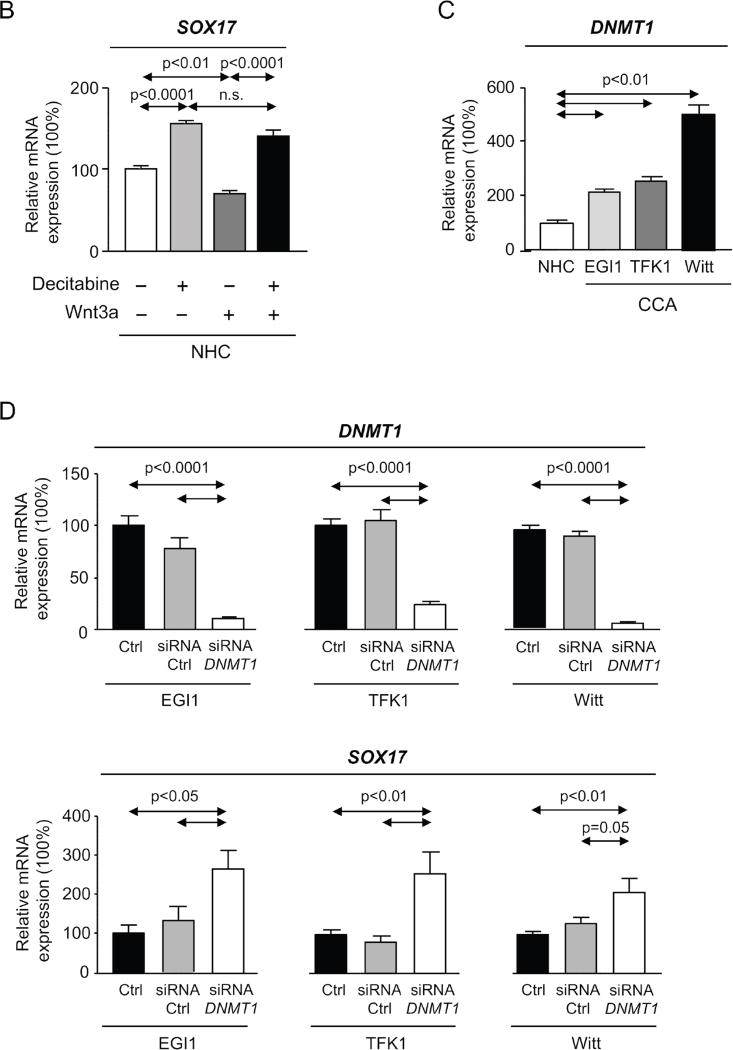
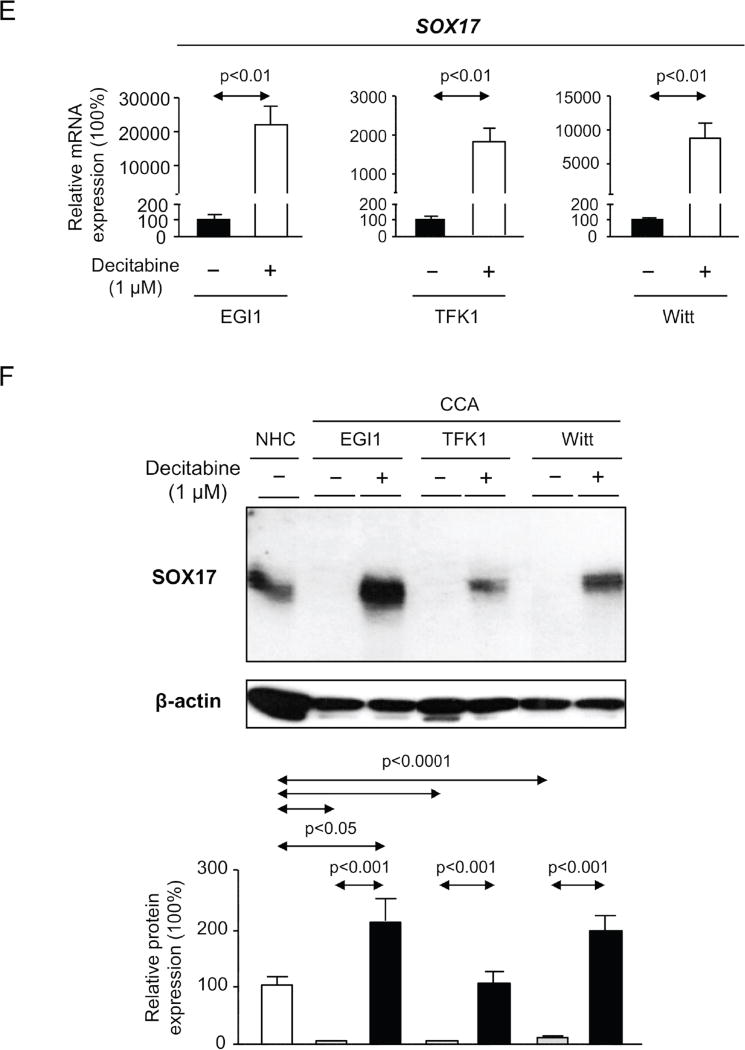
A) Methylation levels of SOX17 promoter in CCA human tissue compared to normal intrahepatic bile ducts (mean±SEM; p-value vs controls, Mann-Whitney t-test) and correlation (nonparametric Spearman test) with SOX17 expression (Copenhagen cohort). Dots=number of patients. B) SOX17 levels in NHC under the presence or absence of Wnt3a and with or without decitabine (n=4–5; mean±SEM; ANOVA+Tukey post hoc test; n.s.: non-significant). C) DNMT1 levels in CCA cell lines and NHC (n=6). D) DNMT1 and SOX17 expression in CCA cells under experimental knock-down of DNMT1 compared to control conditions [n=7–10 for EGI1; n=12–13 for TFK1; and n=15–17 for Witt (pooled data from 2–4 independent experiments)]. SOX17 E) mRNA (n=6–9) and F) protein (n=6–7) levels in CCA cells under the presence or absence of decitabine. NHC were used as control. Panels C-F: mean±SEM; p-value vs controls, Student’s t-test.
In vitro studies aimed to investigate the role of promoter methylation in SOX17 expression revealed that Wnt3a is able to inhibit SOX17 expression in NHC in a DNA methyltransferase (DNMT)-dependent manner; treatment with the DNMT-inhibitor decitabine restored SOX17 expression (Figure 7B). Since DNA methylation is carried out by several DNMT enzymes (DNMT1, 3a and 3b) [20] their expression in three CCA human cell lines (EGI1, TFK1 and Witt) was compared to that found in NHC. Both DNMT1 and DNMT3b were found highly expressed in all three CCA cell lines compared to NHC, whereas no difference of DNMT3a was seen (Figure 7C and Supplementary Figure 5). The knock-down of DNMT1, which is considered the main responsible for constitutive DNA methylation [20], resulted in the upregulation of SOX17 mRNA in all three CCA cell lines compared to controls (Figure 7D). Similarly, pharmacological inhibition of DNMT activity with decitabine increased SOX17 mRNA and protein expression in CCA cells (Figures 7E,F, respectively).
Discussion
The present study supports the concept that SOX17 plays an important role in the regulation of the phenotype of normal human cholangiocytes. Differentiation of iPSCs into cholangiocytes occurs in vitro through a well-established accurate protocol that results in the development of typical biliary features [13]. SOX17 expression, similarly to CK7 and CK19, is highly induced in the last step of this differentiation process and particularly regulates the expression of both biliary markers. These data suggest that this transcription factor is crucial during the last phase of the embryogenic biliary development. SOX17, similar to CK7 and CK19, becomes downregulated during the process of NHC dedifferentiation in vitro and this is associated with increased cellular senescence. Experimental downregulation of SOX17 expression in low passage NHC resulted in decreased levels of biliary markers (CK7 and CK19) and in the downregulation of inhibitors of proliferation and migration, as well as in overexpression of different genes that promote tumorigenesis. However, SOX17 did not regulate cholangiocyte senescence and by contrast, its downregulation did increase the baseline and Wnt3a-dependent proliferation of NHC, events that are associated with decreased expression of the Wnt ligand inhibitor SFRP1. These data are consistent with previous reports indicating that SOX17 may act as a negative regulator of the promitotic Wnt/β-catenin pathway and its downregulation may facilitate the activation of this pathway [9, 11]. Altogether, these results indicate that SOX17 is a key player regulating the differentiation process of cholangiocytes and the maintenance of the biliary phenotype, and its downregulation in cholangiocytes may promote their transformation into a pro-mitotic state.
Based on the aforementioned results and on a previous report showing that SOX17 promoter is hypermethylated in CCA human tissues [2], we investigated in detail the expression of SOX17 in CCA and its potential epigenetic regulation. We found that SOX17 is downregulated in human CCA tissues and cell lines. In addition, we confirmed that the SOX17 promoter is hypermethylated in CCA human tissues and found that its expression inversely correlates with the grade of methylation. These findings seem to constitute a universal event in cholangiocyte-derived CCAs, as CCA human samples independent of their anatomical location (intrahepatic, perihilar or distal) and CCA cell lines independent of being KRAS mutants or wild-types show SOX17 downregulation, pointing out the role of SOX17 in the etiopathogenesis of CCA. Indeed, overexpression of SOX17 reduced the tumorigenic capacity of CCA cells both in vitro and in vivo. These data are consistent with previous reports indicating the potential tumor suppressor capacity of SOX17 [8–11]. The Wnt/β-catenin pathway is very relevant in the promotion of cholangiocarcinogenesis [21, 22]. Wnt ligands are highly present in the tumor microenvironment and are released by both cancer-associated fibroblasts (CAFs) and activated macrophages [23]. Our data showed that Wnt3a ligand promotes proliferation of CCA cells in a dose-dependent manner. However, the Wnt3a-dependent proliferation was inhibited by SOX17 overexpression. SOX17 may inhibit the Wnt signaling by promoting the expression of the Wnt ligand inhibitor SFRP1 and/or by inhibiting β-catenin expression and activity. Our data suggested that SOX17 increases the levels of β-catenin phosphorylated in residues ser33/37/thr41, indicating its inactivation/degradation in CCA cells. Furthermore, SOX17 also downregulated the expression of some genes involved in proliferation and inhibited the anchorage-independent growth and the invasion/migration capacity of CCA cells; this fact was associated with downregulation of different proinvasive/migration genes including S100A4, which is a key driver for CCA dissemination [24]. The analysis of the biliary phenotype in CCA cells revealed that SOX17 stimulates the expression of the biliary marker CK7 and epithelial markers FN and ZO-1, whose deficiency has been related to cancer progression [25]. Moreover, SOX17 normalizes the upregulated CK19 expression in CCA cells.
Mature cholangiocytes have a single primary cilium extending from the apical membrane [26], which in addition to other physiological roles, functions as a structural cell cycle checkpoint. Thus, its loss or shortening induces proliferation in several cholangiopathies [26]. Human CCA cells show loss or decreased primary cilium length, which is dependent on the overexpression of HDAC6 that de-acetylates the cilium scaffold protein α-tubulin [19, 26]. SOX17 overexpression induced the downregulation of HDAC6 together with the restoration of primary cilium structure and size, indicating improved cell polarity and differentiation. In this regard, the phenotype of Sox17+/-mice mimics human biliary atresia [5], which is a cholangiociliopathy that might predispose to the development of CCA [27]. The primary cilia of cholangiocytes in patients with biliary atresia are also shortened and malformed, which could promote the development and progression of the disease [28]. Thus, future studies should evaluate in detail the mechanisms by which SOX17 regulates the primary cilium length in cholangiocytes.
The investigation of the molecular mechanisms triggering the downregulation of SOX17 expression in CCA pointed to a role of Wnt3a. This ligand decreased SOX17 expression in NHC, which was prevented by the presence of the demethylating agent decitabine. These data indicate that molecules present during chronic inflammatory processes and in the CCA tumor microenvironment are able to downregulate the expression of SOX17 in cholangiocytes via a DNA methylation-dependent mechanism and thus promote cell transformation. Moreover, knocking-down of DNMT1 expression or inhibition of DNMT activity with decitabine in CCA cells resulted in the upregulation of SOX17 expression. These data indicate that restoration of SOX17 expression in CCA with demethylating agents could have important therapeutic value. Decitabine was reported to inhibit CCA cell growth in vitro and in a mouse xenograft model [29] and is a drug approved by the food and drug administration (FDA) for the treatment of human diseases such as myelodysplastic syndromes [30]. So, decitabine could be a drug to be considered for CCA. On the other hand, gene therapy might be an alternative approach for overexpressing SOX17 in CCAs and deserves future consideration.
Regarding the potential prognostic value of this transcription factor in patients with CCA, SOX17 expression in the tumor correlates with overall survival after surgical resection. This might be due to its aforementioned properties as a tumor suppressor and as key player in the regulation of cholangiocyte differentiation. Therefore, SOX17 expression in CCA tumors can be considered as a potential prognostic marker but needs to be validated in other independent cohorts of patients. Owing to the urgent need of prognostic biomarkers for CCA, these findings are clinically relevant and should be confirmed in further prospective studies.
In summary, this study provides cellular, molecular and clinical evidence of the key role of SOX17 regulating the differentiation and maintenance of the biliary phenotype and its function as a tumor suppressor in CCA. Moreover, the potential usefulness of measuring SOX17 expression in CCA as prognostic biomarker of patient outcome has been indicated. Genetic, molecular and/or pharmacological restoration of SOX17 expression in CCA may represent a new therapeutic strategy that need to be evaluated in the next future.
Supplementary Material
Acknowledgments
Grant Support: Spanish Ministries of Economy and Competitiveness (J.M. Banales: FIS PI12/00380, FIS PI15/01132; M.J. Perugorria: FIS PI14/00399), and Science and Technology (J.J.G. Marin: SAF2010-15517 and SAF2013-40620-R), and “Instituto de Salud Carlos III” (J.M. Banales, L. Bujanda and J.J.G. Marin: CIBERehd; J.M. Banales: Miguel Servet Program CON14/00129), Spain; National Institute of Health of United States of America (DK100575: R.C. Huebert; R01CA183764: S.A. Gradilone); MM-A was funded by the Zabalduz Program (University of the Basque Country UPV/EHU), AE-B by the UPV/EHU, AS-L by the Basque Government, JMB and OE by the “Asociación Española Contra el Cancer (AECC)”, and NJ-S by a Regenerative Medicine Minnesota fellowship grant (MRM2015PDSCH002).
Abbreviations
- Anova
analysis of variance
- BMIQ
beta mixture interquartile
- BMP4
bone morphogenic protein 4
- CAFs
cancer-associated fibroblasts
- CCA
cholangiocarcinoma
- CDK4
cyclin-dependent kinase 4
- CK7
cytokeratin 7
- CK19
cytokeratin 19
- cMYC
myc protoncogene protein
- CTAT
transparent accurate and timely account
- DAPI
4’,6-diamidino-2-phenylindole
- DE
definitive endoderm
- DMEM
dulbecco's modified eagle's medium
- DMPs
differentially methylated probes
- DNMTs
DNA methyltransferases
- FDA
food and drug administration
- FGF2
fibroblast growth factor 2
- FN
fibronectin
- GAPDH
glyceraldehyde-3 phosphatase dehydrogenase
- HDAC6
histone deacetylase 6
- HP
hepatic progenitor
- HS
hepatic specification
- iDC
iPSC-derived cholangiocytes
- IMF
immunofluorescence
- iPSC
induced pluripotent stem cells
- KLF4
kruppel-like factor 4
- LMD
laser microdissection
- MOI
multiplicity of infection
- NHC
normal human cholangiocytes
- OCT4
octamer-binding transcription factor 4
- P/S
penicillin/streptomycin
- qPCR
quantitative polymerase chain reaction
- ROS
reactive oxygen species
- RPMI
Roswell Park Memorial Institute
- S100A4
S100 calcium-binding protein A4
- SFRP1
secreted frizzled-related protein 1
- SHH
sonic hedgehog
- shRNA
small hairpin RNA
- siRNA
small interference RNA
- SOX2
SRY-related HMG-box 2
- SOX17
SRY-related HMG-box 17
- Sox17−/−
Sox17-deficient mice
- Sox17+/−
Sox17-haploinsuficient mice
- TCGA
the cancer genome atlas
- TGFβ
transforming growth factor beta
- TV
tumor volume
- Wnt3a
wingless-type MMTV integration site family member 3A
- ZO-1
zonula occluding 1
Footnotes
Conflict of interest: authors disclose no conflicts.
Transcript Profiling: the repository accession numbers for microarray gene expression data are GSE77984 and GSE26566.
Author Contributions:
MM-A, EL, MJP, AE-B, OE, AS-L, CJO´R, JBA, RJ-A, AL, MD, OB, NJ-S, RCH, KMT, SAG, AMA, JLL, MGF-B, LB, JJGM, JMB: study concept and design, analysis and interpretation of data, drafting of the manuscript. AM, MM, GJG: Interpretation of data, drafting and critical revision of the manuscript for important intellectual content. MM-A, EL, MJP, AE-B, OE, AS-L, CJO´R, JBA, OB, NJ-S, RCH, KMT, SAG, AMA, JLL, MGF-B, LB, JJGM, JMB: acquisition of data. MM-A, EL, MJP, AE-B, OE, AS-L, CJO´R, JBA, OB, NJ-S, RCH, KMT, SAG, AMA, JLL, MGF-B, LB, JJGM, JMB: statistical analysis. LB, JJGM, JMB: obtained funding.
References
- 1.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13(5):261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 2.Goeppert B, Konermann C, Schmidt CR, Bogatyrova O, Geiselhart L, Ernst C, et al. Global alterations of DNA methylation in cholangiocarcinoma target the Wnt signaling pathway. Hepatology. 2014;59(2):544–554. doi: 10.1002/hep.26721. [DOI] [PubMed] [Google Scholar]
- 3.Kamachi Y, Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140(20):4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 4.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 5.Uemura M, Ozawa A, Nagata T, Kurasawa K, Tsunekawa N, Nobuhisa I, et al. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development. 2013;140(3):639–648. doi: 10.1242/dev.086702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17(1):62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo SD, Sanchez-Cespedes M. The SOX family of genes in cancer development: biological relevance and opportunities for therapy. Expert Opin Ther Targets. 2012;16(9):903–919. doi: 10.1517/14728222.2012.709239. [DOI] [PubMed] [Google Scholar]
- 8.Du YC, Oshima H, Oguma K, Kitamura T, Itadani H, Fujimura T, et al. Induction and down-regulation of Sox17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology. 2009;137(4):1346–1357. doi: 10.1053/j.gastro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Yang Y, Liu S, Herman JG, Lu F, Guo M. SOX17 antagonizes WNT/beta-catenin signaling pathway in hepatocellular carcinoma. Epigenetics. 2010;5(8):743–749. doi: 10.4161/epi.5.8.13104. [DOI] [PubMed] [Google Scholar]
- 10.Kuo IY, Wu CC, Chang JM, Huang YL, Lin CH, Yan JJ, et al. Low SOX17 expression is a prognostic factor and drives transcriptional dysregulation and esophageal cancer progression. Int J Cancer. 2014;135(3):563–573. doi: 10.1002/ijc.28695. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Lv Z, He G, Wang J, Zhang X, Lu G, et al. The SOX17/miR-371-5p/SOX2 axis inhibits EMT, stem cell properties and metastasis in colorectal cancer. Oncotarget. 2015;6(11):9099–9112. doi: 10.18632/oncotarget.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021–1031. doi: 10.1053/j.gastro.2011.12.005. e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Assuncao TM, Sun Y, Jalan-Sakrikar N, Drinane MC, Huang BQ, Li Y, et al. Development and characterization of human-induced pluripotent stem cell-derived cholangiocytes. Lab Invest. 2015;95(6):684–696. doi: 10.1038/labinvest.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banales JM, Saez E, Uriz M, Sarvide S, Urribarri AD, Splinter P, et al. Up-regulation of microRNA 506 leads to decreased Cl-/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012;56(2):687–697. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz-Garrido P, Marin JJ, Perugorria MJ, Urribarri AD, Erice O, Saez E, et al. Ursodeoxycholic acid inhibits hepatic cystogenesis in experimental models of polycystic liver disease. J Hepatol. 2015;63(4):952–961. doi: 10.1016/j.jhep.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urribarri AD, Munoz-Garrido P, Perugorria MJ, Erice O, Merino-Azpitarte M, Arbelaiz A, et al. Inhibition of metalloprotease hyperactivity in cystic cholangiocytes halts the development of polycystic liver diseases. Gut. 2014;63(10):1658–1667. doi: 10.1136/gutjnl-2013-305281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220(2):186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mbom BC, Siemers KA, Ostrowski MA, Nelson WJ, Barth AI. Nek2 phosphorylates and stabilizes beta-catenin at mitotic centrosomes downstream of Plk1. Mol Biol Cell. 2014;25(7):977–991. doi: 10.1091/mbc.E13-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73(7):2259–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest. 2015;125(3):1269–1285. doi: 10.1172/JCI76452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang GL, Zhang W, Ren HY, Shen XY, Chen QX, Shen DY. Retinoid X receptor alpha enhances human cholangiocarcinoma growth through simultaneous activation of Wnt/beta-catenin and nuclear factor-kappaB pathways. Cancer Sci. 2015;106(11):1515–1523. doi: 10.1111/cas.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A, et al. Activated macrophages promote Wnt/beta-catenin signaling in cholangiocarcinoma cells. Tumour Biol. 2014;35(6):5357–5367. doi: 10.1007/s13277-014-1698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54(3):890–899. doi: 10.1002/hep.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao RK, Samak G. Bile duct epithelial tight junctions and barrier function. Tissue Barriers. 2013;1(4):e25718. doi: 10.4161/tisb.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gradilone SA, Lorenzo Pisarellob MJ, LaRussob NF. Primary Cilia in Tumor Biology: The Primary Cilium as a Therapeutic Target in Cholangiocarcinoma. Curr Drug Targets. 2015 doi: 10.2174/1389450116666150223162737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuda A, Sakamoto S, Kanazawa H, Shigeta T, Karaki C, Hamano I, et al. Incidentally detected cholangiocarcinoma in an explanted liver with biliary atresia after Kasai operation. Pediatr Transplant. 2013;17(2):E62–66. doi: 10.1111/petr.12036. [DOI] [PubMed] [Google Scholar]
- 28.Chu AS, Russo PA, Wells RG. Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia. Mod Pathol. 2012;25(5):751–757. doi: 10.1038/modpathol.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang B, Li H, Yang R, Zhou S, Zou S. Decitabine inhibits the cell growth of cholangiocarcinoma in cultured cell lines and mouse xenografts. Oncol Lett. 2014;8(5):1919–1924. doi: 10.3892/ol.2014.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik P, Cashen AF. Decitabine in the treatment of acute myeloid leukemia in elderly patients. Cancer Manag Res. 2014;6:53–61. doi: 10.2147/CMAR.S40600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.