Abstract
Background
AP2 is a clathrin-based endocytic adaptor complex comprising α, β2, μ2 and σ2 subunits. μ2 regulates CFTR endocytosis. The α subunit interacts with CFTR in the intestine but its physiologic significance is unclear.
Methods
CFTR short circuit current was measured in intestinal T84 cells following shRNA knock down of AP2α (AP2αKD). Clathrin-coated structures (CCS) were immunolabeled and quantified in AP2αKD intestinal Caco2BBe (C2BBe) cells. GST tagged human AP2α appendage domain was cloned and its interaction with CFTR determined by GST pull down assay.
Result
AP2αKD in T84 cells resulted in higher CFTR current (57%) compared to control, consistent with increased functional CFTR and delayed endocytosis. Depletion of AP2α reduced CCS in C2BBe cells. Pull down assays revealed an interaction between human AP2α appendage domain and CFTR.
Conclusion
AP2 α interacts with and modulates CFTR function in the intestine by participating in clathrin assembly and recruitment of CFTR to CCS.
Keywords: AP2α, CFTR, clathrin
1. Introduction
Cystic fibrosis transmembrane conductance regulator (CFTR) is the principal chloride channel in epithelial cells. It maintains fluid homeostasis in the underlying tissues by secreting Cl− and HCO3− that is accompanied by water transport [1, 2]. CFTR-mediated fluid secretion is required for proper function of epithelial cells in many organs including the airway, sweat gland, intestinal tract and pancreatic duct [3]. Increased activity of CFTR channels at the plasma membrane of enterocytes in the intestine results in increased secretion of Cl− and HCO3− and secretory diarrhea. Conversely, mutations that lead to decreased CFTR channel expression at the plasma membrane leads to intestinal obstruction and cystic fibrosis [4]
Clathrin-mediated endocytosis plays a crucial role in maintaining cell surface expression of CFTR [5, 6]. This is reflected by observations that abundant CFTR is detected in clathrin-coated vesicles isolated either from epithelial cells grown in culture or native epithelial cells [7] but not in caveolae (a non clathrin-mediated endocytic organelle) isolated from airway epithelial cells [8]. The number of CFTR channels on the apical membrane surface is maintained by endocytosis and its recycling back to plasma membrane [9]. Under physiologic conditions, apical recycling controls CFTR ion transport in a cell and tissue-specific manner that is directed by adaptor proteins [10–12]. For example, Dab2 is the primary CFTR endocytic adaptor in airway [13] but in the intestine, Dab2 plays an indirect role in conjunction with AP2 α being the predominant adaptor [14]. AP2 is a clathrin-mediated heterotetrameric adaptor complex consisting of α, β2, μ2, σ2, of which α and β are large subunits, μ2 medium and σ2 is the small subunit. The N terminal domain of α and β along with μ2 and σ2 subunits form the core of the complex while the C terminal domains of α and β protrude out from the core to form an ear appendage (Figure 1A) [15]. AP2 clusters cargo proteins destined for endocytosis and connects them to the clathrin coat beneath the plasma membrane [16].
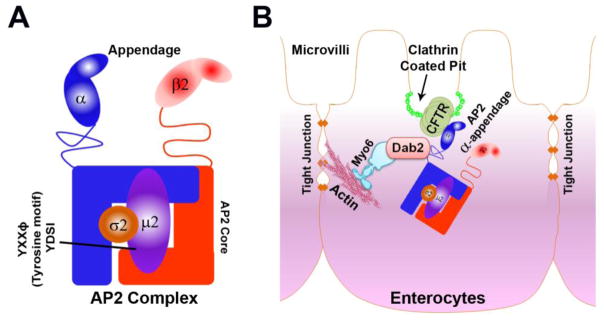
Figure 1. Schematic representation of AP2 complex and model depicting interaction of CFTR, AP2 α, Dab2 and Myosin VI in intestine.
A. AP2 core consists of N terminal domain of α and β along with μ2 and σ2 subunits while the C terminal domain of α and β protrudes out from the core like ear appendage. The μ2 subunit possesses a tyrosine motif. B. AP2 α appendage domain binds to CFTR and Dab2 connecting them like a bridge. Dab2 binds directly to the actin based motor protein Myosin VI.
Mature enterocytes are highly polarized cells with well-developed brush borders, enriched with endocytic machinery. Cargo proteins destined for endocytosis are first recruited by adaptor proteins to clathrin-coated pits (CCP) that are formed at the inter-microvillar region and pinched off to form clathrin-coated vesicles (CCVs). CCVs are recruited away from the plasma membrane by the minus end directed actin motor Myosin VI [16–18]. Myosin VI promotes endocytosis of LDL surface receptors through its adaptor protein Dab2 (Disabled 2). Dab2 recognizes and binds to LDL receptors using an endocytic sorting motif via its phosphotyrosine-binding (PTB) domain [19]. Myosin VI also regulates CFTR endocytosis from the brush border membrane in the intestine [20]. Previous studies from our laboratory revealed that in the absence of a PTB binding domain to facilitate its binding to Dab2, CFTR binds to AP2 α in the intestine[21]. Here, AP2 α serves as a bridge to link Dab2 and CFTR [Figure 1B] while Dab2 binds directly to Myosin VI. Depletion of AP2 α reduced CFTR endocytosis in the human HEK293T, CFTR expressing cells [14]. However, the mechanistic detail of AP2 α mediated regulation of CFTR endocytosis is not clear. To further understand this, we investigated the effect of AP2 α depletion on CCS formation in polarized endogenous CFTR expressing intestinal cells. We also confirmed an interaction between human AP2α appendage and CFTR in HEK293T cells by GST pull down assay. Finally, to determine whether AP2 α is physiologically relevant for CFTR function in the intestine, CFTR ion transport was examined in polarized intestinal cells following shRNA silencing of AP2α.
2. Materials and Methods
2.1 Cloning of AP2 α ear appendage
Primers were designed to amplify human AP2 alpha ear appendage using the human AP2 α sequence retrieved from NCBI (reference sequence NM_012305.3). cDNA was synthesized by reverse transcriptase polymerase chain reaction (RT-PCR) from total RNA isolated from C2BBe cells by TRIzol RNA isolation reagent (Invitrogen, Carlsbad, CA, USA). AP2 α appendage domain was amplified by PCR from cDNA template. The resulting 713 bp AP2 α appendage domain was cloned in Bam H1 and EcoR1 site in PGEX4T-LP acceptor vector (Addgene, Cambridge, MA, USA) to yield GST-tagged AP2 α ear appendage construct. The primers used for cloning AP2 α were as follows:
5′CGCTGGATCCTCCGAAGACAACTTTGCCAGG3′
5′TCCTCAGAATTCAAACTGCGCTGAGAGCAATTCAC 3′.
2.2 Expression and purification of AP2 α ear appendage
The GST tagged AP2 α construct was transformed in Escherichia coli Rosetta strain. Cells were grown in 250 ml of Luria Broth at 37°C for 2 hours. The GST tagged protein was induced by 0.5 mM IPTG (Isopropyl β-D-1-thiogalactopyranoside) and cells were grown overnight at 18°C. The protein was purified using GST beads affinity chromatography and eluted with 10mM reduced glutathione. The eluted fractions were analyzed to detect GST-AP2 α protein by resolving on SDS PAGE 4–15% TGX gel (Biorad, Hercules, CA, USA). All the fractions containing GST-AP2 α protein were pooled, dialyzed and stored at −80°C.
2.3 GST pull down assay
500 μg of purified GST-AP2 α appendage protein was incubated with 40 μl of 50% glutathione Sepharose 4 B bead slurry (Amersham, Biosciences, NJ, USA) on end over end rotator for 2 hours at 4°C. This was followed by washing three times with 1 ml of cold 1X assay buffer (2.5 mM HEPES, 12.5 mM potassium acetate, 0.5 mM magnesium acetate, 0.2 mM EDTA, 0.2 mM EGTA). 1.65 mg of total protein isolated from CFTR expressing HEK293T cells was added to the fusion protein/bead and allowed to bind end over end on a rotator for 4 hours at 4°C followed by four times washing with 1X PBS. The pellet was resuspended in 4X SDS sample buffer (Biorad, Hercules, CA, USA) and resolved using 4–20% precast SDS-PAGE gels (Biorad, Hercules, CA, USA).
2.4 Immunoblotting
Cells were lysed with TGH buffer supplemented with protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) followed by incubation on ice for 20 minutes and centrifugation at 13,500 rpm for 30 minutes at 4°C as previously described [21]. Supernatants were collected and quantified for protein content by Bradford assay and equal amounts of protein were loaded, resolved on precast SDS gel (Biorad, Hercules, CA, USA) and transferred to PVDF membrane (Biorad, Hercules, CA, USA). The blots were blocked in 5% skimmed milk or bovine serum albumin (BSA) at room temperature followed by overnight incubation with primary antibody at 4°C. Blots were incubated with secondary antibody at room temperature and antigen antibody complexes were detected by chemiluminescence with ECL substrate (Biorad, Hercules, CA, USA) and supersignal (Thermoscientific, IL, USA).
2.5 Cell culture
C2BBe, T84 and HEK293T cells were purchased from ATCC and grown at 37°C in 5% CO2-90% air atmosphere. C2BBe and HEK293T cells were grown in high glucose with L-glutamine Dulbecco’s modified Eagle’s medium, supplemented with 7 % FBS, 10 μg/ml apo-transferrin (Millipore, Billerica, MA, USA), 1 mM sodium pyruvate and 1X Antibiotic-Antimycotic. T84 cells were grown in DMEM/F12 medium supplemented with 7% FBS and 1X Antibiotic-Antimycotic. Cells were passaged at 70–80% confluency.
2.6 AP2αKD cells
AP2 α shRNA targeting AP2 α was designed using AP2 α RNA sequence (NM_012305) delivered by the PLKO.1-Puro-based lentiviral system and maintained by a selection with puromycin (14 μg/ul). Silencing of AP2 α (knock down, KD) was achieved by transduction with lentiviral medium containing AP2 shRNA targeting AP2 α as recommended in the protocol (Addgene, Cambridge, MA, USA). Transduced cells were selected with 14μg/μl of puromycin until confluency was achieved in a 12.5 mm dish. Cells were analyzed by immunoblot for knock down efficiency of the target protein using GAPDH as an internal control. The transduced T84 cells were seeded on snapwell filters (Corning, Tewksbury, MA, USA) for transepithelial current measurement studies and resistance was monitored each day using EVOM voltammeter (World Precision Instruments, Sarasota, FL, USA) until transepithelial resistance (TEER) was above 1000 ohms/cm2. C2BBe cells were seeded on the desired transwell filters (Corning, Tewksbury, MA, USA) depending on the nature of experiment.
2.7 CFTR mediated transepithelial Cl− current measurement
T84 cells grown on snapwell filters possessing a TEER above 1000 ohms/cm2 were mounted on the Ussing chamber and Cl− current was measured as described before [22].
2.8 Immunofluorescence Labeling and Confocal Microscopy of C2BBe Cells
Confluent cells grown on transwell filters were fixed in 2% paraformaldehyde (PFA) for 10 minutes at room temperature. Fixed cells were stored at 4 °C prior to immunolabeling. Briefly, cells were labeled as follows: All the steps were carried out at room temperature except for overnight incubation with primary antibody to detect clathrin CP45 (EMD Biosciences, La Jolla, CA, USA) at 4 °C. Cells were washed three times with 100 mM Tris-Cl pH 7.4 followed by incubation with 40 mM DTT for 10 minutes. Cells were rehydrated by washing with PBS-BSA 0.5%-Glycine 0.15%. Nonspecific proteins were blocked by incubating the cells with 2% BSA in phosphate buffered saline (PBS) for 1 hour followed by incubation with primary antibody. Cells were incubated with goat anti mouse (GAM) Alexa 488 secondary antibody for 1 hour to detect clathrin followed by washes with PBS. Cells were incubated with phalloidin Alexa 555 for 30 minutes to detect F-actin, followed by washing. Staining with DAPI was performed to detect nuclei and immunolabeled cells were mounted in Prolong Gold mounting medium (Thermo Fisher, Waltham, MA, USA). Control cells were labeled in the absence of primary antibodies. Immunolabeled cells were stored at 4°C and examined on Zeiss LSM 710-META laser scanning confocal microscope outfitted with Mai-Tai laser and electronics capable of spectrally separating fluorescence emission profiles. Image acquisition and processing were performed using ZEN (Zeiss Efficient Navigation) software.
2.9 Image Processing
Image stacks were taken with oversampling rate of 40 nm/pixel in the XY-dimension and 150 nm/pixel in the Z-dimension, followed by deconvolution and analysis using Volocity software (PerkinElmer, Waltham, MA, USA). Unsaturated raw confocal images were standardized for intensity threshold of the green channel with the adjustment of black level according to instructions in the Volocity user guide (PerkinElmer, Waltham, MA, USA). Fluorescence of the red channel was adjusted such that thickness of brush border (BB) was between 1 and 1.3μm. Brightness was adjusted such that staining of the BB was visible but not oversaturated. For quantification of labeled CCS green spots in the intermicrovillar region (pit) and underneath the BB (vesicle) were counted manually (Figure 3B). Three 40X fields of view were quantified for each WT, control (Scrambled) and AP2αKD. Settings were chosen such that maximum CCSs were visible with exclusion of non-specific structures.
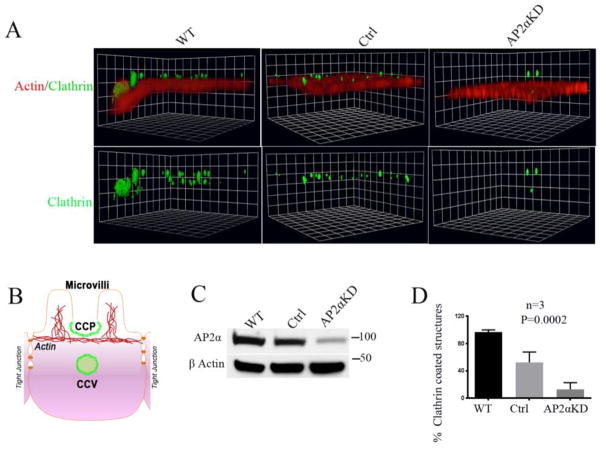
Figure 3. Actin and clathrin IFL in polarized AP2αKD C2BBe cells.
A. A representative 3 D reconstruction image shows the concurrent distribution of CCS relative to the brush border (BB) (marked by actin label). gSTED Z-stack images were captured by confocal microscopy and processed by volocity software as described in material and methods to visualize the CCS (pits in the plane of BB and vesicles below the plane of BB as shown in panel B). XY planes are at the mid-BB level. B. Schematic representation shows BB, clathrin coated pit (CCP) and clathrin coated vesicle (CCV) in a mature polarized enterocyte. C. Immunoblot reveals efficiency of AP2αKD in cells prior to fixation for IFL labeling. D. Quantification of CCS in WT, control and AP2αKD C2BBe cells. N= 3 images quantified for each condition.
3. Results
3.1 Depletion of AP2 α leads to increased cAMP-activated CFTR mediated anion secretion in human intestinal T84 cells
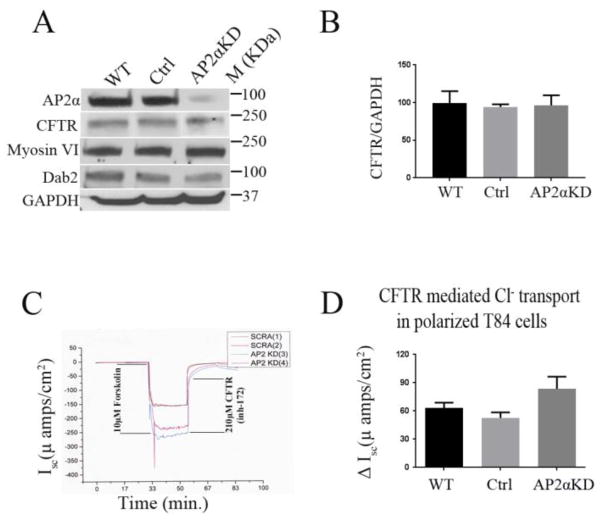
To understand the physiologic role of AP2 α in the human intestine, the T84 cells were employed and targeted AP2 α for shRNA mediated KD. T84 cells resemble secretory crypt cells of the native intestine and express abundant endogenous CFTR that can be assessed functionally using electrophysiologic approaches [7]. Immunoblot analysis revealed about 90 % KD efficiency for AP2 α (Figure 2A-). These cells were grown to achieve complete polarization (TEER>1000 ohms/cm2) as described in materials and methods. Ussing chamber measurement of CFTR mediated currents in polarized AP2αKD cells were 57% higher compared to control cells (Figure 2D), where ΔIsc was calculated by subtracting the maximum and the minimum response obtained from the Ussing chamber tracing (Figure 2D). We confirmed by immunoblot that KD of AP2 α did not alter the expression of other components of the AP-2 complex (Supplemental Figure 1), endocytic proteins (Myosin VI, Dab2) or CFTR (Figure 2A, 2B). CFTR mediated short-circuit current (Isc) response to forskolin was measured in polarized AP2αKD and scrambled (control) T84 cells. CFTR anion secretion was confirmed by CFTR (inh)-172 inhibitor (Figure 2C). These data indicate that AP2 α is important for CFTR ion transport in the human intestine.
Figure 2. cAMP-activated CFTR anion secretion is increased in AP2αKD T84 cells.
A. Immunoblot analysis shows GAPDH corrected expression of AP2 α, CFTR, Dab2 and Myosin VI in T84 cell lysates. B. Histogram shows comparative GAPDH corrected CFTR expression of WT, Control and AP2αKD T84 cells. Cells were analyzed following Isc measurements C, D. CFTR short-circuit current (Isc) and ΔIsc respectively were measured in polarized AP2αKD, WT and scrambled (control) T84 cells. CFTR secretion was stimulated by forskolin and confirmed by CFTR (inh)-172 inhibitor. N= 8 sets were measured for each condition.
3.2 Depletion of AP2 α leads to reduction in CCS in C2BBe cells
AP2 plays a critical role in CCS formation [23, 24] and CFTR is internalized by clathrin-mediated endocytosis [7]. Since we observed that AP2 α depletion increased CFTR anion secretion in T84 cells, we sought to understand whether increased CFTR plasma membrane function was associated with reduction in the number of CCS in AP2 α depleted cells that would account for the observed reduced endocytosis of apical CFTR [14].
AP2 α was targeted for shRNA-mediated KD in C2BBe cells because this cellular model resembles mature enterocytes of the small intestine where endocytosis activity is robust. Immunoblot analysis revealed about 70% KD efficiency (Figure 3C). AP2αKD C2BBe cells were transferred to transwell filters and resistance was monitored daily. When TEER of cells reached 200 ohms/cm2, they were designated day 0 (D0) and allowed to grow for 21 days to achieve full polarization at 21 days (D21). Polarized C2BBe cells at D21 were fixed in PFA and immunolabeled to detect clathrin as described in materials and methods. Images were captured by confocal microscopy, Z stack images were captured and at least 3 fields were used to quantify CCS using volocity software as described in material and methods. The diagram in Figure 3B illustrates the sites of clathrin accumulation (green) in CCP on the enterocyte inter-microvillar region and in CCV. Representative images show reduced CCS in AP2 α depleted cells compared to control (Figure 3A). Quantification confirmed a 75 % reduction in CCS in AP2 α depleted cells compared to control (Figure 3D). These findings align with the previously reported role of AP2 in clathrin assembly [25] and reduced endocytosis of CFTR in the absence of AP2 α.
3.3 Human AP2 α interacts with CFTR in HEK293Tcells
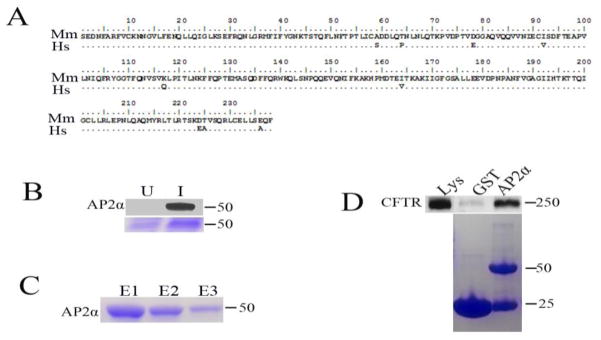
Our previous study demonstrated an interaction between mouse AP2 α and CFTR in rat jejunum. Furthermore, AP2αKD in HEK293T cells expressing CFTR led to decreased endocytosis of CFTR [14]. These observations, and the observation in the present study that depletion of human AP2 α leads to an increase in CFTR anion secretion in human T84 cells prompted us to examine whether human AP2 α directly interacts with CFTR in human cells. To test this, we cloned and expressed GST tagged human AP2 α appendage domain as described in materials and methods. Sequence comparison of mouse and human AP2 α appendage domain revealed 9 amino acid differences, of which 4 are synonymous (Figure 4A). The identity of AP2 α protein was confirmed by western blotting using an antibody that recognizes human AP2 α (Sigma, USA). The AP2 α protein along with GST tag was observed at an expected size of 54.3 KD (Figure 4B). The GST tagged protein was purified (Figure 4C) and used for GST pull down assay. Consistent with our previous findings, GST pull down assays revealed binding of human AP2 α appendage domain with CFTR in human HEK293T cells (Figure 4D). This data supports the interaction of AP2 α directly with CFTR in human.
Figure 4. Human AP2 α interacts with CFTR in HEK293T cells.
A. Sequence alignment of Homo sapiens (Hs) and Mus musculus (Mm) AP2 α appendage domain. Sequence homology is represented by dots in Hs sequence. B. Immunoblot analysis confirms the identity of AP2 α protein in induced (I) and uninduced (U) E. coli culture (top panel) with corresponding Coomassie stained gel images (bottom panel). C. Purification of GST tagged AP2 α protein, the protein was purified with GST beads affinity chromatography and eluted with 10mM reduced glutathione. Elution fractions (E1, E2 and E3) were resolved on SDS-PAGE gels and stained with Coomassie Blue. D. Interaction of CFTR with GST tagged human AP2 α by GST pull down assay. GST-AP2 α appendage or GST interaction with CFTR expressing HEK293T cells was performed as described in materials and methods. One-fourth of each pellet was resolved on SDS-PAGE gels and stained with Coomassie Blue (bottom panel) or transferred to polyvinylidene difluoride nitrocellulose (PVDF) membrane (Top panel) and analyzed by immunoblot to detect CFTR. Coomassie stained gel image shows GST tagged AP2α protein and free GST at their corresponding size (Bottom panel).
4. Discussion
This study provides the first direct evidence of a physiological role for AP2 α in modulating CFTR function in the human intestine. The current study is consistent with and supports the findings of our previous study demonstrating that depletion of AP α in HEK293T cells leads to decreased internalized CFTR, and increased surface CFTR [14]. However, assessment of CFTR ion transport function was not possible in non-polarized HEK293T cells. Human colonic T84 cells can fully polarize and are widely used in intestinal transepithelial Cl− transport studies of CFTR function in the intestine [7]. Using depletion of human AP α, our results reveal marked increase (57%) in transepithelial CFTR anion secretion in depleted T84 cells compared to control, confirming that AP α physiologically modulates CFTR function in the human intestine.
C2BBe cells are well suited to examine structures in the endocytic pathway as they possess a well developed brush border under fully mature conditions, similar to villus enterocytes of the small intestine [26]. Examination of AP2 α depleted cells revealed a reduction in CCS compared to control. These results are consistent with the previous report by Motley et al [27] that examined the role of AP2 in clathrin assembly. The authors found a 12-fold decrease in CCS in μ2 depleted Hela M cells. Our results also align with another report by Boucrot et al [25] that found marked reduction in assembly of clathrin coated pits in μ2 depleted BSC1 and Hela cells. Given that CFTR is internalised by clathrin mediated endocytosis [7, 28], our data suggest that reduction in CCS formation could lead to the reduced amount of internalised CFTR in AP2 α depleted cells resulting in an increased functional pool of CFTR on the cell surface. This notion is supported by our earlier finding of a reduction in internalised CFTR in AP2 α depleted HEK293T cells[14].
Consistent with our previous report that showed an interaction between mouse AP2 α and CFTR in rat jejunum [14] here we confirmed that the human AP2 α appendage domain interacts with CFTR in human derived cells. This result suggests that indeed AP2 α mediates CFTR endocytosis [14] and modulates its function in the intestine by a direct interaction. In summary, we propose that AP2 α modulates CFTR function by two independent but related events: first by interacting with CFTR directly and second by taking part in clathrin assembly (Figure 5). AP2 α depletion affects CFTR sorting and clathrin assembly leading to decreased endocytosis and increased anion secretion in AP2 α depleted cells due to increased surface CFTR.
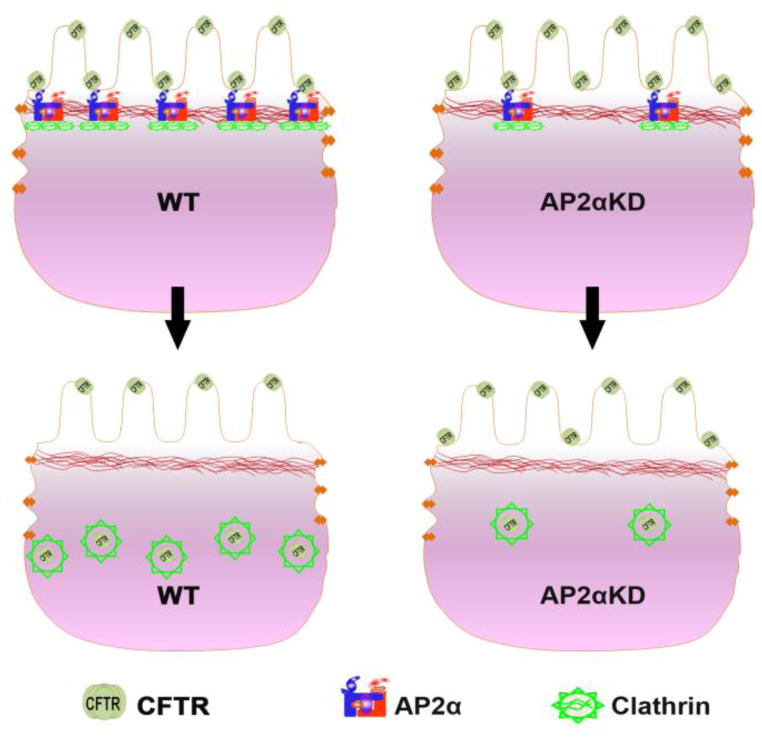
Figure 5. Model depicting AP α-dependent modulation of CFTR function in the enterocyte.
AP2 α simultaneously interacts with CFTR and participates in clathrin assembly. CFTR is recruited to CCP and internalized in CCV resulting in reduction in the functional pool of CFTR on the BB. In contrast to the WT condition, AP2 α depleted cells display reduced number of CFTR recruited to fewer assembled CCV, resulting in increased functional pool of surface CFTR and anion secretion.
Supplementary Material
Highlights.
The physiological function of AP2 α and CFTR interaction in intestine is shown
AP2 αKD in T84 cells increased CFTR current.
The CCS were depleted in C2BBe cells when AP2 α was knockdown.
AP2 α appendage domain and CFTR interact with each other.
AP2 α modulated CFTR function by interacting with CFTR
Acknowledgments
This study was supported by NIH grant DK 077065 to NA. The authors thank Dr. Md Kaimul Ahsan for providing technical assistance.
Abbreviations
- AP2αKD
Knock down of AP2α
- CCS
Clathrin-coated structures
- C2BBe
Caco2BBe
Footnotes
No conflicts of interests exist.
Contributions: V.K. and S.D. performed experiments and analyzed data with supervision from N.A. V.K. wrote first draft of the manuscript. N.A. V.K. and S.D. designed and conceived the experiments. S.D. and N.A. wrote the final revised manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Loughlin EV, Hunt DM, Bostrom TE, Hunter D, Gaskin KJ, Gyory A, et al. X-ray microanalysis of cell elements in normal and cystic fibrosis jejunum: evidence for chloride secretion in villi. Gastroenterology. 1996;110:411–8. doi: 10.1053/gast.1996.v110.pm8566587. [DOI] [PubMed] [Google Scholar]
- 2.Pratha VS, Hogan DL, Martensson BA, Bernard J, Zhou R, Isenberg JI. Identification of transport abnormalities in duodenal mucosa and duodenal enterocytes from patients with cystic fibrosis. Gastroenterology. 2000;118:1051–60. doi: 10.1016/s0016-5085(00)70358-1. [DOI] [PubMed] [Google Scholar]
- 3.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.Field M, Semrad CE. Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol. 1993;55:631–55. doi: 10.1146/annurev.ph.55.030193.003215. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury NA. Intracellular CFTR: localization and function. Physiol Rev. 1999;79:S175–91. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- 6.Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem J. 1997;328( Pt 2):353–61. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–8. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 8.Bradbury NA, Clark JA, Watkins SC, Widnell CC, Smith HSt, Bridges RJ. Characterization of the internalization pathways for the cystic fibrosis transmembrane conductance regulator. Am J Physiol. 1999;276:L659–68. doi: 10.1152/ajplung.1999.276.4.L659. [DOI] [PubMed] [Google Scholar]
- 9.Ameen N, Silvis M, Bradbury NA. Endocytic trafficking of CFTR in health and disease. J Cyst Fibros. 2007;6:1–14. doi: 10.1016/j.jcf.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, et al. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell. 2009;20:2337–50. doi: 10.1091/mbc.E08-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–36. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 12.Naren AP, Di A, Cormet-Boyaka E, Boyaka PN, McGhee JR, Zhou W, et al. Syntaxin 1A is expressed in airway epithelial cells, where it modulates CFTR Cl(−) currents. J Clin Invest. 2000;105:377–86. doi: 10.1172/JCI8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cihil KM, Ellinger P, Fellows A, Stolz DB, Madden DR, Swiatecka-Urban A. Disabled-2 protein facilitates assembly polypeptide-2-independent recruitment of cystic fibrosis transmembrane conductance regulator to endocytic vesicles in polarized human airway epithelial cells. J Biol Chem. 2012;287:15087–99. doi: 10.1074/jbc.M112.341875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaco A, Jakab R, Hegan P, Mooseker M, Ameen N. Alpha-AP-2 directs myosin VI-dependent endocytosis of cystic fibrosis transmembrane conductance regulator chloride channels in the intestine. J Biol Chem. 2010;285:17177–87. doi: 10.1074/jbc.M110.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–91. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- 16.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–8. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apodaca G. Endocytic traffic in polarized epithelial cells: Role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–59. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 18.Buss F, Spudich G, Kendrick-Jones J. Myosin VI: Cellular functions and motor properties. Annu Rev Cell Dev Bi. 2004;20:649–76. doi: 10.1146/annurev.cellbio.20.012103.094243. [DOI] [PubMed] [Google Scholar]
- 19.Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–26. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8:998–1006. doi: 10.1111/j.1600-0854.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 21.Weixel KM, Bradbury NA. The carboxyl terminus of the cystic fibrosis transmembrane conductance regulator binds to AP-2 clathrin adaptors. J Biol Chem. 2000;275:3655–60. doi: 10.1074/jbc.275.5.3655. [DOI] [PubMed] [Google Scholar]
- 22.Kravtsov DV, Ahsan MK, Kumari V, van Ijzendoorn SC, Reyes-Mugica M, Kumar A, et al. Identification of intestinal ion transport defects in microvillus inclusion disease. Am J Physiol Gastrointest Liver Physiol. 2016;311:G142–55. doi: 10.1152/ajpgi.00041.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehrlich M, Boll W, van Oijen A, Hariharan R, Chandran K, Nibert ML, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Saffarian S, Cocucci E, Kirchhausen T. Distinct dynamics of endocytic clathrin-coated pits and coated plaques. PLoS Biol. 2009;7:e1000191. doi: 10.1371/journal.pbio.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucrot E, Saffarian S, Zhang R, Kirchhausen T. Roles of AP-2 in clathrin-mediated endocytosis. PLoS One. 2010;5:e10597. doi: 10.1371/journal.pone.0010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102( Pt 3):581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- 27.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162:909–18. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weixel KM, Bradbury NA. Endocytic adaptor complexes bind the C-terminal domain of CFTR. Pflug Arch Eur J Phy. 2001;443:S70–S4. doi: 10.1007/s004240100648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.