Abstract
Objectives
To determine if a re-TUR in the presence or absence of muscle at the first TUR in T1-high grade (HG)/G3 bladder cancer patients makes a difference in recurrence, progression, cancer specific (CSS) and overall survival (OS).
Methods
In a large retrospective multi-centre cohort of 2451 T1-HG/G3 patients initially treated with BCG, 935 (38%) had a re-TUR. According to the presence or absence of muscle in the specimen of the primary TUR, patients were divided in 4 groups: group 1 (no muscle, no re-TUR), group 2 (no muscle, re-TUR), group 3 (muscle, no re-TUR) and group 4 (muscle, re-TUR). Clinical outcomes were compared across the 4 groups.
Results
Re-TUR had a positive impact on recurrence, progression, CSS and OS only if muscle was not present in the primary specimen. Adjusting for the most important prognostic factors, re-TUR in the absence of muscle had a borderline significant effect on time to recurrence (HR = 0.67, p = 0.08), progression (HR = 0.46, p = 0.06), CSS (HR = 0.31; p = 0.07) and OS (HR = 0.48, p = 0.05). Re-TUR in the presence of muscle in the primary specimen did not improve the outcome for any of the endpoints.
Conclusions
Our retrospective analysis suggests that re-TUR may not be necessary in T1-HG/G3 patients if muscle is present in the specimen of the primary TUR.
Keywords: bladder cancer, T1G3, high grade, reTUR, recurrence, progression
Introduction
High risk NMIBCs encompass all high grade (HG)/G3 papillary tumors, tumors with lamina propria invasion (T1), carcinoma in situ, as well as multiple recurrent large low grade lesions, all tumors whose common denominator is their high risk to progress to muscle invasive disease (1). This especially holds true for T1G3, where their long term progression and cancer specific death rates of up to 40% and 30%, respectively, identifies a subgroup of NMIBC of particular aggressiveness (2). TUR, the first diagnostic and therapeutic approach for NMIBC, is currently viewed as potentially the most critical step affecting the prognosis of the disease (3). A number of quality issues, some potentially linked to the experience of the surgeon, suggest that the initial TUR may be inadequate in a high percent of high risk NMIBC patients. First, dramatically higher rates of residual disease at a second look TUR, which approach 50% in Ta and 70% in T1 disease, have been reported (4). More importantly, up to 30% of T1 disease will be upstaged to muscle invasive disease at the re-TUR (5). These figures have led recent guidelines to strongly recommend repeat TUR within 4–6 weeks any time lamina propria involvement (1, 6) or high grade disease (1) is documented at the initial TUR.
Several issues make this recommendation at least debatable. The risk of upstaging to muscle invasive disease at re-TUR remained significant but did not exceed 7% in two recent series (7, 8). Furthermore BCG, which has proved to be effective in marker lesion studies (9), may ablate residual disease. In a small retrospective cohort of T1G3, long term outcomes were not improved by re-TUR (8). The benefit of immediate re-TUR may be different according to the presence or absence of detrusor muscle in the primary TUR specimen, the former being linked to a significant reduction of the risk of up staging and better prognosis (10). All these figures taken together suggest that the potential benefits of re-TUR should be carefully weighed taking into account the health care burden of the procedure (11) and its side effects (8).
In the current study, we retrospectively compared the long term outcomes of a large series of T1-HG/G3 patients treated with BCG who did not or did receive a re-TUR within 4 to 6 weeks.
Patients and methods
Study population
Patients with primary T1G3 (WHO 1973) / T1-HG (ISUP 1998/WHO 2004) or secondary T1-HG/G3 disease from a previously BCG naïve non T1-HG/G3 NMIBC tumor formed the retrospective study cohort provided they received at least a full induction course of BCG between 1990 and 2011. Patients who previously received BCG for a tumor that was not T1-HG/G3 or did not receive BCG as the initial intravesical treatment for a T1-HG/G3 tumor were excluded. Patients with a history of muscle invasive disease (≥ T2), upper tract urothelial cancer, or a non-urothelial carcinoma were also excluded. Details are provided in a previous publication (12).
Data collection: clinical-pathological variables and re-TUR information
The following patient and tumor characteristics were included in the database: age, gender, smoking history and intensity, exposure to chemical compounds, tumor status (primary or recurrent), previous intravesical chemotherapy, tumor size (< versus ≥ 3 cm), tumor focality (solitary versus multiple), presence of CIS, prostatic urethra involvement with or without stromal invasion, presence of muscle in the tissue specimen, BCG dose and total number of instillations. Any instillation beyond the 6 induction instillations was defined as maintenance BCG. Information on re-TUR (defined as a second TUR performed within 4–6 weeks after an initial macroscopically complete TUR and before BCG administration) was also recorded. Results of pathology at re-TUR were categorized into: no evidence of disease, persistent disease with down staging (Ta), or persistent T1 disease. Patients with muscle invasive disease at re-TUR did not match the study inclusion criteria and were therefore excluded upfront.
Statistical Considerations
Patients were divided into 4 groups according to whether or not a re-TUR was carried out and the presence or absence of muscle in the specimen of the primary TUR: group 1 (no muscle, no re-TUR), group 2 (no muscle, re-TUR), group 3 (muscle, no re-TUR) and group 4 (muscle, re-TUR).
The presence of muscle (no/yes) and re-TUR (no/yes) according to the presence of muscle (no/yes) were compared for the following end points: time to first recurrence, progression to muscle invasive disease, and the duration of cancer specific survival (CSS) and overall survival (OS).
Times to events were calculated taking the date of starting BCG as time zero. Overall survival was estimated using the Kaplan-Meier technique. To take into account patients who died prior to observing the event of interest (competing risk), times to the other events were estimated using cumulative incidence functions. Patients without an event or death prior to the event were censored at the last date of follow up. Times to events were compared with the Cox univariable and multivariable proportional hazards regression model using the variables previously identified (12).
Results
Information on whether or not a re-TUR had been performed was available in 2277 (92.9%) of the 2451 eligible patients. 935 (41.1%) of the 2277 patients underwent re-TUR and 1342 (58.9%) did not. Baseline patient and disease characteristics according to re-TUR and no re-TUR are reported in Table 1. Patients were more likely to have undergone a re-TUR if they had tumors ≥ 3 cm (34.1% versus 22.6%), multifocal tumors (50.3% versus 23.3%) or did not receive maintenance BCG (41.2% versus 33.2%).
Table 1.
Baseline characteristics of patients according to re-TUR status
| Variable | No Re-TUR (%) | Re-TUR (%) | Re-TUR Unknown (%) | All patients (%) |
|---|---|---|---|---|
|
| ||||
| N | 1342 | 935 | 174 | 2451 |
|
| ||||
| Age | ||||
| < 70 yrs | 726 (52.4) | 549 (39.6) | 110 (7.9) | 1385 (56.5) |
| ≥ 70 yrs | 616 (57.8) | 386 (36.2) | 64 (6.0) | 1066 (43.5) |
| Median (yrs) | 69 | 67 | 67 | 68 |
| Interquartile range (yrs) | 61 – 75 | 59 – 74 | 60 – 72 | 60 – 74 |
|
| ||||
| Sex | ||||
| Male | 1112 (55.3) | 756 (37.6) | 144 (7.2) | 2012 (82.1) |
| Female | 230 (52.4) | 179 (40.8) | 30 (6.8) | 439 (17.9) |
|
| ||||
| Tumor status | ||||
| Primary T1G3 | 1175 (53.9) | 848 (38.9) | 156 (7.2) | 2179 (88.9) |
| Recurrent after non T1G3 | 167 (61.4) | 87 (32.0) | 18 (6.6) | 272 (11.1) |
|
| ||||
| Previous intravesical chemotherapy | ||||
| No | 1261 (54.4) | 895 (38.6) | 164 (7.1) | 2320 (94.7) |
| Yes | 81 (61.8) | 40 (30.5) | 10 (7.6) | 131 (5.3) |
|
| ||||
| Muscle in primary TUR specimen | ||||
| No | 130 (31.3) | 276 (66.4) | 10 (2.4) | 416 (17.0) |
| Yes | 1092 (61.8) | 624 (35.3) | 52 (2.9) | 1768 (72.1) |
| Missing/Unknown | 120 (72.3) | 35 (21.1) | 112 (6.6) | 267 (10.9) |
|
| ||||
| Tumor grade | ||||
| WHO 1973 grade 3 | 1090 (64.0) | 442 (26.0) | 171 (10.0) | 1703 (69.5) |
| WHO 2004 high grade | 978 (54.9) | 799 (44.9) | 3 (0.2) | 1780 (72.6) |
| Grade 3 and/or high grade | 1342 (54.8) | 935 (38.1) | 174 (7.1) | 2451 (100) |
|
| ||||
| Tumor focality | ||||
| Solitary | 618 (64.1) | 225 (23.3) | 121 (12.6) | 964 (39.3) |
| Multiple | 631 (46.2) | 687 (50.3) | 47 (3.4) | 1365 (55.7) |
| Missing/Unknown | 93 (76.2) | 23 (18.9) | 6 (4.9) | 122 (5.0) |
|
| ||||
| Largest tumor diameter | ||||
| < 3 cm | 806 (70.9) | 257 (22.6) | 74 (6.5) | 1137 (46.4) |
| ≥ 3 cm | 326 (58.2) | 191 (34.1) | 43 (7.7) | 560 (22.8) |
| Missing/Unknown | 210 (27.9) | 487 (64.6) | 57 (7.6) | 754 (30.8) |
|
| ||||
| Concomitant CIS | ||||
| No | 1019 (55.0) | 694 (37.5) | 139 (7.5) | 1852 (75.6) |
| Yes | 323 (53.9) | 241 (40.2) | 35 (5.8) | 599 (24.4) |
|
| ||||
| Invasion of prostatic urethra | ||||
| No | 936 (70.0) | 401 (30.0) | 0 | 1337 (54.6) |
| Yes, without stromal invasion | 26 (59.1) | 18 (40.9) | 0 | 44 (1.8) |
| Yes, with stromal invasion | 2 (40.0) | 3 (60.0) | 0 | 5 (0.2) |
| Missing/Unknown | 378 (35.5) | 513 (48.2) | 174 (16.3) | 1065 (43.4) |
|
| ||||
| Pathology at restaging TUR* | ||||
| No residual tumor | NA | 267 (28.6) | NA | 267 (28.6) |
| Ta | NA | 378 (40.4) | NA | 378 (40.4) |
| T1 | NA | 289 (30.9) | NA | 289 (30.9) |
| CIS | NA | NA** | NA | NA** |
| Missing/Unknown | NA | 1 (0.1) | NA | 1 (0.1) |
|
| ||||
| Maintenance BCG | ||||
| No | 753 (49.7) | 624 (41.2) | 138 (9.1) | 1515 (61.8) |
| Yes | 589 (62.9) | 311 (33.2) | 36 (3.9) | 936 (38.2) |
Separate information on CIS at re-TUR was not available.
Muscle was present in the original TUR specimen in 1768 (72.1%) of the 2451 patients, not present in 416 (17.0%) patients and unknown in 267 (10.9%). 276 (66.4%) of 416 patients underwent a re-TUR if muscle was not present in the specimen as compared to 624 (35.3%) of 1768 patients when muscle was present.
Table 2 presents the patient baseline characteristics according to re-TUR status and the presence or absence of muscle in the specimen of the first TUR. Multiple tumors were more likely to undergo a re-TUR when muscle was not present in the primary TUR, 83.2%, versus 44.7% when muscle was present. Patients without muscle in primary TUR were more likely to have received maintenance BCG if no re-TUR was done.
Table 2.
Baseline characteristics of patients according to re-TUR status and the presence or absence of muscle in the specimen of the first TUR
| Variable | No muscle, no reTUR (%) | No muscle, reTUR (%) | Muscle, no reTUR (%) | Muscle, reTUR (%) | Unknown (%) | All Patients (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| N | 130 | 276 | 1092 | 624 | 329 | 2451 |
|
| ||||||
| Age | ||||||
| < 70 yrs | 74 (5.3) | 173 (12.5) | 582 (42.0) | 361 (26.1) | 195 (14.1) | 1385 (56.5) |
| ≥ 70 yrs | 56 (5.3) | 103 (9.7) | 510 (47.8) | 263 (24.7) | 134 (12.6) | 1066 (43.5) |
| Median (yrs) | 68 | 65 | 69 | 68 | 67 | 68 |
| Interquartile range (yrs) | 61–75 | 57–73 | 61–75 | 60–74 | 61–74 | 60–74 |
|
| ||||||
| Sex | ||||||
| Male | 101 (5.0) | 217 (10.8) | 907 (45.1) | 512 (25.5) | 275 (13.7) | 2012 (82.1) |
| Female | 29 (6.6) | 59 (13.4) | 185 (42.1) | 112 (25.5) | 54 (12.3) | 439 (17.9) |
|
| ||||||
| Tumor status | ||||||
| Primary T1G3 | 110 (5.1) | 244 (11.2) | 967 (44.4) | 575 (26.4) | 283 (13.0) | 2179 (88.9) |
| Recurrent after non T1G3 | 20 (7.4) | 32 (11.8) | 125 (46.0) | 49 (18.0) | 46 (16.9) | 272 (11.1) |
|
| ||||||
| Previous intravesical chemotherapy | ||||||
| No | 118 (5.1) | 263 (11.3) | 1031 (44.4) | 598 (25.8) | 310 (13.4) | 2320 (94.7) |
| Yes | 12 (9.2) | 13 (9.9) | 61 (46.6) | 26 (19.9) | 19 (14.5) | 131 (5.3) |
|
| ||||||
| Tumor focality | ||||||
| Solitary | 47 (4.9) | 35 (3.6) | 539 (55.9) | 178 (18.5) | 165 (17.1) | 964 (39.3) |
| Multiple | 48 (3.5) | 238 (17.4) | 541 (39.6) | 438 (32.1) | 100 (7.3) | 1365 (55.7) |
| Missing/Unknown | 35 (28.7) | 3 (2.5) | 12 (9.8) | 8 (6.6) | 64 (52.5) | 122 (5.0) |
|
| ||||||
| Largest tumor diameter | ||||||
| < 3 cm | 65 (5.7) | 35 (3.1) | 684 (60.2) | 210 (18.5) | 143 (12.6) | 1137 (46.4) |
| ≥ 3 cm | 24 (4.3) | 29 (5.2) | 291 (52.0) | 152 (27.1) | 64 (11.4) | 560 (22.8) |
| Missing/Unknown | 41 (5.4) | 212 (28.1) | 117 (15.5) | 262 (34.8) | 122 (16.2) | 754 (30.8) |
|
| ||||||
| Concomitant CIS | ||||||
| No | 103 (5.6) | 215 (11.6) | 814 (44.0) | 454 (24.5) | 266 (14.4) | 1852 (75.6) |
| Yes | 27 (4.5) | 61 (10.2) | 278 (46.4) | 170 (28.4) | 63 (10.5) | 599 (24.4) |
|
| ||||||
| Invasion of prostatic urethra | ||||||
| No | 82 (6.1) | 53 (4.0) | 812 (60.7) | 342 (25.6) | 48 (3.6) | 1337 (54.6) |
| Yes, without stromal invasion | 0 | 2 (4.6) | 25 (56.8) | 16 (36.4) | 1 (2.3) | 44 (1.8) |
| Yes, with stromal invasion | 0 | 1 (20.0) | 2 (40.0) | 2 (40.0) | 0 | 5 (0.2) |
| Missing/Unknown | 48 (4.5) | 220 (20.7) | 253 (23.8) | 264 (24.8) | 280 (26.3) | 1065 (43.4) |
|
| ||||||
| Pathology at restaging TUR* | ||||||
| No residual tumor | NA | 39 (14.1) | NA | 217 (34.8) | 11 (3.3) | 267 (28.6) |
| Ta | NA | 126 (45.7) | NA | 240 (38.5) | 12 (3.6) | 378 (40.4) |
| T1 | NA | 111 (40.2) | NA | 166 (26.6) | 12 (3.6) | 289 (30.9) |
| CIS | NA | NA** | NA | NA** | NA** | NA** |
| Missing/Unknown | NA | 0 | NA | 1 (.02) | NA | 1 (0.1) |
|
| ||||||
| Maintenance BCG | ||||||
| No | 89 (5.9) | 245 (16.2) | 627 (41.4) | 373 (24.6) | 181 (12.0) | 1515 (61.8) |
| Yes | 41 (4.4) | 31 (3.3) | 465 (49.7) | 251 (26.8) | 148 (15.8) | 936 (38.2) |
Separate information on CIS at re-TUR was not available.
Persistent disease at re-TUR was documented in 85.9% of patients in the absence of muscle in primary TUR as compared to 65.2% when muscle had been reported in the primary TUR. Similarly, the rate of persistent T1 disease was higher when no muscle was reported in the first TUR (40.2%) as compared to that of a primary TUR with muscle in the specimen (26.6%).
The median duration of follow up was 5.2 years. Table 3 shows the distribution of clinical outcomes across the 4 groups according to the presence or absence of muscle in the primary TUR and whether or not a re-TUR was done.
Table 3.
Clinical outcome according to re-TUR status and the presence or absence of muscle in the specimen of the first TUR
| Variable | No muscle, no reTUR (%) | No muscle, reTUR (%) | Muscle, no reTUR (%) | Muscle, reTUR (%) | Unknown (%) | All Patients (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| N | 130 | 276 | 1092 | 624 | 329 | 2451 |
|
| ||||||
| Recurrence | ||||||
| No | 52 (40.0) | 115 (41.7) | 561 (51.4) | 281 (45.0) | 198 (60.2) | 1207 (49.3) |
| Yes | 60 (60.0) | 161 (58.3) | 531 (48.6) | 343 (55.0) | 131 (39.8) | 1244 (50.7) |
|
| ||||||
| Progression | ||||||
| No | 102 (78.5) | 238 (86.2) | 871 (79.8) | 504 (80.8) | 271 (82.4) | 1986 (81.0) |
| Yes | 28 (21.5) | 38 (13.8) | 221 (20.2) | 120 (19.2) | 58 (17.6) | 465 (19.0) |
|
| ||||||
| Cancer specific mortality | ||||||
| No | 115 (88.5) | 257 (93.1) | 998 (91.4) | 563 (90.2) | 297 (90.3) | 2230 (91.0) |
| Yes | 15 (11.5) | 19 (6.9) | 94 (8.6) | 61 (9.8) | 32 (9.7) | 221 (9.0) |
|
| ||||||
| Overall Survival | ||||||
| Alive | 89 (68.5) | 219 (79.4) | 812 (74.4) | 485 (77.7) | 250 (76.0) | 1855 (75.7) |
| Dead | 41 (31.5) | 57 (20.6) | 280 (25.6) | 139 (22.3) | 79 (24.0) | 596 (24.3) |
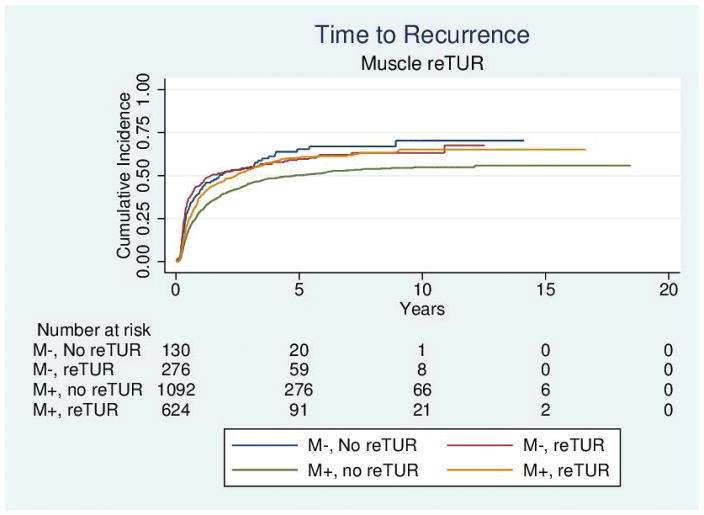
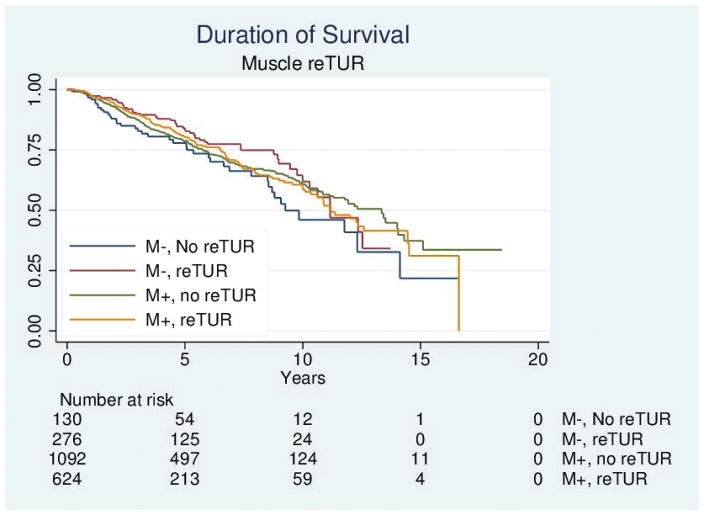
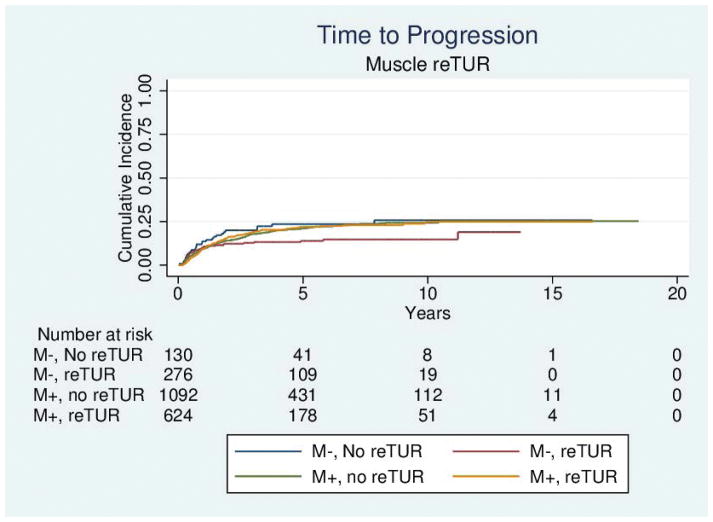
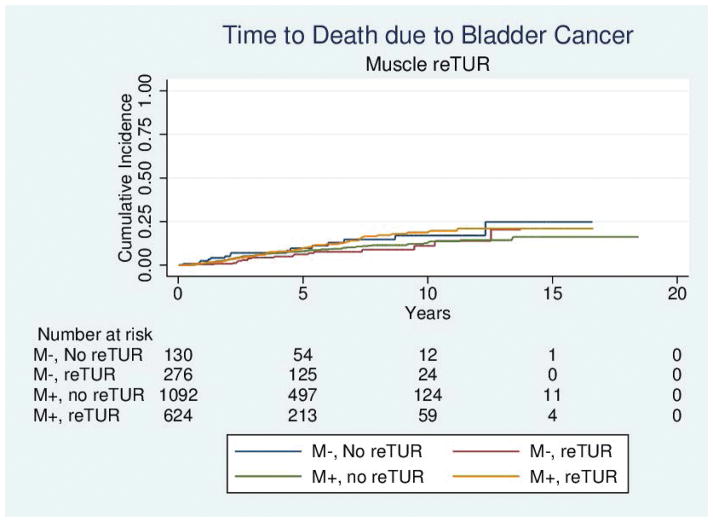
Table 4 reports the results of the univariable and multivariable analyses of the effect of re-TUR on recurrence, progression, bladder cancer specific and overall survival according to whether or not muscle was present in the primary TUR. Adjusting for the most important prognostic factors including age, number of tumors, tumor size, the presence of CIS and the use of maintenance BCG, re-TUR in the absence of muscle had a borderline significant positive impact on time to recurrence (HR = 0.67, 95%CI: 0.42–1.04, p = 0.08), time to progression (HR = 0.46, 95%CI: 0.20–1.03, p=0.06), duration of CSS (HR = 0.31, 95%CI: p=0.07) and OS (HR = 0.48, 95%CI: 0.23–1.00, p=0.05). Re-TUR in presence of muscle in the primary specimen did not improve the outcome for any of the endpoints after adjusting for prognostic factors. Time to event curves of the 4 groups for the time to recurrence, progression, and the duration of bladder cancer specific and overall survival are given in Figures 1–4.
Table 4.
Univariable and Multivariable Analyses of Time to Recurrence, time to progression, duration of Bladder Cancer Specific and overall survival
| Univariable | Multivariable | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p value | HR (95%CI) | p value | |
|
| ||||
| Recurrence* | ||||
| Muscle (no/yes) | 0.95 (0.92–0.98) | 0.001 | 0.95 (0.91–0.99) | 0.04 |
| reTUR (no/yes), No muscle | 0.97 (0.74–1.27) | 0.80 | 0.67 (0.42–1.04) | 0.08 |
| reTUR (no/yes), Muscle | 1.30 (1.14–1.49) | < 0.001 | 1.38 (1.17–1.64) | < 0.001 |
| reTUR (no/yes), All patients | 1.28 (1.14–1.43) | < 0.001 | 1.27 (1.09–1.49) | 0.002 |
|
| ||||
| Progression** | ||||
| Muscle (no/yes) | 0.99 (0.94–1.03) | 0.56 | 1.00 (0.94–1.06) | 0.97 |
| reTUR (no/yes), No muscle | 0.59 (0.36–0.96) | 0.03 | 0.46 (0.20–1.03) | 0.06 |
| reTUR (no/yes), Muscle | 1.01 (0.81–1.26) | 0.95 | 1.20 (0.93–1.55) | 0.17 |
| reTUR (no/yes), All patients | 0.92 (0.76–1.11) | 0.39 | 1.10 (0.86–1.39) | 0.45 |
|
| ||||
| Disease Specific Survival*** | ||||
| Muscle (no/yes) | 1.03 (.97–1.09) | 0.28 | 1.08 (1.01–1.15) | 0.03 |
| reTUR (no/yes), No muscle | 0.56 (0.29–1.11) | 0.10 | 0.31 (0.09–1.08) | 0.07 |
| reTUR (no/yes), Muscle | 1.29 (0.93–1.78) | 0.13 | 1.60 (1.11–2.31) | 0.01 |
| reTUR (no/yes), All patients | 1.09 (0.82–1.43) | 0.56 | 1.32 (0.95–1.84) | 0.10 |
|
| ||||
| Overall Survival*** | ||||
| Muscle (no/yes) | 0.99 (0.95–1.03) | 0.62 | 1.02 (0.98–1.07) | 0.98 |
| reTUR (no/yes), No muscle | 0.66 (0.44–0.99) | 0.05 | 0.48 (0.23–1.00) | 0.05 |
| reTUR (no/yes), Muscle | 0.99 (0.81–1.22) | 0.95 | 1.23 (0.97–1.56) | 0.09 |
| reTUR (no/yes), All patients | 0.92 (0.78–1.10) | 0.37 | 1.09 (0.88–1.35) | 0.45 |
- HR < 1: better outcome when muscle is present, better outcome when a reTUR is done
- HR > 1: worse outcome when muscle is present, worse outcome when a reTUR is done
Recurrence adjusted for number of tumors, tumor size and maintenance BCG
Progression adjusted for age, tumor size, CIS and maintenance BCG
Survival adjusted for age, tumor size and maintenance BCG
Figure 1.
Time to recurrence according to the presence or absence of muscle in the primary specimen and whether or not a reTUR was carried out.
Figure 4.
Duration of survival according to the presence or absence of muscle in the primary specimen and whether or not a reTUR was carried out.
Discussion
The importance of restaging TUR has been supported by accumulating evidence in high risk NMIBC, mainly in the worst subgroup of T1G3 tumors (4, 13). The finding of muscle invasive disease in up to 50% of clinical T1G3 tumors undergoing cystectomy suggests that this disease category is often under staged (14). Even more strikingly, up to 80% of T1 NMIBCs had persistent disease at re-TUR, of whom 30% were muscle invasive (4).
Our results, generated on a large multi-center series of primary T1-HG/G3 patients receiving BCG, are in line with these figures: out of a total of 935 (38.1%) patients undergoing re-TUR, 71.3% had residual disease, with 30.9% stage T1 and 40.4% stage Ta.
In this context, re-TUR has been strongly advocated in an attempt to overcome quality issues of the initial TUR, some of them occurring even in expert hands (4, 15) and to improve disease outcome (4, 7). In a randomized study, patients with T1 disease at first TUR exhibited a two-fold increased risk of recurrence and a four-fold increased risk of progression when re-TUR was not done (7). In a large retrospective series of Ta and T1 high risk NMIBC treated with BCG, the 3-month recurrence rate was as low as 9% for those with a re-TUR (87% of 1021 patients) as compared to 55% for the group treated with a single resection (13).
Others have recently questioned the necessity for all high risk NMIBC patients to undergo a repeat TUR within 4–6 weeks. In a retrospective series of 200 T1G3 patients, re-TUR impacted positively on short-term outcomes but did not play a role on the long term risk of recurrence, progression and CSS (8). Another study pointed out that staging inaccuracies of the primary TUR may depend upon the presence or absence of detrusor muscle in the specimen, the latter indicating that the resection was not deep enough to exclude involvement of the muscolaris propria (15). The risk of downstaging at second TUR was dramatically decreased when muscle was present in the primary TUR (5, 16). Absence of muscle in the primary specimen was also acknowledged as an independent predictor of progression to muscle invasive disease in a small retrospective series in T1G3 (10). Tumor status at second resection was found to be significantly associated with risk of recurrence, treatment failure and borderline significant for cancer-specific death in a prospective, randomized study conducted by the Nordic Association of Urology on 250 T1 patients randomized to BCG or epirubicin plus interferon-a2b (17).
In our series, 1768 of the 2184 (81.0%) patients for whom the information was available had detrusor muscle in the primary TUR specimen. This proportion varied between 89.4% for those who did not have a second resection and 69.3% for those with a re-TUR, suggesting that absence of muscle in the primary TUR may have played a role in the decision for re-TUR. Detrusor muscle documented in the primary TUR specimen was associated with a lower, yet still consistent, rate of any residual disease (65.1% vs 85.9%) and chiefly of T1 NMIBC (26.6% vs 40.2%) at re-TUR as compared to absence of muscle.
Re-TUR impacted favorably on the endpoints of recurrence, progression, CSS and OS only in the absence of muscle at first TUR. We failed to show an advantage of a re-TUR after a first TUR that included detrusor muscle in the pathological specimen. Even more strikingly, such patients showed a higher risk of recurrence even after adjusting for prognostic factors such as tumor multiplicity, tumor size and the use of maintenance BCG. We cannot provide a rational explanation for this paradoxical finding.
We can speculate that the effect of BCG, which has been shown to ablate residual disease in a marker lesion study (9) and to reduce the progression rate (18), could account for the lack of improvement in outcome with re-TUR as compared to no re-TUR when muscle was included in the first TUR. Notably, in the only study (7) reporting a significantly higher risk of disease progression in T1 NMIBC not receiving immediate re-TUR, patients were treated with intravesical chemotherapy, a treatment which has never been proven to prevent disease progression (1). Nonetheless, BCG may not be effective enough to replace the need for a second TUR when muscle has not been included in the first resection, potentially because of the significant amount of residual disease.
Our results better refine those of a recent retrospective series of 210 untreated T1G3 patients where an early re-TUR did not appear to change the long term prognosis (8). In this latter study, muscle in the specimen was not taken into account. The main clinical implication of our findings is that re-TUR may be avoided in T1-HG/G3 patients where the first TUR was deep enough to include muscle in the resection specimen. This is likely to translate into a positive impact on the burden of health care costs of bladder cancer (11) while avoiding exposure of the patient to the risks of additional surgery (8).
Some limitations of our study are to be acknowledged. The first is the lack of knowledge about the proportion of patients diagnosed with muscle invasive disease at re-TUR that were excluded by our study design. The rate of T2 disease at re-TUR, which has been reported to be between 7% and 30% (4, 7), represents the main strength in support of a re-TUR (1). The risk of downstaging is significantly reduced but not eliminated by inclusion of muscle in the specimen of the first TUR (16). Similarly, the non re-TUR patients in our study almost certainly included a higher proportion of “downstaged” T1G3 disease as compared to the re-TUR patients. However, the finding that re-TUR improved prognosis only when muscle was not present in primary TUR suggests that the risk of downstaging at primary TUR is probably minimal when muscle is included, making re-TUR unnecessary in this latter instance. Secondly, as re-TUR was not determined by randomization, a number of selection biases that the retrospective design of this study cannot address may have accounted for the decision to do a re-TUR. In addition, a non-complete TUR at first TUR may also have influenced the urologists to schedule a patient for a re-TUR.
Conclusions
Our analysis on a large retrospective series of T1-HG/G3 patients treated with BCG suggests that immediate re-TUR does not improve long term disease outcomes of patients with detrusor muscle included in the specimen of the primary TUR. A prospective study would allow confirming whether immediate re-TUR may thus be spared in such cases.
Figure 2.
Time to progression according to the presence or absence of muscle in the primary specimen and whether or not a reTUR was carried out.
Figure 3.
Time to death due to bladder cancer according to the presence or absence of muscle in the primary specimen and whether or not a reTUR was carried out.
Acknowledgments
Grant Support: P30 CA008748
Footnotes
Conflicts of Interest: None disclosed.
References
- 1.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, Rouprêt M. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013 Oct;64(4):639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997;158(1):62–7. doi: 10.1097/00005392-199707000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Herr HW, Donat SM. A re-staging transurethral resection predicts early progression of superficial bladder cancer. BJU Int. 2006;97(6):1194–8. doi: 10.1111/j.1464-410X.2006.06145.x. [DOI] [PubMed] [Google Scholar]
- 4.Herr HW. Role of re-resection in non-muscle-invasive bladder cancer. Scientific World Journal. 2011 Feb 3;11:283–8. doi: 10.1100/tsw.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herr HW. The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol. 1999 Jul;162(1):74–6. doi: 10.1097/00005392-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS, Jr, Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007 Dec;178(6):2314–30. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F. Impact of routine second transurethral resection on the long-term outcome of patients with newly diagnosed pT1 urothelial carcinoma with respect to recurrence, progression rate, and disease-specific survival: a prospective randomised clinical trial. Eur Urol. 2010;58(2):185–90. doi: 10.1016/j.eururo.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Angulo JC, Palou J, García-Tello A, de Fata FR, Rodríguez O, Villavicencio H. Second transurethral resection and prognosis of high-grade non-muscle invasive bladder cancer in patients not receiving bacillus Calmette-Guérin. Actas Urol Esp. 2014;38(3):164–71. doi: 10.1016/j.acuro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Mack D, Höltl W, Bassi P, Brausi M, Ferrari P, de Balincourt C, Sylvester R European Organization for Research and Treatment of Cancer Genitourinary Group. The ablative effect of quarter dose bacillus Calmette-Guerin on a papillary marker lesion of the bladder. J Urol. 2001 Feb;165(2):401–3. doi: 10.1097/00005392-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Shindo T, Masumori N, Kitamura H, Tanaka T, Fukuta F, Hasegawa T, Yanase M, Miyake M, Miyao N, Takahashi A, Matsukawa M, Taguchi K, Shigyo M, Kunishima Y, Tachiki H, Tsukamoto T. Clinical significance of definite muscle layer in TUR specimen for evaluating progression rate in T1G3 bladder cancer: multicenter retrospective study by the Sapporo Medical University Urologic Oncology Consortium (SUOC) World J Urol. 2013 Nov 5; doi: 10.1007/s00345-013-1205-1. [DOI] [PubMed] [Google Scholar]
- 11.Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, Lotan Y. The Economics of Bladder Cancer: Costs and Considerations of Caring for This Disease. Eur Urol. 2014 Jan 21; doi: 10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Gontero P, Sylvester R, Pisano F, Joniau S, Van der Eeckt K, Serretta V, Larré S, Di Stasi S, Van Rhijn B, Witjes AJ, Grotenhuis AJ, Kiemeney LA, Colombo R, Briganti A, Babjuk M, Malmstrom PU, Oderda M, Irani J, Malats N, Baniel J, Mano R, Cai T, Cha EK, Ardelt P, Varkarakis J, Bartoletti R, Spahn M, Johansson R, Frea B, Soukup V, Xylinas E, Dalbagni G, Karnes RJ, Shariat SF, Palou J. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with bacillus Calmette-Guérin: results of a retrospective multicenter study of 2451 patients. Eur Urol. 2015;67:74–82. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Sfakianos JP, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guérin therapy for urothelial carcinoma not invading muscle of the bladder. BJU Int. 2011;108(4):526–30. doi: 10.1111/j.1464-410X.2010.09874.x. [DOI] [PubMed] [Google Scholar]
- 14.Fritsche HM, Burger M, Svatek RS, Jeldres C, Karakiewicz PI, Novara G, Skinner E, Denzinger S, Fradet Y, Isbarn H, Bastian PJ, Volkmer BG, Montorsi F, Kassouf W, Tilki D, Otto W, Capitanio U, Izawa JI, Ficarra V, Lerner S, Sagalowsky AI, Schoenberg M, Kamat A, Dinney CP, Lotan Y, Shariat SF. Characteristics and outcomes of patients with clinical T1 grade 3 urothelial carcinoma treated with radical cystectomy: results from an international cohort. Eur Urol. 2010;57(2):300–9. doi: 10.1016/j.eururo.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Ritch CR, Clark PE, Morgan TM. Restaging transurethral resection for non-muscle invasive bladder cancer: who, why, when, and how? Urol Clin North Am. 2013;40(2):295–304. doi: 10.1016/j.ucl.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Dutta SC, Smith JA, Jr, Shappell SB, Coffey CS, Chang SS, Cookson MS. Clinical under staging of high risk nonmuscle invasive urothelial carcinoma treated with radical cystectomy. J Urol. 2001 Aug;166(2):490–3. [PubMed] [Google Scholar]
- 17.Hemdan T, Johansson R, Jahnson S, Hellström P, Tasdemir I, Malmström PU. Members of the Urothelial Cancer Group of the Nordic Association of Urology. 5-Year outcome of a randomized prospective study comparing bacillus Calmette-Guérin with epirubicin and interferon-α2b in patients with T1 bladder cancer. J Urol. 2014;191(5):1244–9. doi: 10.1016/j.juro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002 Nov;168(5):1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]