Abstract
Conclusion
vOCR can detect loss of otolith-ocular function without specifying the side of vestibular loss. Since vOCR is measured with a simple head tilt maneuver, it can be potentially used as a bedside clinical test in combination with video head impulse test.
Objective
Video-oculography (VOG) goggles are being integrated into the bedside assessment of patients with vestibular disorders. Lacking, however, is a method to evaluate otolith function. This study validated a VOG test for loss of otolith function.
Methods
VOG was used to measure ocular counter-roll (vOCR) in 12 healthy controls, 14 patients with unilateral vestibular loss (UVL), and six patients with bilateral vestibular loss (BVL) with a static lateral head tilt of 30°. The results were compared with vestibular evoked myogenic potentials (VEMP), a widely-used laboratory test of otolith function.
Results
The average vOCR for healthy controls (4.6°) was significantly different from UVL (2.7°) and BVL (1.6°) patients (p < 0.0001). The vOCR and VEMP measurements were correlated across subjects, especially the click and tap oVEMPs (click oVEMP R = 0.45, tap oVEMP R = 0.51; p < 0.0003). The receiver operator characteristic (ROC) analysis showed that vOCR and VEMPs detected loss of otolith function equally well. The best threshold for vOCR to detect vestibular loss was at 3°. The vOCR values from the side of vestibular loss and the healthy side were not different in UVL patients (2.53° vs 2.8°; p = 0.59).
Keywords: OCR, ocular counter-roll, vestibular, otolith, clinical test, video-oculography, VOG
Introduction
Video-oculography (VOG) goggles are being integrated into the bedside assessment of patients with vestibular disorders, using primarily the video head impulse test (vHIT) to evaluate semicircular canal function [1]. Lacking, however, is a comparable method to evaluate otolith function. The goal here was to validate a VOG test for detecting loss of otolith-ocular function.
Ocular counter-roll (OCR) is a vestibulo-ocular reflex (VOR) characterized by torsional rotations of the eye in response to lateral tilt of the head. During head tilt, the dynamic OCR is mainly driven by activity from both the semicircular canals and the otolith organs, and consists of a torsional nystagmus with slow phases away from and quick phases toward the head tilt [2]. With a sustained head tilt, the static OCR is driven by the otolith organs (mainly the utricles), which results in a static change in torsional eye position in the direction away from the head tilt with a gain (eye movement/head movement) of ~10–25% [3].
Various methods have been reported to measure OCR, using either VOG or scleral search coils [4–8]. However, OCR measurement using VOG has not been previously reported as a clinical test for vestibular loss. Here we used a VOG method that can track torsional eye position in real time to measure static OCR (vOCR) [9]. We measured vOCR in patients with known vestibular loss and in healthy controls to determine whether it can detect loss of otolith-ocular function. The vOCR results were compared with vestibular evoked myogenic potentials (VEMPs), a widely-used laboratory test of otolith function.
Methods
Participants
We enrolled 32 participants; 12 healthy controls with normal vestibular examination and no prior history of dizziness, 14 patients with unilateral vestibular loss (UVL) due to vestibular neuritis (two patients) or VIII nerve tumor resection (12 patients), and six patients with bilateral vestibular loss (BVL) (idiopathic). The vestibular loss was confirmed by calorics (unilateral weakness of 100%) in UVL patients with vestibular neuritis, and by rotational chair testing (absent VOR response at constant velocities of 60°/s and 240°/s) and calorics in all BVL patients. All patients had a chronic course (BVL average = 5 years, UVL average = 5.9 years). The average ages for healthy controls (3 female), UVL (5 female), and BVL patients (3 female) were 40, 53, and 60 years, respectively. The experiments were approved by the Johns Hopkins institutional review board, and informed written consent was obtained from all participants.
vOCR
VOG was captured with RealEyes xDVR goggles (Micromedical Technologies Inc., Chatham, IL). We used a torsion method based on iris pattern recognition in order to track and measure torsional eye position. This method operates in real time at 100 Hz with a noise level less than 0.1°. Further technical details about this method have been published previously [9]. Participants sat upright in dim light fixing on a red dot (diameter = 0.33°) on a CRT monitor (1280 px by 1024 px) 135 cm away. We used a molded bite-bar mounted on a rotary motor (Zaber Technologies Inc., Vancouver, BC) to passively move the head and measure head position. The head was upright for 60 s and then tilted laterally 30° towards the shoulder and remained tilted for 60 s. We tracked ocular torsion simultaneously and measured vOCR as the difference between the average ocular torsion in upright position and during head tilt (Figure 1). To avoid the effect of dynamic vOCR, we only used torsion values from 20–60 s after the head was tilted. The vOCR measurements were repeated three times for both right and left head tilts.
Figure 1.
(A) Example of vOCR to the right (blue trace), induced by a lateral head tilt to the left (red trace). (B) The zoom-in view shows the dynamic and static components of vOCR, which are the OCR during the head movement and the OCR during the static head tilt, respectively. The dynamic OCR consists of torsional slow phases away and quick phases toward the head tilt and is driven by activity from both the semicircular canals and the otoliths. The static OCR shows a change in ocular torsion in the opposite direction of head tilt and is driven by the otoliths. (C) Example of three repeated right and left vOCR measurements in one participant. Each color shows one repetition with the vOCR measurement from both eyes. There are minimal differences among the three repeated measurements.
Vemp
We performed ocular VEMP (oVEMP), a measure of utricular function, and also cervical VEMP (cVEMP), a measure of saccular function, in all participants. For VEMP measurements we used the methods previously described by Zuniga et al. [10]. To record oVEMP, electrodes were placed on the cheek, one ~3mm below the eye, and one 2 cm underneath the other electrode. The ground electrode was placed on the sternum. Participants lay with the upper body elevated 30° from earth-horizontal and fixed on a target 20° above the horizontal meridian. To record cVEMP, electrodes were placed at the midpoint of the sternocleidomastoid (SCM) muscle and on the sternoclavicular junction. The ground electrode was placed on the sternum. Participants first lay with upper bodies elevated at 30° from horizontal, and then lifted their heads up from the head rest to provide tonic SCM activity during stimulation and recording. Stimuli consisted of 500 Hz tone bursts (125 dB SPL with 4 ms pulses at 5 Hz) for both cVEMP and oVEMP, and sound clicks (105 dB SPL with 0.1 ms pulses at 5 Hz) and head taps (50 manual taps at mid forehead hairline with a reflex hammer and inertial microswitch trigger, Viasys Inc., Madison, WI, Care Fusion Inc., Dublin, OH) for oVEMP. The oVEMP was measured as the peak-to-peak amplitude between the n10 and p16 in the EMG waveform, and the cVEMP as the peak-to-peak amplitude between the p13 and n23. cVEMP values were normalized by the baseline EMG activity from 10 ms prior to the auditory stimulus. If a VEMP response was too small and the waveform could not be identified, we assigned the average background EMG activity at the expected response latency as the small VEMP value instead of using zero value for the VEMP response. This was to avoid having overlapping zero values from subjects with absent VEMPs in the correlation plots (see Figure 2).
Figure 2.
(A) Correlation between VEMP (right and left ears) and vOCR measurements (right and left head tilts). The click and tap oVEMPs show robust correlations. (B) The ROC curves for VEMP measurements from both ears and vOCR measurements from both head tilt directions (64 data points for each test) show similar area under the curve (AUC) for all tests, and thus similar performances for detecting vestibular loss. * The best vOCR threshold to detect vestibular loss was at 3°.
Data analysis
We used Pearson method to calculate correlations between VEMP and vOCR values. For comparisons, t-test and analysis of variance (ANOVA) were used with a significant p-value less than 0.05. We also calculated an asymmetry ratio for VEMP and vOCR measurements as the absolute difference between the values measured from both sides divided by the sum of the absolute values for each test. Therefore, the asymmetry ratio close to zero means minimum asymmetry in the measurements from the right and left side and the asymmetry ratio close to one means maximum asymmetry. To compare the performance of each test in discriminating between vestibular loss and normal function, we used receiver operator characteristic (ROC) analysis. In an ROC curve the sensitivity (true positive rate) is plotted against 1-Specificity (false positive rate) for different thresholds of a test parameter. Thus, each point on the ROC curve corresponds to the true and false positive rates at a particular decision threshold. The best threshold would yield a point in the upper left corner of the ROC space, representing 100% sensitivity (no false negatives) and 100% specificity (no false positives). A completely random result would give a point along a diagonal line on the ROC plot (line of no-discrimination). The area under the ROC curve (AUC) in ROC analysis is a measure of how well a parameter can distinguish between two diagnostic categories (disease vs normal).
Results
In all participants, there were minimal differences in vOCR among three repeated measurements for both head tilt directions (average standard deviation = 0.51°) and between both eyes (average standard deviation = 0.47°). vOCR was correlated with click and tap oVEMPs and also tone burst cVEMP across subjects (click oVEMP; R = 0.45; p = 0.0002, tap oVEMP; R = 0.51; p < 0.0001, and tone burst cVEMP; R = 0.37; p = 0.0029) (Figure 2(A)).
The click, tone burst, and tap oVEMPs showed significant differences between the side of vestibular loss (click oVEMP = 0.67 ± 0.17 µV; tap OVEMP = 3.54 ± 0.6 µV; tone burst oVEMP = 1.14 ± 0.31 µV) and the healthy side (click oVEMP = 2.73 ± 0.75 µV; tap oVEMP = 10.29 ± 1.1 µV; tone burst oVEMP = 3.05 ± 0.67 µV) (t-test; click oVEMP, p = 0.007, tap OVEMP, p = 0.0003, and tone burst oVEMP, p = 0.01). The tone burst cVEMP was also different between the side of vestibular loss (0.27 ± 0.07) and the healthy side (0.79 ± 0.1) (t-test; p = 0.0004). The vOCR did not differ between the side of vestibular loss (2.53 ± 0.31°) and the healthy side (2.8 ± 0.26°) (t-test; p = 0.59). The average vOCR asymmetry ratio in UVL, BVL, and healthy controls groups were 0.18 ± 0.04, 0.17 ± 0.05, and 0.14 ± 0.04, respectively. The average VEMP asymmetry ratios in UVL, BVL, and healthy control groups were, respectively, click oVEMP = 0.56 ± 0.09, 0.44 ± 0.12, 0.28 ± 0.07; tap oVEMP = 0.51 ± 0.06, 0.41 ± 0.14, 0.14 ± 0.03; tone burst oVEMP = 0.53 ± 0.08, 0.54 ± 0.15, 0.31 ± 0.08; and tone burst cVEMP = 0.56 ± 0.09, 0.62 ± 0.15, 0.30 ± 0.06 (Table 1). Overall, there were no significant correlations between vOCR and VEMP asymmetry ratios (vOCR and click oVEMP, R = −0.04, p = 0.84; vOCR and tap oVEMP, R = 0.07, p = 0.71; vOCR and tone burst oVEMP, R = 0.2, p = 0.28; and vOCR and tone burst cVEMP, R = 0.12, p = 0.53).
Table 1.
Mean vOCR and asymmetry ratio (AR) are shown with SEM for vOCR and VEMPs in healthy control and patient subgroups.
| vOCR | Click oVEMP | Tap oVEMP | Tone burst oVEMP | Tone burst cVEMP | |
|---|---|---|---|---|---|
| Healthy Control | |||||
| Both sides (mean) | 4.65 ± 0.38° | 4.30 ± 0.87 µV | 17.11 ± 2.5 µV | 3.42 ± 0.8 µV | 1.78 ± 0.3 |
| UVL | |||||
| Healthy side | 2.8 ± 0.26° | 2.73 ± 0.75 µV | 10.29 ± 1.1 µV | 3.05 ± 0.67 µV | 0.79 ± 0.1 |
| Side of loss | 2.53 ± 0.31° | 0.67 ± 0.17 µV | 3.54 ± 0.6 µV | 1.14 ± 0.31 µV | 0.27 ± 0.07 |
| BVL | |||||
| Both sides (mean) | 1.56 ± 0.44° | 0.84 ± 0.24 µV | 5.45 ± 2.8 µV | 1.02 ± 0.18 µV | 0.68 ± 0.2 |
| Asymmetry Ratio (AR) | |||||
| Healthy Control | 0.14 ± 0.04 | 0.28 ± 0.07 | 0.14 ± 0.03 | 0.31 ± 0.08 | 0.30 ± 0.06 |
| UVL | 0.18 ± 0.04 | 0.56 ± 0.09 | 0.51 ± 0.06 | 0.53 ± 0.08 | 0.56 ± 0.09 |
| BVL | 0.17 ± 0.05 | 0.44 ± 0.12 | 0.41 ± 0.14 | 0.54 ± 0.15 | 0.62 ± 0.15 |
AR values close to zero means minimum asymmetry between the right and left side measurements and AR values close to one means maximum asymmetry.
We used ROC analysis to compare the performance of each test in discriminating between vestibular loss and the normal function. Overall, VEMP and vOCR detected vestibular loss comparably, shown by AUC (Figure 2(B)). The best vOCR threshold to detect vestibular loss was at 3°, the point where the first derivative of the ROC curve is closest to one (Figure 2(B)). At this threshold, the sensitivity and specificity of vOCR in detecting vestibular loss were 80% and 81%, respectively.
The average vOCR values from both sides for healthy controls, UVL and BVL, were 4.65° ± 0.38, 2.67° ± 0.25 and 1.56° ± 0.44, respectively. These average values were significantly different (ANOVA; p < 0.0001), and the post-hoc analysis with Bonferroni method showed a difference between UVL patients and heathy controls (p < 0.0001), BVL patients and healthy controls (p < 0.0001), and a trend between UVL and BVL patients (p = 0.07). Thus, overall, the vOCR values from the healthy controls were significantly different from the patients with vestibular loss.
Discussion
We used a real-time VOG method to measure OCR as a clinical test of otolith function. Our findings show that vOCR (1) is reliable with repeated measurements, (2) detects loss of otolith-ocular function in patients with vestibular loss, and (3) is as sensitive as VEMPs for showing an abnormality with comparable ROC curves. Among VEMPs, the click and tap oVEMPs strongly correlated with vOCR, consistent with all being measures of otolith-ocular function.
This study was the first step to validate vOCR as a diagnostic test for detecting loss of otolith-ocular function. The results show ranges of vOCR values that can be associated with normal vestibular function and vestibular loss (Figure 3). The overlap between these groups reflects the extent of vestibular loss detectable using vOCR. Overall there was no difference in vOCR values between the right and left head tilts in either patients or healthy controls. Thus, although vOCR detected loss of otolith function, it could not identify the side of vestibular loss in UVL patients. This finding could be related to the effect of head tilt on otolith-ocular function from both ears as opposed to the auditory stimuli in VEMPs which are essentially monaural. Since all patients in this study had chronic loss, the symmetric vOCR might also be due to long-term adaptive changes that can modify vestibular responses on both sides [11,12]. In such cases the symmetry in vOCR values between right and left sides may be useful to differentiate between chronic central and peripheral lesions, as patients with cerebellar or brainstem lesions are more likely to have asymmetric OCR gains [13]. The asymmetry in OCR gains may also be present in patients with acute peripheral loss [6,11].
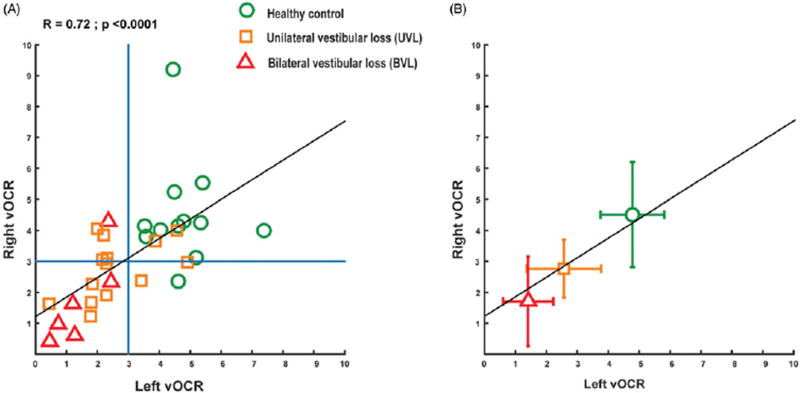
Figure 3.
Correlation between the right and left vOCR in patients with vestibular loss and healthy controls (black lines). (A) The blue lines show the detection threshold for vestibular loss obtained from the ROC curve (3°) and most patients with vestibular loss fall below this threshold. (B) The average vOCR values for BVL, UVL, and healthy controls. The error bars correspond to the standard deviation for the right and left vOCR.
The maneuver in vOCR is a simple head tilt and can be easily performed at the bedside. Here we chose 30° head tilt as it is large enough to induce OCR and yet it is a comfortable neck position to maintain. We used a bite bar to control and measure head position, but the head tilt can be controlled and monitored with other methods; for example, using a head position signal from VOG goggles. Future studies must examine the feasibility of such a method. In addition, it would be helpful to combine vOCR with vHIT as part of the same VOG platform. With such an approach, vOCR would detect loss of otolith function and vHIT would detect loss of semicircular canal function as well as the side of vestibular loss.
In conclusion, in the present study vOCR was used as a clinical test of otolith function. The results show vOCR can detect loss of otolith function with an accuracy comparable with VEMPs. Since vOCR is measured with a simple head tilt maneuver, it can potentially be used as a bedside test to examine otolith-ocular function.
Acknowledgments
Funding
This work was supported by National Institute on Deafness and Other Communication Disorders.
Footnotes
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leigh R, Zee D. The neurology of eye movements. 5. New York: Oxford University Press; 2015. [Google Scholar]
- 3.Bockisch CJ, Haslwanter T. Three-dimensional eye position during static roll and pitch in humans. Vision Res. 2001;41:2127–37. doi: 10.1016/s0042-6989(01)00094-3. [DOI] [PubMed] [Google Scholar]
- 4.Diamond SG, Markham CH. Ocular counterrolling as an indicator of vestibular otolith function. Neurology. 1983;33:1460–9. doi: 10.1212/wnl.33.11.1460. [DOI] [PubMed] [Google Scholar]
- 5.Kingma H, Stegeman P, Vogels R. Ocular torsion induced by static and dynamic visual stimulation and static whole body roll. Eur Arch Otorhinolaryngol. 1997;254:S61–S3. doi: 10.1007/BF02439726. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Priscoveanu A, Straumann D, Böhmer A, Obzina H. Vestibulo-ocular responses during static head roll and three-dimensional head impulses after vestibular neuritis. Acta Otolaryngol. 1999;119:750–7. doi: 10.1080/00016489950180379. [DOI] [PubMed] [Google Scholar]
- 7.Schworm HD, Ygge J, Pansell T, Lennerstrand G. Assessment of ocular counterroll during head tilt using binocular video oculography. Invest Ophthalmol Vis Sci. 2002;43:662–7. [PubMed] [Google Scholar]
- 8.Zingler VC, Kryvoshey D, Schneider E, Glasauer S, Brandt T, Strupp M. A clinical test of otolith function: static ocular counterroll with passive head tilt. Neuroreport. 2006;17:611–15. doi: 10.1097/00001756-200604240-00011. [DOI] [PubMed] [Google Scholar]
- 9.Otero-Millan J, Roberts DC, Lasker A, Zee DS, Kheradmand A. Knowing what the brain is seeing in three dimensions: a novel, noninvasive, sensitive, accurate, and low-noise technique for measuring ocular torsion. J Vis. 2015;15:11. doi: 10.1167/15.14.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuniga MG, Janky KL, Nguyen KD, Welgampola MS, Carey JP. Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol. 2013;34:121–6. doi: 10.1097/MAO.0b013e31827136b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez C, Borel L, Magnan J, Lacour M. Torsional optokinetic nystagmus after unilateral vestibular loss: asymmetry and compensation. Brain. 2005;128:1511–24. doi: 10.1093/brain/awh504. [DOI] [PubMed] [Google Scholar]
- 12.Devèze A, Montava M, Lopez C, Lacour M, Magnan J, Borel L. Vestibular compensation following vestibular neurotomy. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:197–203. doi: 10.1016/j.anorl.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Chandrakumar M, Blakeman A, Goltz HC, Sharpe JA, Wong AM. Static ocular counterroll reflex in skew deviation. Neurology. 2011;77:638–44. doi: 10.1212/WNL.0b013e3182299f71. [DOI] [PMC free article] [PubMed] [Google Scholar]