Abstract
Background
Individuals with cerebral palsy have less lean body mass, greater relative adiposity, and lower fitness and physical activity participation; and yet, the prevalence of age-related multimorbidity in this population has yet to be established.
Purpose
To examine the prevalence of lifestyle-related chronic conditions and multimorbidity in a sample of middle-aged adults with cerebral palsy.
Methods
A clinic-based sample of middle-aged adults with cerebral palsy was examined using Electronic Medical Records Search Engine (EMERSE) software. Our cohort included n= 435 individuals aged 40–60 years old, with an ICD-9/10-CM Diagnosis Code for cerebral palsy. Prevalence of 12 chronic conditions were evaluated, including existing diagnoses or historical record of: osteopenia/osteoporosis, myocardial infarction, stroke, coronary artery disease, impaired glucose tolerance/type 2 diabetes, other cardiovascular conditions, rheumatoid arthritis, osteoarthritis, asthma, emphysema, pre-hypertension/hypertension, and hyperlipidemia. Multivariate logistic models were used to estimate adjusted mulitmorbidity (i.e., ≥2 chronic conditions), adjusting for age, sex, smoking status, obesity, and Gross Motor Function Classification System (GMFCS).
Results
There were 137 unique multimorbidity combinations. Multimorbidity was significantly more prevalent among obese versus non-obese individuals for both GMFCS I–III (75.8% vs. 53.6%) and GMFCS IV–V (79.0% vs 64.2%), but was also significantly higher in non-obese individuals with GMFCS IV–V (64.2%) compared to individuals with non-obese individuals with GMFCS I–III (53.6%). Both obesity status (OR: 2.20; 95% CI 1.32–2.79) and the GMFCS IV–V category (OR: 1.81; 95% CI 1.32–3.68) were independently associated with multimorbidity.
Conclusion
Middle-aged adults with cerebral palsy have high estimates of multimorbidity, and both obesity and higher GMFCS levels are independently associated with greater risk.
Keywords: cerebral palsy, multimorbidity, obesity, diabetes, osteoporosis, hypertension
Introduction
Cerebral Palsy is the most common pediatric-onset physical disability, with an incidence between 2–3 cases per 1,000 births.1,2 Cerebral palsy is caused by an insult to or malformation of the developing brain that affects motor control centers and leads to alterations in growth and development.3 Although by definition, cerebral palsy is a non-progressive disorder, it affects overall health and especially mobility throughout the lifespan. Despite the fact that many individuals with mild to moderate cerebral palsy may experience near-normal life expectancies,4 no standard of follow-up exists for individuals after the age of 18 years, and there is very little clinical focus on individuals with cerebral palsy who live into middle and later adulthood.5 The vast majority of research focuses on interventions for children and adolescents with cerebral palsy, and little is known about long-term health trajectories among adults in this population.
However, as more individuals with cerebral palsy live longer lives, it is becoming increasingly evident that they may be subject to secondary health risks due to exaggerated sedentary behavior and excess adipose tissue.5,6 Indeed, there is mounting evidence that individuals with cerebral palsy have lower fitness, less muscle mass, diminished bone density, neuromuscular inefficiency and reduced functional reserve throughout adulthood.7–13 These factors place middle-aged adults with cerebral palsy at a heightened risk for numerous secondary health concerns such as frailty and obesity-related cardiometabolic diseases.14 Mortality records demonstrate a 2- to 3- fold increase of coronary artery disease among aging adults with cerebral palsy as compared with typically-developed adults.15 In order to facilitate the development of appropriate prevention strategies and allotment of treatment resources, it is critical to elucidate the extent to which chronic diseases are elevated among aging adults with cerebral palsy, as well as to identify which factors are associated with heightened risk for multimorbidity (i.e., presence of multiple chronic diseases).
A recent report of the Medical Expenditure Panel Survey demonstrated a higher risk for various lifestyle-related chronic diseases in adults with cerebral palsy of all ages.16 Despite the novelty of those findings, the data were derived from primarily high-functioning adults with cerebral palsy, and obtained via self-report from a household member. Thus, what remains to be determined is the extent to which multiple chronic conditions are present in a heterogeneous clinical sample of middle-aged adults with cerebral palsy. The objectives of this study were to build upon previous findings by analyzing correlates and types of multimorbidity in an established clinical registry of middle-aged adults with cerebral palsy. We hypothesized that adults with cerebral palsy with greater mobility restrictions would have a high prevalence of multiple chronic diseases such as diabetes, asthma, hypertension, stroke, emphysema, joint pain, and arthritis, as well as increased risk factors, such as obesity, and lipid profiles.
Methods
Subject Identification
A cohort query tool (Data Direct: https://datadirect.med.umich.edu) was used to identify adults with cerebral palsy that had clinical appointments at the University of Michigan Health System. Inclusion criteria included a diagnosis of cerebral palsy using ICD 9 code 343.x or ICD 10 codes G80, age 18 and older, and having an encounter with the medical system (inpatient or outpatient) during a 5 year period of 01/01/2011 to 12/31/2015. Patients were excluded if they did not have a definitive diagnosis of cerebral palsy. Out of the 1,883 eligible adults with cerebral palsy aged ≥18 years, a subgroup of n=435 subjects aged 40–60 years was extracted for this study. Approval to conduct this study was received by the Institutional Review Board, and all analyses were performed in 2016.
Electronic Medical Record Search Engine
We annotated the dataset using the Electronic Medical Records Search Engine (EMERSE) software. This internally developed web application provides an interface for searching the electronic medical record with a bundle of word iterations and phrases for variables of interest.17 Specifically, information for each variable was collected by developing a bundle of key words (including synonyms, abbreviations, and common misspellings) and then using the search functions of EMERSE to identify patient charts for those key words. When a key word was discovered in a patient chart using EMERSE, the chart was manually examined in order to confirm that the word was used to describe a diagnosis or information pertaining to the subject (i.e. not a family member).
Demographic Data
Demographic data included race, age, and gender. Data for these variables were collected directly from the subject list in EMERSE.
Cerebral Palsy Phenotype Data
Cerebral palsy phenotype data included distribution and Gross Motor Function Classification System (GMFCS).18 Distribution refers to the degree of paralysis (i.e. quadriplegic, hemiplegic, diplegic). For many subjects, distribution data were collected directly from patient charts. For other subjects, distribution was determined by examining history and physical exam information in patient charts. GMFCS refers to the 5-level grading scale that assesses gross motor function activity limitations in individuals with cerebral palsy. Distinctions between levels are based on functional abilities, the need for assistive technology, and, to a lesser extent, quality of movement. Individuals with GMFCS level I generally walk without restrictions but may have more limited advanced motor skills. GMFCS level V describes individuals who require extensive assistive technology and physical assistance.19 For some subjects, GMFCS was collected directly from patient charts. For many subjects, GMFCS was determined by examining history and physical exam information in patient charts. GMFCS scores I–V are listed in Table 1. We stratified GMFCS into 2 categories for our data collection and analysis, in order to reflect higher versus lower functional status: (1) GMFCS I–III and (2) GMFCS IV–V.
Table 1.
Descriptive characteristics of the study population by gender.
| Men | Women | p-value | |
|---|---|---|---|
|
|
|
||
| n=201 | n=234 | ||
| Age, years | 48.46 (5.75) | 49.83 (5.64) | 0.01 |
| Body Mass Index (BMI), kg/m2 | 25.61 (7.38) | 28.71 (9.30) | <0.001 |
| Obesity (BMI >30), % | 15.2 | 34.8 | <0.001 |
| Smoker, % | 11.0 | 12.0 | 0.74 |
| GMFCS Level | |||
| Level I, % | 13.5 | 12.4 | 0.91 |
| Level II, % | 18.1 | 16.8 | 0.89 |
| Level III, % | 23.4 | 24.4 | 0.63 |
| Level IV, % | 23.9 | 21.1 | 0.22 |
| Level V, % | 21.1 | 25.3 | 0.88 |
| CP Subtype | |||
| Quadriplegia, % | 58.9 | 51.1 | 0.22 |
| Hemiplegia, % | 26.8 | 24.8 | 0.73 |
| Diplegia, % | 14.3 | 24.1 | 0.04 |
Chronic Conditions
Clinical data were included for existing diagnosis or historical record of: (1) osteopenia/osteoporosis, (2) myocardial infarction, (3) stroke, (4) coronary artery disease, (5) impaired glucose tolerance/type 2 diabetes, (6) various other cardiovascular conditions (e.g., heart valve disorders, peripheral artery disease, aortic aneurysm, heart failure, etc.), (7) rheumatoid arthritis, (8) osteoarthritis, (9) asthma, (10) emphysema, (11) pre-hypertension/hypertension, and (12) hyperlipidemia. In addition to using the EMERSE search functions, certain variables required additional data collection. For blood pressure, the most recent measurement was used. The blood pressure data were then organized into categories: normal (SBP ≥90 mmHg but <120 mmHg, or DBP ≥60 mmHg but < 80 mmHg), pre-hypertension (SBP ≥120 mmHg but <140 mmHg, or DBP ≥80 mmHg but <90 mmHg), or hypertension (SBP ≥140 mmHg or DBP ≥90 mmHg). For hyperlipidemia, in addition to using the EMERSE search function, data were collected by searching for total cholesterol measurements and/or triglyceride measurements above 240 mg/dL or 200 mg/dL, respectively. Lastly, since perinatal strokes can cause cerebral palsy, and our goal was to analyze chronic conditions in adults with cerebral palsy, a clinical history of stroke was only included in the analyses if it occurred at age 18 or older.
Body mass index (BMI) was recorded from the most recent measurement, and calculated as weight divided by height in meters squared (kg/m2). BMI measurements were then organized into categories: underweight (BMI <18.5), normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), and obese (BMI ≥30.0).
Multimorbidity
Multimorbidity was defined as the presence of at least 2 chronic conditions among a list of the 12 aforementioned chronic conditions. These conditions were selected in accordance with guidance from the literature pertaining to older adults and adults with disabilities,20,21 as well as based on potential burden to physical functioning, and availability and reliability of the diagnosis within our clinical data.
Statistical Analyses
Descriptive characteristics are provided as means, standard errors, and percentages. Differences in these characteristics were tested using two samples t-tests and chi-squared tests for continuous and categorical variables, respectively. A similar strategy was used to test differences for outcomes across GMFCS categories. To assess the odds of multimorbidity in the sample, a multivariate logistic regression modeling approach was used. All models were adjusted for age, gender, smoking status, obesity, and GMFCS category (reference: GMFCS I–III). All statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC) with 2-sided 95% confidence intervals to determine significance.
Results
Descriptive characteristics are presented as means, standard deviations, and percentages by gender in Table 1. Women with cerebral palsy were older, had higher BMIs, and a greater prevalence of obesity (all p=0.01). There were no significant differences in GMFCS distribution or cerebral palsy subtype distribution between women and men.
Differences by Gender and GMFCS
For the full sample, the mean number of chronic condition was 1.9 (SD = 1.5), with pre-hypertension/hypertension as the most prevalent individual condition (50%), followed by osteopenia/osteoporosis (40%), osteoarthritis (27%), and hyperlipidemia (19%). Women with cerebral palsy had higher BMIs and prevalences of obesity within both GMFCS categories (GMFCS I–III and GMFCS IV–V), compared to men with cerebral palsy. Women with GMFCS I–III had higher prevalences of osteopenia/osteoporosis (40.2% vs 22.3%) and asthma (26.8% vs 16%) than men with GMFCS I–III. Men with GMFCS IV–V had a higher prevalence of stroke (5.2% vs 0%) than women with GMFCS IV–V; however, women with GMFCS IV–V had higher prevalences of diabetes (14.7% vs 1.3%), pre-hypertension/hypertension (58.1% vs 36%), rheumatoid arthritis (6.2% vs 1.3%), and osteoarthritis (30.9% vs 20.8%) than men with GMFCS IV–V (Table 2).
Table 2.
Demographic characteristics chronic disease prevalence by gender and GMFCS category.
| GMFCS I–III | GMFCS IV–V | |||
|---|---|---|---|---|
|
|
|
|||
| Men | Women | Men | Women | |
|
|
|
|
|
|
| n=94 | n=112 | n=77 | n=97 | |
| Age, years | 47.62 (5.84) | 49.85 (5.67)* | 49.87 (5.58)† | 50.37 (5.49) |
| Body Mass Index (BMI), kg/m2 | 27.18 (5.93)† | 30.66 (8.24)*† | 23.84 (7.77) | 27.19 (10.55)* |
| Obesity (BMI >30), % | 18.3 | 44.1*† | 13.2 | 29.5* |
| Smoker, % | 16.0† | 14.3 | 6.5 | 9.3 |
|
| ||||
| Dependent Variables: Prevalence, % | ||||
|
| ||||
| a Diabetes | 10.8† | 15.2 | 1.3 | 14.7* |
| Osteopenia or Osteoporosis | 22.3 | 40.2* | 55.8† | 58.8† |
| Myocardial Infarction | 3.2 | 3.6 | 3.9 | 4.1 |
| Stroke | 4.3 | 5.4† | 5.2* | 0.0 |
| Coronary Artery Disease | 1.1 | 4.5 | 0.0 | 2.1 |
| Pre-hypertension or Hypertension | 53.3† | 51.8 | 36.0 | 58.1* |
| Other Cardiovascular Disease | 12.8 | 15.2 | 18.2 | 16.5 |
| Rheumatoid Arthritis | 4.3 | 1.8 | 1.3 | 6.2*† |
| Osteoarthritis | 30.9† | 33.9 | 20.8 | 30.9* |
| Emphysema | 2.1 | 1.8 | 5.2 | 2.1 |
| Asthma | 16.0 | 26.8* | 19.5 | 21.7 |
| Hyperlipidemia | 24.5† | 17.9 | 15.6 | 21.7 |
Significant difference between men and women in the equivalent GMFCS category.
Significant difference between GMFCS I–III vs IV–V within the same sex: Denoted as group with higher risk.
Diabetes: Impaired Glucose Tolerance or Diabetes
Men with GMFCS I–III had higher prevalences of diabetes (10.8% vs 1.3%), pre-hypertension/hypertension (53.3% vs 36.0%), osteoarthritis (30.9% vs 20.8%), and hyperlipidemia (24.5% vs 15.6%) than men with GMFCS IV–V; however, men with GMFCS IV–V had a higher prevalence of osteopenia/osteoporosis (55.8%) than men with GMFCS I–III (22.3%). Women with GMFCS I–III had a higher prevalence of stroke (5.4%) than women with GMFCS IV–V (0%); however, women with GMFCS IV–V had higher prevalences of osteopenia/osteoporosis (58.8% vs 40.2%) and rheumatoid arthritis (6.2% vs 1.8%) than women with GMFCS I–III (Table 2).
Multimorbidity
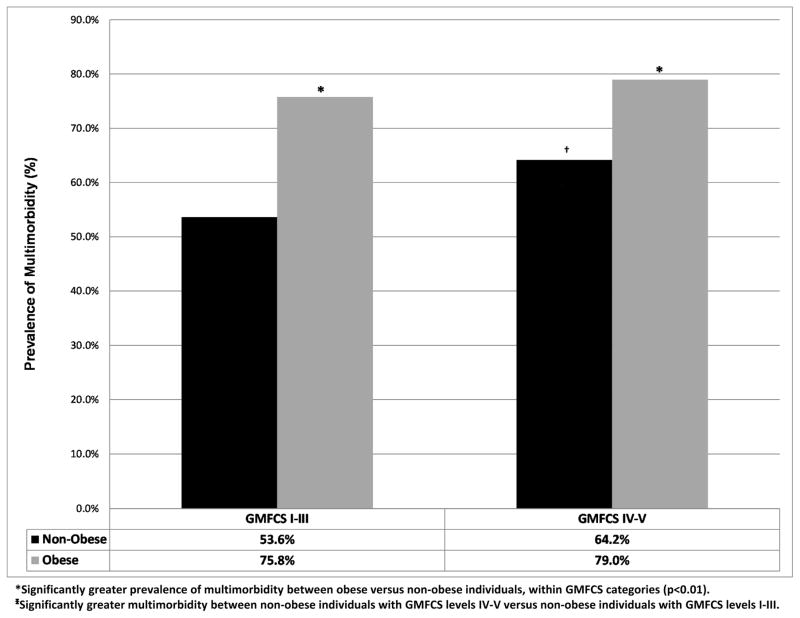
Prevalent multimorbidity was found in 252 patients (57.8%). Moreover, we found 137 unique multimorbidity combinations, and the most prevalent combinations comprising multimorbidity were: (a) pre-hypertension/hypertension and osteopenia/osteoporosis (n = 84); (b) osteoarthritis and osteopenia/osteoporosis (n = 73); (c) pre-hypertension/hypertension and osteoarthritis (n = 54); and (d) pre-hypertension/hypertension and asthma (n = 36). The prevalence of multimorbidity was significantly higher among obese versus non-obese individuals for both GMFCS I–III (75.8% vs. 53.6%) and GMFCS IV–V (79.0% vs 64.2%), but was also significantly higher in non-obese individuals with GMFCS IV–V (64.2%) compared to individuals with GMFCS I–III (53.6%), respectively (Figure 1) (all p<0.05).
Figure 1.
Prevalence of multimorbidity for the entire cohort stratified by obesity and GMFCS category.
Of the 435 adults with cerebral palsy in the cohort, n=381 had complete data for the multivariate analysis. After adjusting for gender, age, and smoking status, both obesity status (OR: 2.20; 95% CI 1.32–2.79) and the GMFCS IV–V category (OR: 1.81; 95% CI 1.32–3.68) were significantly associated with multimorbidity (Table 3). Age (OR: 1.05; 95% CI 1.01–1.10) and smoking status (OR: 1.18; 95% CI 1.42–7.5) were also significantly associated with multimorbidity.
Table 3.
Multiple logistic regression models for independent predictors of multimorbidity status in all adults with cerebral palsy.
| Model Predictor(s) | Estimate* | SE | Odds Ratio | 95% CL | Pr > ChiSq | |
|---|---|---|---|---|---|---|
|
|
||||||
| aMultimorbidity | ||||||
| Sex (Reference: Males) | 0.20 | 0.23 | 1.22 | 0.77–1.92 | 0.40 | |
| Age | 0.05 | 0.02 | 1.05 | 1.01–1.09 | 0.02 | |
| Smoking Status (Reference: Non-Smoker) | 1.18 | 0.43 | 3.26 | 1.42–7.5 | <0.01 | |
| Obesity Status (Reference: Not Obese) | 0.78 | 0.28 | 2.17 | 1.24–3.79 | <0.01 | |
| GMFCS Category (Reference: GMFCSI-III) | 0.28 | 0.09 | 1.33 | 1.12–1.57 | <0.01 | |
≥2 chronic conditions
Discussion
The principal findings of the current study were that middle-aged (40–60 years old) individuals with cerebral palsy that had worse functional impairments or were obese had a significantly higher prevalence of multimorbidity than those with better functional impairements or were not obese. Multimorbidity, the coexistence of 2 or more chronic conditions, has become increasingly burdensome through the third phase of the epidemiologic transition, which is characterized by a compression of mortality rates combined with an expansion of the older-adult population.
Indeed, the overall prevalence of multimorbidity in our cohort was nearly 60%, which is only slightly lower than that recently reported for adults 65 years old and older (67%) without cerebral palsy,22 and significantly higher than that reported for middle-aged men (40–49 years: 20.4%; 50–59 years: 38.1%) and women (40–49 years: 19.7%; 50–59 years: 35.8%) without cerebral palsy.23 However, we caution the comparability between studies, because there is no current consensus on the number and type of conditions to be considered, the source of diagnoses, and the window of capture for chronic disease clustering. The results showing substantially increased adjusted odds of multimorbidity among obese individuals raise critical questions about preventable health complications in cerebral palsy through the lifespan. Previous reports have demonstrated higher rates of lifestyle-related chronic conditions in adults with cerebral palsy;16,24 however, this is the first study to examine the predictors of multimorbidity in a heterogeneous, clinic-based sample of middle-aged adults with cerebral palsy. The three most prevalent combinations of multimorbidity were pre-hypertension/hypertension and osteopenia/osteoporosis; osteoarthritis and osteopenia/osteoporosis; pre-hypertension/hypertension and osteoarthritis; and pre-hypertension/hypertension and asthma. We and others have proposed rationale for population surveillance to better understand age- and lifestyle-related chronic disease risk in the cerebral palsy population.5,11,14 Thus, future efforts are needed not only to better understand the healthcare utilization associated with these coexisting conditions, but more importantly, to identify early-onset, modifiable factors that potentiate chronic health risks in this population.
Increased sedentary behavior, decreased muscle volumes, and weakness have been identified even in ambulatory children with cerebral palsy25–27 which may track into adulthood, and thus result in an accumulation for chronic disease risk over a lifetime. Despite the widespread support of activity participation as an instrumental therapeutic tool for children with cerebral palsy to prevent secondary conditions,28 to date there is little evidence to explicate the value of health-related physical activity to confer benefits for cardiometabolic health, musculoskeletal integrity, mental health, or functional preservation through the lifespan.
As with all cross-sectional investigations, a limitation of this study is the inability to unravel the cause-effect relationship between exposures and outcomes. Whether having worse functional limitations in cerebral palsy “causes” an increased risk for multimorbidity, or if secondary conditions such as pulmonary dysfunction and joint pain are drivers of greater sedentary lifestyles and higher risk of chronic diseases, is an interesting and complex topic. Secondly, these results were derived from a single hospital/clinic-based sample, which may have excluded healthy, symptom-free individuals. Lastly, we did not include a matched control group for comparison of prevalence estimates, as this was not the primary aim of the study; and yet, formal statistical comparison with an age-, gender- and BMI-matched cohort of typically developed adults would provide subsequent support for increased risk in this population. Subsequent research is also needed using longitudinal data sources such as claims data to examine the patterns of healthcare utilization, chronic disease risk, and survival among individuals with cerebral palsy, with a priority focus on how these outcomes fluctuate during transition into and throughout adulthood.
Although previous research has discounted the use of BMI as an indicator of chronic disease in cerebral palsy,29 these current findings revealed that obese adults (i.e., as determined by BMI ≥ 30) with cerebral palsy had significantly higher prevalence of multimorbidity. BMI cutoffs are known to have excellent specificity (i.e. accurately classify individuals who are not obese, as not obese), and yet sensitivity is very poor, especially among adults with physical disabilities.30 Therefore, many individuals with cerebral palsy who are normal or overweight according to BMI may actually be at increased risk for cardiometabolic abnormalities and multimorbidity. Thus, it is important to gain greater understanding of the drivers of weight gain and obesity in cerebral palsy, in order to improve early clinical screening, provide better information to patients and their caregivers, and for future design of targeted, preventive medicine interventions. Recognizing the burden of multiple chronic conditions for health through the lifespan should provide rationale for primary care practitioners to adopt a patient-centered focus on multimorbidity treatment and prevention in cerebral palsy care.
Conclusions
Middle-aged adults with cerebral palsy have a high prevalence of multimorbidity, and these estimates are greater for individuals with GMFCS IV-V, as well as for obese individuals. Future translational research and surveillance efforts are absolutely vital to better characterize the etiology and temporal sequence of these multi-morbid conditions in cerebral palsy, and more importantly, to inform the development of early health-related physical activity and nutrition interventions that may lessen the burden of preventable diseases through the lifespan.
Acknowledgments
Funding Sources
Nicole Cremer is funded by the University of Michigan Medical School (UMMS) Summer Biomedical Research Program. Dr. Peterson is funded by the National Institutes of Health (NIH) (1KO1 HD074706) and the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) (90IF0102-01).
Footnotes
Conflict of Interest:
Nicole Cremer has no financial disclosures.
Edward A. Hurvitz has no financial disclosures.
Mark D. Peterson has no financial disclosures.
Role of the Sponsors: The funders had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
All authors had access to the data and a role in writing the manuscript.
References
- 1.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clinics in perinatology. 2006;33(2):251–267. doi: 10.1016/j.clp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 3.Green LB, Hurvitz EA. Cerebral palsy. Physical medicine and rehabilitation clinics of North America. 2007;18(4):859–882. vii. doi: 10.1016/j.pmr.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Hutton JL. Outcome in cerebral palsy: life-expectancy. Paediatrics and Child Health. 2008;18(9):419–422. [Google Scholar]
- 5.Peterson MD, Gordon PM, Hurvitz EA, Burant CF. Secondary muscle pathology and metabolic dysregulation in adults with cerebral palsy. Am J Physiol Endocrinol Metab. 2012;303(9):E1085–1093. doi: 10.1152/ajpendo.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson MD, Zhang P, Haapala HJ, Wang SC, Hurvitz EA. Greater Adipose Tissue Distribution and Diminished Spinal Musculoskeletal Density in Adults With Cerebral Palsy. Arch Phys Med Rehab. 2015;96(10):1828–1833. doi: 10.1016/j.apmr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson MD, Lukasik L, Muth T, et al. Recumbent cross-training is a feasible and safe mode of physical activity for significantly motor-impaired adults with cerebral palsy. Archives of physical medicine and rehabilitation. 2013;94(2):401–407. doi: 10.1016/j.apmr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreau NG, Li L, Geaghan JP, Damiano DL. Fatigue resistance during a voluntary performance task is associated with lower levels of mobility in cerebral palsy. Archives of physical medicine and rehabilitation. 2008;89(10):2011–2016. doi: 10.1016/j.apmr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampe R, Grassl S, Mitternacht J, Gerdesmeyer L, Gradinger R. MRT-measurements of muscle volumes of the lower extremities of youths with spastic hemiplegia caused by cerebral palsy. Brain & development. 2006;28(8):500–506. doi: 10.1016/j.braindev.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan KJ. Osteoporosis in adults with cerebral palsy. Developmental medicine and child neurology. 2009;51(Suppl 4):38–51. doi: 10.1111/j.1469-8749.2009.03432.x. [DOI] [PubMed] [Google Scholar]
- 11.Shortland A. Muscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders? Developmental medicine and child neurology. 2009;51(Suppl 4):59–63. doi: 10.1111/j.1469-8749.2009.03434.x. [DOI] [PubMed] [Google Scholar]
- 12.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Developmental medicine and child neurology. 2005;47(5):329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 13.Stackhouse SK, Stapleton MR, Wagner DA, McClure PW. Voluntary activation of the infraspinatus muscle in nonfatigued and fatigued states. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons … [et al.] 2010;19(2):224–229. doi: 10.1016/j.jse.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Peterson MD, Gordon PM, Hurvitz EA. Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(2):171–182. doi: 10.1111/j.1467-789X.2012.01052.x. [DOI] [PubMed] [Google Scholar]
- 15.Strauss D, Cable W, Shavelle R. Causes of excess mortality in cerebral palsy. Developmental medicine and child neurology. 1999;41(9):580–585. doi: 10.1017/s001216229900122x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic Conditions in Adults With Cerebral Palsy. JAMA : the journal of the American Medical Association. 2015;314(21):2303–2305. doi: 10.1001/jama.2015.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) J Biomed Inform. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum P, Palisano R, Bartlett D, Galuppi B, Russel D. Development of the Gross Motor Functional Classification System for cerebral palsy. Developmental Medicine and Child Neurology. 2008;50:249–253. doi: 10.1111/j.1469-8749.2008.02045.x. [DOI] [PubMed] [Google Scholar]
- 19.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revided Gross Motor Function Classification System. Developmental medicine and child neurology. 2008;(50):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed]
- 20.Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity Combinations and Disability in Older Adults. J Gerontol a-Biol. 2016;71(6):823–830. doi: 10.1093/gerona/glw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salive ME. Multimorbidity in Older Adults. Epidemiologic reviews. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 22.Jindai K, Nielson C, Vorderstrasse B, Quiñones A. Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005–2012. Prev Chronic Dis. 2016;13(E151) doi: 10.5888/pcd13.160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population: patterns by age, sex, and race/ethnicity. Mayo Clin Proc. 2014;89(10):1336–1349. doi: 10.1016/j.mayocp.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinh A, Wong P, Fahey MC, et al. Musculoskeletal and Endocrine Health in Adults With Cerebral Palsy: New Opportunities for Intervention. The Journal of clinical endocrinology and metabolism. 2016;101(3):1190–1197. doi: 10.1210/jc.2015-3888. [DOI] [PubMed] [Google Scholar]
- 25.Obeid J, Balemans AC, Noorduyn SG, Gorter JW, Timmons BW. Objectively measured sedentary time in youth with cerebral palsy compared with age-, sex-, and season-matched youth who are developing typically: an explorative study. Phys Ther. 2014;94(8):1163–1167. doi: 10.2522/ptj.20130333. [DOI] [PubMed] [Google Scholar]
- 26.Noble JJ, Charles-Edwards GD, Keevil SF, Lewis AP, Gough M, Shortland AP. Intramuscular fat in ambulant young adults with bilateral spastic cerebral palsy. BMC musculoskeletal disorders. 2014;15:236. doi: 10.1186/1471-2474-15-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan JM, Gormley J. Associations of sedentary behaviour, physical activity, blood pressure and anthropometric measures with cardiorespiratory fitness in children with cerebral palsy. PloS one. 2015 doi: 10.1371/journal.pone.0123267. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler EG, Kolobe TH, Damiano DL, et al. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: section on pediatrics research summit proceedings. Phys Ther. 2007;87(11):1495–1510. doi: 10.2522/ptj.20060116. [DOI] [PubMed] [Google Scholar]
- 29.Peterson MD, Haapala HJ, EAH Predictors of cardiometabolic risk among adults with cerebral palsy. Arch Phys Med Rehabil. 2012;93(5):816–821. doi: 10.1016/j.apmr.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Peterson MD, Al Snih S, Stoddard J, Shekar A, Hurvitz EA. Obesity misclassification and the metabolic syndrome in adults with functional mobility impairments: Nutrition Examination Survey 2003–2006. Preventive medicine. 2014;60:71–76. doi: 10.1016/j.ypmed.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]