Abstract
The French heartworm Angiostongylus vasorum is found in European red fox (Vulpes vulpes) and dog populations, where it appears to be spreading geographically. Once introduced into new areas, it establishes in local fox populations, typically to over 50% prevalence in a few years. High susceptibility and constant excretion of first stage larvae (L1) by the definitive hosts are prerequisites for sustaining high parasite biomass in a particular habitat. The present study explores the hypothesis that repeated ingestion of gastropods in nature will result in accumulation of adult worms and elevated excretion of L1 in feces. Experimentally infected foxes were subsequently inoculated via stomach tube once (9 weeks post initial inoculation) or twice (9 and 13 weeks post inoculation (wpi)) with 100 third stage A. vasorum larvae (L3) previously isolated from aquatic snails infected with L1 from a naturally infected dog. Despite large variation in fecal larval excretion for the individual animals within the groups, excretion of L1 was significantly higher in foxes twice inoculated as compared to foxes inoculated only once. With an outlier in the once inoculated group removed, excretion became significantly higher in the three times inoculated group. Establishment of adult worms varied and only a trend to higher worm burdens was found in the group of foxes inoculated three times. However, this became significant with the same single outlier removed. Overall, it appears that protective immunity to A. vasorum does not appear to occur in V. vulpes with animals exhibiting high infection intensities without obvious clinical signs. The increasing larval excretion in foxes being repeatedly exposed to A. vasorum L3 support the hypothesis that foxes under natural conditions may repeatedly ingest infected gastropods and remain a source of environmental contamination for several months, potentially contributing to the establishment of endemic foci through increasing L1 excretion.
Keywords: Foxes, Angiostrongylus vasorum, Experimental inoculation, Immunology, Larval excretion, Worm burden
Graphical abstract
Highlights
-
•
Foxes were experimentally infected with 100 Angiostrongylus vasorum larvae (L3).
-
•
Two groups were later challenged once or twice.
-
•
Increasing larval counts linked to time post infection and repeated inoculation.
-
•
Data suggests role of foxes in rapid establishment of the parasite in new areas.
1. Introduction
The parasitic nematode Angiostrongylus vasorum infects dogs and other canids, in particular the red fox (Vulpes vulpes). Adult worms are located in the pulmonary arteries and the right ventricle of the heart producing eggs that are carried by the arterioles to the lungs. In the lungs, the eggs hatch and the first stage larvae (L1) penetrate the capillaries and alveoli, enter the bronchial system and are subsequently coughed up, swallowed and excreted in feces. Gastropod intermediate hosts become infected after ingesting L1 in feces and third stage larvae (L3) subsequently develop in their tissue within 16–18 days (Guilhon and Cens., 1973). Life-cycle completion occurs when a canid definitive host ingests infective snails or slugs (Guilhon, 1963, Rosen et al., 1970, Guilhon and Cens, 1973), or perhaps ingests L3 released from gastropods (Barcante et al., 2003). The prepatant period in dogs is around 40–60 days (Bolt et al., 1994). Frogs and birds may become intermediate and paratenic hosts (Bolt et al., 1993, Mozzer and Lima, 2015).
Genetic analyses have identified shared parasite haplotypes between different end hosts such as dogs, foxes and coyotes (Jefferies et al., 2010). The frequent occurrence of canine angiostrongylosis in areas with contemporaneous and high (up to 50% and more) prevalence in wild foxes (Jeffery et al., 2004, Saeed et al., 2006, Al-Sabi et al., 2013, Taylor et al., 2015) support the important role of wildlife, particularly foxes, in the epidemiology of the parasite for the establishment of endemic foci. Several studies in dogs have contributed with information on clinical (Chapman et al., 2004, Schnyder et al., 2010), diagnostic (Schnyder et al., 2015), immunological/hematological (Cury et al., 2002, Cury et al., 2005), pathological (Bourque et al., 2008, Schnyder et al., 2010) and epidemiological (Morgan et al., 2009) aspects as well as response to anthelmintic treatment (Conboy, 2004, Willesen et al., 2006, Willesen et al., 2007, Willesen et al., 2009, Schnyder et al., 2009). In contrast, notwithstanding their important role as reservoirs, little is known from foxes.
The geographic range of A. vasorum is apparently increasing in Europe, with new autochthonous infections being reported from areas not previously known to be endemic, e.g. Sweden (Ablad et al., 2003), the Netherlands (van Doorn et al., 2009), Poland (Demiaszkiewicz et al., 2014) and Slovakia (Miterpáková et al., 2014), although with predicted climatic suitability (Morgan et al., 2009). The first evidence for the endemic presence of A. vasorum in Europe originated from France (Serres, 1854), Ireland (Roche and Kelliher, 1968) and the UK (Simpson and Neal, 1982). In Denmark, prior to 1991, necropsy findings demonstrated infection in just 2 dogs that had visited France. Two years later, a considerable number of Danish dogs (which had never left Denmark) in the north of Copenhagen were found infected and exhibiting clinical signs. It was therefore concluded that an endemic focus was present. A field survey conducted after this finding revealed for the first time A. vasorum infection in wild Danish foxes (Bolt et al., 1992), confirming the establishment of important endemic foci. These findings support the possibility that the movement of infected dogs may facilitate the introduction of the parasite to new areas, infecting local gastropod populations and leading to a rapid increase in prevalence. Considering the important role of the fox in the maintenance of A. vasorum and the apparent propensity of the parasite to be transmitted between dogs and foxes (via gastropods), it would be useful from an epidemiological perspective to determine the course of A. vasorum infections in foxes. Repeated ingestion of L3 is likely to occur in foxes but it is not possible to quantify these infections from cross-sectional wildlife studies. We have recently shown that severe clinical signs in response to A. vasorum infections are apparently lacking in foxes. Foxes also showed limited age resistance and their infections are long-lasting with apparently restricted impact on their health status (Webster et al., 2017). This could indicate a high level of immunological tolerance in this species and may consequently result in cumulative infections, thus contributing to the parasite's rapid local increase in prevalence.
The objective of the present study was to assess the effect in red foxes of repeated infections with A. vasorum L3 on worm burdens, parasite fecundity, eosinophil counts and larval output in feces. Repeated infections in an experimental setting enable a more systematic method of investigating the course of A. vasorum infections mimicking natural conditions. We hypothesize that the establishment of A. vasorum in new areas of Europe, once introduced, is substantially due to the cumulative effects of repeated infection in local red foxes with consistent excretion of L1 and corresponding environmental contamination.
2. Materials and methods
2.1. Experimental design
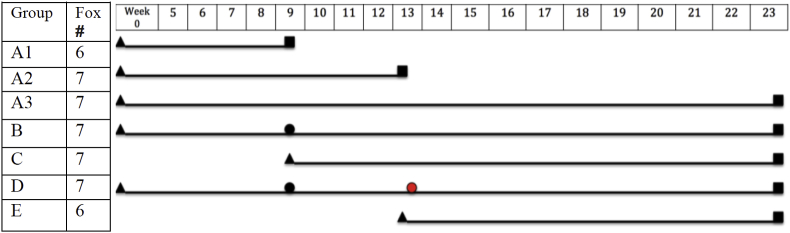
A total of 48 female foxes (38 cubs and 9 juveniles) were obtained from a fur farmer in Møldrup, Jutland, Denmark. Prior to arrival all foxes were cage-reared and exclusively fed heat-processed fishmeal. Upon arrival foxes were placed in cages above ground with a mesh floor to allow for fecal collection underneath cages. The foxes were allocated into seven groups, A1 (n = 6), A2 (7), A3 (7), B (7), C (7), D (7) and E (6). Six juveniles were placed in group A1 while the remaining juveniles (n = 3) were randomly allocated. Prior to inoculation all animals were anaesthetized by intra-muscular injection of Zoletil®50 (a mixture of zolazepam (5 mg/kg body weight (BW)) and tiletamin (5 mg/kg BW) (ChemVet, Silkeborg, Denmark) in combination with medetomidin (80 μg/kg BW, Domitor®Vet) (Orion Pharma, Espoo, Finland). Groups A (1, 2 and 3) (once inoculated group), B (twice inoculated group) and D (group inoculated three times) were inoculated with 100 L3 A. vasorum at day 0.
Third stage larvae of A. vasorum were obtained from Biomorhalaria glabrata previously infected with L1s from a naturally infected dog. Infections of snails were established by placing 5–6 snails in small containers with 100–200 L1 per snail in 20 ml tap water (enough to cover the snails) for 24 h under a constant light source. The snails were subsequently transferred to larger water tanks and kept there for 6 weeks. Infective L3 were obtained by tissue digestion: the snails were crushed and digested in 1% pepsin (1:10 000 IU) dissolved in 1% HCl (37%) in tap water at 37 °C for 10–20 min on a magnetic stirrer. The fluid was then passed through a 180 μm sieve and allowed to settle for 30 min before the supernatant was discarded and the sediment washed 2–3 times in tap water. The recovered L3 were used to inoculate four farm bred foxes, resulting in chronic infections and thus maintaining a supply of L1 for further snail infections and production of L3. Finally, prior to inoculation of the experimental foxes, the number of L3 in a subsample was counted and individual inoculation doses were prepared. At 9 weeks post inoculation (wpi) groups B and D were inoculated with 100 L3. Group C was inoculated with the same dose as a control group to assess larval viability. At the same time, group A1 was euthanized. At 13 wpi group D was inoculated for a third time with 100 L3, with group E receiving the same dose as a control for this third inoculation. A further 7 foxes from group A were euthanized (A2). At 23 wpi all remaining foxes were euthanized (Fig. 1). The study was conducted under Danish experimental animal license no. 2005/561–1060.
Fig. 1.
Experimental design: inoculations with 100 third stage larvae (L3) of Angiostrongylus vasorum are indicated by triangles, challenge inoculations (also with 100 L3) by circles and the differing group termination times by squares.
2.2. Laboratory analyses
Fresh fecal samples were collected one day per week from 5 wpi to 1 week prior to necropsy at 23 wpi, either from the cages or from the floor immediately beneath the cages. Samples were processed within 24 h after deposit using a modified Baermann method (ca. 10 g of feces), and the sediments were transferred to tubes which were examined for L1 within a week. The number of L1 per gram of feces (LPG) was determined. In group C at 11 wpi, L1 were detected in feces from two foxes subsequently observed to be negative for the subsequent 5 weeks; these data points were left out as sample contamination was suspected.
Blood plasma was taken at 0, 5, 12, 14, 16, 17, 20 and 22 wpi, isolated by centrifugation and kept at −20 °C until use. Eosinophil counts were performed using an ADVIA120 hematology analyzer (Siemens, Berlin, Germany) using canine settings. Clinical signs and well-being were monitored daily during feeding, and animals were examined more thoroughly at blood sampling, dosing and necropsies.
2.3. Post mortem examination
A reverse perfusion of the cardio-pulmonary vascular system was developed for the study to improve the recovery of intact live worms at necropsy: Anaesthetized foxes (see above) were given heparin intravenously (350 IU/kg BW) in order to prevent blood clotting during the following procedures. Three minutes later a lethal pentobarbital dose (100 mg/kg BW intravenously) was given, the thorax opened and thoracic organs perfused in situ after aorta, vena azygos and both venae cavae were clamped off. Approximately 3 L of isotonic perfusion fluid (15 g sodium citrate + 8.6 g NaCl dissolved in 1 L tap water) were pumped through a 16G needle into the left atrium, through pulmonary veins, lung capillaries and pulmonary arteries to the pulmonary trunk from which it was led via a plastic cylinder (5 mm diameter) on to a 200 μm sieve for worm collection. Recovered worms were directly transferred to RPMI 1640 medium (Life Technologies, Waltham, Massachusetts, USA) until counting. The perfusion procedure was followed by removal of lungs and heart, dissection of both ventricles and pulmonary arteries and slicing of the lung tissue with fine scissors to obtain worms captured in nodules. Finally, the sliced lung tissue was placed floating in normal saline (Baermann technique), from where additional worms were collected the following day. All worms recovered were counted and determined to sex under a dissection microscope (×40).
2.4. Statistical analysis
Worm fecundity was determined by larval excretion (LPG) on the final week prior to necropsy divided by the total number of female worms recovered post-mortem. For LPG data, groups A3, B and D were analyzed via a two-way analysis of variance (ANOVA) in a linear mixed effects model (accounting for repeated measures) with wpi included as a variable in a restricted maximum likelihood adjustment. Individual time points were also analyzed via linear regression. All blood parameters were analyzed within each group separately via ANOVA against wpi with Tukey HSD post hoc analysis used to ascertain differences between individual time points. Analysis of blood parameters (overall values with time points pooled) between groups was also performed by one-way ANOVA. Reference ranges (means ± 2 × standard deviation, SD) were established by using values of all animals in groups A1-A3, B and D from week 0 (n = 35). Worm burden, fecundity and the number of male and female worms were analyzed by linear regression analysis with the group as the main factor and final body-weight included as a covariate. All data were tested for normality prior to statistical analysis. Parameters were found to be normally distributed, with the exception of larval excretion data for which log (base-e) transformation was needed prior to analysis. Data were analyzed in R. Values were considered significant if p = ≤ 0.05.
3. Results
Patent infections were established in all foxes, and once patent L1 excretion continued throughout the experiment in all foxes. The cause of death of a single fox in group E during the trial was not determined, and this individual was excluded from the final analysis. Foxes in groups A1 (5/6), B (7/7) and D (6/7) reached patency 7 wpi (i.e. less than 49 days pre-patency), with all animals excreting L1 the following week.
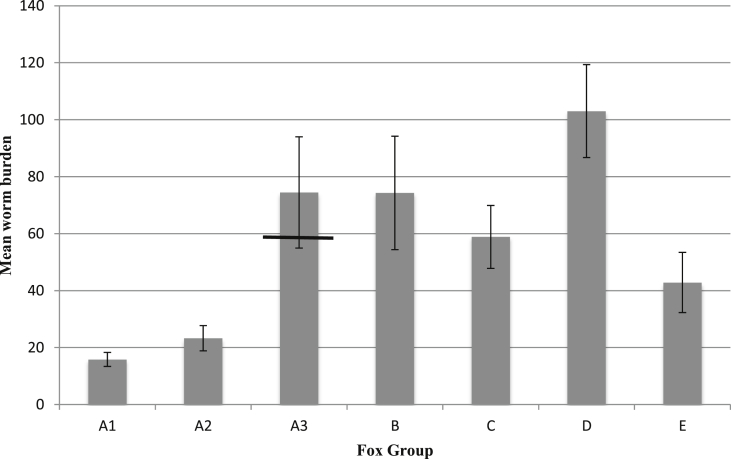
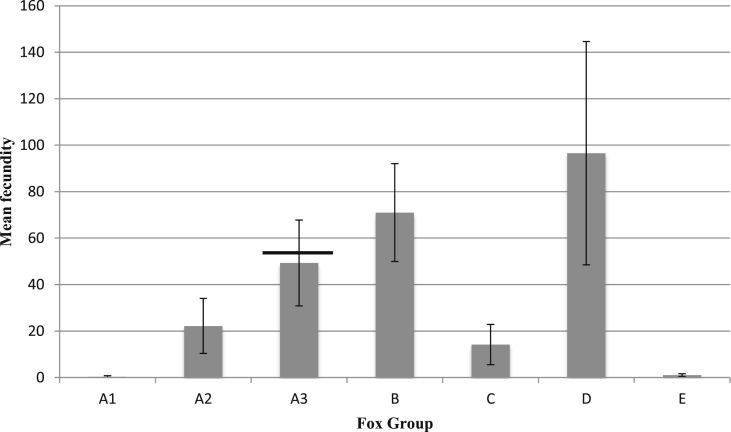
Adult worms were recovered from all foxes at necropsy. Mean (±SD) worm burdens were 15.8 ± 5.9, 23.3 ± 11.7, 74.4 ± 51.7, for groups A1 (once inoculated), A2 (once inoculated) and A3 (once inoculated) respectively; 74.2 ± 52.7 and 58.9 ± 29.2 for groups B (twice inoculated) and C (twice inoculated) respectively and 103.0 ± 43.1 and 42.8 ± 25.9 for groups D (inoculated three times) E (inoculated three times) respectively (Fig. 2). However, worm burdens were not significantly different between groups A3-B-D with the same being true for fecundity (Fig. 3), nor were the numbers of male and female worms. As inoculation doses were administered via aliquots, some variation in the number of L3 the individual foxes eventually received was expected. A single fox in group A3 had a worm burden of 173 and this was deemed to be beyond acceptable variation. Linear regression analysis was therefore re-run on fecundity, the number of isolated female, male and total worm burdens without this fox. While the majority of parameters remained non-significant with this fox removed, group D had significantly greater numbers of isolated male worms and also total number of worms, compared to group A3 (p = 0.02 and p = 0.05 respectively). The majority of the worms recovered in this study were through the perfusion technique, while lower numbers were obtained through dissection and floatation of the lungs (Bearmann technique). Worm recovery rates overall for perfusion, dissection and floatation of lung tissue were 63.9%, 23% and 13.6% respectively.
Fig. 2.
Arithmetic mean worm burden at necropsy per group. Foxes of groups A were inoculated once and necropsied at 9 wpi (A1), 13 wpi (A2) or 23 wpi (A3), while foxes of groups challenged once (B) or twice (D) and the control foxes of the first (C) and second challenge (E) were necropsied 23 wpi. Bar indicates mean with outlier excluded. Error bars = S.E.M.
Fig. 3.
Arithmetic mean worm fecundity (expressed as final LPG/number female worms at necropsy) per group. Foxes of groups A were inoculated once and necropsied at 9 wpi (A1), 13 wpi (A2) or 23 wpi (A3), while foxes of groups challenged once (B) or twice (D) and the control foxes of the first (C) and second challenge (E) were necropsied 23 wpi. Bar indicates mean with outlier excluded. Error bars = S.E.M.
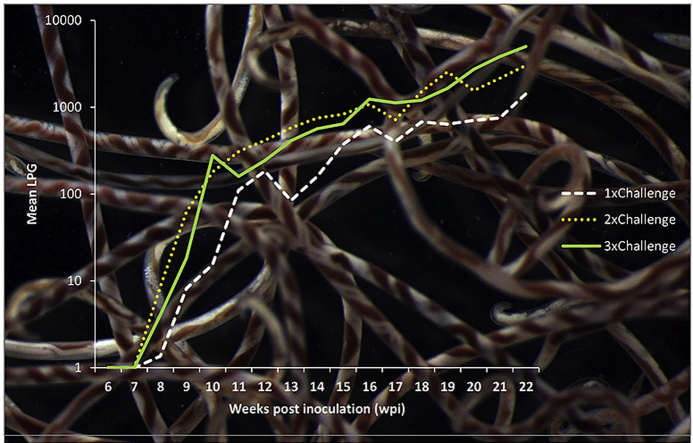
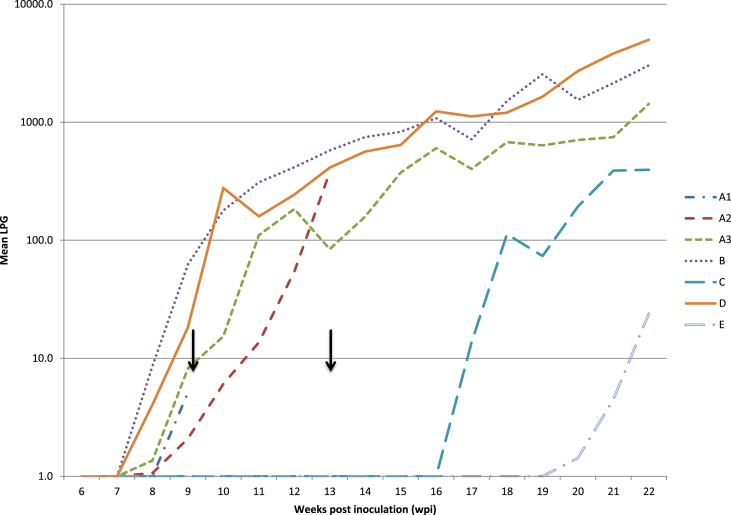
Larval outputs increased with increasing total dose (A3 versus B versus D) but overall larval output (as determined by the repeated measures ANOVA) was only significantly different (Fig. 4) between group A3 and B, with A3 having lower overall LPG than group B (p = 0.01), while overall larval output between A3 and D did not differ significantly (p = 0.08). With the removal of the high worm burden fox in group A3, both groups B and D had significantly higher larval output than group A3 (p=<0.01 and p = 0.01 respectively). Mean larval counts of group B were not significantly different from group D (p = 0.09). Individual time point analysis indicated significantly lower LPG in group A3 at 9 wpi compared to group B (p = 0.04) but not with group D (p = 0.12). At 13 wpi this relationship was maintained (A3-B p = 0.04, A3– D p = 0.23). At 20 wpi, group D had significantly higher LPG than A3 (p=<0.01) but not group B (p = 0.11). The final LPGs obtained from 22 wpi however demonstrated no significant difference between any of these three groups.
Fig. 4.
Arithmetic mean larval counts (first stage larvae per gram of feces) of foxes dogs experimentally inoculated at 0 wpi and challenged once (group B) or twice (group D) with 100 third stage larvae of Angiostrongylus vasorum from 6 to 22 wpi. Y-axis log10 transformed. Arrows indicate the challenge time points.
Significantly lower worm burdens were recorded in groups A1 (mean ± SD) and A2 necropsied at 9 and 13 wpi after a single inoculation as compared to the single inoculated A3 group followed for the entire study period (necropsied 23 wpi) (both p = 0.01) (Fig. 3). Foxes of group C and E (all necropsied at 23 wpi of the study but 14 and 10 weeks after the actual inoculation), representing controls for twice and three times inoculated, respectively, all developed infections demonstrating the viability of the infective L3 at this stage of the experiment, and therefore validating the repeat inoculation methodology. There was no significant difference in worm burden between groups C and E against group A3 (p = 0.43 and p = 0.13 respectively), nor between groups C and E themselves (p = 0.32).
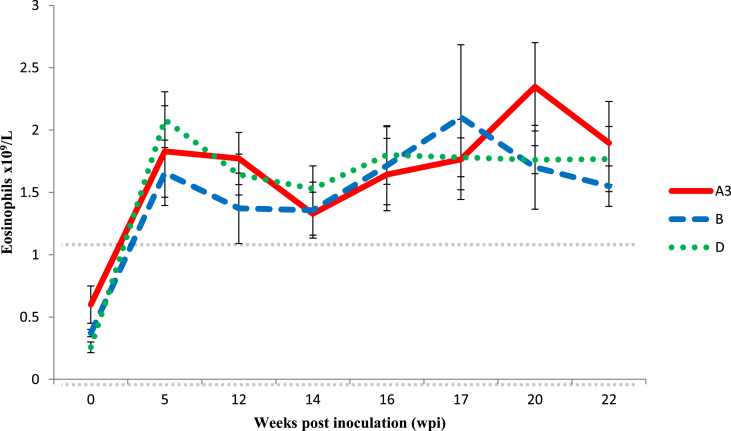
Statistical comparison within each group revealed no significant differences in mean eosinophil counts in group A3, B or D (data not shown). All groups demonstrated an overall significant increase in eosinophils (Fig. 5) over the course of the experiment (group A3 p = 0.01, group B p = 0.02 and group D p < 0.01), with all groups exceeding the reference range from 5wpi, but the groups were not significantly different from each other. Analysis of individual wpi for group A3 revealed significantly higher numbers of eosinophils at 20 and 22 wpi compared to 0 wpi (p = 0.05 and D p < 0.01, respectively), while for group B there were no significant differences in counts between time points, and for group D there were significant differences between wpi 0 counts and those at all other sampling points (p=<0.01). WBC counts and absolute lymphocyte numbers did not vary significantly over time or between groups (data not shown), supporting the hypothesis that foxes exhibit a rather weak non-specific innate immune response to A. vasorum infection.
Fig. 5.
Absolute counts of Eosinophils. The dotted horizontal lines represent reference ranges based on average values ± 2 × S.D. of values of foxes from groups A3, B and D at 0 wpi. Error bars (S.E.M).
Despite the repeated clinical assessments, it is possible that subtle signs were missed but the well-known signs associated with clinical canine angiostrongylosis such as coughing, dyspnoea or bleeding abnormalities were not observed at any time during the study.
4. Discussion
This study is the first to report on the effect of repeated inoculation with A. vasorum in red foxes, allowing insights into the role of this host as a wildlife reservoir for the parasite.
Infections in red foxes persisted for the duration of the study with LPG steadily increasing in group A3 (once inoculated). For the vast majority of foxes, the pre-patency period was <7wpi, with all foxes being patent by 8wpi. This suggests that foxes that ingest infected gastropod intermediate hosts are likely to harbor the infection for at least 6 months, and environmental contamination is likely to occur for at least 5 months, with high probability for longer patency, potentially as long as one or more years. In dogs, A. vasorum infections have been shown to persist up to five years if left untreated (Rosen et al., 1970). No significant difference between twice inoculated (B and C) and once inoculated (A3) groups was observed in female worm burden or fecundity. The fecundity data indicate that higher worm burden is not reflected in reduced fecundity. This is the opposite of what has been observed in other nematode parasites such as Ascaris lumbricoides in humans and Trichostrongylus colubriformis in sheep (Dobson et al., 1990, Hall and Holland, 2000) and also in the fox and dog hookworm Ancylostoma caninum (Krupp, 1961). The removal of the fox in group A3 with a worm burden of 173, resulted in a significant difference in worm burden between group A3 and group D (inoculated three times) but no significant difference in fecundity, indicating that in this study increasing A. vasorum burden in foxes did not result in reduced parasite fecundity.
Our experimental setting demonstrates a trend toward increasing LPG in response to repeated infections. The lack of a statistically significant difference between group D (inoculated three times) against group A3 (inoculated once) and B (inoculated twice) could possibly be explained by the high variation in LPG results (and therefore potentially by the different individual responses of foxes) throughout the study. With the outlier in group A3 removed, larval output in both the repeatedly inoculated groups B and D is significantly higher compared to the once inoculated group. Thus, it is not fully clear to what extent increasing LPG is due to repeated inoculation or to the natural propensity of LPG to increase in this host. Either way, the data suggest that once wild foxes are exposed to gastropods infected with A. vasorum, they will continue to excrete increasing numbers of L1 over a long period, and that environmental contamination correspondingly increases, causing increased likelihood of infection of intermediate and, consequently, of definitive hosts. A rapid establishment of A. vasorum, once introduced into new areas, likely depends on many factors, but evidence from this study clearly suggests increasing environmental contamination as a function of increased risk of infection for foxes and plays an important epidemiological role. The reasons behind persistent infections and lack of expulsion of adult worms might lie in reduced immunological reactions of foxes to A. vasorum, as suggested by the limited hematological changes also presented in this study. The absence of any severe clinical signs in experimentally infected foxes (Webster et al., 2017) certainly suggests an advanced symbiosis of the parasite with the fox and a high degree of tolerance to the parasite. Similarly, clinical data from foxes under natural conditions harboring relatively large worm burdens have not been associated with emaciation in necropsied foxes (Jeffery et al., 2004, Morgan et al., 2008, Al-Sabi et al., 2014). These results contrast with the numerous cases of severe to lethal canine angiostrongylosis, suggesting fundamental differences in pathogenesis, clinical outcomes and immunological reactions between foxes and dogs (Chapman et al., 2004, Staebler et al., 2005, Denk et al., 2009, Yamakawa et al., 2009).
As in a previous study in foxes (Webster et al., 2017), eosinophilia was observed in all inoculated groups A3, B and D, independently of the number of inoculations. Endoparasitism with prolonged host-tissue contact is frequently reported to induce eosinophilia, particularly repeated exposure to the same parasite which can elicit a more immediate and drastic eosinophilia (Schultze, 2000). The lack of difference in eosinophil levels between fox groups receiving single or repeated inoculations is interesting, and may contribute to the ability of A. vasorum infections to accumulate in foxes. Eosinophilia (and leucocytosis) have been reported in naturally A. vasorum infected dogs (Chapman et al., 2004, Willesen et al., 2009), and in experimental infections occur around 3–7 wpi (Neff, 1971, Cury et al., 2002, Schnyder et al., 2010).
Quantification of adult A. vasorum is difficult because in dead hosts the majority of adult worms are no longer located in the heart but in the peripheral branches of the pulmonary arteries and thus not easily accessible. Dissection and rinsing of the lung tissue and heart are the gold standard for worm isolation from wildlife or dogs found dead (Jeffery et al., 2004, Morgan et al., 2008, Tolnai et al., 2015): they give a rough estimate of worm burden but frequently result in damaged worms leading to difficulty in differentiation from other lungworms. Positive cases may also be missed as this method's sensitivity is reported as approximately 85% (Houpin et al., 2016). The reverse perfusion technique, including injection of heparin (to prevent blood clotting in small vessels) prior to euthanasia, and perfusion immediately after death offers great advantages for counting and for isolating live intact worms for e.g. worms for culture, or for evaluating anthelmintic efficacy (Schnyder et al., 2009, Böhm et al., 2014). However, this method is not applicable to wild foxes as administration of heparin and immediate dissection in hunted foxes is typically not possible as they are frequently frozen prior to necropsy. Thus, obtaining accurate worm burdens is more difficult in wild animals which may explain the low average worm burdens previously reported in the wild, for example, means of 7.7 worms from 364 infected foxes (Saeed et al., 2006), 17.7 from 56 infected foxes in Denmark (Al-Sabi et al., 2013) and 6.3 from 76 infected animals from the UK (Taylor et al., 2015). However, it is important to appreciate that doses used in this study might exceed those in the majority of natural infections. From 28 infected slugs (from a total of 298 collected) belonging to 4 species (Arion lusitanicus, A. ater, A. ater rufus and Limax maximus) collected around Copenhagen, 82% harbored fewer than 10 larvae with just 14% harboring over 100 (Ferdushy et al., 2009). The majority of the worms recovered in our study were through the perfusion technique, while lower numbers were obtained through dissection and floatation of the lungs. It is not possible to know how effective dissection and floatation would have been, if perfusion had not been performed prior, but dog dissection data sustain the efficacy of the reverse lung perfusion technique (Schnyder et al., 2009). Mean worm burdens from groups A3, B and D in this study were therefore in excess of what is typically observed in naturally infected foxes. This is also the case with LPG values, which were greater than those described by Al-Sabi et al. (2010) who reported LPG values of 1–15 in 23 naturally infected foxes, although in this latter study LPG was determined from sieving feces and as such not directly comparable.
The increasing LPG within the study period and the potential increase in LPG as a result of repeated inoculations (ostensibly due to the limited protective immune response to this parasite in foxes) found in this study provides compelling evidence for the ability for rapid establishment of this parasite into new areas where foxes are present. Furthermore, the increasing LPGs from 13 wpi through to 23 wpi in groups A3, B, and D suggest that LPG may have further markedly increased over time if the experiment had continued.
5. Conclusions
Although the complete effects of repeated inoculations on worm burden and fecundity could not be fully determined in the present study, the most important finding in terms of potential environmental contamination is the persistent L1 excretion with feces. This study demonstrates that regardless of repeated exposure to A. vasorum, once the animals are infected, the number of L1 produced per fox will increase for a certain time period (currently not known but at least several months). This is likely to facilitate, once introduced in the intermediate gastropod host population, the rapid establishment of the parasite where climatic conditions are favorable. In contrast to dogs, which may be given anthelmintic treatments and therefore get rid of the infection, fox larval excretion in feces could ensure continuous infection of the local gastropod populations. As a result, infected foxes could be the major source of L1s and be key to a rapid build-up of infection in a naïve fox population. The wide distribution of foxes, including in urban areas (Gloor et al., 2001), help to explain the highly successful establishment of this parasite in wide areas of Europe and potentially also in additional areas where foxes are present.
Conflict of interest
Authors declare no conflict of interest.
References
- Ablad B., Christensson D., Lind E.O., Agren E., Morner T. Angiostrongylus vasorum etablerad i Sverige. Sven. Veterinärtidning. 2003;12:11–15. [Google Scholar]
- Al-Sabi M.N., Deplazes P., Webster P., Willesen J.L., Davidson R.K., Kapel C.M.O. PCR detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol. Res. 2010;107:135–140. doi: 10.1007/s00436-010-1847-5. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N.S., Chriél M., Jensen T.H., Enemark H.L. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012–A comparative study. Int. J. Parasitol. Parasites Wildl. 2013;2:144–151. doi: 10.1016/j.ijppaw.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabi M.N., Halasa T., Kapel C.M.O. Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol. 2014;59:98–107. doi: 10.2478/s11686-014-0214-6. [DOI] [PubMed] [Google Scholar]
- Barcante T.A., Barcante J.M.P., Dias S.R.C., Lima W.S. Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905: emergence of third-stage larvae from infected Biomphalaria glabrata snails. Parasitol. Res. 2003;91:471–475. doi: 10.1007/s00436-003-1000-9. [DOI] [PubMed] [Google Scholar]
- Bolt G., Monrad J., Henriksen P., Dietz H.H., Koch J., Bindseil E., Jensen A.L. The fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark. Field survey and experimental infections. Acta Vet. Scand. 1992;33:357–362. doi: 10.1186/BF03547302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt G., Monrad J., Frandsen F., Henriksen P., Dietz H.H. The common frog (Rana temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol. Res. 1993;79:428–430. doi: 10.1007/BF00931834. [DOI] [PubMed] [Google Scholar]
- Bolt G., Monrad J., Koch J., Jensen A.L. Canine angiostrongylosis: a review. Vet. Rec. 1994;135:447–452. doi: 10.1136/vr.135.19.447. [DOI] [PubMed] [Google Scholar]
- Bourque A.C., Conboy C., Miller L.M., Whitney H. Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador. Canada. J. Vet. Diagn Invest. 2008;20:11–20. doi: 10.1177/104063870802000103. [DOI] [PubMed] [Google Scholar]
- Böhm C., Schnyder M., Thamsborg S.M., Thompson C.M., Trout C., Wolken S., Schnitzler B. Assessment of the combination of spinosad and milbemycin oxime in preventing the development of canine Angiostrongylus vasorum infections. Vet. Parasitol. 2014;199:272–277. doi: 10.1016/j.vetpar.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Chapman P.S., Boag A.K., Guitian J., Boswood A. Angiostrongylus vasorum infection in 23 dogs. J. Small Anim. Pract. 2004;45:435–440. doi: 10.1111/j.1748-5827.2004.tb00261.x. 1999-2002. [DOI] [PubMed] [Google Scholar]
- Conboy G. Natural infections of Crenosoma vulpis and Angiostrongylus vasorum in dogs in Atlantic Canada and their treatment with milbemycin oxime. Vet. Rec. 2004;155:16–18. doi: 10.1136/vr.155.1.16. [DOI] [PubMed] [Google Scholar]
- Cury M.C., Lima W.S., Guimaraes M.P., Carvalho M.G. Hematological and coagulation profiles in dogs experimentally infected with Angiostrongylus vasorum (Baillet, 1866) Vet. Parasitol. 2002;104:139–149. doi: 10.1016/S0304-4017(01)00616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury M.C., Guimaraes M.P., Lima W.S., Caldeira M.C., Couto T.R., Murta K., Carvalho M.G., Baptista J.M. Biochemical serum profiles in dogs experimentally infected with Angiostrongylus vasorum (Baillet, 1866) Vet. Parasitol. 2005;128:121–127. doi: 10.1016/j.vetpar.2004.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk D., Matiasek K., Just F.T., Hermanns W., Baiker K., Herbach N., Fischer A. Disseminated angiostrongylosis with fatal cerebral haemorrhages in two dogs in Germany: a clinical case study. Vet. Parasitol. 2009;160:100–108. doi: 10.1016/j.vetpar.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Demiaszkiewicz A.W., Pyziel A.M., Kuligowska I., Lachowicz J. The first report of Angiostrongylus vasorum (Nematoda; Metastrongyloidea) in Poland, in red foxes (Vulpes vulpes) Acta Parasitol. 2014;59:758–762. doi: 10.2478/s11686-014-0290-7. [DOI] [PubMed] [Google Scholar]
- Dobson R.J., Waller P.J., Donald A.D. Population dynamics of Trichostrongylus colubriformis in sheep: the effect of infection rate on the establishment of infective larvae and parasite fecundity. Int. J. Parasitol. 1990;20:347–352. doi: 10.1016/0020-7519(90)90150-l. [DOI] [PubMed] [Google Scholar]
- Ferdushy T., Kapel C.M.O., Webster P., Al-Sabi M.N.S., Grønvold J. The occurance of Angiostrongylus vasorum in terrestrial slugs from forests and parks in the Copenhagen area, Denmark. J. Helminthol. 2009;22:379–383. doi: 10.1017/S0022149X09377706. [DOI] [PubMed] [Google Scholar]
- Gloor S., Bontadina F., Hegglin D., Deplazes P., Breitenmoser U. The rise of urban fox populations in Switzerland. Mamm. Biol. 2001;66:155–164. [Google Scholar]
- Guilhon J. Recherches sur le cycle évolutif du Strongle des vaisseaux du chien. Bull. Acad. Vét. 1963;36:431–442. [Google Scholar]
- Guilhon J., Cens B. Angiostrongylus vasorum (Baillet, 1866): Étude biologique et morphologique. Ann. Parasitol. Hum. Comp. 1973;48:567–596. [PubMed] [Google Scholar]
- Hall A., Holland C. Geographical variation in Ascaris lumbricoides fecundity and its implications for helminth control. Parasitol. Today. 2000;16:540–544. doi: 10.1016/s0169-4758(00)01779-8. [DOI] [PubMed] [Google Scholar]
- Houpin E., McCarthy G., Ferrand M., De Waal T., O'Neill E.J., Zintl A. Comparison of three methods for the detection of Angiostrongylus vasorum in the final host. Vet. Parasitol. 2016;220:54–58. doi: 10.1016/j.vetpar.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Jefferies R., Shaw S.E., Willesen J., Viney M.E., Morgan E.R. Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Infect Gen Evol. 2010;10:561–568. doi: 10.1016/j.meegid.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Jeffery R.A., Lankester M.W., McGrath M.J., Whitney H.G. Angiostrongylus vasorum and Crenosoma vulpis in red foxes (Vulpes vulpes) in Newfoundland, Canada. Can. J. Zool. 2004;82:66–74. [Google Scholar]
- Krupp I.M. Effects of crowding and of superinfection on habitat selection and egg production in Ancylostoma caninum. J. Parasitol. 1961;47:957–961. [PubMed] [Google Scholar]
- Miterpáková M., Hurníková Z., Zalewski A.P. The first clinically manifested case of angiostrongylosis in a dog in Slovakia. Acta Parasitol. 2014;59:661–665. doi: 10.2478/s11686-014-0289-0. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Tomlinson A., Hunter S., Nichols T., Roberts E., Fox M.T., Taylor M.A. Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2008;154:48–57. doi: 10.1016/j.vetpar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Jefferies R., Krajewski M., Ward P., Shaw S.E. Canine pulmonary angiostrongylosis: the influence of climate on parasite distribution. Parasitol. Int. 2009;58:406–410. doi: 10.1016/j.parint.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Mozzer L.R., Lima W.S. Gallus gallus domesticus: paratenic host of Angiostrongylus vasorum. Vet. Parasitol. 2015;207:81–84. doi: 10.1016/j.vetpar.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Neff H. Universität Zürich; Zürich: 1971. Experimentelle Infektionen von Hunden mit Angiostrongylus vasorum (Nematoda) Dissertation. [Google Scholar]
- Roche M.M., Kelliher D.J. Angiostrongylus vasorum infestation in the dog: a case report. Ir. Vet. J. 1968;22:108–113. [Google Scholar]
- Rosen L., Ash L.R., Wallace G.D. Life history of the canine lungworm Angiostrongylus vasorum (Baillet) Am. J. Vet. Res. 1970;31:131–143. [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Schultze A.E. Interpretation of canine leukocyte responses. In: Feldman B.F., Zinkl J.G., Jain N.C., editors. Schalm's Veterinary Hematology. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 1155–1160. [Google Scholar]
- Schnyder M., Fahrion A., Ossent P., Kohler L., Webster P., Heine J., Deplazes P. Larvicidal effect of imidacloprid/moxidectin spot-on solution in dogs experimentally inoculated with Angiostrongylus vasorum. Vet. Parasitol. 2009;166:326–332. doi: 10.1016/j.vetpar.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Fahrion A., Riond B., Ossent P., Webster P., Kranjc A., Glausm T., Deplazes P. Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitol. Res. 2010;107:1471–1480. doi: 10.1007/s00436-010-2021-9. [DOI] [PubMed] [Google Scholar]
- Schnyder M., Jefferies R., Schucan A., Morgan E.R., Deplazes P. Comparison of coprological, immunological and molecular methods for the detection of dogs infected with Angiostrongylus vasorum before and after anthelmintic treatment. Parasitol. 2015;142:1270–2127. doi: 10.1017/S0031182015000554. [DOI] [PubMed] [Google Scholar]
- Serres E. Entozoaires trouvés dans l’oreille droite, le ventricule correspondant et l’artère pulmonaire d’un chien. J. Vét Midi. 1854;7:70. [Google Scholar]
- Simpson V.R., Neal C. Angiostrongylus vasorum infection in snails and slugs. Vet. Rec. 1982;111:303–304. doi: 10.1136/vr.111.13.303. [DOI] [PubMed] [Google Scholar]
- Staebler S., Ochs H., Steffen F., Naegeli F., Borel N., Sieber-Ruckstuhl N., Deplazes P. Autochthonous infections with Angiostrongylus vasorum in dogs in Switzerland and Germany (in German) Schweiz Arch. Tierheilkd. 2005;147:121–127. doi: 10.1024/0036-7281.147.3.121. [DOI] [PubMed] [Google Scholar]
- Taylor C.S., Garcia Gato R., Learmount J., Aziz N.A., Montgomery C., Rose H., Coulthwaite C.L., McGarry J.W., Forman D.W., Allen S., Wall R., Morgan E.R. Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitol. 2015;142:1190–1195. doi: 10.1017/S0031182015000463. [DOI] [PubMed] [Google Scholar]
- Tolnai Z., Szell Z., Sreter T. Environmental determinants of the spatial distribution of Angiostrongylus vasorum, Crenosoma vulpis and Eucoleus aerophilus in Hungary. Vet. Parasitol. 2015;207:355–358. doi: 10.1016/j.vetpar.2014.12.008. [DOI] [PubMed] [Google Scholar]
- van Doorn D.C., van de Sande A.H., Nijsse E.R., Eysker M., Ploeger H.W. Autochthonous Angiostrongylus vasorum infection in dogs in The Netherlands. Vet. Parasitol. 2009;162:163–166. doi: 10.1016/j.vetpar.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Webster P., Monrad J., Kapel C.M.O., Kristensen A.T., Jensen A.L., Thamsborg S.M. The effect of host age and inoculation dose on infection dynamics of Angiostrongylus vasorum in red foxes (Vulpes vulpes) Parasit. Vectors. 2017;10:4. doi: 10.1186/s13071-016-1940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willesen J.L., Jensen A.L., Kristensen A.T., Kjelgaard-Hansen M., Jessen R., Koch J. Serum fructosamine concentrations in 59 dogs naturally infected with Angiostrongylus vasorum. J. Vet. Med. Ser. A. 2006;53:266–269. doi: 10.1111/j.1439-0442.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- Willesen J.L., Jensen A.L., Kristensen A.T., Koch J. Haematological and biochemical changes in dogs naturally infected with Angiostrongylus vasorum before and after treatment. Vet. J. 2009;180:106–111. doi: 10.1016/j.tvjl.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Willesen J.L., Kristensen A.T., Jensen A.L., Heine J., Koch J. Efficacy and safety of imidacloprid/moxidectin spot-on solution and fenbendazole in the treatment of dogs naturally infected with Angiostrongylus vasorum (Baillet, 1866) Vet. Parasitol. 2007;147:258–264. doi: 10.1016/j.vetpar.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Yamakawa Y., McGarry J.W., Denk D., Dukes-McEwan J., Macdonald N., Mas A., McConnell F., Tatton B., Valentine E.G., Wayne J., Williams J.M., Hetzel U. Emerging canine angiostrongylosis in northern England: five fatal cases. Vet. Rec. 2009;164:149–152. doi: 10.1136/vr.164.5.149. [DOI] [PubMed] [Google Scholar]