Abstract
Background
TAVI is a percutaneous approach to aortic valve replacement in high surgical risk patients deemed inoperable.
Aim
To evaluate the early and mid-term outcomes for an Irish TAVI cohort over a six-year period at St James's Hospital and Blackrock Clinic, Dublin, Ireland.
Results
In total 147 patients, 56% male with an average age of 82 underwent TAVI between December 2008 and December 2014. Thirty day, one year and two year survival was 90.5%, 83% and 71% respectively. Major vascular complications and renal failure were the biggest predictors of mortality at 30 days (p = 0.02). We observed a pacing rate of 13.5%, the majority in patients who had Medtronic Corevalve implants (p < 0.05). With increasing procedural experience there was a reduction in length of stay from 10 days to 7.5 days.
Conclusion
This review, the first of its kind in Ireland showed favorable rates of 30 day and one year and two year survival post TAVI with procedural success and complication rates similar to international registry data.
1. Background
Aortic Stenosis (AS) is the most common valvular heart disease in the developed world [1]. It affects 2% of people over the age of 65, 3% over 75 and 4% over 85 years of age, and with an ageing population the incidence is projected to increase [2]. Once symptomatic AS has an extremely poor prognosis and left untreated carries a 2-year mortality rate of 50% [3]. Replacement of the valve is the only treatment option that improves symptoms and survival.
Transcatheter Aortic Valve implantation (TAVI) has over the last decade become the standard of care for high surgical risk or inoperable patients with severe aortic stenosis [4]. It facilitates percutaneous implantation of a new aortic valve with the majority of procedures performed via the transfemoral route under local anaesthetic with transapical, transaortic and the subclavian artery access as an alternative, dependent on patient vascular anatomy. The first valve was implanted in man by Cribier in 2002 [5] and currently two main devices are implanted in Ireland; the balloon-expandable Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, California) and the self-expandable CoreValve prosthesis (Medtronic, Minneapolis, Minnesota).
The Irish TAVI program commenced in 2008 and there exists a paucity of data on clinical and procedural outcomes post implantation. We sought to address this dearth of information by analyzing the short and mid-term survival post implantation and factors that influence them.
2. Aim
To define the characteristics of a real-world patient population treated with TAVI and to evaluate their clinical outcome over the short and mid-term.
3. Methods
We analyzed consecutive patients presenting for TAVI at two tertiary referral centers in Dublin, St James's Hospital and Blackrock Clinic. TAVI was first performed in these centers in 2008 and by December 2014, 147 patients had undergone the procedure.
Patient eligibility for the procedure was decided in each center by a multidisciplinary team composed of interventional cardiologists, imaging cardiologists and cardiothoracic surgeons. A transfemoral approach was the default strategy for all patients. The preprocedural demographic, clinical, laboratory, and technical (electrocardiographic and echocardiographic) data were collected. Transthoracic echocardiography was performed before the procedure, within 7 days after device implantation and at follow up clinical visits, with all patients having one or more transthoracic echocardiograms by six months and one-year post procedure.
Separate clinical end points were collected during or immediately after TAVI: death, myocardial infarction, cerebrovascular complications, major vascular and bleeding complications, and acute kidney injury (AKI). The following valve associated end points were recorded: new left bundle branch block (LBBB), new atrioventricular block and new permanent pacemaker implantation.
All cerebrovascular events were evaluated by neurologists who reassessed such patients daily. The serum creatinine and hemoglobin (HB) levels were recorded on day one after the procedure and followed whilst an inpatient. A major bleed was considered as a drop in HB > 2 g/dl. Twelve-lead electrocardiographic recordings were obtained before treatment and 1 day after treatment, after which the electrocardiograms were examined for the occurrence of new left bundle branch block or new atrioventricular block.
Primary care physicians and referring medical teams were contacted post discharge to determine mortality and pacemaker implantation rates after discharge.
3.1. Statistical analysis
Numerical data are presented as means and standard deviations with comparisons performed by using the Student t-test. Categorical data are presented as percentages and comparisons were made by using the χ2 test or the Fisher exact test. Mortality data has been presented using Kaplan Meier survival curves. Univariate analysis of each of the possible predictors of outcome was performed and those that were statistically significant (p < 0.05) presented here. All statistical analysis was undertaken using Graphpad Prism version 6.0.
4. Results
Between December 2008 and December 2014 we reviewed the outcomes of 147 patients who underwent TAVI. Table 1 shows the baseline demographics for the entire cohort. The average age for was 82 ± 5 years, with 56% male patients. As shown in Table 1 we analyzed our data in three cohorts by year of implant. There were no significant differences by age, sex, and New York Heart Association (NYHA) or Canadian Cardiovascular Society (CCS) class across all cohorts. The ejection fraction was considered as being low normal across the three cohorts but did not differ significantly.
Table 1.
Baseline patient demographics.
| Variable | Entire cohort | 2008–2009 | 2010–2011 | 2012–2014 | p value |
|---|---|---|---|---|---|
| Age (years) | 82 ± 5 | 83 ± 4 | 82 ± 4 | 81 ± 5 | 0.6 |
| Male | 83 (56%) | 25 (64%) | 19 (47%) | 39 (61%) | 0.05 |
| NYHA | 3.3 ± 0.6 | 3.4 ± 0.2 | 3.2 ± 0.5 | 3.3 ± 0.7 | 0.8 |
| CCS | 0.4 ± 0.8 | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.4 ± 1.0 | 0.7 |
| GRF ml/min | 58 ± 22 | 54 ± 19 | 66 ± 22 | 57 ± 25 | 0.07 |
| GFR < 30 ml/min | 29 (20%) | 7 (19%) | 5 (14%) | 17 (25%) | 0.2 |
| Pacemaker | 17 (12%) | 7 (18%) | 3 (8%) | 7 (11%) | 0.2 |
| Atrial fibrillation | 25 (17%) | 6 (15%) | 3 (8%) | 16 (25%) | 0.3 |
| Ejection fraction | 50 ± 9 | 51 ± 10 | 49 ± 9 | 50 ± 8 | 0.6 |
| AVA | 0.8 ± 0.7 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.9 ± 0.9 | 0.2 |
| AR grade | 1.1 ± 0.9 | 0.9 ± 0.6 | 0.8 ± 0.6 | 1.3 ± 1.1 | 0.02 |
| MR grade | 1.1 ± 0.8 | 1.3 ± 0.5 | 1.0 ± 0.3 | 1.4 ± 0.7 | 0.5 |
| EURO II score | 9.1 ± 5 | 9.1 ± 6.3 | 9.4 ± 3.2 | 8.2 ± 3.7 | 0.4 |
Data is expressed as mean ± standard deviation or number of patients (%).
AVA is aortic valve area-cm2; AR is Aortic Regurgitation; MR is Mitral Regurgitation; EF is ejection fraction measured by Simpsons.
The majority of patients were in sinus rhythm prior to valve implant with 12% having a prior pacemaker implant and 17% in atrial fibrillation at the time of procedure. The average EURO II score was 9.1 and did not differ significantly between the various cohorts.
Femoral access was used in 92% of cases with conscious sedation as the default for non-surgical femoral access. 59% of implants were CoreValve implants with a predominance of Corevalve usage in the first four years of the program (p < 0.05). There was no valve in valve implants. Whilst the average length of stay was 9.7 days for the entire patient cohort by 2012–2014 the length of stay had reduced to an average of 7.5 days.
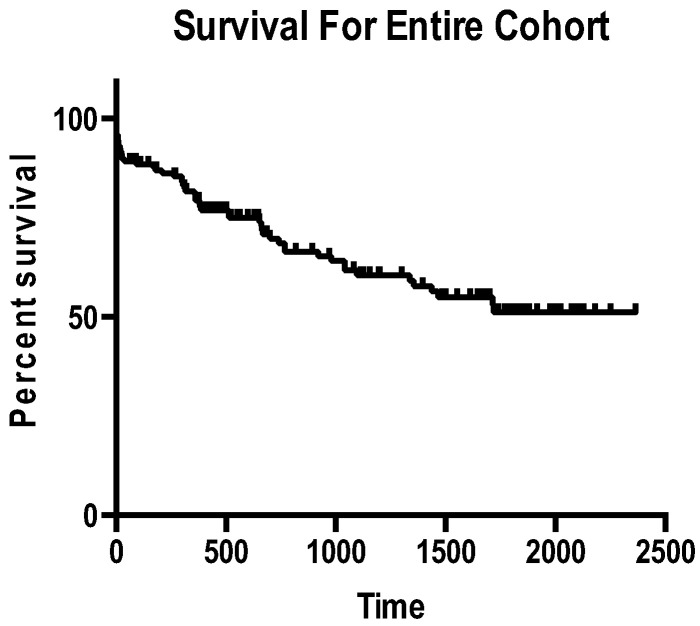
At 30 days, 14 (9.5%) of patients had died, with 12 (86%) attributable to cardiac death. At one and two years respectively, mortality rates were 17% and 29%. Whilst at one year 19/25(76%) deaths were attributable to primary cardiac etiologies we noted attrition in the rate of cardiac death by two years with 57% of deaths primarily cardiac. Fig. 1 shows the Kaplan Meier survival curve for the entire cohort with a three-year survival rate of 55%. When survival was compared between cohorts at 30 days, one year and two years there were no statistically significant differences.
Fig. 1.
Kaplan Meier survival curve (time in days).
Univariate predictors of mortality at 30 days and one year were a GFR < 30 mls/min and major vascular complications (p = 0.02). Left ventricular dysfunction, diabetes mellitus, prior cardiac surgery and atrial fibrillation were not related to 30 day and one year outcomes in our population. EURO II score, which estimates the 30-day mortality after cardiac surgery, was not a predictor of poorer outcomes in our cohort.
When we analyzed our data for 30 day and one-year mortality by valve type, there was no significant divergence in mortality rates. As detailed in Table 2, Table 3 the pacing rate at one year was 13.5% with significant differences in pacing rates for Corevalve and Edwards implants with 18/20 (90%) pacemakers implanted in patients who had a Corevalve implanted (p < 0.05). New LBBB occurred in 12 patients who had a Corevalve implant versus 4 whom had an Edwards implant.
Table 2.
Procedural characteristics.
| Entire cohort | 2008–2009 | 2010–2011 | 2012–2014 | p value | |
|---|---|---|---|---|---|
| Valve type | |||||
| Edwards | 60 (41%) | 14 (36%) | 10 (26%) | 36 (53%) | 0.01 |
| Corevalve | 87 (59%) | 25 (64%) | 29 (74%) | 33 (48%) | 0.002 |
| Access | |||||
| Femoral | 135 (92%) | 34 (87%) | 36 (90%) | 65 (95%) | |
| Other | 12 (8%) | 5 (13%) | 4 (10%) | 3 (5%) | 0.01 |
| Length of stay (days) | 9.7 ± 19 | 10 ± 35 | 9.7 ± 12 | 7.5 ± 8.4 | 0.24 |
Data is expressed as mean ± standard deviation or number of patients (%).
Table 3.
Outcomes.
| Variable | Entire cohort | 2008–2009 | 2010–2011 | 2012–2014 | |
|---|---|---|---|---|---|
| 30 day survival | 133 (90.5%) | 36 (92%) | 37 (94%) | 60 (94%) | 0.2 |
| 1 yr survival | 122 (83%) | 33 (85%) | 36 (90%) | 53 (82%) | 0.3 |
| 2 yr survival | 104 (71%) | 27 (69%) | 29 (73%) | 48 (75%) | 0.2 |
| Stroke | 7 (4.7%) | 3 (8%) | 2 (5%) | 2 (3%) | 0.4 |
| Vascular complications | 18 (12%) | 7 (17%) | 6 (15%) | 5 (8%) | 0.15 |
| Pacemaker | 20 (13.6%) | 6 (15%) | 7 (18%) | 7 (11%) | 0.5 |
| Ejection fraction | 50 ± 8 | 50 ± 8 | 51 ± 9 | 49 ± 8 | 0.4 |
| AR | 0.9 ± 0.7 | 1.1 ± 0.7 | 1.2 ± 0.8 | 0.8 ± 0.6 | 0.2 |
| MR | 1.0 ± 0.8 | 1.2 ± 0.8 | 1.1 ± 0.6 | 0.9 ± 0.6 | 0.6 |
| Improvement in creatinine | 9.2% | 8% | 9% | 10% | 0.2 |
Data is expressed as mean ± standard deviation or number of patients (%).
AR is Aortic Regurgitation; MR is Mitral Regurgitation; EF measured by Simpsons.
Average creatinine improvement by discharge.
Major vascular complications occurred in 12% of patients and did not differ by valve type. We observed 7 strokes in our cohort, which all occurred intraprocedurally or within the first day post implant; 6 of these were non-disabling events and did not significantly prolong discharge. However, six of the seven strokes were in patients who had a Corevalve implant. We observed an improvement in Aortic Regurgitation (AR) grade from 1.1 to 0.9 with 6 patients (4%) having grade 3 or 4 AR post procedure. This did not influence mortality. Similarly we observed an improvement in both Mitral Regurgitation (MR) and ejection fraction (EF) post valve implantation.
Finally we addressed the issue of a learning curve by analyzing the rate of major vascular complications, 30 day and one-year mortality with increasing procedural experience. Whilst there was an observed decrease in major vascular complications between the three cohorts it was not of statistical significance (p = 0.15) with no differences in mortality between groups. Additionally there have been no significant differences in pacing rates or minor vascular complications with increasing experience to date.
5. Discussion
This review represents the first in the Republic of Ireland of an unselected TAVI cohort. We present here six-year data on the observed trends in patient selection, procedural and post procedural complications and report on the short and mid-term mortality. The average age of our cohort was 82 with 56% male patients. The average age for the three groupings decreased slightly with time but is comparable to UK TAVI registry data, which reported an average age of 81 and 47% male patients [6].
We achieved mortality tracking for all of our patients over the six-year period and demonstrated a 30-day mortality of 9.5%. This rate was similar to the UK TAVI data of 7.1% and also was similar to rates reported in the Canadian TAVI registry of 10.4% [6], [7]. We compared favorably with a one-year and two-year survival of 83% and 71% respectively versus 82% and 73% in the UK TAVI registry [6]. Whilst we demonstrated a marginal improvement in 30-day mortality with increasing procedural experience this did not translate to improved survival at either one or two years. The majority of early deaths were cardiac in nature related to acute arrhythmic events or cardiac failure. Beyond the initial 30 days we noted a decrease in deaths related to cardiac etiologies with mortality driven by cancer and sepsis similar to that described in the Dutch TAVI registry [8].
The average EURO II score of 9 for the entire cohort represented a high-risk patient grouping, which had been turned down for conventional surgical valve replacement. We did not find a correlation between the EURO II score and 30-day mortality post implantation. This is not dissimilar to other published series, which found the EURO score was predictive only in the highest quartile [6]. Lung et al. in analyzing their data found the biggest predictors of early death to be age, severity of symptoms and other comorbidities [9]. In our series we noted a GFR < 30 mls/min as the strongest predictor of 30 day and one year mortality.
We observed a pacing rate of 13.5% that did not differ by preprocedural rhythm or access site but was significantly different by valve type with Corevalve implants having a significantly higher pacing rate post procedure. The mechanisms of which are well described in the published literature [10]. Our stroke rate of 4.7% was similar to the observed rate of 6.1% in the PARTNER trial but higher than the 2.1% in the recent UK TAVI registry [6]. We believe this reflects our default approach of transfemoral TAVI that is associated with a higher stroke rate when compared to transapical access [11].
Observed major vascular complications occurred in 12% of patients and were a driver of 30-day mortality. There was a reduction in the rate of major vascular complications with increasing procedural experience and smaller sheath sizes with the newer valve designs. The association between postprocedural AR and outcomes has been widely reported but given that only 6 patients had moderate to severe AR we were unable to show a mortality difference. There was also no significant difference in the rates of AR by valve type.
In conclusion we have shown that TAVI in Ireland has blossomed from its inception and is outcomes are comparable to other international registries. With increasing procedural experience there has been a reduction in length of stay and comparable 30-day, one year and two year mortality. Short-term outcome continues to be difficult to predict. The inception of a national database will help us better understand our population and consolidate our data going forward.
Contributor Information
A. Bajrangee, Email: amritbajrangee@rcsi.ie.
J.J. Coughlan, Email: jjcoughlan@gmail.com.
S. Teehan, Email: steehan@stjames.ie.
C. O'Connor, Email: COconnor@stjames.ie.
R.T. Murphy, Email: rtmurphy@stjames.ie.
B. Foley, Email: bfoley@stjames.ie.
C. Daly, Email: cdaly@stjames.ie.
D. Burke, Email: dburke@stjames.ie.
A.O. Maree, Email: andrew.maree@gmail.com.
P.A. Crean, Email: pacrean@stjames.ie.
References
- 1.Manning W. Asymptomatic aortic stenosis in the elderly. JAMA. 2013;310(14):1490. doi: 10.1001/jama.2013.279194. [DOI] [PubMed] [Google Scholar]
- 2.Czarny M., Resar J. Diagnosis and management of valvular aortic stenosis. CMC. 2014;15 doi: 10.4137/CMC.S15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaccarotella C., Mongiardo A., Indolfi C. Pathophysiology of aortic stenosis and approach to treatment with percutaneous valve implantation. Circ. J. 2011;75(1):11–19. doi: 10.1253/circj.cj-10-1105. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Wahab M., Jose J., Richardt G. Transfemoral TAVI devices: design overview and clinical outcomes. EuroIntervention. 2015;14(W):W114–W118. doi: 10.4244/EIJV11SWA33. [DOI] [PubMed] [Google Scholar]
- 5.Cribier A. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 6.Ludman P., Moat N., de Belder M., Blackman D., Duncan A., Banya W. Transcatheter aortic valve implantation in the United Kingdom clinical perspective. Circulation. 2015;131(13):1181–1190. doi: 10.1161/CIRCULATIONAHA.114.013947. [DOI] [PubMed] [Google Scholar]
- 7.Rodés-Cabau J., Dumont E., De La Rochelliére R., Doyle D., Lemieux J., Bergeron S. Feasibility and initial results of percutaneous aortic valve implantation including selection of the transfemoral or transapical approach in patients with severe aortic stenosis. Am. J. Cardiol. 2008;102(9):1240–1246. doi: 10.1016/j.amjcard.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Van Mieghem N., van der Boon R., Nuis R., Schultz C., van Geuns R., Serruys P. Cause of death after transcatheter aortic valve implantation. Catheter. Cardiovasc. Interv. 2013;83(7):E277–E282. doi: 10.1002/ccd.24597. [DOI] [PubMed] [Google Scholar]
- 9.Lung B., Laouenan C., Himbert D., Eltchaninoff H., Chevreul K., Donzeau-Gouge P. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100(13):1016–1023. doi: 10.1136/heartjnl-2013-305314. [DOI] [PubMed] [Google Scholar]
- 10.Khawaja M., Rajani R., Cook A., Khavandi A., Moynagh A., Chowdhary S. Permanent pacemaker insertion after CoreValve transcatheter aortic valve implantation: incidence and contributing factors (the UK CoreValve collaborative) Circulation. 2011;123(9):951–960. doi: 10.1161/CIRCULATIONAHA.109.927152. [DOI] [PubMed] [Google Scholar]
- 11.Dewey T., Bowers B., Thourani V., Babaliaros V., Smith C., Leon M. Transapical aortic valve replacement for severe aortic stenosis: results from the nonrandomized continued access cohort of the PARTNER trial. Ann. Thorac. Surg. 2013;96(6):2083–2089. doi: 10.1016/j.athoracsur.2013.05.093. [DOI] [PubMed] [Google Scholar]