Abstract
Introduction
For women with an increased breast cancer risk, reducing excess weight and increasing physical activity are believed to be important approaches for reducing their risk. This study tested a weight loss intervention that combined commercially available technology-based self-monitoring tools with individualized phone calls.
Design
Women were randomized to a weight loss intervention arm (n=36) or a usual care arm (n=18).
Setting/Participants
Participants were women with a BMI ≥ 27.5 kg/m2 and elevated breast cancer risk recruited from the mammography clinic at the Moores Cancer Center at the University of California San Diego.
Intervention
Intervention participants used the MyFitnessPal website and phone app to monitor diet and a Fitbit to monitor physical activity. Participants received 12 standardized coaching calls with trained counselors over 6 months. Usual care participants received the U.S. Dietary Guidelines for Americans at baseline and two brief calls over the 6 months.
Main outcome measures
Weight and accelerometer-measured physical activity were assessed at baseline and 6 months. Data were collected in San Diego, CA, from 2012 to 2014 and analyzed in 2015.
Results
Participants (n=54) had a mean age of 59.5 (SD=5.6) years, BMI of 31.9 (SD=3.5), and a mean Gail Model score of 2.5 (SD=1.4). At 6 months, intervention participants had lost significantly more weight (4.4 kg vs 0.8 kg, p=0.004) and a greater percentage of starting weight (5.3% vs 1.0%, p=0.005) than usual care participants. Across arms, greater increases in moderate-to-vigorous physical activity resulted in greater weight loss (p=0.01).
Conclusions
Combining technology-based self-monitoring tools with phone counseling supported weight loss over 6 months in women at increased risk for breast cancer.
Introduction
Breast cancer is the most common cancer among U.S. women, with an estimated 230,000 new cases diagnosed in the U.S. in 2015.1 Two modifiable risk factors for breast cancer are excess weight and physical inactivity. Obesity has been shown to have a dose–response association with post-menopausal breast cancer risk: higher BMI correlates with greater risk.2–5 Similarly, research suggests that engaging in healthy lifestyle behaviors, including being physically active, may lower breast cancer risk by 12%–40%.6,7 The major mechanism linking obesity with breast cancer risk is elevated estrogen production by adipose tissue.8 However, obesity is also linked with higher insulin levels and chronic adipose tissue inflammation, which are both postulated to increase cancer risk.8 Promoting weight loss and increased physical activity may be important for reducing breast cancer risk, especially among women with elevated risk due to family history, reproductive factors, or previous breast biopsies. Many successful weight loss interventions are time consuming and resource intensive. Both eHealth and mHealth technology have the potential to reduce the cost through the use of free, commercially available websites and apps, as well as through wearable activity monitors, such as the Fitbit, which are less expensive than a gym membership or many types of exercise equipment. These technologies also have the potential to be time saving, as they can reduce participants’ time spent self-monitoring through automatic calculations of calories from amount eaten, scanning of products to get calorie information, and automatic tracking of physical activity. Finding effective ways to incorporate the key aspects of traditional weight loss programs with newer eHealth and mHealth technology that could be time saving and disseminated cost-effectively is important for weight loss interventions.9
Technology has the potential to make self-monitoring of diet and physical activity less burdensome and more effective. Self-monitoring, when used to make positive changes to diet and physical activity, has been shown to be an effective part of behavior modification programs that have resulted in successful weight loss.10–13 There are many eHealth options that can help the process for monitoring diet quicker and easier than traditional methods. Such eHealth interventions as MyFitnessPal, Lose It!, and SuperTracker have been defined as any treatment delivered via the Internet, including interventions delivered through mobile devices or tablet computers.14 Dietary monitoring options include tools such as searchable databases of calorie counts of thousands of foods, bar code readers to scan in packaged food, calculating calories per amount eaten and the total the number of calories eaten per day, and the ability to inform the user of the number of calories they have left to consume to stay within their daily calorie goal. In addition, wearable activity monitors automatically track activity and can sync via websites and apps with very little effort from the user. Although studies have found higher levels of compliance of self-monitoring using technology-based tools,15 this does not necessarily translate into greater weight loss or behavior change. In a trial with overweight primary care patients, simply introducing a smartphone app for tracking diet and physical activity (MyFitnessPal) did not result in significantly greater weight loss than a control group.16 A recent meta-analysis found that even when eHealth-only interventions produce statistically significantly weight loss, participants in eHealth programs frequently had less weight loss than has been seen in traditional group-based weight loss programs.9
Integrating technology for self-monitoring into traditional weight loss interventions, such as group- and individual-based counseling, may have great potential to support weight loss. The meta-analysis of Hutchesson et al.9 reported more weight loss in eHealth interventions that incorporated face-to-face sessions than in interventions that utilized only eHealth components. In addition, they found greater weight loss when objective measurement devices, such as wearable physical activity monitors, were added to traditional weight loss interventions. Many of the studies in their review used study-specific eHealth components (e.g., website developed only for study participants) and physical activity monitors that are not commercially available, limiting the potential “real-world” applicability of the interventions.
The current study sought to combine new commercially available technology with traditional weight loss counseling to test an intervention with potential for dissemination. The aim of the current study was to examine the efficacy of a weight loss intervention, with technology-based self-monitoring of diet and physical activity and phone counseling, compared with a control group among women at increased risk of breast cancer. It was hypothesized that participants in the intervention arm would lose more weight and engage in more minutes of moderate-to-vigorous intensity physical activity (MVPA) per week than women in the usual care arm.
Methods
This RCT tested a weight loss intervention that combined use of MyFitnessPal and a Fitbit One activity tracker with traditional phone-based counseling, versus a usual care group, among middle-aged and older women at elevated risk for developing breast cancer. Data were collected from 2012 to 2014 and all procedures were approved by the University of California, San Diego Human Research Protections Program.
Study Setting and Participants
To be eligible, women had to have a BMI ≥ 27.5 kg/m2 and be aged 40–75 years. They also had to be at increased risk of breast cancer, defined as having a Gail Model score of ≥ 1.7 (indicating a 5-year incident breast cancer risk of ≥ 1.7%),17,18 or a previous history of ductal or lobular carcinoma in situ. Women were excluded if they reported performing > 150 minutes/week of MVPA, were enrolled in another dietary or physical activity trial, did not have regular access to high-speed Internet, were not fluent in English, or had any medical/psychological condition or other problem that would interfere with participation.
Participants were recruited through the mammography clinic at the Moores Cancer Center at the University of California (UC) San Diego. Identification of potential participants was facilitated by the Athena UC San Diego Breast Imaging Registry, a project of the UC Athena Breast Health Network. Those patients from the registry who met the criteria for age, BMI, and breast cancer risk (or history of ductal or lobular carcinoma in situ) were contacted by phone to solicit their interest and eligibility. Those who were both eligible and interested were then scheduled for a baseline visit at the UC San Diego Moores Cancer Center.
At the baseline visit, the study coordinator obtained written informed consent from the participants, measured height and weight, and provided materials for the completion of the web-based baseline questionnaire. Participants were taught how to wear the ActiGraph accelerometer, were asked to wear it for at least 12 hours/day for 7 days, and provided with a return mailer.
A web-based application was used to randomly assign each participant with 2:1 probability to either the technology plus phone-based intervention group or to the usual care group. The allocation sequence was developed by the Moores Cancer Center Statistics Shared Resource. Staff members who randomized participants were blinded to the allocation sequence. Participants were notified of their group assignment via phone and received intervention materials by postal mail.
Intervention
Participants assigned to the intervention group received a 6-month weight loss intervention that focused on the development and practice of self-monitoring and self-regulatory skills. Participants were given a weight loss goal to lose 10% of their starting weight. To achieve this goal, they were encouraged to engage in at least 150 minutes/week of MVPA and restrict calories at a level sufficient to induce initial weight loss of 1–2 lb/week (approximate deficit of 500 kcal/day). Dietary goals emphasized increased intake of fruits, vegetables, and fiber, and decreased intake of unhealthy fats and refined grains.
To assist with reducing calories, participants were instructed to self-monitor their diet with MyFitnessPal, which is a popular and free electronic calorie-counting tool. It can be accessed online or through a smartphone and has a database of >3 million foods with an easy-to-use interface. MyFitnessPal asks the user to enter their current weight, goal weight, and goal rate of weight loss (limited to 0.5–2 lb/week) to provide the user with a personalized daily calorie goal. The calorie goal automatically adjusts when a participant updates their weight in the website or phone app. MyFitnessPal makes self-monitoring easier by saving common foods, calculating calories from recipes, and the app includes a barcode scanner for store-bought foods. Users are encouraged to enter consumed foods in real time, and are provided immediate feedback on how many calories are left until their daily goal is reached. Real-time reports can also be generated to show weight trend, caloric intake in the past week, and nutritional summaries of their diet.
To support changes in physical activity, each participant was given a Fitbit One clip-on tracker. The Fitbit is an accelerometer-based activity meter that provides real-time feedback of number of steps taken and minutes of moderate-intensity activity. The Fitbit wirelessly uploads data to a website that provides graphical visualizations of daily activity patterns. Fitbit and MyFitnessPal accounts can be connected so that all collected information can be conveniently assessed from one web-based portal.
Participants also received 12 phone calls (30 minutes each) over the 6-month intervention period. These calls were delivered by trained lay coaches following a protocol previously shown to be effective in achieving major dietary change, 19–21 physical activity promotion,22 and short-term weight loss.22 Each participant was matched with a single coach to provide continuity throughout the intervention. The schedule of these sessions was designed to provide maximum support and training during the early phase of behavior change, followed by a gradual transition to greater self-reliance. Participants received weekly calls for the first 2 months, biweekly calls for Month 3, and then monthly calls for Months 4 and 5. Coaches would try calling a participant three times before a call was considered missed. The phone counseling was based on Social Cognitive Theory23 and followed a phased, step-wise approach focused on: (1) helping the participant to establish a series of short-term goals; and (2) assisting the participant to evaluate performance in a manner that would maintain or improve self-efficacy. Each call included a specific behavioral focus. Example topics include meal planning, increasing vegetable intake, reducing refined carbohydrates, increasing fiber, increasing daily steps, and increasing moderate-intensity physical activity. Each participant received a manual that included detailed information on these topics.
Usual Care Group
Participants assigned to the usual care group received a copy of the U.S. Dietary Guidelines for Americans. To maintain engagement with the study and reduce loss to follow-up, they also received two brief 15-minute phone calls, one during Month 2 and one during Month 5. During the calls, participants were asked if they wanted to set a weight loss or exercise goal or if they had any nutrition-related questions. These calls did not include in-depth coaching or recommendations for diet or physical activity change.
Outcome Measures
A standard stadiometer was used to measure height to the nearest 0.1 cm. Weight was measured on a digital scale to the nearest 0.1 kg. The measurement was taken twice and the mean of both readings was used. BMI was calculated based on these measurements.
Participants completed questionnaires online prior to randomization and again at the 6-month follow-up. The baseline questionnaire included demographics, technology use, and medical history.
Prior to randomization and again at 6 months, each participant wore an ActiGraph GT3X+ accelerometer (ActiGraph, Pensacola, FL) during their waking hours for 7 consecutive days. At both time points, data were promptly downloaded after the 7-day wear period and screened for completeness and irregularities. Participants were asked to re-wear the accelerometer if it was not worn for at least 10 hours/day for 5 days. Ninety consecutive zeroes were designated as non-wear time and standard calibration thresholds were used to aggregate data into minutes spent in sedentary, light, moderate, and vigorous activity using the Freedson cut points.24 The accelerometer also provided data for amount of time spent in bouts (≥10 continuous minutes) of MVPA.
Statistical Analysis
Baseline characteristics between the two groups were assessed using t-tests for continuous variables and chi-square or two-tailed Fisher’s exact tests (when warranted by small cell counts) for categorical variables. Physical activity measures between groups were analyzed using a longitudinal model of the subject’s day level activity, time, and time-by-intervention interaction, controlling for ActiGraph wear time. In these models, individual day–level activity was nested within each assessment time point. This model was used to provide estimates for baseline differences as well as differences in the change (6 months to baseline) between groups. All longitudinal analyses were carried out with a subject-level random intercept using an unstructured covariance structure, as determined by model Akaike Information Criterion comparisons. All outcome variables were modeled on a linear, continuous scale with one exception. Physical activity in bouts was analyzed using a negative binomial regression model to account for the heavy right skew and overdispersed nature of the variable, due to the high number of participants with 0 minutes of MVPA in ≥ 10-minute bouts.
Linear regression models examined the difference in weight between the two groups at baseline and at 6 months, controlling for baseline weight in the 6-month model. Change in weight between groups was analyzed using a longitudinal model. The analyses were carried out with a subject-level random intercept using a standard variance components covariance structure, determined by model Akaike Information Criterion comparisons. As a sensitivity analysis, change in weight between groups was also analyzed using a linear regression model of change in weight by intervention group, controlling for baseline weight. As the linear regression model of change was not meaningfully different from the longitudinal model, longitudinal model results are presented.
Percentage weight loss between groups was analyzed using a linear regression model of weight by intervention group, controlling for baseline weight. Comparison of weight loss > 5% was analyzed using a logistic regression model of weight loss > 5% (yes/no) by intervention group, controlling for baseline weight.
In the analysis of physical activity and percentage weight loss, total MVPA at the 6-month follow-up was used at the outcome variable and percentage weight loss as the independent variable because of the repeated nature of the day-level activity measurements. This model was then used to assess significance of the association between activity and percentage weight change and was used to obtain model estimates for activity at varying levels of weight loss. These estimates were used to construct a graph of percentage weight loss by activity. Percentage weight loss was calculated as the percentage change in weight at 6 months versus baseline. This model controlled for mean-centered baseline MVPA, mean-centered baseline weight, and intervention group.
Demographic characteristics were not included as covariates because baseline analysis revealed that the randomization had been successful in eliminating demographic differences by treatment group. This non-inclusion was confirmed by sensitivity analysis.
Missing data were assumed missing at random and were accounted for in the longitudinal random effects models by using a likelihood-based estimation method, which uses all available data and does not ignore subjects with missing data. In analysis models that used only a single time point, a complete cases analysis was carried out, with those missing data dropped from the analysis. All statistical analyses were conducted using SAS, version 9.4 in 2015.
Results
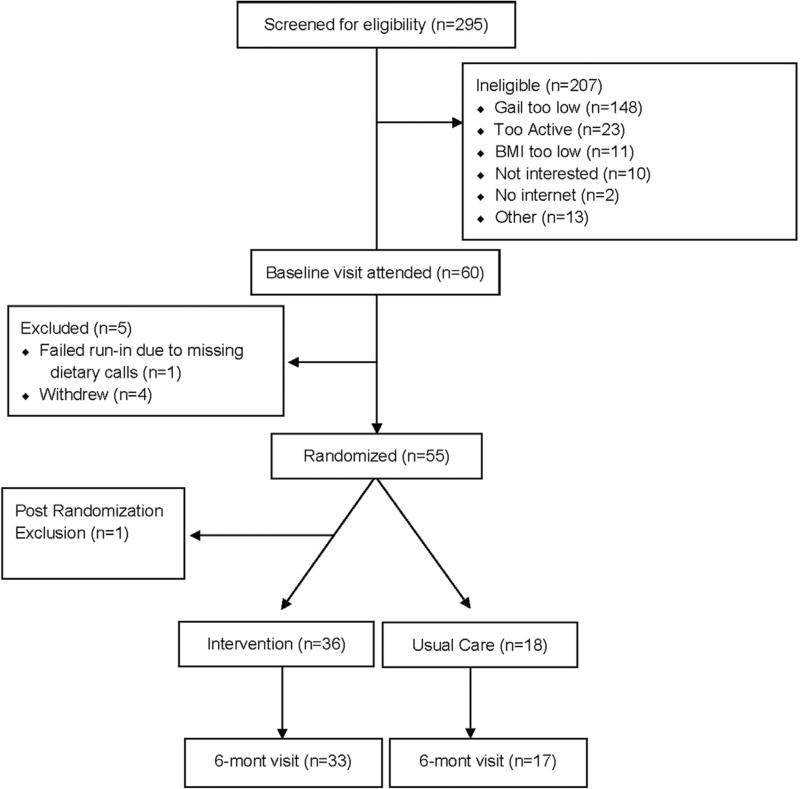
A total of 295 women were screened for eligibility; of those, 88 were eligible and 60 attended the baseline visit. A total of 54 women were enrolled in the study (intervention, n=36; usual care, n=18). At 6 months, three participants in the intervention group and two in the usual care group were lost to follow-up (87% retention rate). Reasons for loss to follow-up were related to lack of time due to family issues (n=3) and being unable to contact (n=1). A CONSORT diagram is provided in Figure 1. Adherence to the intervention was high, with 92% of the intervention arm completing at least ten of 12 counseling phone calls. Self-reported adherence to using the Fitbit was high, with 77% reporting wearing it every day, and 19% reporting wearing it 4–6 days/week throughout the 6-month intervention.
Figure 1.
CONSORT diagram.
Participants were, on average, aged 59.5 (SD=5.6; range, 47–69) years. The mean Gail Model score was 2.5 (SD=1.4), indicating a mean 2.5% likelihood of developing invasive breast cancer within 5 years. Fifty percent of participants had undergone at least one biopsy and 37% had at least one first-degree relative with breast cancer. Mean BMI was 31.9 kg/m2 (SD=3.5; range, 27–40). At baseline, participants were performing 14.4 (SD=17.7) minutes/day of accumulated MVPA and 46.3% of participants had at least one 10-minute bout of MVPA. The intervention group had significantly more minutes of MVPA in 10-minute bouts at baseline than the usual care group (p<0.0001). Table 1 provides baseline characteristics.
Table 1.
Baseline Characteristics of Women at Increased Risk for Breast Cancer Who Were Enrolled in San Diego, CA From 2012–2014 (n=54)
| Characteristics | Intervention group | Usual care group | p-value |
|---|---|---|---|
| n | 36 | 18 | – |
| Demographics | |||
| Age, years, M (SD) | 59.4 (5.6) | 59.8 (5.9) | 0.787 |
| College degree or higher, % | 39.4 | 66.7 | 0.836 |
| White, % | 91.7 | 100 | 0.288 |
| Non-Hispanic, % | 19.44 | 0 | 0.082 |
| Adiposity, M (SD) | |||
| Weight, kg | 86.3 (10.2) | 85.3 (10.5) | 0.744 |
| BMI, kg/m2 | 32.2 (3.4) | 31.3 (3.7) | 0.351 |
| Physical activity (minutes/day), M (SD) | |||
| Daily MVPA | 17 (20.3) | 10 (10.2) | 0.149 |
| Daily MVPA in 10-minute bouts | 7 (18.6) | 1 (5.3) | < 0.0001 |
| Breast cancer risk | |||
| Gail model score, M (SD) | 2.6 (1.7) | 2.5 (0.6) | 0.798 |
| ≥ 1 Biopsy, % | 52.8 | 44.4 | 0.564 |
| ≥ 1 FDR with breast cancer, % | 33.3 | 44.4 | 0.425 |
Note: Boldface indicates statistical significance (p<0.05).
FDR, first-degree relative; MVPA, moderate-to-vigorous intensity physical activity.
At 6 months, the intervention group lost a mean of 4.4 (SD=4.3) kg, compared with a loss of 0.8 (SD=3.8) kg in the usual care group (between-group, p=0.005). As a proportion of starting body weight, the intervention group lost 5.3% of baseline weight by 6 months versus a 1% loss in the usual care group (p=0.005). Significantly more women in the intervention group lost 5% of their starting weight than in the control group (42.4% vs 11.8%, p=0.028) (Table 2).
Table 2.
Changes in Weight and Physical Activity by Study Arm (Baseline, n=54, 6 Months, n = 50)
| Outcomes | Intervention group | Usual care group | p-value |
|---|---|---|---|
| Weight (kg), M (SD) | |||
| Baseline | 86.3 (10.2) | 85.3 (10.5) | 0.744 |
| 6 months | 82.1 (12.0) | 85.1 (11.3) | 0.005 |
| 6 months to baseline | −4.4 (4.3) | −0.5 (3.8) | 0.005 |
| Percent weight loss from baseline | |||
| 6 months, M (SD) | −5.3 (5.3) | −1.0 (4.9) | 0.005 |
| Lost >5%, n(%) | 14 (42.4) | 2 (11.8) | 0.028 |
| Daily MVPA, minutes/day, M (SD) | |||
| Baseline | 17 (20.3) | 10 (10.2) | 0.149 |
| 6 months | 32 (25.7) | 22 (18.9) | 0.020 |
| 6 months to baseline | 15 (14.2) | 11 (10.1) | 0.131 |
| Daily MVPA in 10-minute bouts, M (SD) | |||
| Baseline | 7 (18.6) | 1 (5.3) | < 0.0001 |
| 6 months | 13(24) | 4 (10.2) | <0.0005 |
| 6 months to baseline | 6 (12.9) | 2 (4.9) | 0.941 |
Note: Boldface indicates statistical significance (p<0.05).
MVPA, moderate-to-vigorous intensity physical activity.
The intervention group increased total MVPA by 15.01 (SD=14.2) minutes/day versus an increase of 10.9 (SD=10.1) minutes/day in the usual care group. Although the difference at 6 months was statistically significant (p=0.02), the difference between the changes in each group was not (p=0.l3). For MVPA accumulated in bouts of at least 10 minutes, the intervention group increased by 6.1 (SD=12.9) minutes/day versus an increase of 1.8 (SD=4.9) minutes/day in the usual care group. However, this difference in change between groups was not statistically significant, given the large difference in this variable at baseline (p=0.94) (Table 2).
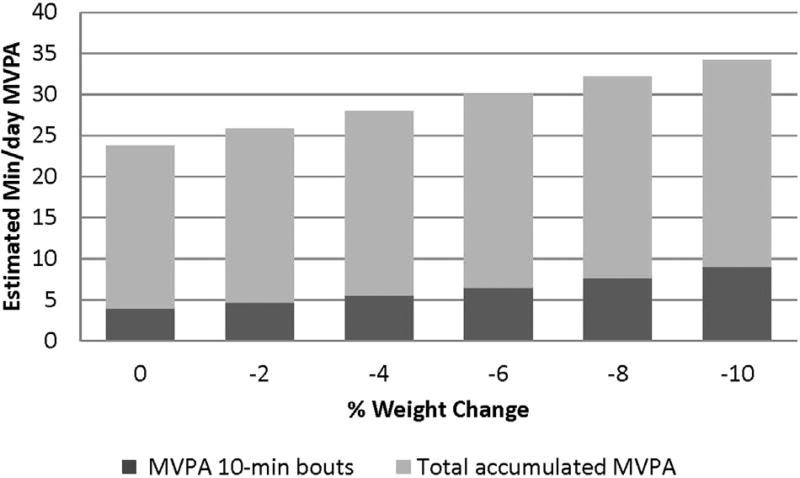
Across both groups, percentage weight loss at 6 months was significantly associated with minutes/day of accumulated MVPA (p=0.0l) and minutes/day of MVPA in 10-minute bouts (p=0.028) (Figure 2), after controlling for baseline MVPA, baseline weight, and intervention group. Greater weight loss was associated with more total MVPA and MVPA in 10-minute bouts. Specially, 10% weight loss was associated with engaging in 34.26 minutes/day of total MVPA and 9.04 minutes/ day of MVPA in 10-minute bouts.
Figure 2.
Percent weight loss at 6-months by estimated MVPA; controlling for intervention group, baseline physical activity, and baseline weight (n=50).
MVPA, moderate-to-vigorous intensity physical activity.
Discussion
Combining technology-based self-monitoring tools with traditional phone counseling was successful in helping women at increased risk for breast cancer lose weight. The combined intervention achieved had a greater proportion of participants that achieved clinically meaningful weight loss (≥ 5% of starting weight) compared with usual care. Five percent weight loss is associated with many health benefits, including reduced risk of postmenopausal breast cancer, cardiovascular disease, and Type 2 diabetes.25–27 Although the intervention group significantly increased their daily MVPA and engaged in more MVPA at 6 months than the usual care group, the change in MVPA was not statistically different between the two groups. As there were no group differences in the change in MVPA, it was hypothesized that the group differences in weight loss were due to changes in diet. This postulation is consistent with previous research that has shown that dietary changes are more effective for weight loss than changes in physical activity.28 However, in both arms, greater weight loss was associated with more MVPA and bouts of MVPA, suggesting that greater physical activity may have been needed to get participants to the study goal of 10% weight loss.
Findings are consistent with previous research indicating that combining technology with traditional weight loss components is effective for losing weight. The current study achieved a mean 4.4-kg weight loss, which is similar to a meta-analysis that found a mean difference weight loss of 3.7 kg among eHealth interventions that incorporated more-traditional weight loss intervention components.9 However, only a few of the interventions use publicly available technologies.9,29 Having each study create their own eHealth components can be time consuming and expensive, with little promise for updating and dissemination. Commercially available websites like myfitnesspal.com and fitbit.com are free to use and are continually updated with new features and better-designed consumer interfaces. Surprisingly, in the current study, there was not a significant between-group difference in change in MVPA. However, it was hypothesized that wearing a Fitbit may have provided a sense of accountability and a reminder of their weight loss effort throughout the day. The findings from the current study suggest that capitalizing on existing technology and combining it with traditional phone counseling can help women achieve meaningful weight loss while maintaining potential for dissemination.
Limitations
Although the use of commercially available technology could be considered a strength of this study, there are limitations inherent in using commercially available products. The products are updated over time with new added features, so there is less direct control over the intervention than is typical in traditional intervention trials. For example, the Fitbit app added a feature that allows use of the phone’s GPS to track activity, which is helpful for self-monitoring when someone forgets to wear their Fitbit. Another limitation of using commercially available products was that participants’ use of the websites or the Fitbit was not able to be directly measured. However, Fitbit and MyFitnessPal do have an open application program interface, which means that future research studies could build in the capability of pulling user data from the websites. Other limitations include the lack of adherence data regarding using MyFitnessPal and the lack of valid data on energy intake. In addition, activity monitors such as the Fitbit promote aerobic and non-sedentary activity, but do not address strength training or balance, which can have important roles in reducing health risks in overweight/obese adults. It should also be noted that the small sample size of this pilot study may have limited the ability to detect a significant between-group difference for change in MVPA. Also, as this was a pilot study, the combined intervention was not compared with phone counseling only; therefore, the benefit of the technology-based self-monitoring tools could not be teased apart from phone counseling. A final limitation concerns generalizability of the findings, given that the study sample was largely non-Hispanic white and well educated.
Conclusions
Combining technology-based self-monitoring tools with phone counseling supported women at increased risk for breast cancer to lose weight over 6 months. Commercially available self-monitoring tools have continued to improve and grow in popularity since the initiation of this trial in 2012. These tools provide a less-burdensome way to track diet and physical activity than paper diaries, as evidenced by greater adherence to self-monitoring,30,31 which could make them important adjuncts to traditional weight loss counseling. More research is needed on the integration of commercially available technology into weight loss research, especially in populations at increased risk of developing cancer.
Acknowledgments
This study was funded by the University of California Athena Breast Health Network, via the Safeway Foundation and University of California Office of the President, and by a gift from Carol Vassiliadis and family. Dr. Cadmus-Bertram is supported by grant K07CA178870 and Dr. Hartman by grant K07CA181323, both from the National Cancer Institute.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015;1(5):611–621. doi: 10.1001/jamaoncol.2015.1546. http://dx.doi.org/10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123(3):641–649. doi: 10.1007/s10549-010-1116-4. http://dx.doi.org/10.1007/s10549-010-1116-4. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status —a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. http://dx.doi.org/10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. http://dx.doi.org/10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137(3):869–882. doi: 10.1007/s10549-012-2396-7. http://dx.doi.org/10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 7.McTiernan A. Behavioral risk factors in breast cancer: can risk be modified? Oncologist. 2003;8(4):326–334. doi: 10.1634/theoncologist.8-4-326. http://dx.doi.org/10.1634/theoncologist.8-4-326. [DOI] [PubMed] [Google Scholar]
- 8.Rose DP, Gracheck PJ, Vona-Davis L. The interactions of obesity, inflammation and insulin resistance in breast cancer. Cancers (Basel) 2015;7(4):2147–2168. doi: 10.3390/cancers7040883. http://dx.doi.org/10.3390/cancers7040883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchesson MJ, Rollo ME, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta-analysis. Obes Rev. 2015;16(5):376–392. doi: 10.1111/obr.12268. http://dx.doi.org/10.1111/obr.12268. [DOI] [PubMed] [Google Scholar]
- 10.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. http://dx.doi.org/10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eat Behav. 2009;10(4):220–227. doi: 10.1016/j.eatbeh.2009.07.004. http://dx.doi.org/10.1016/j.eatbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166(15):1620–1625. doi: 10.1001/archinte.166.15.1620. http://dx.doi.org/10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 13.Tate DF, Wing RR, Winett RA. Using Internet technology to deliver a behavioral weight loss program. JAMA. 2001;285(9):1172–1177. doi: 10.1001/jama.285.9.1172. http://dx.doi.org/10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 14.Eysenbach G CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of web-based and mobile health interventions. J Med Internet Res. 2011;13(4):e126. doi: 10.2196/jmir.1923. http://dx.doi.org/10.2196/jmir.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self-monitoring, but not dietary quality, improves with use of smartphone app technology in an 8-week weight loss trial. J Nutr Educ Behav. 2014;46(5):440–444. doi: 10.1016/j.jneb.2014.04.291. http://dx.doi.org/10.1016/j.jneb.2014.04.291. [DOI] [PubMed] [Google Scholar]
- 16.Laing BY, Mangione CM, Tseng CH, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161(10 suppl):S5–S12. doi: 10.7326/M13-3005. http://dx.doi.org/10.7326/M13-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. http://dx.doi.org/10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 18.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99(23):1782–1792. doi: 10.1093/jnci/djm223. http://dx.doi.org/10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 19.Newman VA, Thomson CA, Rock CL, et al. Achieving substantial changes in eating behavior among women previously treated for breast cancer—an overview of the intervention. J Am Diet Assoc. 2005;105(3):382–391. doi: 10.1016/j.jada.2004.12.008. quiz 488. http://dx.doi.org/10.1016/j.jada.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Madlensky L, Natarajan L, Flatt SW, Faerber S, Newman VA, Pierce JP. Timing of dietary change in response to a telephone counseling intervention: evidence from the WHEL study. Health Psychol. 2008;27(5):539–547. doi: 10.1037/0278-6133.27.5.539. http://dx.doi.org/10.1037/0278-6133.27.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce JP, Newman VA, Natarajan L, et al. Telephone counseling helps maintain long-term adherence to a high-vegetable dietary pattern. J Nutr. 2007;137(10):2291–2296. doi: 10.1093/jn/137.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadmus-Bertram L, Wang JB, Patterson RE, Newman VA, Parker BA, Pierce JP. Web-based self-monitoring for weight loss among overweight/obese women at increased risk for breast cancer: the HELP pilot study. Psychooncology. 2013;22(8):1821–1828. doi: 10.1002/pon.3219. http://dx.doi.org/10.1002/pon.3219. [DOI] [PubMed] [Google Scholar]
- 23.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 24.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. http://dx.doi.org/10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 25.Harvie M, Howell A, Vierkant RA, et al. Association of gain and loss of weight before and after menopause with risk of postmenopausal breast cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2005;14(3):656–661. doi: 10.1158/1055-9965.EPI-04-0001. http://dx.doi.org/10.1158/1055-9965.EPI-04-0001. [DOI] [PubMed] [Google Scholar]
- 26.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. http://dx.doi.org/10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Cardiology/American Heart Association Task Force on Practice Guidelines OEP. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring) 2014;22(suppl 2):S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 28.Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P Behavioural Weight Management Review Group. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557–1568. doi: 10.1016/j.jand.2014.07.005. http://dx.doi.org/10.1016/j.jand.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine DM, Savarimuthu S, Squires A, Nicholson J, Jay M. Technology-assisted weight loss interventions in primary care: a systematic review. J Gen Intern Med. 2015;30(1):107–117. doi: 10.1007/s11606-014-2987-6. http://dx.doi.org/10.1007/s11606-014-2987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med. 2012;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. http://dx.doi.org/10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieffers JR, Hanning RM. Dietary assessment and self-monitoring with nutrition applications for mobile devices. Can J Diet Pract Res. 2012;73(3):e253–e260. doi: 10.3148/73.3.2012.e253. http://dx.doi.org/10.3148/73.3.2012.e253. [DOI] [PubMed] [Google Scholar]