Abstract
Purpose
To report the influence of radiation therapy (RT) dose and surgical pathology variables on disease control and overall survival (OS) in patients treated for high-risk neuroblastoma at a single institution.
Methods and Materials
We conducted a retrospective study of 67 high-risk neuroblastoma patients who received RT as part of definitive management from January 2003 until May 2014.
Results
At a median follow-up of 4.5years, 26patients (38.8%) failed distantly; 4 of these patients also failed locally. One patient progressed locally without distant failure. Local control was 92.5%, and total disease control was 59.5%. No benefit was demonstrated for RT doses over 21.6 Gy with respect to local relapse—free survival (P = .55), disease-free survival (P = .22), or OS (P= .72). With respect to local relapse—free survival, disease-free survival, and OS, no disadvantage was seen for positive lymph nodes on surgical pathology, positive surgical margins, or gross residual disease. Of the patients with gross residual disease, 75% (6 of 8) went on to have no evidence of disease at time of last follow-up, and the 2 patients who failed did so distantly.
Conclusions
Patients with high-risk neuroblastoma in this series maintained excellent local control, with no benefit demonstrated for radiation doses over 21.6 Gy, and no disadvantage demonstrated for gross residual disease after surgery, positive surgical margins, or pathologic lymph node positivity. Though the limitations of a retrospective review for an uncommon disease must be kept in mind, with small numbers in some of the subgroups, it seems that dose escalation should be considered only in exceptional circumstances.
Introduction
Outcomes for high-risk neuroblastoma have improved significantly in recent years. Cure was a rarity in historical series (1–3), but with advancement of aggressive multimodal therapy, rates of progression-free survival often approach or exceed 50% (4–9). Given the inherent toxicities of radiating pediatric patients, and because systemic failure remains the obstacle to cure in the vast majority of failures (4, 5, 10, 11), minimizing dosage of consolidative radiation therapy (RT) to the primary site after surgical resection remains a valid concern.
Modern series, consisting of varying radiation doses and fractionation schema, report excellent local control rates that range from 84% to 97% (4–8, 11, 12).As a seemingly intuitive means of increasing local control probability, many of these experiences incorporate what is generally a nonstandardized pattern of dose escalation for patients with gross residual disease at the primary site after induction chemotherapy and surgery. Some authors have explicitly suggested utility of increased radiation dosage to residual disease after surgery (12, 13).Others have conceded that definitively establishing the role of RT for any one subgroup of high-risk neuroblastoma patients will be impossible even in a national trial setting (14). With that in mind, current practice, bolstered by inclusion into the guidelines from the recently completed Children’s Oncology Group (COG) phase 3 high-risk neuroblastoma trial, ANBL0532, shifted to treating the postinduction chemotherapy, preoperative tumor volume to 21.6 Gy, with a 14.4-Gy boost to unresected gross residual disease at the primary site. ANBL0532 closed to accrual in February 2012, and although results are unreported to date, this radiation protocol is now taken as the standard of care by most institutions.
With a significant portion of treated children now surviving into adulthood, there is now a cohort for whom the incidence of late side effects of radiation has been documented, with an apparent threshold for increased late toxicities above 30 Gy (12, 15, 16). Thus, dose escalation in children is not inconsequential in terms of increased toxicity. We sought to offer another large cohort of patients, treated at our institution via a modern multimodality paradigm, to help identify whether a subset of patients may benefit from RT dose escalation based on unfavorable surgical, pathologic, or radiologic characteristics.
Methods and Materials
With institutional review board approval, we conducted a retrospective chart review of all high-risk neuroblastoma patients who received radiation therapy as part of definitive management from January 2003 until May 2014. All 67 patients were diagnosed with high-risk neuroblastoma. Patients were either International Neuroblastoma Staging System stage 3 or 4, and were high-risk as defined by the COG Neuroblastoma Risk Stratification System. This COG high-risk definition consisted of: (1) stage 4 disease in patients older than 1 year; (2) stage 3 disease with unfavorable histology in patients older than 1 year; (3) MYCN-amplified stage 2, 3, or 4S disease in patients older than 1 year; or (4) MYCN-amplified stage 4 disease in patients younger than 1 year (17).
Staging workup included computed tomography (CT) of the chest, abdomen, and pelvis; 123I-meta-iodobenzylguanidine (MIBG) scan; technetium-99 bone scan; and bilateral bone marrow aspirates and biopsy. Occasionally positron emission tomography—CT was used, particularly for patients with MIBG non-avid disease.
After intensive multiagent induction chemotherapy (18), response was typically evaluated by bone scan, MIBG, CT, and sampling of the bone marrow. Maximum safe surgical resection of the primary site was attempted on all patients except in the uncommon instance of complete or near-complete response to chemotherapy per imaging (2 patients). Gross total resection (GTR) was defined as meeting 2 criteria: (1) visible removal of all macroscopically viewable tumor per surgeon’s operative note; and (2) no clearly stated residual disease greater than 1 cm3 per postoperative CT and MIBG radiology/nuclear medicine reports. The operative note was reviewed to determine whether dedicated lymph node dissection was performed; if no explicit lymph node dissection was performed, surgeons typically sent several incidentally found lymph nodes as part of the surgical specimen. Surgical margin status and lymph node involvement was determined per review of surgical pathology reports. Postsurgical, pre-radiation imaging reports were reviewed for the presence of residual gross disease at the primary site.
After recovery from surgery, patients underwent single or tandem autologous stem cell transplantation after myeloablative chemotherapy conditioning. No patients underwent total body irradiation as part of transplant conditioning. After recovery from myelosuppression, all patients were treated with external beam radiation therapy to the primary tumor site. A significant portion of patients also underwent concurrent RT to metastatic sites as part of postconsolidation local control. Computed tomography—based treatment planning was used to deliver megavoltage (MV) photons via external beam radiation therapy in all cases. For consolidative RT to the primary site, the gross tumor volume consisted of the post-induction chemotherapy, pre-surgery tumor volume, which inherently encompassed any remaining post-surgery residual disease or adjacent gross lymphadenopathy. Clinical target volume expansions were variable, and in a proportion of patients included elective nodal coverage; expansions were modified as needed to account for anatomic constraints of tumor spread. The planning target volume was typically created by expanding the clinical target volume by 1 to 2 cm; later, with the availability of on-board imaging and highly conformal techniques, including intensity modulated RT and volumetric modulated arc therapy, reduced subcentimeter planning target volume margins were sometimes used. Fraction size to the primary site was 1.5 or 1.8 Gy daily; 1 patient was treated in 1.2-Gy twice-daily fractions.
After the conclusion of RT, patients typically received maintenance cis-retinoic acid for at least 6 cycles, or 6 cycles of immunotherapy with the monoclonal anti-GD2 antibody Unituxin/interleukin-2/granulocyte-macrophage colony-stimulating factor in addition to cis-retinoic acid (19). Patients were followed after definitive treatment in pediatric hematology/oncology and radiation oncology clinics, as part of our large tertiary referral center. Only a small number of patients were lost to follow-up after a relatively short interval, mostly if parents chose to transfer care back to local facilities.
All disease relapses were correlated with: (1) status of gross residual disease or positive margins at the primary site after surgery; (2) surgical management of lymph nodes; (3) involvement of lymph nodes per surgical pathology; and (4) RT parameters.
For statistical analysis, overall survival was defined as time from date of diagnosis to death or last follow-up. Disease-free survival was defined as time from diagnosis to death, relapse (any), or last follow-up. Local relapse-free survival was defined as time from diagnosis to local relapse or last follow-up. Survival was estimated using the Kaplan-Meier method and was compared across groups using logrank tests. Additionally, Cox proportional hazards models were constructed to obtain hazard ratios and 95% confidence intervals for each variable of interest (20). In the event of small sample sizes and event totals, notably with local relapse, Firth’s penalized maximum likelihood estimation was used to reduce bias in the parameter estimates and confidence intervals, as well as handle any empty cells (21).
Results
From January 2003 until May 2014, 67 patients received RT to the primary site as part of definitive management of high-risk neuroblastoma. Patient characteristics are summarized in Table 1. Sixty-five patients underwent resection of the primary tumor. Two patients did not undergo a resection of the primary because of complete response to induction chemotherapy. Pathology reports were unavailable for 6 of the patients. Eight patients had gross disease left behind after surgical resection. Seven patients had positive surgical margins on final surgical pathology. Eight patients had dedicated lymph node dissection stated in the operative note. An additional 28 patients did not have dedicated lymph node dissections but did have incidentally excised lymph nodes sent as part of surgical pathology. Twenty-seven patients had positive lymph nodes on final surgical pathology. Number of lymph nodes examined on the surgical pathology specimens was not considered owing to the high number of instances in which conglomeration of nodal masses was seen, which precluded counting individual lymph nodes.
Table 1.
Patient characteristics (n = 67)
| Age (y) | |
| Median | 3.3 |
| Range | 9 mo-20.7 y |
| Sex | |
| Male | 40 |
| Female | 27 |
| INSS stage | |
| 3 | 3 |
| 4 | 64 |
| MYCN status | |
| Amplified | 31 |
| Nonamplified | 25 |
| Unknown | 11 |
| Primary site | |
| Abdomen/pelvis | 61 |
| Thorax | 4 |
| Combined | 2 |
| Resection extent | |
| GTR | 44 |
| STR/positive margins | 15 |
| No surgery | 2 |
| Unavailable report | 6 |
| Surgical treatment of lymph nodes | |
| Dissection | 8 |
| Sampling | 28 |
| None sent | 25 |
| Unknown | 6 |
Abbreviations: GTR = gross total resection; INSS = International Neuroblastoma Staging System; STR = subtotal resection.
Values are number of patients unless otherwise noted.
See Table 2 for radiation patterns. Median RT dose was 21.6 Gy (range, 19.8–36.0 Gy). The patient who received 19.8 Gy was intended to receive 21.6 Gy but did not receive the final fraction. Although fraction size was typically 1.8 Gy (56 patients), 10 patients were treated with 1.5-Gy fractions, and 1 patient was treated with 1.2-Gy twice-daily fractions to a total dose of 24 Gy.
Table 2.
Radiation patterns
| Radiation variable | n (%) |
|---|---|
| Primary site dose in Gy (n = 67) | |
| ≤21.6 | 48 (71.6) |
| >21.6 | 19 (28.4) |
| 19.8 | 1 (1.5) |
| 21.0* † ‡ | 7 (10.4) |
| 21.6§‖ | 40 (59.7) |
| 22.5 | 1 (1.5) |
| 23.4ঠ| 7 (10.4) |
| 24.0 | 1 (1.5) |
| 25.2*† | 3 (4.5) |
| 25.5† | 1 (1.5) |
| 30.0 | 2 (3.0) |
| 30.6† | 2 (3.0) |
| 36.0¶ | 2 (3.0) |
| Concurrent metastatic sites irradiated (range, 1–3 metastatic sites) | |
| Yes | 25 (37.3) |
| No | 42 (62.7) |
| Technique | |
| AP/PA | 39 (58.2) |
| 3D-CRT | 6 (9.0) |
| IMRT | 3 (4.5) |
| VMAT | 19 (28.4) |
| Elective lymph node coverage | |
| Yes | 25 (37.3) |
| No | 42 (62.7) |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; AP/PA = anterior—posterior/posterior—anterior beams; IMRT = intensity modulated radiation therapy; VMAT = volumetric modulated arc therapy.
One patient who failed locally received corresponding dose.
One patient with gross residual disease received corresponding dose.
One patient with positive margins received corresponding dose.
Five patients with positive margins received corresponding dose.
Three patients who failed locally received corresponding dose.
Two patients with gross residual disease received corresponding dose.
For results of the univariate analysis examining relationships between covariates and disease outcomes, please refer to Table 3. Figures 1–3 show Kaplan-Meier curves for the impact of RT dose on local relapse-free survival, disease-free survival, and overall survival. At a median follow-up of 4.5 years, 26 patients (38.8%) failed at a distant site; 4 of these patients who failed in a distant site also developed local failure within the radiation treatment field. One patient (1.5%) progressed locally without failure elsewhere. Local control was 95.5% at 2 years, 94.0% at 5 years, and 92.5% overall. Overall disease control was 59.5%. Of the patients with gross residual disease, 75% (6 of 8) went on to have no evidence of disease at time of last follow-up, and the 2 patients who failed did so distantly, with the primary site remaining controlled.
Table 3.
Outcome associations
| Variable outcome | HR | CI | P |
|---|---|---|---|
| Dose ≥21.6 Gy | |||
| LRFS | 1.45 | 0.19–11.17 | .55 |
| DFS | 1.39 | 0.68–4.02 | .22 |
| OS | 1.19 | 0.22–2.61 | .72 |
| Positive nodes | |||
| LRFS | 1.42 | 0.20–10.18 | .72 |
| DFS | 1.39 | 0.63–3.11 | .41 |
| OS | 1.19 | 0.32–4.46 | .82 |
| Dedicated lymph node dissection | |||
| LRFS | 1.88 | 0.24–14.43 | .76 |
| DFS | 1.20 | 0.43–3.37 | .87 |
| OS | 1.43 | 0.32–6.36 | .80 |
| Residual disease per CT | |||
| LRFS | 1.80 | 0.30–10.68 | .57 |
| DFS | 1.26 | 0.58–2.69 | .60 |
| OS | 1.18 | 0.35–4.03 | .85 |
| Residual disease per MIBG | |||
| LRFS | 1.90 | 0.27–13.47 | .52 |
| DFS | 0.97 | 0.43–2.14 | .88 |
| OS | 0.24 | 0.04–1.47 | .06 |
| Gross residual or positive margins | |||
| LRFS | 1.75 | 0.29–10.46 | .59 |
| DFS | 1.18 | 0.51–2.70 | .76 |
| OS | 0.61 | 0.14–2.69 | .40 |
Abbreviations: CI = confidence interval; CT = computed tomography; DFS = disease-free survival; HR = hazard ratio; LRFS = local relapse—free survival; MIBG = 123I-meta-iodobenzylguanidine; OS = overall survival.
Fig. 1.
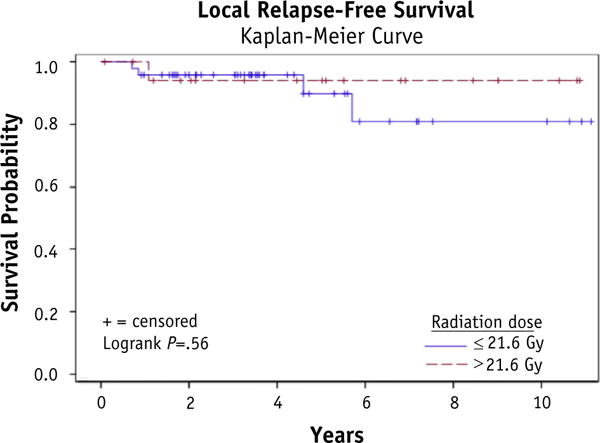
Local relapse—free survival.
Fig. 3.
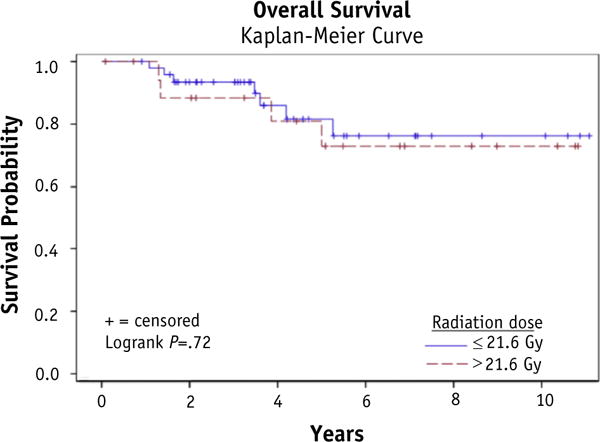
Overall survival.
Each instance of local failure was evaluated in additional detail. Two of the in-field failures occurred after lengthy interval from RT (5.1 and 3.8 years later) in the setting of diffuse relapsed disease, possibly suggesting coincidental reseeding of tumor within the prior RT field. One of these patients who failed after lengthy interval from RT initially had a diffuse paraspinal mass extending from T3 to S2 and was treated with large anterior—posterior/posterior—anterior fields covering this region after surgical resection; ultimately she relapsed not truly in the paraspinal area that was the site of her resected primary, but instead in the adjacent osseous spine. This was considered local failure because of the close proximity to the original disease and the fact that it did receive full RT dose initially, having been encompassed by the 100% isodose line; however, being years later in the setting of diffuse relapse it seems also possible that this area of disease was from secondary tumor seeding or progression of vertebral bone marrow involvement versus true in-field progression of residual tumor unsterilized from RT. The other patient eventually developed a recurrent inguinal lymph node mass that seemed to originate out of the initial radiation field but ultimately grew upward to a large extent, where it reached an in-field area of the midline retro-peritoneum and thus was counted as a local failure.
The patient who was considered to have failed in-field without distant failure had a metastasis at the base of skull that was treated with an 18-MV vertex field and 6-MV lateral fields to 21.6 Gy at 1.8 Gy per fraction. Three months later, because the soft tissue mass had not adequately decreased in size/activity and remained suspicious, the area was treated again using similar fields to 21.6 Gy in 1.2-Gy twice-daily fractions. He went on to have durable disease control.
Excluding the aforementioned patients, there were only 2 patients (3.0%) who clearly had local failure at the primary site despite consolidative radiation. One of these patients died relatively soon after treatment, with MIBG activity and magnetic resonance imaging showing probable recurrent disease in the vicinity of the treated primary site, which had received 21.6 Gy; this patient had diffuse progression of disease as her cause of death. The other patient clearly showed progression in-field in the area of the treated primary site, which had received 25.2 Gy; he simultaneously relapsed distally. Neither of these patients had gross residual disease after surgery, or positive surgical margins.
Discussion
High-risk neuroblastoma is a highly aggressive yet curable disease—increasingly so with modern treatment paradigms. Outcomes from our series, with local control of approximately 92.5% and total disease control at 59.5%, are generally consistent with the recent literature (49), representing a significant improvement from discouraging historical reports (1–3). Local control is inherently a part of curative therapy, and RT following surgical resection is a necessary component. Radiation therapy is most commonly tasked with controlling microscopic disease, because GTR is achievable in the majority of cases, and for this purpose, RT doses between 21 and 24 Gy seem to be effective (4, 6–9, 11, 22). Historically, consensus favored improved outcomes with either greater than 90% resection or GTR (23–26), though other authors have found no relation to extent of resection (5, 27–29), leaving uncertainty regarding how to compensate when residual tissue remains. Thus dose escalation to residual disease has emerged as a reasonable manner in which outcomes might be improved for this population that may be at greater risk (12).
In our series, 75% of patients (6 of 8) with gross residual disease following surgery went on to have no evidence of disease at time of last follow-up, and the 2 patients who failed did so distantly. Only 2 of these patients were treated recently enough so that guidelines were followed for dose escalation to 36 Gy. Although local control was achieved in these 2 cases, there is no way of knowing whether a lower dose would have sufficed. Conversely, there is no way of knowing whether the 5 patients with local failure in our series, who received RT doses from 21.0 to 25.2 Gy, may have been controlled had they received further dose escalation. Because a small number of patients received subtotal resection, and because none of these patients failed locally, we grouped together patients with gross residual disease and patients with positive margins in our analysis, in an attempt to create an increased sample size for a population of patients that might be at higher risk. Our data show no association between subtotal resection or positive margins and worse patient outcomes. On the basis of our series, we would anticipate excellent local control when results are reported for COG ANBL0532, including in the presence of gross residual disease meeting size criteria for boosting to 36 Gy. Because boost was not randomized on this protocol, a cohort of patients who had gross residual disease but did not receive boost to 36 Gy will not be available for comparison. Our series, though containing small numbers of such patients, did not show any local failures among patients with gross residual disease, including in the 6 patients with gross residual disease who were not boosted to 36 Gy. As such, even in the likely scenario that favorable local control for patients with gross residual disease is demonstrated on COG ANBL0532, we believe some question may remain as to whether boost to 36 Gy leads to a quantifiable gain in local control, especially considering that further improvements in systemic multimodal therapy may also contribute to improved local disease control.
The most frequent in-field late effects of RT in neuroblastoma are musculoskeletal abnormalities (12, 16). Inan older study describing patients treated with orthovoltage equipment, Mayfield et al (30) report significant spinal deformity in 76% of children surviving more than 5 years after therapy, associated with doses over 30 Gy. In the MV era, Halperin et al (15) reported a smaller 29.4% rate of scoliosis or kyphosis in irradiated children surviving more than 36 months. More recently, Kushner et al (12) report an 8.6% incidence of short stature from decreased growth of irradiated vertebrae. Ducassou et al (16) report at 5 years a 50% rate of late effects within the RT field and a 31.8% incidence of musculoskeletal abnormalities; patients experiencing musculoskeletal abnormalities and multiple infield late effects all received RT doses of greater than 31 Gy/1.5-Gy fractions. It seems from the limited evidence that late toxicities tend to increase above a threshold dose of approximately 30 Gy. From our series it would seem that in the majority of instances the radiosensitivity of neuroblastoma allows for treatment with RT doses well short of this apparent cutoff around 30 Gy.
Although normal tissue constraints for organs at risk are not well defined or robustly supported by toxicity outcomes in the treatment of high-risk neuroblastoma (31), the dosimetric advantages of modern conformal techniques theoretically minimize the incidence of certain late effects. Intensity modulated RT seems superior in sparing kidney dose for midline neuroblastoma and may be capable of sparing vertebral bodies from the high-dose region, but this is at the expense of increased lower dose wash particularly to the liver, stomach, and spleen (32). Pai Panandiker et al (22) even suggest that high-quality intensity modulated RT might have the capacity to improve locoregional control, even in the case of subtotal resection, because of improved target volume coverage, and as such dose escalation beyond 30.6 Gy might not be needed.
Hyperfractionation (9, 12) and proton therapy (33) have also been means by which attempts have been made to mitigate late toxicities. With the sort of radiosensitivity that our series and others suggest, allowing for most RT courses to be limited to 21.6 Gy, it may be that modern, more-expensive techniques would practically provide no clinical advantage.
Kushner et al (12) suggest that dedicated coverage of uninvolved regional lymph nodes with the RT fields may improve results. In our series, 37.3% of patients had dedicated coverage of elective regional lymph nodes, at the discretion of the treating radiation oncologist, and these patients did not seem to have improved outcomes. Similarly, no benefit was demonstrated for dedicated lymph node dissection at the time of surgery, though this was in a small number of patients (n= 8). Because neuroblastoma is thought to be primarily a hematogenously spread disease, perhaps these findings are not surprising. Nevertheless, there is a relatively high incidence of pathologically positive lymph nodes, 45.8% in our series, and so consideration of how to address the lymph nodes was warranted. On the basis of our series, it does not seem as though RT target volumes should be expanded to include coverage of uninvolved elective nodal regions.
On the basis of this study and the available literature, it seems that RT to 21 to 24 Gy is sufficient for most cases of high-risk neuroblastoma, including in the presence of gross residual disease, positive margins, or nodal involvement. In the future, results of COG ANBL0532 will serve to further clarify the role for dose escalation, because as it stands the evidence to support routine dose escalation to unresected gross disease to 36 Gy remains insufficient. Currently there remains a need for further exploration of the optimal dose for achieving local control in high-risk neuroblastoma without significant increase in toxicity, particularly in light of modern improvements in systemic therapy.
Conclusion
Patients with high-risk neuroblastoma in this large retrospective series maintained excellent local control, with no benefit demonstrated for RT doses over 21.6 Gy. Local control was not impacted by presence of residual disease or positive margins, extent of lymph node dissection, or lymph node positivity. Systemic disease control remains the obstacle to improved outcomes in high-risk neuroblastoma for the majority of instances. The results from COG ANBL0532 will provide further insight into whether accepting the added toxicities of RT dose escalation to gross residual disease is necessary to improve outcomes.
Fig. 2.
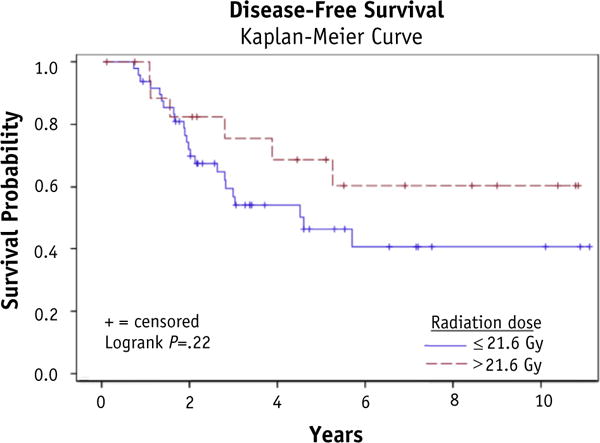
Disease-free survival.
Summary.
Dose escalation to gross residual disease in high-risk neuroblastoma is now common. The benefit remains unclear, and the limited evidence suggests increased toxicity at doses above 30 Gy. Here we offer a series of 67 patients treated with radiation as part of definitive management of high-risk neuroblastoma and demonstrate no benefit for radiation doses over 21.6 Gy, and no disadvantage of gross residual disease, positive margins, or pathologically involved lymph nodes.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and the National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: none.
References
- 1.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 2.Rosen EM, Cassady JR, Frantz CN, et al. Improved survival in neuroblastoma using multimodality therapy. Radiother Oncol. 1984;2:189–200. doi: 10.1016/s0167-8140(84)80059-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosen EM, Cassady JR, Frantz CN, et al. Neuroblastoma: The Joint Center for Radiation Therapy/Dana-Farber Cancer Institute/Children’s Hospital experience. J Clin Oncol. 1984;2:719–732. doi: 10.1200/JCO.1984.2.7.719. [DOI] [PubMed] [Google Scholar]
- 4.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–1554. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 5.Browne M, Kletzel M, Cohn SL, et al. Excellent local tumor control regardless of extent of surgical resection after treatment on the Chicago Pilot II protocol for neuroblastoma. J Pediatr Surg. 2006;41:271–276. doi: 10.1016/j.jpedsurg.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Mazloom A, Louis CU, Nuchtern J, et al. Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2014;90:858–862. doi: 10.1016/j.ijrobp.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolden SL, Gollamudi SV, Kushner BH, et al. Local control with multimodality therapy for stage 4 neuroblastoma. Int J Radiat Oncol Biol Phys. 2000;46:969–974. doi: 10.1016/s0360-3016(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 8.Marcus KJ, Shamberger R, Litman H, et al. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J Pediatr Hematol Oncol. 2003;25:934–940. doi: 10.1097/00043426-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Casey DL, Kushner BH, Cheung NK, et al. Local control with 21-Gy radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2016;96:393–400. doi: 10.1016/j.ijrobp.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda H, August CS, Goldwein JW, et al. Sites of relapse in patients with neuroblastoma following bone marrow transplantation in relation to preparatory “debulking” treatments. J Pediatr Surg. 1992;27:1438–1441. doi: 10.1016/0022-3468(92)90195-d. [DOI] [PubMed] [Google Scholar]
- 11.Bradfield SM, Douglas JG, Hawkins DS, et al. Fractionated low-dose radiotherapy after myeloablative stem cell transplantation for local control in patients with high-risk neuroblastoma. Cancer. 2004;100:1268–1275. doi: 10.1002/cncr.20091. [DOI] [PubMed] [Google Scholar]
- 12.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 13.Robbins JR, Krasin MJ, Pai Panandiker AS, et al. Radiation therapy as part of local control of metastatic neuroblastoma: The St Jude Children’s Research Hospital experience. J Pediatr Surg. 2010;45:678–686. doi: 10.1016/j.jpedsurg.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremens B, Klingebiel T, Herrmann F, et al. High-dose consolidation with local radiation and bone marrow rescue in patients with advanced neuroblastoma. Med Pediatr Oncol. 1994;23:470–475. doi: 10.1002/mpo.2950230604. [DOI] [PubMed] [Google Scholar]
- 15.Halperin EC, Cox EB. Radiation therapy in the management of neuroblastoma: The Duke University Medical Center experience 1967–1984. Int J Radiat Oncol Biol Phys. 1986;12:1829–1837. doi: 10.1016/0360-3016(86)90326-3. [DOI] [PubMed] [Google Scholar]
- 16.Ducassou A, Gambart M, Munzer C, et al. Long-term side effects of radiotherapy for pediatric localized neuroblastoma: Results from clinical trials NB90 and NB94. Strahlenther Onkol. 2015;191:604–612. doi: 10.1007/s00066-015-0837-z. [DOI] [PubMed] [Google Scholar]
- 17.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Park JR, Scott JR, Stewart CF, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high-risk neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:4351–4357. doi: 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57:114–119. doi: 10.1111/j.0006-341x.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 22.Pai Panandiker AS, Beltran C, Billups CA, et al. Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer. 2013;60:761–765. doi: 10.1002/pbc.24350. [DOI] [PubMed] [Google Scholar]
- 23.Adkins ES, Sawin R, Gerbing RB, et al. Efficacy of complete resection for high-risk neuroblastoma: A Children’s Cancer Group study. J Pediatr Surg. 2004;39:931–936. doi: 10.1016/j.jpedsurg.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 24.La Quaglia MP, Kushner BH, Su W, et al. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004;39:412–417. doi: 10.1016/j.jpedsurg.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 25.von Allmen D, Grupp S, Diller L, et al. Aggressive surgical therapy and radiotherapy for patients with high-risk neuroblastoma treated with rapid sequence tandem transplant. J Pediatr Surg. 2005;40:936–941. doi: 10.1016/j.jpedsurg.2005.03.008. discussion 941. [DOI] [PubMed] [Google Scholar]
- 26.Englum BR, Rialon KL, Speicher PJ, et al. Value of surgical resection in children with high-risk neuroblastoma. Pediatr Blood Cancer. 2015;62:1529–1535. doi: 10.1002/pbc.25504. [DOI] [PubMed] [Google Scholar]
- 27.Pai Panandiker AS, McGregor L, Krasin MJ, et al. Locoregional tumor progression after radiation therapy influences overall survival in pediatric patients with neuroblastoma. Int J Radiat Oncol Biol Phys. 2010;76:1161–1165. doi: 10.1016/j.ijrobp.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg. 2002;12:402–409. doi: 10.1055/s-2002-36952. [DOI] [PubMed] [Google Scholar]
- 29.Bagatell R, McHugh K, Naranjo A, et al. Assessment of primary site response in children with high-risk neuroblastoma: An international multicenter study. J Clin Oncol. 2016;34:740–746. doi: 10.1200/JCO.2015.63.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayfield JK, Riseborough EJ, Jaffe N, et al. Spinal deformity in children treated for neuroblastoma. J Bone Joint Surg Am. 1981;63:183–193. [PubMed] [Google Scholar]
- 31.Kandula S, Sutter A, Prabhu RS, et al. Reassessing dose constraints of organs at risk in children with abdominal neuroblastoma treated with definitive radiation therapy: A correlation with late toxicity. Pediatr Blood Cancer. 2015;62:970–975. doi: 10.1002/pbc.25372. [DOI] [PubMed] [Google Scholar]
- 32.Paulino AC, Ferenci MS, Chiang KY, et al. Comparison of conventional to intensity modulated radiation therapy for abdominal neuroblastoma. Pediatr Blood Cancer. 2006;46:739–744. doi: 10.1002/pbc.20456. [DOI] [PubMed] [Google Scholar]
- 33.Hattangadi JA, Rombi B, Yock TI, et al. Proton radiotherapy for high-risk pediatric neuroblastoma: Early outcomes and dose comparison. Int J Radiat Oncol Biol Phys. 2012;83:1015–1022. doi: 10.1016/j.ijrobp.2011.08.035. [DOI] [PubMed] [Google Scholar]


