Abstract
Global motion perception is often used as an index of dorsal visual stream function in neurodevelopmental studies. However, the relationship between global motion perception and visuomotor control, a primary function of the dorsal stream, is unclear. We measured global motion perception (motion coherence threshold; MCT) and performance on standardized measures of motor function in 606 4.5-year-old children born at risk of abnormal neurodevelopment. Visual acuity, stereoacuity and verbal IQ were also assessed. After adjustment for verbal IQ or both visual acuity and stereoacuity, MCT was modestly, but significantly, associated with all components of motor function with the exception of gross motor scores. In a separate analysis, stereoacuity, but not visual acuity, was significantly associated with both gross and fine motor scores. These results indicate that the development of motion perception and stereoacuity are associated with motor function in pre-school children.
Keywords: motion perception, motor function, extrastriate visual cortex, neonatal hypoglycemia, prenatal drug exposure
1. Introduction
A primary role of human vision is the guidance of motor functions such as reaching, grasping and locomotion. In fact, a dominant theory of visual processing posits two parallel streams; one specialized for object recognition (the ventral stream) and one for visuomotor control or “vision for action” (the dorsal stream) (Goodale & Milner, 1992). The ventral stream receives predominantly parvocellular input from the lateral geniculate nucleus and projects to the inferior temporal lobe via V1 and ventral extrastriate areas such as V4 (Livingstone & Hubel, 1988; Van Essen & Gallant, 1994). Conversely, the dorsal stream receives magnocellular input and projects from V1 to regions of the posterior parietal lobe involved in visuomotor control. The dorsal stream involves motion-sensitive extrastriate areas such as V3a, and V5 (Braddick et al., 2001; Goodale & Milner, 1992).
The dorsal stream is of interest from a child development perspective because it may be particularly susceptible to the effects of abnormal neurodevelopment (the dorsal stream vulnerability hypothesis) (Braddick, Atkinson, & Wattam-Bell, 2003; Grinter, Maybery, & Badcock, 2010; Spencer et al., 2000). Furthermore, visuomotor control is a key domain of preschool development that is related to later performance in areas such as learning, writing and mathematics (Becker, Miao, Duncan, & Mcclelland, 2014; Kurdek & Sinclair, 2001).
Visual areas that are typically included in definitions of the dorsal stream (V1, V3A and V5, also known as the middle temporal area or MT) form a motion processing hierarchy within the visual cortex (Movshon & Newsome, 1996). This involves the representation of local motion signals in V1 and the integration of these signals in V3A and V5 to enable perception of coherent or global motion.
Global motion perception is typically measured using random dot kinematograms (RDKs), which consist of two populations of moving dots; signal dots that move in a common coherent direction and noise dots that move randomly. The observer’s task is to detect the direction of the coherent signal dots and the relative proportion of signal to noise in the stimulus can be varied to estimate a motion coherence threshold (the percentage of signal dots required for a particular level of task performance) (Newsome & Pare, 1988). It is well established that V5, a component of the dorsal stream, plays a central role in detecting coherent motion in RDKs (Cai, Chen, Zhou, Thompson, & Fang, 2014; Chen, Cai, Zhou, Thompson, & Fang, 2016; Newsome & Pare, 1988; Rudolph & Pasternak, 1999). However, the extent to which performance on a global motion task reflects the function of higher-level dorsal stream areas such as the posterior parietal cortex is unclear.
Visuomotor control refers to the visual guidance of motor actions, a function that is supported by the posterior parietal lobe (Cavina-Pratesi et al., 2010; Tunik, Frey, & Grafton, 2005). Examples of visually guided actions include reaching, grasping and object manipulation. The accuracy and kinematics of visually guided actions can be assessed with a high degree of accuracy using infrared motion tracking systems (Grant, Melmoth, Morgan, & Finlay, 2007; Melmoth, Finlay, Morgan, & Grant, 2009). Alternatively, there are several well-validated standardized clinical test batteries of motor function that include visuomotor tasks. One example is the Beery-Buktenica Developmental Test of Visual–Motor Integration (Beery VMI) (Beery & Beery, 2010). The visuomotor control aspect of this test (referred to as the visual motor integration component) involves copying visually presented stimuli using a pencil and paper. Other examples are the Movement Assessment Battery for Children (M-ABC) (Schoemaker, Niemeijer, Flapper, & Smits-Engelsman, 2012; Smits-Engelsman, Fiers, Henderson, & Henderson, 2008) and the Peabody Developmental Motor Scales – 2 (PDMS-2) (Rasa, Rashedi, Hosseini, & Sazmand, 2011; Tavasoli, Azimi, & Montazari, 2014). The assessment of fine motor skills within these test batteries involves activities such as bead threading, coin posting and using building blocks. Gross motor function assessment includes ball catching along with predominately motor tasks such as balancing and jumping.
The primary aim of this study was to examine the relationship between global motion perception and standardized measures of motor function in preschool children. An association between global motion perception and motor function would indicate a link between the development of abilities supported by V5 and the posterior parietal lobe respectively, in accordance with the concept of a dorsal processing stream. Such an association would also support the current practice of using global motion perception as an index of general dorsal stream function (Atkinson et al., 1997; Chakraborty, Anstice, Jacobs, LaGasse, et al., 2015; Guzzetta et al., 2009; MacKay et al., 2005; Manning, Charman, & Pellicano, 2013; Palomares & Shannon, 2013).
Several studies have reported concurrent global motion and motor function deficits in children with abnormal neurodevelopment, for example due to William’s syndrome (Atkinson et al., 1997) or Dravet syndrome (Ricci et al., 2014). In these studies, motor function was assessed using a card posting task (Atkinson et al., 1997) or the Beery VMI (Ricci et al., 2014). These findings are consistent with an association between global motion perception and visuomotor control. In some cases, the same children exhibited normal performance on tests of global form perception that were designed to target the ventral processing stream (Atkinson et al., 1997). This suggests the presence of a specific dorsal stream impairment in certain neurodevelopmental disorders (Braddick et al., 2003; Grinter et al., 2010).
More recently, the relationship between global motion perception and visuomotor control has been investigated in a group of normally developing children (Braddick et al., 2016). Braddick et al. measured global motion perception, global form perception and performance on the visual-motor integration component of the Beery VMI in 154 children aged 5–12 years. Greater sensitivity to global motion perception was modestly but significantly associated with better visuomotor control (R2 = 0.06). No such relationship was found for global form perception. Furthermore, an association between greater sensitivity to global motion and a greater proportion of cortical area devoted to the posterior parietal lobe was evident from structural MRI data. Global motion perception was also associated with measures of mathematical ability that involve the posterior parietal lobe. These results support the idea that global motion perception provides at least a partial index of overall dorsal stream function.
In this study, we assessed the relationship between global motion perception and performance across a range of well validated, standardized tests of motor function (see section 2.4, neurodevelopmental assessment). The study involved a large group of pre-school children born with neurodevelopmental risk factors and assessed in a consistent fashion at a consistent age, and with a wide range of motor function outcomes. The children were recruited from two large-scale multidisciplinary studies; Children with Hypoglycemia and their Later Development (CHYLD) and Infant Development Environment and Lifestyle (IDEAL). The studies were designed to investigate the impact of neonatal hypoglycemia (CHYLD) and prenatal drug exposure (IDEAL) on child development. Both study protocols involved a comprehensive assessment of neurodevelopment at 4.5 years of age, including visual-motor integration and fine and gross motor function.
We expected that motor function would vary considerably within this group of children because both neonatal hypoglycemia (Lucas, Morley, & Cole, 1988) and prenatal drug exposure (Wouldes et al., 2014) affect motor development. Specifically, moderate levels of neonatal hypoglycaemia (glucose concentration < 2.6 mm/l) have been associated with lower gross motor scores on the Bayley motor scale at 18 months of age (Lucas et al., 1988). In addition, 1 to 3-year-old children with prenatal exposure to methamphetamine had lower scores on the Peabody Developmental Motor scale than closely matched children without methamphetamine exposure (Wouldes et al., 2014). We reasoned that this variability would allow for the detection of any association between global motion perception and visuomotor control that may exist. Additional benefits of studying this group included the ability to control for the effect of age (all children were assessed at 4.5 years) and the fact that the children had not yet started school, which may substantially influence development (Camilli, Vargas, Ryan, & Barnett, 2010). Furthermore, verbal IQ (V-IQ) scores from the Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III (Welscher, 2002) were available and could be used to control for the potentially confounding effect of cognitive development on performance of the global motion task (Jakobson, Frisk, & Downie, 2006; Manning et al., 2013). The V-IQ score was chosen as it does not involve any measures of visual processing (Jarrold, Baddeley, & Hewes, 1998).
The secondary aim of this study was to investigate the relationships between clinical measures of visual function (stereopsis and visual acuity) and visuomotor control in the same group of children. Disorders of binocular vision such as amblyopia and strabismus disrupt normal development of visually guided reaching and grasping (Grant, Suttle, Melmoth, Conway, & Sloper, 2014; Simon Grant & Conway, 2014; Simon Grant, Melmoth, Morgan, & Finlay, 2007; Simon Grant & Moseley, 2011b; Mazyn, Lenoir, Montagne, Delaey, & Savelsbergh, 2007; Melmoth, Finlay, Morgan, & Grant, 2009; O’Connor, Birch, Anderson, & Draper, 2010b; Suttle, Melmoth, Finlay, Sloper, & Grant, 2011) and impair performance on standardized tests of fine motor function (Caputo et al., 2007; Drover, Stager, Morale, Leffler, & Birch, 2008; Hrisos, Clarke, Kelly, Henderson, & Wright, 2006; Rogers, Chazan, Fellows, & Tsou, 1982; Webber, Wood, Gole, & Brown, 2008, Webber, Wood, & Thompson, 2016). However, the relationship between stereoacuity and fine motor function in normally developing children may be limited to specific tasks. In particular, within a group of visually normal 5 to 13-year-old children, binocular viewing enabled better fine motor performance than monocular viewing on a bead-threading task, but not a peg-board task, despite the fact that both tasks require accurate visuomotor control (Alramis, Roy, Christian, & Niechwiej-Szwedo, 2016).
Visual acuity has also been associated with visuomotor control, although the nature of the relationship varies across studies and patient populations. In children with a history of unilateral cataract, better distance visual acuity in the stronger eye, but not interocular acuity difference, was associated with better visuomotor control (Celano, Hartmann, DuBois, & Drews-Botsch, 2015). Conversely, better distance visual acuity in the poorer seeing eye and interocular acuity difference were both associated with visuomotor control in a study of children and adults with varied levels of visual function (O’Connor & Birch, 2010; O’Connor, Birch, Anderson, & Draper, 2010a). In addition, visuomotor control was weakly (R2 = 0.01) associated with better eye near visual acuity, and unrelated to stereoacuity, in a group of 174 developmentally normal pre-school age children (Ho, Tang, Fu, Leung, & Pang, 2015). However, a strong association between age and visuomotor control (R2 = 0.34) may have masked relationships involving visual acuity and stereopsis in this latter study.
In summary, the primary aim of this study was to test the hypothesis that global motion perception is associated with visuomotor control in preschool children because both abilities involve a common neural substrate (the dorsal visual processing stream). The secondary aim was to investigate whether motor function was associated with stereopsis and/or visual acuity.
2. Materials and methods
2.1. Participants
Six hundred and six participants were recruited from two large-scale multidisciplinary studies; the Children with Hypoglycemia and their Later Development (CHYLD) study and the Infant Development Environment and Lifestyle (IDEAL) study. Both study protocols involved a comprehensive assessment of neurodevelopment at 4.5 years of age, including visual-motor integration and fine and gross motor function. The Northern Y Regional Health and Disability Ethics Committee approved the study protocols of the CHYLD study, whereas the Auckland and the Waitemata District Health Boards and their Māori ethics committees approved the IDEAL study. All caregivers gave informed consent and the study conformed to the principles of the Declaration of Helsinki.
The CHYLD study was designed to assess the longitudinal outcomes of children who were born with one or more of the following risk factors for neonatal hypoglycemia; child of a diabetic mother, small (< 2.5 Kg or < 10th centile) or large (> 4.5 Kg or > 90th centile) at birth or late preterm birth (≥ 32 weeks’ gestation) (McKinlay et al., 2015). The IDEAL study investigated the effect of prenatal methamphetamine exposure on subsequent neurodevelopment. It included a group of children who had experienced prenatal methamphetamine exposure and a non-methamphetamine exposed control group matched for socio-economic status, parental education, birth weight and gestational age (Wouldes et al., 2013). The majority of children (140 out of 165) recruited from the IDEAL cohort had been prenatally exposed to one or more drugs, such as marijuana, nicotine and alcohol. The 25 non-exposed children were still at risk due to low socio-economic status.
2.2. Global Motion Perception
The protocol used to assess global motion perception has been described elsewhere (Chakraborty, Anstice, Jacobs, LaGasse, et al., 2015; Chakraborty, Anstice, Jacobs, Paudel, et al., 2015). Global motion perception was assessed using RDKs, which consisted of 100 circular dots (dot diameter 0.24°, dot density 1.27 dot/deg2) presented within a circular aperture (10° diameter) at a viewing distance of 60 cm (Chakraborty, Anstice, Jacobs, Paudel, et al., 2015). Dot speed was 6°/second (dots were displaced 0.1° every 17 ms) and the presentation time was 1 second. Similar dot speeds have been used in previous studies of global motion perception in children (Gunn et al., 2002; Manning, Dakin, Tibber, & Pellicano, 2014), as higher dot speeds may not be as sensitive to individual differences in global motion perception (Lewis & Maurer, 2005; Narasimhan & Giaschi, 2012; Meier, Sum, & Giaschi, 2016), while dot speeds lower than 2 deg/sec may fall below the optimal processing speed for MT/V5 neurons (Maunsell & Van Essen, 1983). Dots had a limited lifetime, whereby each dot had a 5% chance of disappearing on each frame and being redrawn in a random location. Bright dots (137 cd/m2) were presented on a grey background (45 cd/m2) and dot contrast was 0.51 defined using the Michelson equation: (Ldots − Lbackground)/(Ldots + Lbackground). Signal dots moved coherently upwards or downwards and noise dots moved in random directions. Stimuli were presented on a 15″ Dell cathode ray tube monitor (model: E771p) with a 120 Hz refresh rate and 1024×768 resolution. Stimuli used for motion coherence threshold measurement were generated using MATLAB 2013a and psychtoolbox-3 (Pelli, 1997).
Prior to threshold measurement, children were trained to be familiar with the stimuli and task. In the training session, 100% coherent signal dots were presented until the children gave 4 successive correct responses, following which the experimenter manually varied the coherence level (in 5% steps) at least 3 times to make the child familiar with the testing protocol. The training program was terminated and the testing halted if a child could not give 4 successive correct responses within 25 trials at 100% coherence.
In the test session, a 2-down-1-up adaptive staircase varied the coherence of the RDK to measure a motion coherence threshold. The staircase began at 100% coherence and had a proportional step size of 50% until the first reversal and 25% thereafter. The staircase was terminated after 5 reversals and the threshold was calculated by averaging the last 4 reversals. Data from only those children who passed the training session were included in the final analysis. Children (n = 18) who passed the training session but were unable to complete the staircase procedure (due to fatigue or loss of attention) were assigned a motion coherence threshold of 100%.
2.3. Visual Acuity and Stereoacuity
Vision screening was conducted to rule out significant ocular pathology. Both monocular and binocular visual acuities were measured, with habitual refractive correction in place if worn, using the crowded linear Lea symbol test in the CHYLD cohort and crowded Keeler logMAR test in the IDEAL cohort. Subsequent references to visual acuity should be interpreted as habitual monocular visual acuity of the better eye. Stereoacuity was measured using the graded circles portion of the stereo fly test at a distance of 40 cm. This test uses polarized plates to present contour stereopsis cues at varying levels of disparity. Children who had no measureable stereoacuity were assigned a score of 3000 seconds of arc (corresponding to the largest disparity presented in the test), to indicate that they had poor depth perception. Further details of the vision screening protocol can be found elsewhere (Chakraborty, Anstice, Jacobs, Paudel, et al., 2015).
2.4. Neurodevelopmental Assessment
Trained developmental assessors under the supervision of an experienced developmental psychologist performed all neurodevelopmental tests. Assessors were blinded to the child’s medical history and/or prenatal drug exposure.
Motor function
Children completed the Beery VMI, a reliable and well-validated paper and pencil based test that involves copying geometric shapes of varying complexity by hand (Beery & Beery, 2010). In addition to the main visual motor integration component of the Beery VMI, there are two standardized sub-tests that are designed to isolate visual perception (VP) and motor coordination (MC). The VP test involves shape matching and the MC test involves tracing geometric shapes.
Motor function was also assessed using the Movement Assessment Battery for Children (M-ABC 2) in the CHYLD study and the Peabody Developmental Motor Scales (PDMS-2) in the IDEAL study.
The M-ABC is a well validated (Schoemaker et al., 2012; Smits-Engelsman et al., 2008) assessment battery designed to evaluate fine and gross motor function in children. The M-ABC includes a performance test and a teachers’ observational checklist. Only the performance test was used in the CHYLD study. This has three subcomponents; 1) manual dexterity (MD), 2) aiming and catching (A&C) and, 3) balance.
The PDMS-2 is also well validated (Rasa et al., 2011; Tavasoli et al., 2014) and has six subscales targeting gross motor (GM) and fine motor (FM) function; 1) reflex (only used with infants ≤ 12 months of age), 2) stationary balance, 3) locomotion, 4) object manipulation, 5) grasping, and, 6) visual-motor integration. Scores for each of the tests are summed to give GM, FM and total motor (TM) scores.
Verbal IQ
The Wechsler Preschool and Primary Scale of Intelligence (WPPSI)-III was administered following published guidelines (Welscher, 2002). The verbal IQ composite score generated by the WPPSI-III includes tests of acquired knowledge, verbal reasoning and attention to verbal stimuli.
2.5. Statistical Analyses
Data from the M-ABC and the PDMS-2 tests of motor development were combined as reported previously (Waelvelde, Peersman, & Leuven, 2007) using the following approach. Gross motor function: GM percentile rank from the PDMS-2 or the mean of the A&C and balance percentile ranks from the M-ABC. Fine motor function: FM percentile rank from the PDMS-2 or the MD percentile rank from the M-ABC. Total motor function: The TM percentile rank from the M-ABC or the PDMS-2.
Linear regression (SPSS v22, IBM Corp, Armonk, NY, USA) was used to assess the association between motion coherence thresholds and each of the measures provided by the standardized motor function tests (visual motor integration, motor coordination, gross motor, fine motor and total motor) separately. Analyses were repeated excluding those participants who could not complete the motion coherence threshold staricase; however, the outcomes remained unchanged. Additional regression models were constructed to adjust for 1) visual acuity and stereoacuity and 2) verbal IQ. Motor variables and MCT were converted to z-scores, while stereoacuity (≤100 secs of arc = 0, >100 to 800 secs of arc = 1, >800 secs of arc = 2) and visual acuity (≤ 0.3 logMAR = 1, > 0.3 logMAR = 2) were categorically coded, because the skewness (stereoacuity = 3.19, visual acuity = 1.61) and kurtosis (stereoacuity = 8.35, visual acuity = 5.06) of those distributions exceeded the recommended limits for parametric tests (Tabachnick & Fidell, 2012). Verbal IQ was coded as a binary variable to identify children who did and did not have cognitive impairment. Children were classified as having below normal verbal IQ (coded as 1) if their composite score was below 85 (corresponding to the 15th percentile in the normative dataset used to standardize the test). All other children were classified as having normal verbal IQ (coded as 0).
Separate post hoc exploratory regression analyses were conducted to assess the effect of preterm birth (binary variable; 1 = term, 2 = preterm) and prenatal drug exposure (binary variable; 1 = unexposed, 2 = exposed) on the relationship between motion coherence threshold and each motor function.
3. Results
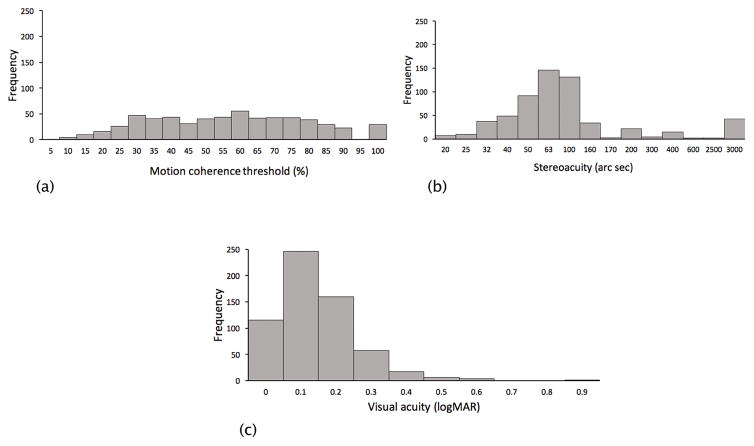
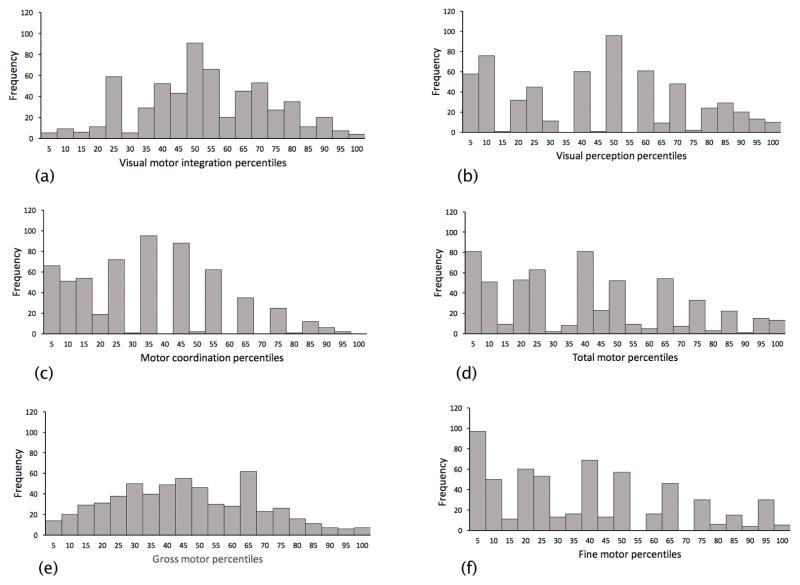
Motor and vision data were collected from 606 participants (CHYLD, n = 441; IDEAL, n = 165). There were 37 children (CHYLD: 24, IDEAL: 13) with more than 0.1 logMAR interocular difference in habitual visual acuity of whom 14 children (CHYLD: 12, IDEAL: 2) had a diagnosis of amblyopia. Eight children (CHYLD: 6, IDEAL: 2) had strabismus. Overall task compliance for tests of visual function was between 97% (motion coherence threshold) and 99% (visual acuity). Compliance for motor testing was between 97% (gross motor function) and 99% (visual-motor integration). In addition, 99% of children completed the verbal IQ test. The baseline demographics, motor and vision scores of the 606 children are described in Table 1 and Table 2, respectively. The distributions of each variable are shown in Figure 1 (vision) and Figure 2 (motor). The CHYLD and IDEAL cohorts differed in the prevalence of neonatal risk factors for neonatal hypoglycaemia (100% CHYLD, 4.2% IDEAL) and prenatal drug exposure (29% CHYLD, 85% IDEAL) because of the individual study designs.
Table 1.
Cohort details:
| N‡ | ||
|---|---|---|
|
| ||
| Maternal background | ||
| Ethnicity, N(%) | ||
| European | 323 (53.65) | |
| Maori | 602 | 217 (36.04) |
| Pacific | 32 (5.31) | |
| Other | 30 (4.98) | |
|
| ||
| Household income categories, N(%) | ||
| < NZD 10,000 | 13 (2.83) | |
| > NZD 10,000 – 20,000 | 50 (10.91) | |
| > NZD 20,000 – 30,000 | 66 (14.41) | |
| > NZD 30,000 – 40,000 | 458 | 59 (12.88) |
| > NZD 40,000 – 50,000 | 13 (2.83) | |
| > NZD 50,000 | 257 (56.11) | |
|
| ||
| Maternal substance use, N(%) | ||
| alcohol | 521 | 137 (26.29) |
| marijuana | 527 | 79 (14.99) |
| tobacco | 475 | 221 (46.52) |
| methamphetamine and other drugs | 528 | 79 (14.96) |
|
| ||
| Child characteristics | ||
|
| ||
| Gender female, N(%) | 606 | 287 (47.35) |
|
| ||
| Gestational age in weeks, mean (SD) | 600 | 38.8 (2.42) |
|
| ||
| Birth weight in kg, mean (SD) | 600 | 3.2 (0.73) |
|
| ||
| Preterm (>32 weeks’ gestation), N(%) | 600 | 156 (26) |
|
| ||
| Small (< 2.5 Kg birth weight), N(%) | 600 | 71 (11.83) |
|
| ||
| Large (> 4.5 Kg birth weight), N(%) | 600 | 41 (6.83) |
|
| ||
| Verbal IQ¶, N(%) | ||
| ≥85 | 600 | 430 (70.95) |
| <85 | 170 (28.05) | |
N: the number of children for whom data were available. Not all study participants provided responses in all categories.
Verbal IQ – verbal intelligence quotient.
Table 2.
Vision and motor outcomes:
| N‡ | ||
|---|---|---|
|
| ||
| Visual outcomes | ||
|
| ||
| Motion coherence threshold %, mean (SD) | 606 | 55 (22.0) |
|
| ||
| Stereoacuity, N(%) | ||
| <100 secs of arc | 476 (78.54) | |
| >100 – 800 secs of arc | 606 | 84 (13.86) |
| >800# secs of arc | 46 (7.59) | |
|
| ||
| Visual acuity, N(%) | ||
| ≤ 0.3 logMAR | 606 | 578 (95.37) |
| > 0.3 logMAR | 28 (4.62) | |
|
| ||
| Motor outcomes (mean, SD) | ||
|
| ||
| Beery VMI percentile | 600 | 51.3 (20.14) |
|
| ||
| Beery VP percentile | 597 | 42.4 (28.11) |
|
| ||
| Beery MC percentile | 593 | 33.9 (22.44) |
|
| ||
| Fine motor control percentile§ | 594 | 36.3 (27.78) |
|
| ||
| Gross motor control percentile§ | 587 | 43.8 (22.12) |
|
| ||
| Total motor control percentile§ | 594 | 37.8 (26.89) |
Abbreviations:
N: number of children for whom data were available. Not all study participants provided responses in all categories.
includes children who had no measureable stereoacuity. VMI – visual motor integration, VP – visual perception, MC – motor coordination.
See main text for the details of this calculation.
Figure 1.
Distributions of (A) motion coherence thresholds, (B) stereoacuity and (C) visual acuity. Data are combined across the two cohorts of children.
Figure 2.
Distributions of (A) visual motor integration, (B) visual perception and (C) motor coordination (D) total motor function (E) gross motor function, and (F) fine motor function. Data are combined across the two cohorts of children.
MCT was modestly, but significantly associated with visual motor integration, motor coordination, gross motor and total motor scores (Table 3). A decrease (improvement) of 1 standard deviation (SD) in MCT was associated with a 0.13 to 0.19 SD increase (improvement) in each of these motor functions. MCT remained significantly associated with each of these motor functions when the regression model was adjusted to include both visual acuity and stereoacuity or verbal IQ. MCT was not significantly associated with fine motor score but was significantly associated with the Beery visual perception score in all models. Moreover, the associations between motion coherence thresholds and the various components of motor function were similar for children who had verbal IQ <15th percentile (n = 170, R2 ranged from 0.015 to 0.04) and ≥15th percentile (n = 430, R2 ranged from 0.02 to 0.04).
Table 3.
The associations between motion coherence threshold and motor functions with and without adjustment for both visual acuity and stereoacuity or verbal IQ
| Visual motor integration | Visual perception | Motor coordination | Gross motor | Fine motor | Total motor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β [95% CI] (p) | R2 | β [95% CI] (p) | R2 | β [95% CI] (p) | R2 | β [95% CI] (p) | R2 | β [95% CI] (p) | R2 | β [95% CI] (p) | R2 | |
| Motion coherence threshold | −0.17 [−0.25, −0.09] (< 0.001)* | 0.03 | −0.19 [−0.27, −0.11] (< 0.001)* | 0.04 | −0.15 [−0.24, −0.07] (< 0.001)* | 0.02 | −0.13 [−0.18, −0.04] (0.002)* | 0.02 | −0.07 [−0.15, −0.01] (0.087) | 0.003 | −0.15 [−0.24, −0.07] (< 0.001)* | 0.02 |
| Motion coherence threshold adjusting for visual acuity and stereoacuity | −0.14 [−0.23, −0.07] (< 0.001)* | 0.05 | −0.17 [−0.26, −0.09] (< 0.001)* | 0.05 | −0.14 [−0.22, −0.06] (0.001)* | 0.03 | −0.10 [−0.16, −0.02] (0.011)* | 0.04 | −0.05 [−0.13, −0.04] (>0.250) | 0.02 | −0.13 [−0.22, −0.05] (0.001)* | 0.05 |
| Motion coherence threshold adjusting for verbal IQ | −0.14 [−0.22, −0.06] (< 0.001)* | 0.06 | −0.17 [−0.25, −0.09] (< 0.001)* | 0.05 | −0.13 [−0.21, −0.05] (0.002)* | 0.05 | −0.09 [−0.15, −0.01] (0.023)* | 0.05 | −0.04 [−0.13, −0.04] (>0.250) | 0.03 | −0.12 [−0.20, −0.04] (0.003)* | 0.08 |
indicates statistically significant associations.
β: represents standardized coefficients
The regression model including only MCT as an independent variable explained 2–4% of the variation in motor function (Table 3). The model also including visual acuity and stereoacuity as independent variables explained 2–5% of the variation in motor function and the model including verbal IQ explained 5–8%. Therefore, as expected, models including a measure of cognitive function explained a greater proportion of the variance in motor function than models including measures of visual function alone. In addition, children with poor verbal IQ (below 15th percentile) had significantly poorer global motion perception, visual acuity, stereoacuity, and motor function (all p < 0.005 and t > −2.95) than those with good verbal IQ (above 15th percentile).
The regression models involving MCT that adjusted for visual acuity and stereoacuity revealed that both of these visual functions were independently and significantly associated with a sub-set of motor functions. To further investigate these associations, two separate regression analyses were conducted that included either stereoacuity or visual acuity as the independent variable and each motor function separately as the dependent variable (Table. 4). Unstandardized B-coefficients were used to quantify associations within these models because visual functions and motor functions were expressed in different units. Visual acuity and stereoacuity explained between 1–2% and 1–3% of variance in motor function, respectively. For statistically significant associations, a one-step increase in visual acuity grade (i.e. worse visual acuity; e.g. > 0.3 logMAR vs. ≤ 0.3 logMAR) was associated with a −0.49 to −0.63 percentile decrease (reduction) in motor function. Similarly, a one-step increase in stereoacuity grade (i.e. worse stereoacuity; e.g. 100 – 800 secs of arc vs. < 100 secs of arc) was associated with a −0.14 to −0.28 percentile decrease (reduction) in motor function.
Table 4.
The associations between stereoacuity or visual acuity and motor functions
| Visual motor integration | Visual perception | Motor coordination | Gross motor | Fine motor | Total motor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B [95% CI] (p) | R2 | B [95% CI] (p) | R2 | B [95% CI] (p) | R2 | B [95% CI] (p) | R2 | B [95% CI] (p) | R2 | B [95% CI] (p) | R2 | |
| Visual acuity | −0.54 [−0.92, −0.16] (0.006)* | 0.01 | −0.63 [−1.02, −0.25] (0.001)* | 0.02 | −0.19 [−0.58, −0.20] (>0.250) | 0.001 | −0.33 [−0.66, −0.008] (0.062) | 0.005 | −0.39 [−0.77, −0.006] (0.064) | 0.005 | −0.49 [−0.88, −0.11] (0.013)* | 0.01 |
| Stereo acuity | −0.24 [−0.37, −0.10] (0.001)* | 0.02 | −0.15 [−0.29, −0.03] (0.031)* | 0.006 | −0.20 [−0.39, −0.06] (0.004)* | 0.01 | −0.14 [−0.38, −0.10] (< 0.001)* | 0.03 | −0.22 [−0.36, −0.08] (0.002)* | 0.02 | −0.28 [−0.42, −0.15] (< 0.001)* | 0.03 |
indicates statistically significant associations.
B: represents unstandardized coefficients
Post hoc exploratory analyses did not reveal any significant interactions between preterm birth or prenatal drug exposure and the association between motion coherence threshold and motor functions.
4. Discussion
Global motion perception is linked to the function of V5; a key dorsal stream area (Born & Tootell, 1992; Braddick et al., 2001; Goodale & Milner, 1992). However, the relationship between the development of global motion perception and visuomotor control, a primary function of the dorsal stream (Goodale, 2011), is not well understood. The presence of such a relationship in preschool children would provide evidence a link between visual and motor development and support the use of global motion perception as a measure of dorsal stream function (Atkinson et al., 1997; Brieber et al., 2010; Chakraborty, Anstice, Jacobs, LaGasse, et al., 2015; Gummel, Ygge, Benassi, & Bolzani, 2012; Gunn et al., 2002; Guzzetta et al., 2009; Hansen, Stein, Orde, Winter, & Talcott, 2001; MacKay et al., 2005; Manning et al., 2013; Palomares & Shannon, 2013; Reiss, Hoffman, & Landau, 2005). We addressed this issue by investigating whether global motion perception was related to performance on standardized tests of motor function in a large group of 4.5-year-old children born with developmental risk factors.
Motion coherence thresholds were moderately but statistically significantly associated with visual motor integration, motor coordination, gross motor and total motor scores, whereby lower (better) motion coherence thresholds were associated with higher (better) motor scores. These associations remained after controlling for other visual and cognitive functions. Therefore, our data indicate that global motion perception and, by extension, V5 function is related to multiple aspects of motor control in pre-school children. This is consistent with previous studies of adults involving neuropsychological patients (Goodale & Milner, 1992), non-invasive brain stimulation (Whitney et al., 2007) or fMRI (Oreja-Guevara et al., 2004) that have demonstrated an association between V5 function and visuomotor control.
Our results are also in agreement with a recent study of normally developing children that reported an association between global motion perception and visuomotor control (R2 = 0.06) (Braddick et al., 2016). The association reported by Braddick et al. in a normal population was slightly stronger than those that we observed, even though our sample included children at risk of abnormal motor development. Braddick et. al. tested older children (range 5–12 years, mean age ~8 years) than those tested in this study (4.5 years). Older children are likely to provide less variable threshold estimates and this may have increased the strength of the association detected by Braddick et. al. Supporting this possibility, Braddick et al. reported that variability in motion coherence thresholds reduced substantially with increasing age in their sample, presumably due to maturation of global motion processing and/or cognition.
Although the associations we report between motion coherence thresholds and motor functions are statistically significant and cannot be explained by visual acuity, stereopsis or verbal IQ, they account for only a modest portion of the variance within the regression models. Individual differences in the maturation of dorsal stream areas at the age of 4.5 years might account for some of this variability (Braddick et al., 2016). In addition, other aspects of neurodevelopment such as visuospatial cognition, which was not measured in this study, might account for a larger portion of the variance.
The associations between motion coherence threshold and motor functions that we observed were not significantly influenced by preterm birth or prenatal drug exposure. This is important because preterm birth and prenatal drug exposure have been found to independently affect motion coherence thresholds (Chakraborty, Anstice, Jacobs, LaGasse, et al., 2015; Guzzetta et al., 2009) and motor function (Moreira, Magalhães, & Alves, 2014; Wouldes et al., 2014). Therefore, our data suggest that there is a true association between motion coherence threshold and motor function, including among children with a range of developmental risk factors.
Twenty-five of the children in the IDEAL cohort did not experience prenatal drug exposure, but were matched for socioeconomic risk factors with the rest of the cohort. Their motion coherence thresholds (58 ± 23%) did not differ from those of the drug exposed children (57 ± 22%). It is possible that socioeconomic risk factors, that were common to all children, had a more detrimental effect on the development of global motion perception than did drug exposure. A detailed analysis of the impact of prenatal drug exposure on motion coherence thresholds in the IDEAL cohort is available elsewhere (Chakraborty, Anstice, Jacobs, LaGasse, et al., 2015).
The average motion coherence thresholds we observed were higher (poorer) than those reported in some previous studies involving children of a comparable age (MacKay et al., 2005; Parrish, Giaschi, Boden, & Dougherty, 2005) and lower (better) than others (Hadad, Maurer, & Lewis, 2011). Differences in testing protocols and stimulus parameters are likely to contribute to differences in absolute motion coherence thresholds between studies of preschool age children (Narasimhan & Giaschi, 2012; Pilly & Seitz, 2009).
Within a secondary analysis, we also assessed the relationship between motor functions and two other measures of visual function, visual acuity and stereoacuity, that have previously been linked to motor development (Piano & O’Connor, 2013). In agreement with previous work (Ho et al., 2015), visual acuity was significantly but weakly related to the Beery visual motor integration and visual perception scores as well as total motor scores. However, we did not observe a relationship between visual acuity and motor coordination, gross motor or fine motor scores. The relationship between visual acuity and the Beery visual perception score is expected as the visual perception test is designed to detect spatial vision impairments that might complicate the interpretation of visual motor integration scores (Subramanian, Morale, Wang, & Birch, 2012). It is important to note that <5% of the children in our study had abnormal visual acuity for their age and therefore our results do not bear upon the relationship between impaired visual acuity and motor function. Associations between visual acuity and a range of motor functions have been reported in populations that include children with visual impairment (Celano et al., 2015).
Stereoacuity was significantly associated with all measures of motor function that were included in this study. The strongest associations were with visual motor integration and total motor scores. This is consistent with recent reports that stereopsis plays an important role in the development of specific fine motor skills in children with normal vision (Alramis et al., 2016) and visuomotor control in patients with amblyopia or strabismus (Caputo et al., 2007; Drover et al., 2008; Grant et al., 2007; Melmoth et al., 2009; Rogers et al., 1982).
The strengths of this study include a large sample size studied at a consistent age. This allowed us to detect subtle relationships. Furthermore, we had access to verbal IQ scores that allowed us to control for factors that may have influenced the ability of preschool children to comprehend our psychophysical global motion task. However, the study also has weaknesses. Although our study team was experienced in pediatric data collection, the young age of our participants is likely to have increased the variability within our dataset and this may have contributed to the relatively weak associations that we observed. Furthermore, because we assessed children at 4.5 years of age when global motion perception and motor function are not yet fully mature (Hadad, Maurer, & Lewis, 2011), we do not know if a relationship between these two factors persists during later development. Studies of older children such as the experiment recently conducted by Braddick et al. are required to address both of these issues (Braddick et al., 2016).
Because this was a correlational study, we cannot directly quantify the functional significance of the associations we observed. Dorsal stream processing has been implicated in skills such as reading (Solan, Shelley-Tremblay, Hansen, & Larson, 2007) and therefore there may be a link between motion coherence thresholds, motor function and academic achievement. However, well-controlled interventional studies using techniques such as perceptual learning to improve global motion perception (Law & Gold, 2008; Sasaki, Nanez, & Watanabe, 2010; Sasaki & Watanabe, 2001; Watanabe et al., 2002) are required to assess whether changes in global motion perception result in functionally significant developmental gains.
In conclusion, our data indicate that global motion perception is related to motor function in 4.5-year-old children. This is consistent with the concept of a dorsal processing stream that involves motion sensitive area V5 and projects to areas of the posterior parietal lobe involved in visual motor control. Furthermore, our results highlight the importance of stereoacuity and motion perception for motor development.
Highlights.
Global motion perception is modestly associated with motor development
Stereoacuity is also associated with both gross and fine motor control
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD0692201, National Institutes on Drug Abuse grants 2RO1DA014948 and RO1DA021757 and the Auckland Medical Research Foundation. We acknowledge the contribution of all members of the CHYLD Study team: Coila Bevan, Jessica Brosnahan, Ellen Campbell, Tineke Crawford, Kelly Fredell, Karen Frost, Claire Hahnhaussen, Safayet Hossin, Greg Gamble, Anna Gsell, Yannan Jiang, Kelly Jones, Sapphire Martin, Neil Micklewood, Chris McKinlay, Grace McKnight, Christina McQuoid, Janine Paynter, Raquel O. Rodrigues, Jenny Rogers, Kate Sommers, Heather Stewart, Anna Timmings, Jess Wilson, Rebecca Young, from the Liggins Institute, University of Auckland, New Zealand; Jo Arthur, Susanne Bruder, Gillian Matheson, Tzu-Ying (Sandy) Yu from the School of Optometry and Vision Science, University of Auckland, New Zealand Nataliia Burakevych, Department of Paediatrics; Child and Youth Health, University of Auckland, New Zealand. Judith Ansell, Ryan San Diego, Department of Psychological Medicine, University of Auckland, New Zealand Matthew Signal, Aaron Le Compte, Department of Engineering, University of Canterbury, New Zealand. Max Berry, Arun Nair, Ailsa Tuck, Alexandra Wallace, Phil Weston from the Department of Paediatrics, Waikato Hospital, Hamilton, New Zealand. The CHYLD Steering Group: Jane Alsweiler, Department of Paediatrics; Child and Youth Health, University of Auckland, J. Geoffery Chase, Department of Engineering, University of Canterbury, Jane Harding, Liggins Institute, University of Auckland, Deborah Harris, Newborn Intensive Care Unit, Waikato District Health Board, Benjamin Thompson, Department of Optometry and Vision Science, University of Auckland, Trecia Ann Wouldes, Department of Psychological Medicine, University of Auckland, Auckland, New Zealand. International Advisory Group: Heidi Feldman, Stanford University School of Medicine, USA; William Hay, University of Colorado School of Medicine, USA; Darrell Wilson, Stanford University School of Medicine, USA; Robert Hess, McGill Vision Research Unit, Department of Ophthalmology, McGill University, USA. We also acknowledge the members of NZ IDEAL study team: Jenny Rogers, Josephine Cliffe, Suzanne Cumming, and Heather Stewart.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alramis F, Roy E, Christian L, Niechwiej-Szwedo E. Contribution of binocular vision to the performance of complex manipulation tasks in 5–13 years old visually-normal children. Human Movement Science. 2016;46:52–62. doi: 10.1016/j.humov.2015.12.006. http://doi.org/10.1016/j.humov.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport. 1997;8(8):1919–22. doi: 10.1097/00001756-199705260-00025. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9223077. [DOI] [PubMed] [Google Scholar]
- Becker DR, Miao A, Duncan R, Mcclelland MM. Early Childhood Research Quarterly Behavioral self-regulation and executive function both predict visuomotor skills and early academic achievement. Early Childhood Research Quarterly. 2014;29(4):411–424. http://doi.org/10.1016/j.ecresq.2014.04.014. [Google Scholar]
- Beery K, Beery N. The Beery-Buktenica Developmental Test of Visual-Motor Integration. 6. Bloomington, MN: Pearson Education; 2010. [Google Scholar]
- Born RT, Tootell RBH. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357(6378):497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Newman E, Akshoomoff N, Kuperman JM, Bartsch H, … Jernigan TL. Global Visual Motion Sensitivity: Associations with Parietal Area and Children’s Mathematical Cognition. Journal of Cognitive Neuroscience. 2016;28(12):1897–1908. doi: 10.1162/jocn_a_01018. http://doi.org/10.1162/jocn_a_01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and “dorsal-stream vulnerability. Neuropsychologia. 2003;41(13):1769–1784. doi: 10.1016/s0028-3932(03)00178-7. http://doi.org/10.1016/S0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30(1):61–72. doi: 10.1068/p3048. http://doi.org/10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48(6):1644–51. doi: 10.1016/j.neuropsychologia.2010.02.007. http://doi.org/10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Cai P, Chen N, Zhou T, Thompson B, Fang F. Global versus local: double dissociation between MT+ and V3A in motion processing revealed using continuous theta burst transcranial magnetic stimulation. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-4084-9. Braddick 1993 http://doi.org/10.1007/s00221-014-4084-9. [DOI] [PubMed]
- Camilli G, Vargas S, Ryan S, Barnett WS. Meta-analysis of the effects of early education interventions on cognitive and social development. The Teachers College Record. 2010;112(3):579–620. [Google Scholar]
- Caputo R, Tinelli F, Bancale A, Campa L, Frosini R, Guzzetta A, … Cioni G. Motor coordination in children with congenital strabismus: Effects of late surgery. European Journal of Paediatric Neurology. 2007;11(5):285–291. doi: 10.1016/j.ejpn.2007.02.002. http://doi.org/10.1016/j.ejpn.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Monaco S, Fattori P, Galletti C, McAdam TD, Quinlan DJ, … Culham JC. Functional magnetic resonance imaging reveals the neural substrates of arm transport and grip formation in reach-to-grasp actions in humans. The Journal of Neuroscience. 2010;30(31):10306–10323. doi: 10.1523/JNEUROSCI.2023-10.2010. http://doi.org/10.1523/JNEUROSCI.2023-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celano M, Hartmann EE, DuBois LG, Drews-Botsch C. Motor skills of children with unilateral visual impairment in the Infant Aphakia Treatment Study. Developmental Medicine & Child Neurology. 2015;58(2):154–159. doi: 10.1111/dmcn.12832. http://doi.org/10.1111/dmcn.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Anstice NS, Jacobs RJ, LaGasse LL, Lester BM, Wouldes TA, Thompson B. Prenatal exposure to recreational drugs affects global motion perception in preschool children. Scientific Reports. 2015;5 doi: 10.1038/srep16921. http://doi.org/10.1038/srep16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Anstice NS, Jacobs RJ, Paudel N, Lagasse LL, Lester BM, … Thompson B. Global motion perception is independent from contrast sensitivity for coherent motion direction discrimination and visual acuity in 4.5-year- old children. Vision Research. 2015;115:83–91. doi: 10.1016/j.visres.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Cai P, Zhou T, Thompson B, Fang F. Perceptual learning modifies the functional specializations of visual cortical areas. Proceedings of the National Academy of Sciences. 2016;113(20):5724–5729. doi: 10.1073/pnas.1524160113. http://doi.org/10.1073/pnas.1524160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover JR, Stager DR, Morale SE, Leffler JN, Birch EE. Improvement in motor development following surgery for infantile esotropia. Journal of AAPOS. 2008;12(2):136–140. doi: 10.1016/j.jaapos.2007.08.013. http://doi.org/10.1016/j.jaapos.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA. Transforming vision into action. Vision Research. 2011;51(13):1567–1587. doi: 10.1016/j.visres.2010.07.027. http://doi.org/10.1016/j.visres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(I):20–25. doi: 10.1016/0166-2236(92)90344-8. http://doi.org/10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grant S, Conway ML. Reach-to-precision grasp deficits in amblyopia: Effects of object contrast and low visibility. Vision Research. 2014 doi: 10.1016/j.visres.2014.11.009. http://doi.org/10.1016/j.visres.2014.11.009. [DOI] [PubMed]
- Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Investigative Ophthalmology & Visual Science. 2007;48(3):1139. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus. 2011;19(3):119–128. doi: 10.3109/09273972.2011.600423. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21870915. [DOI] [PubMed] [Google Scholar]
- Grant S, Suttle C, Melmoth DR, Conway ML, Sloper JJ. Age- and Stereovision-Dependent Eye-Hand Coordination Deficits in Children With Amblyopia and Abnormal Binocularity. Investigative Ophthalmology & Visual Science. 2014;55(9):5687–57015. doi: 10.1167/iovs.14-14745. http://doi.org/10.1167/iovs.14-14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter EJ, Maybery MT, Badcock DR. Vision in developmental disorders: is there a dorsal stream deficit? Brain Research Bulletin. 2010;82(3–4):147–60. doi: 10.1016/j.brainresbull.2010.02.016. http://doi.org/10.1016/j.brainresbull.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Gummel K, Ygge J, Benassi M, Bolzani R. Motion perception in children with foetal alcohol syndrome. Acta Paediatrica (Oslo, Norway: 1992) 2012;101(8):e327–32. doi: 10.1111/j.1651-2227.2012.02700.x. http://doi.org/10.1111/j.1651-2227.2012.02700.x. [DOI] [PubMed] [Google Scholar]
- Gunn A, Cory E, Atkinson J, Braddick O, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002;13(6):843–7. doi: 10.1097/00001756-200205070-00021. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11997698. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Tinelli F, Del Viva MM, Bancale A, Arrighi R, Pascale RR, Cioni G. Motion perception in preterm children: role of prematurity and brain damage. Neuroreport. 2009;20(15):1339–43. doi: 10.1097/WNR.0b013e328330b6f3. http://doi.org/10.1097/WNR.0b013e328330b6f3. [DOI] [PubMed] [Google Scholar]
- Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Developmental Science. 2011;14(6):1330–1339. doi: 10.1111/j.1467-7687.2011.01078.x. http://doi.org/10.1111/j.1467-7687.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Developmental Science. 2011;14(6):1330–1339. doi: 10.1111/j.1467-7687.2011.01078.x. http://doi.org/10.1111/j.1467-7687.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- Hansen PC, Stein JF, Orde SR, Winter JL, Talcott JB. Are dyslexics’ visual deficits limited to measures of dorsal stream function? Neuroreport. 2001;12(7):1527–1530. doi: 10.1097/00001756-200105250-00045. http://doi.org/10.1097/00001756-200105250-00045. [DOI] [PubMed] [Google Scholar]
- Ho W, Tang MM, Fu C, Leung K, Pang PC. Relationship between Vision and Visual Perception in Hong Kong Preschoolers. Optometry and Vision Science. 2015;92(5):623–631. doi: 10.1097/OPX.0000000000000569. [DOI] [PubMed] [Google Scholar]
- Hrisos S, Clarke MP, Kelly T, Henderson J, Wright CM. Unilateral visual impairment and neurodevelopmental performance in preschool children. The British Journal of Ophthalmology. 2006;90(7):836–838. doi: 10.1136/bjo.2006.090910. http://doi.org/10.1136/bjo.2006.090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson LS, Frisk V, Downie ALS. Motion-defined form processing in extremely premature children. Neuropsychologia. 2006;44(10):1777–1786. doi: 10.1016/j.neuropsychologia.2006.03.011. http://doi.org/10.1016/j.neuropsychologia.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Jarrold C, Baddeley AD, Hewes AK. Verbal and nonverbal abilities in the Williams syndrome phenotype: evidence for diverging developmental trajectories. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39(4):511–523. http://doi.org/Doi10.1017/S0021963098002443. [PubMed] [Google Scholar]
- Kurdek LA, Sinclair RJ. Predicting reading and mathematics achievement in fourth-grade children from kindergarten readiness scores. Journal of Educational Psychology. 2001;93(3):451–455. http://doi.org/10.1037/0022-0663.93.3.451. [Google Scholar]
- Law C-T, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nature Neuroscience. 2008;11(4):505–513. doi: 10.1038/nn2070. http://doi.org/10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Developmental Psychobiology. 2005;46:163–183. doi: 10.1002/dev.20055. http://doi.org/10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240(4853):740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. Bmj. 1988;297(6659):1304–1308. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay TL, Jakobson LS, Ellemberg D, Lewis TL, Maurer D, Casiro O. Deficits in the processing of local and global motion in very low birthweight children. Neuropsychologia. 2005;43(12):1738–1748. doi: 10.1016/j.neuropsychologia.2005.02.008. http://doi.org/10.1016/j.neuropsychologia.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Manning C, Charman T, Pellicano E. Processing Slow and Fast Motion in Children With Autism Spectrum Conditions. Autism Research. 2013;6(6):531–541. doi: 10.1002/aur.1309. http://doi.org/10.1002/aur.1309. [DOI] [PubMed] [Google Scholar]
- Manning C, Dakin SC, Tibber MS, Pellicano E. Averaging, not internal noise, limits the development of coherent motion processing. Developmental Cognitive Neuroscience. 2014;10:1–13. doi: 10.1016/j.dcn.2014.07.004. http://doi.org/10.1016/j.dcn.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. Journal of Neurophysiology. 1983;49(5):1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- Mazyn LIN, Lenoir M, Montagne G, Delaey C, Savelsbergh GJP. Stereo vision enhances the learning of a catching skill. Experimental Brain Research. 2007;179(4):723–726. doi: 10.1007/s00221-007-0957-5. http://doi.org/10.1007/s00221-007-0957-5. [DOI] [PubMed] [Google Scholar]
- McKinlay CJD, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, … Harding JE. Neonatal Glycemia and Neurodevelopmental Outcomes at 2 Years. New England Journal of Medicine. 2015;373(16):1507–1518. doi: 10.1056/NEJMoa1504909. http://doi.org/10.1056/NEJMoa1504909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmoth DR, Finlay AL, Morgan MJ, Grant S. Grasping deficits and adaptations in adults with stereo vision losses. Investigative Ophthalmology and Visual Science. 2009;50(8):3711–3720. doi: 10.1167/iovs.08-3229. http://doi.org/10.1167/iovs.08-3229. [DOI] [PubMed] [Google Scholar]
- Moreira RS, Magalhães LC, Alves CRL. Effect of preterm birth on motor development, behavior, and school performance of school-age children: a systematic review. Jornal de Pediatria. 2014;90(2):119–134. doi: 10.1016/j.jped.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Newsome WT. Visual Response Properties of Striate Cortical Neurons Projecting to Area MT in Macaque Monkeys. The Journal of Neuroscience. 1996;16(23):7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. Retrieved from http://www.jneurosci.org/content/16/23/7733.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Giaschi D. The effect of dot speed and density on the development of global motion perception. Vision Research. 2012;62:102–107. doi: 10.1016/j.visres.2012.02.016. http://doi.org/10.1016/j.visres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Newsome T, Pare EB. A Selective Impairment of Motion Perception Following Lesions of the Middle Temporal Visual Area (MT) The Journal of Neuroscience. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optometry & Vision Science. 2010a;87(12):942–947. doi: 10.1097/OPX.0b013e3181fd132e. [DOI] [PubMed] [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H. The functional significance of stereopsis. Investigative Ophthalmology and Visual Science. 2010b;51(4):2019–2023. doi: 10.1167/iovs.09-4434. http://doi.org/10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C, Kleiser R, Paulus W, Kruse W, Seitz RJ, Hoffmann KP. The role of V5 (hMT+) in visually guided hand movements: an fMRI study. European Journal of Neuroscience. 2004;19(11):3113–3120. doi: 10.1111/j.0953-816X.2004.03393.x. [DOI] [PubMed] [Google Scholar]
- Palomares M, Shannon MT. Global dot integration in typically developing children and in Williams Syndrome. Brain and Cognition. 2013;83(3):262–270. doi: 10.1016/j.bandc.2013.09.003. http://doi.org/10.1016/j.bandc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vision Research. 2005;45(7):827–837. doi: 10.1016/j.visres.2004.10.005. http://doi.org/10.1016/j.visres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10(4):437–442. Retrieved from http://booksandjournals.brillonline.com/content/journals/10.1163/156856897x00366. [PubMed] [Google Scholar]
- Piano MEF, O’Connor AR. The effect of degrading binocular single vision on fine visuomotor skill task performance. Investigative Ophthalmology & Visual Science. 2013;54(13):8204–8213. doi: 10.1167/iovs.12-10934. http://doi.org/10.1167/iovs.12-10934. [DOI] [PubMed] [Google Scholar]
- Pilly PK, Seitz AR. What a difference a parameter makes: a psychophysical comparison of random dot motion algorithms. Vision Research. 2009;49(13):1599–1612. doi: 10.1016/j.visres.2009.03.019. http://doi.org/10.1016/j.visres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasa AR, Rashedi V, Hosseini SA, Sazmand AH. Validity and reliability of peabody developmental motor scales (PDMS) in infants of tehran. Iranian Rehabilitation Journal. 2011;10(13):31–33. [Google Scholar]
- Reiss JE, Hoffman JE, Landau B. Motion processing specialization in Williams syndrome. Vision Research. 2005;45(27):3379–3390. doi: 10.1016/j.visres.2005.05.011. http://doi.org/10.1016/j.visres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ricci D, Chieffo D, Battaglia D, Brogna C, Contaldo I, De Clemente V, … Guzzetta F. A prospective longitudinal study on visuo-cognitive development in Dravet syndrome: Is there a “dorsal stream vulnerability”? Epilepsy Research. 2014 doi: 10.1016/j.eplepsyres.2014.10.009. http://doi.org/http://dx.doi.org/10.1016/j.eplepsyres.2014.10.009. [DOI] [PubMed]
- Rogers GL, Chazan S, Fellows R, Tsou BH. Strabismus Surgery and Its Effect Upon Infant Development in Congenital Esotropia. Ophthalmology. 1982;89(5):479–483. doi: 10.1016/s0161-6420(82)34766-1. http://doi.org/http://dx.doi.org/10.1016/S0161-6420(82)34766-1. [DOI] [PubMed] [Google Scholar]
- Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cerebral Cortex. 1999;9(1):90–100. doi: 10.1093/cercor/9.1.90. http://doi.org/10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nature Reviews. Neuroscience. 2010;11(1):53–60. doi: 10.1038/nrn2737. http://doi.org/10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Watanabe T. Perceptual learning without perception. Nature. 2001;413(October):844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Schoemaker MM, Niemeijer AS, Flapper BCT, Smits-Engelsman BCM. Validity and reliability of the Movement Assessment Battery for Children-2 Checklist for children with and without motor impairments. Developmental Medicine and Child Neurology. 2012;54(4):368–375. doi: 10.1111/j.1469-8749.2012.04226.x. http://doi.org/10.1111/j.1469-8749.2012.04226.x. [DOI] [PubMed] [Google Scholar]
- Smits-Engelsman BC, Fiers MJ, Henderson SE, Henderson L. Interrater reliability of the Movement Assessment Battery for Children. Physical Therapy. 2008;88(2):286–294. doi: 10.2522/ptj.20070068. http://doi.org/10.2522/ptj.20070068. [DOI] [PubMed] [Google Scholar]
- Solan Ha, Shelley-Tremblay JF, Hansen PC, Larson S. Is there a common linkage among reading comprehension, visual attention, and magnocellular processing? Journal of Learning Disabilities. 2007;40(3):270–8. doi: 10.1177/00222194070400030701. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17518218. [DOI] [PubMed] [Google Scholar]
- Spencer J, O’Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport. 2000;11(12):2765–2767. doi: 10.1097/00001756-200008210-00031. http://doi.org/10.1097/00001756-200008210-00031. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Morale SE, Wang YZ, Birch EE. Abnormal radial deformation hyperacuity in children with strabismic amblyopia. Investigative Ophthalmology and Visual Science. 2012;53(7):3303–3308. doi: 10.1167/iovs.11-8774. http://doi.org/10.1167/iovs.11-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Investigative Ophthalmology and Visual Science. 2011;52(3):1851–1864. doi: 10.1167/iovs.10-6341. http://doi.org/10.1167/iovs.10-6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Using multivariate statistics 5th ed. 6. Pearson; 2012. [Google Scholar]
- Tavasoli A, Azimi P, Montazari A. Reliability and Validity of the Peabody Developmental Motor Scales-Second Edition for Assessing Motor Development of Low Birth Weight Preterm Infants. Pediatric Neurology. 2014;51(4):522–526. doi: 10.1016/j.pediatrneurol.2014.06.010. http://doi.org/10.1016/j.pediatrneurol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nature Neuroscience. 2005;8(4):505–511. doi: 10.1038/nn1430. http://doi.org/10.1038/nn1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Gallant JL. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994;13(1):1–10. doi: 10.1016/0896-6273(94)90455-3. http://doi.org/10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Van Waelvelde H, Peersman W, Leuven KU. Convergent Validity Between Two Motor Tests: Movement-ABC and PDMS-2. Adapted Physical Activity Quarterly. 2007;24:59–69. doi: 10.1123/apaq.24.1.59. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Náñez JE, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature Neuroscience. 2002;5(10):1003–1009. doi: 10.1038/nn915. http://doi.org/10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Investigative ophthalmology & visual science. 2008;49(2):594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- Webber AL, Wood JM, Thompson B. Fine motor skills of children with amblyopia improve following binocular treatment. Investigative Ophthalmology and Visual Science. 2016;57(11):4713–4720. doi: 10.1167/iovs.16-19797. http://doi.org/10.1167/iovs.16-19797. [DOI] [PubMed] [Google Scholar]
- Welscher D. Wechsler preschool and primary scale of intelligence. 3. San Antonio, TX: Pearson; 2002. [Google Scholar]
- Whitney D, Ellison A, Rice NJ, Arnold D, Goodale M, Walsh V, Milner D. Visually guided reaching depends on motion area MT+ Cerebral Cortex. 2007;17(11):2644–2649. doi: 10.1093/cercor/bhl172. http://doi.org/10.1093/cercor/bhl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes TA, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, … Lester BM. Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug and Alcohol Dependence. 2013;127(1–3):101–7. doi: 10.1016/j.drugalcdep.2012.06.016. http://doi.org/10.1016/j.drugalcdep.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes TA, LaGasse LL, Huestis MA, DellaGrotta S, Dansereau LM, Lester BM. Prenatal methamphetamine exposure and neurodevelopmental outcomes in children from 1 to 3years. Neurotoxicology and Teratology. 2014;42:77–84. doi: 10.1016/j.ntt.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]