Abstract
Corneal epithelial defects are a common cause of ocular morbidity and can result in corneal scarring if they do not heal properly. Matrix metalloproteinases (MMPs) are extracellular matrix proteinases that regulate multiple aspects of corneal repair. We have previously shown that MMP12 has a protective effect on corneal fibrosis through its regulation of neutrophil and macrophage infiltration and angiogenesis in a chemical injury model involving full thickness damage to the cornea. However, the role of MMP12 in injuries limited to the corneal epithelium is relatively unknown. This study investigates the reparative effects of MMP12 following isolated corneal epithelial injury. Using a corneal epithelial debridement injury model performed on corneas of wild-type (WT) mice, we show that Mmp12 is expressed early following corneal epithelial injury with highest expression levels at 8 hours after injury and lower expression levels at 4 and 8 days after injury. We investigated whether MMP12 has an effect on the rate of epithelial repair and cell migration using in vivo and in vitro scratch assays performed on WT and Mmp12-/- mice. We found that loss of MMP12 results in a slower scratch wound repair rate both in vivo and in vitro. We also found that corneas of Mmp12-/- mice have decreased neutrophil infiltration following injury. Loss of MMP12, however, does not affect cell proliferation in the center of the wounds. These data support a role of MMP12 in promoting early repair processes following corneal epithelial injury by enhancing epithelial cell migration and neutrophil infiltration.
Keywords: Cornea, wound healing, epithelium, matrix metalloproteinases, inflammation
1. Introduction
The cornea functions as an important component of the visual processing system by refracting light through the pupil and lens onto the retina. It also serves as a protective barrier for the internal contents of the eye against external insults. The corneal epithelium is the outermost cellular layer of the cornea and consists of several layers of stratified squamous non-keratinized epithelial cells (Chi and Trinkaus-Randall, 2013). The corneal stromal layer comprises nearly 90% of the thickness of the cornea and consists of keratocytes and a collagen-rich extracellular matrix (ECM) (Hassell and Birk, 2010). The corneal epithelial basement membrane (BM) is positioned between the basal epithelial cells and the corneal stromal layer (Torricelli et al., 2013). The structural simplicity and accessibility of the cornea makes it a good model system for studying basic tissue repair processes.
Corneal epithelial defects are a common cause of ocular morbidity. In the United States, abrasions of the corneal epithelium are the most common type of emergency department-treated eye injuries (Channa et al., 2016; McGwin and Owsley, 2005). Corneal abrasions typically heal quickly and uneventfully, but certain risk factors, including limbal stem cell deficiency, dry eye disease, diabetic keratopathy, and neurotrophic keratopathy, may delay repair. Epithelial defects that do not heal after two weeks of treatment are termed persistent epithelial defects and can lead to permanent corneal scarring from concurrent infection or chronic inflammation (Jeng, 2016; Liu and Kao, 2015).
Corneal epithelial repair is a dynamic process that includes the ECM and cell signaling cascades (Chi and Trinkaus-Randall, 2013; Liu and Kao, 2015). Matrix metalloproteinases (MMPs) are a prominent family of ECM proteinases that regulate various aspects of the repair process including inflammation, neovascularization, and tissue remodeling (Azar, 2006; Chan et al., 2013; Fini et al., 1998; Gordon et al., 2011; Ljubimov and Saghizadeh, 2015). MMPs control these processes through their ability to cleave nearly all components of the ECM (Lu et al., 2011) and by acting on a variety of substrates, including ECM and BM proteins, proteinases, and their inhibitors, to activate growth factors, cytokines, receptors, and adhesion molecules (Sivak and Fini, 2002). MMPs have been found to negatively impact corneal healing through a variety of mechanisms. For example, the over-expression of MMP9 in mice causes failure of corneal re-epithelialization and chronic corneal ulcerations (Fini et al., 1996), and mice deficient in MMP9 have accelerated corneal epithelial wound repair (Mohan et al., 2002). Overexpression of MMP10 and cathepsin F in organ-cultured human corneas results in delayed wound healing and altered basement membrane and integrin patterns (Saghizadeh et al., 2010). More recent studies have found that MMPs can also positively impact corneal healing. The overexpression of MMP14 in injured mouse corneas may prevent corneal fibrosis, and thereby maintain corneal clarity, through the decreased expression of type III collagen and α-smooth muscle actin (Galiacy et al., 2011).
MMP12 (macrophage metalloelastase) is another MMP that is able to protect against corneal fibrosis (Chan et al., 2013). It was first identified and characterized as a secreted protein from mouse peritoneal macrophages through its elastase activity (Werb and Gordon, 1975). Although MMP12 is predominantly expressed by macrophages (Shapiro et al., 1993), its expression is upregulated in rat corneal epithelium (Lyu and Joo, 2005) and rabbit corneal stromal myofibroblasts following corneal injury (Iwanami et al., 2009). Using a chemical injury model that damages both the epithelial and stromal layers of the cornea, we previously observed a protective effect of MMP12 on corneal fibrosis through it regulation of immune cell infiltration and angiogenesis (Chan et al., 2013). In the present study, we investigated whether MMP12 has protective effects on corneal repair following epithelial injury alone using mice deficient in MMP12. We uncovered a role for MMP12 in promoting early corneal epithelial repair through its regulation of epithelial cell migration and neutrophil infiltration.
2. Materials and Methods
2.1. Mice
Mice homozygous for the null allele of MMP12 (Shipley et al., 1996) were genotyped using published protocols and backcrossed to FVB/n. All experiments were performed with 8–12 week old male and female mice and sibling wild-type littermates served as controls. Mice were maintained under pathogen-free conditions in the UCSF barrier facility. All animal experiments were conducted in accordance with procedures approved by the UCSF Institutional Animal Care and Use Committee and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Animal model of injury
Corneal epithelial debridement was performed on mice as previously described (Chan and Werb, 2015). Briefly, mice were anesthetized by isofluorane inhalation (Abbott Laboratories, Alameda, CA) and by topical application of 0.5% Proparacaine (Bausch & Lomb, Rochester, NY) applied to their ocular surface. A 1.5 mm trephine (Beaver-Visitec, Waltham, MA) was used to demarcate the central cornea of the right eye only and the trephine mark was visualized under a stereomicroscope (Leica Biosystems Inc., Wetzlar, Germany). The epithelium within the trephine mark was then removed down to the basement membrane using an Algerbrush II (Katena Products, Inc., Denville, NJ); the left eye was left intact as a contralateral control. After the epithelial debridement, topical 0.5% Proparacaine was again placed onto the ocular surface for anesthesia.
2.3. RNA and qPCR
Corneal tissue was harvested and then freeze-fractured with a mortar and pestle. Total RNA was extracted using homogenization and Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol and then subjected to DNase treatment (New England Biolabs, Ipswich, MA). cDNA was synthesized using the Superscript III RT First Strand Kit (Invitrogen, Carlsbad, CA). qPCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) in an Applied
Biosystems 7500 Instrument (Carlsbad, CA) machine. Ct values were normalized to HPRT, and relative expression was calculated using the 2ΔΔCt method. Primer sequences were: Mmp12-forward, 5′ GAGTCCAGCCACCAACATTAC3′, Mmp12-reverse, 5′ GCGAAGTGGGTCAAAGAC AG3′, Hprt-forward, 5′GCCTAAGATGAGCGCAAGTTG3′, Hprt-reverse, 5′ TACTAGGCAGATGGCCACAGG3′ and were purchased from Integrated DNA Technologies (San Diego, CA).
2.4. Primary cell culture and cell migration assay
Primary cultures of mouse corneal epithelial cells from WT and Mmp12-/- mice were established in supplementary hormonal epithelial medium (SHEM) as previously described (Kawakita et al., 2004; Levin and Verkman, 2006). SHEM consisted of equal volume HEPES-buffered DMEM and F12 medium, containing 0.1 mg/ml calcium chloride, 10 ng/mL mouse-derived EGF, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite, 0.5 μg/mL hydrocortisone, 0.1 μg/mL cholera toxin A subunit (all from Sigma-Aldrich, St. Louis, MO), 5% FBS, 50 μg/mL gentamicin, and 1.25 μg/mL amphotericin B. Enucleated eyes of 6- to 8-week old mice were rinsed briefly in phosphate-buffered saline (PBS) and then washed in SHEM. Whole globes were then enzymatically digested for 18 hours at 4°C in SHEM containing 15 mg/mL Dispase II (Sigma-Aldrich, St. Louis, MO) and 100 mM d-sorbitol (Sigma-Aldrich, St. Louis, MO). Using a stereo dissecting microscope, corneal–limbal epithelial cell sheets were removed intact from each globe using jeweler forceps #1 (Katena, Denville, NJ). Sheets from eyes of 2 individual mice of the same genotype were pooled, centrifuged (5 minutes at 800g), resuspended in SHEM, counted with a hemocytometer, and seeded into a 24-well plate coated with Matrigel basement membrane matrix (BD Biosciences, Franklin Lakes, NJ) at a density of 0.5 × 106 cells/well (for migration studies). Medium was replaced after 24 hours, to remove unattached suprabasal cells and cells were grown to confluency for 5–7 days in supplementary hormonal epithelial medium (SHEM). 8 individual primary cultures were used for each genotype. Un-passaged cells were used for all experiments.
Primary mouse corneal epithelial cells were grown to confluency for the cell migration assay. Cells were synchronized by replacing the medium with SHEM containing low serum (1% FBS) and lacking EGF for 10 hours. Scratch wounds were made with a 10 μL pipette tip. Bright-field, time-lapse movies of wound closure were collected on a Zeiss Axiovert S-100 microscope by using a 10× A-Plan objective lens, a Ludl shutter, a Cohu CCD camera, and a Ludl x-y-z motorized stage. Temperature was held at 37°C and CO2 was held at 5% by using a CTI Controller 3700 and Temperature Control 37.2 combination. Images were acquired every 15 min by using MetaMorph (Molecular Devices, Inc., Sunnyvale CA). Wound area was measured with NIH ImageJ software, and cell migration was determined as percent of the wound area relative to the original wound area.
2.5. Immunofluorescence
Primary cells cultured in chamber slides were fixed in 4% paraformaldehyde in PBS for 10 minutes, permeabilized using blocking buffer (PBS + 0.8% Triton X-100 + 2% goat serum) and incubated with a rabbit polyclonal primary antibody against ZO-1 (Invitrogen, Carlsbad, CA, #61-7300) or mouse monoclonal primary antibody against E-cadherin (BD Biosciences, San Jose, CA; #610181) for one hour at room temperature. Appropriate Alexa 488-conjungated goat anti-rabbit or anti-mouse IgG (Invitrogen, Carlsbad, CA) secondary antibodies were used for one hour at room temperature. Chambers were removed and slides were mounted using Fluoro-gel mounting medium (Electron Microscopy Science, Hatfield, PA). Zo-1 and E-cadherin were imaged using a confocal laser-scanning microscope (LSM 5 Pascal; Zeiss).
2.6. Gr-1 and Ki67 analysis
Eyes were enucleated and the corneas were dissected to remove the lens, iris, and retina. Four incisions were made equal distances apart to aid in flattening the corneas. Corneal tissue was then fixed with 4% paraformaldehyde in PBS at 4°C overnight, washed twice in PBS, postfixed with chilled 100% acetone for 20 minutes at room temperature, and blocked overnight at 4°C in blocking buffer (PBS + 0.8% Triton X-100 + 2% goat serum). Immunostaining was performed using a rabbit polyclonal primary antibody against Ki-67 (Fisher Scientific, Hampton, NH; #PIPA519462), and a rat primary monoclonal antibody against Gr-1 (BD Pharmingen, San Jose, CA; #550291) overnight at 4°C, followed by overnight incubation at 4°C with secondary antibodies Alexa 488-conjungated goat anti-rabbit and Alexa 488-conjungated goat anti-rat (Invitrogen, Carlsbad, CA). Routine protocols included corneas stained with an isotype control or secondary antibodies alone. Corneas were then placed epithelial side-up on a slide, Fluoro-gel mounting medium (Electron Microscopy Science, Hatfield, PA) was added, and coverslips were placed.
A confocal laser-scanning microscope (Gr-1:LSM 5 Pascal; Zeiss; Ki67:C1, Nikon) was used to image the localization of Alexa Fluor 488 in the central cornea. For Gr-1, optical sections of confocal epifluorescence images were acquired sequentially through the full cornea thickness at an interval of 5 μm with a 10× objective lens with image acquisition software (Zeiss). Optical sections were merged and viewed en face. For Ki67, single optical sections containing epithelial cells were imaged using a 10× objective lens and image acquisition software (Zeiss). For both Gr-1 and Ki67, the number of pixels per color was determined using ImageJ (Schindelin et al., 2015).
2.7. Statistical Analysis
Statistical analysis was performed with one-tailed t-tests to compare mean values (Prism, GraphPad Software, La Jolla, CA). Data are considered significant if p values are less than 0.05.
3. Results
3.1. MMP12 is expressed early after corneal epithelial injury
We previously found Mmp12 to be highly expressed 2 and 6 days after corneal alkali injury where both the corneal epithelial and stromal layers are affected (Chan et al., 2013). Therefore, we sought to determine the temporal expression pattern of Mmp12 following epithelial injury alone. Corneal epithelial injuries were performed in WT mice using a 1.5 mm trephine to mark the central cornea of the right eye and the epithelial layer within the mark was removed using an Algerbrush (Chan and Werb, 2015; Stepp et al., 2014) (Fig. 1A). The injuries resulted in large epithelial defects that were almost completely healed by 18 hours after injury (Fig. 1A). In contrast to chemically injured corneas that develop corneal haze and neovascularization, corneas examined 8 days after epithelial injury showed normal transparency and avascularity (Chan et al., 2013) (Fig 1A). We collected injured corneas at 1, 2, 8, and 16 hours, and 1, 2, 4, 6, and 8 days after injury and measured the time course of Mmp12 mRNA expression using quantitative real-time PCR (qPCR) analysis. Increased expression of Mmp12 was observed as early as 2 hours post-injury. Expression peaked at 8 hours after injury (48-fold, P value = 0.028) and gradually declined at 4 and 8 days after injury (3-fold, P value = 0.042 and 7-fold, P value = 0.030, respectively; Fig 1B). These results indicate that epithelial injury alone induces early Mmp12 expression.
Fig. 1. Mmp12 is expressed in a murine model of corneal epithelial injury.
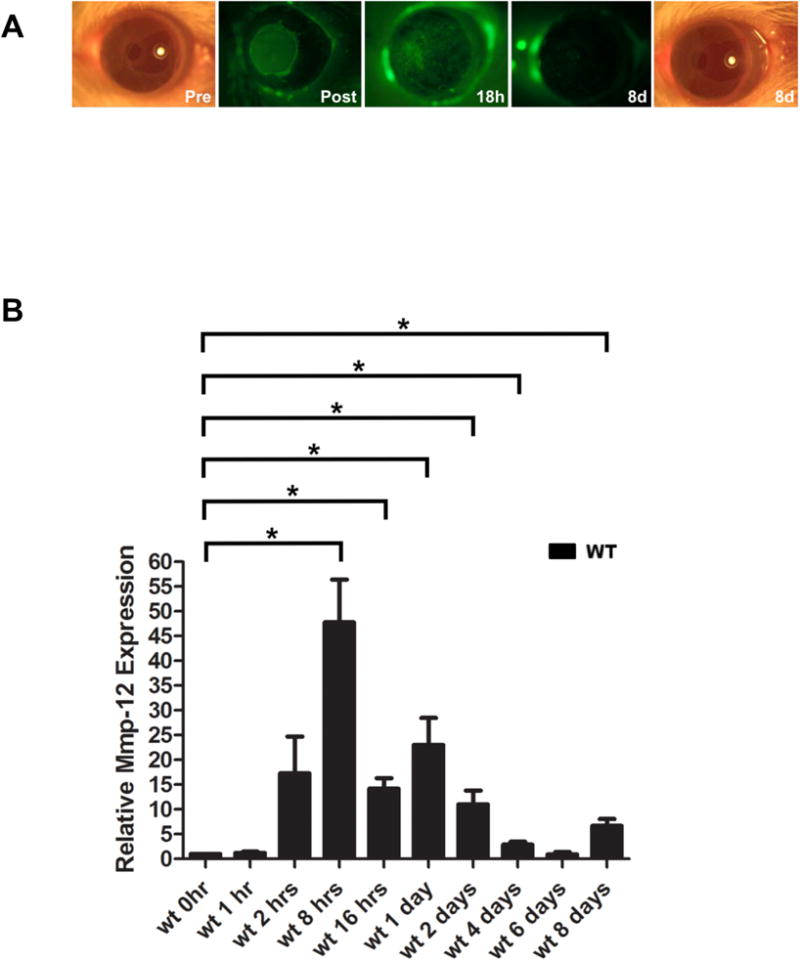
(A) The corneal injury model results in a 1.5 mm central corneal epithelial defect right after injury (Post 0h) seen with fluorescein staining, that is almost completely healed by 18 hours after injury (18h). Photographs of the corneas just prior to injury (Pre 0h) and 8 days after injury (8d) show intact and clear corneas. There is no evidence of corneal scarring or neovascularization 8 days after injury. (B) Quantification of Mmp12 mRNA expression levels in wild-type mouse corneas after corneal epithelial injury. Relative levels (means ± s.e.m.); the means and ranges are: 1.2 (0.8-1.7), 17 (9.6-32.0), 48 (29.0-80.0), 14 (11.0-18.0), 23 (15.0-39.0), 11 (15.0-39.0), 2.9 (2.1-4.0), 0.94 (0.3-1.8), and 6.7 (3.9-8.8) for 1 hour (1h), 2 hours (2h), 8 hours (8h), 16 hours (16h), 2 days (2d), 4 days (4d), 6 days (6d), and 8 days (8d) after epithelial injury, respectively (n = 3), *P<0.05.
3.2. Loss of MMP12 results in delayed corneal epithelial repair
Because we found that MMP12 was highly expressed within the first 24 hours after epithelial injury, we evaluated whether Mmp12-/- mice showed altered epithelial repair as compared to WT mice. Wounds were generated and epithelial healing was monitored and compared by performing fluorescein staining and photographing the corneas immediately after injury, 8 hours, and 18 hours after injury. The Mmp12-/- mice had significantly delayed corneal epithelial closure at both 8 and 18 hours after injury (Fig. 2A,C). Quantitative analysis revealed that at 8 hours post injury, wounds were 42% percent healed in WT mice compared with 31% healed in Mmp12-/- mice (P value = 0.0017; Fig 2B). At 18 hours post injury, healing was completed (99% healed) in WT mice, while only 93% healed in Mmp12-/- mice (P value = 0.048). Corneas were 100% healed in 5 out of 8 wounded WT mice (62.5%), whereas only 1 out 5 wounded Mmp12-/- mice had a healed wound (20%). These results demonstrate delayed corneal epithelial repair in Mmp12-/- mice.
Fig. 2. Delayed epithelial healing in Mmp12-/- mice during corneal wound repair in vivo.
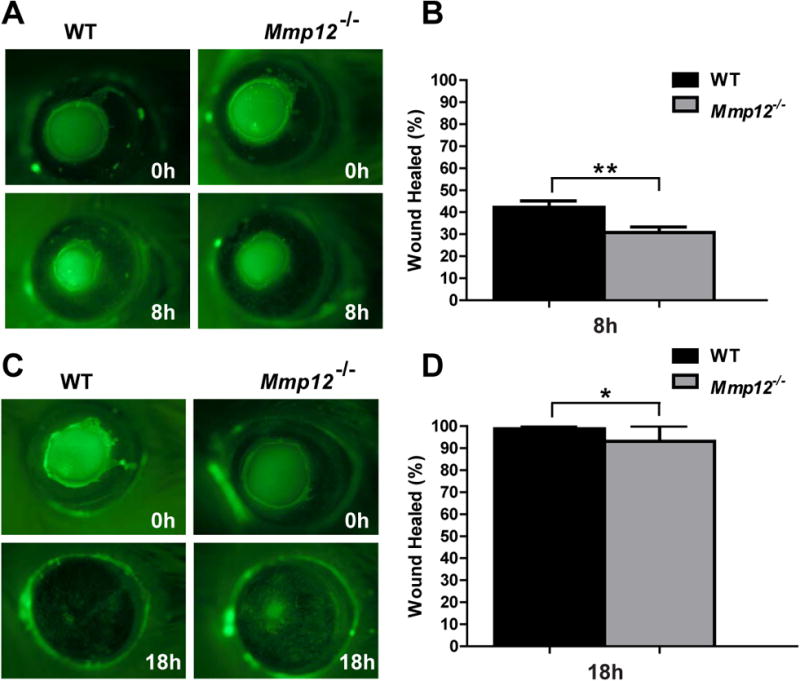
(A) Photographs of scratch injured WT and Mmp12-/- mouse corneas just prior to injury (0h) and 8 hours (8h) after corneal epithelial injury. (B) Quantification of the percent wound area healed 8 hours after injury in WT (42%; n=6) and Mmp12-/- (31%; n=11) mouse corneas. Results are means ± s.e.m.; ** P <0.005. (C) Photographs of scratch injured WT and Mmp12-/- mouse corneas just prior to injury (0h) and 18 hours (18h) after corneal epithelial injury. (D) Quantification of the percent wound area healed 18 hours after injury in WT (99%; n=8) and Mmp12-/- (93%; n=5) mouse corneas. Results are means ± s.e.m.; * P <0.05.
3.3. MMP12 promotes corneal epithelial cell migration
Immediately following a corneal epithelial defect, the neighboring epithelial cells migrate over the affected area until they cover the defect with a cell monolayer (Chi and Trinkaus-Randall, 2013; Richardson et al., 2016; Suzuki et al., 2003). To investigate if MMP12 deficiency affects epithelial cell migration, we performed in vitro scratch assays of mouse corneal epithelial cultures. Primary corneal epithelial cells from WT and Mmp12-/- mice were isolated and cultured to obtain confluent monolayers (Levin and Verkman, 2006). Following scratch injury, cells at the margins of the wound and their neighbors became migratory and closed the wound gaps (Fig. 3A). Mmp12-/- corneal epithelial cells migrated at a reduced rate compared to WT cells and closure of the wound gap was delayed in the Mmp12-/- epithelial cell cultures compared with the WT cell cultures at 2 hours and 6 hours after injury. At 2 hours post injury, the wound area was 63% of the original wound size for WT cells and 75% for Mmp12-/- cells (P value = 0.0037; Fig. 3B). After 6 hours, 4% of the wound area remained for WT cells and 13% remained for Mmp12-/- cells (P value = 0.042; Fig. 3B). These results suggest that MMP12 impacts cell migration rate in culture.
Fig. 3. Delayed healing of Mmp12-/- mouse primary epithelial cells in vitro.
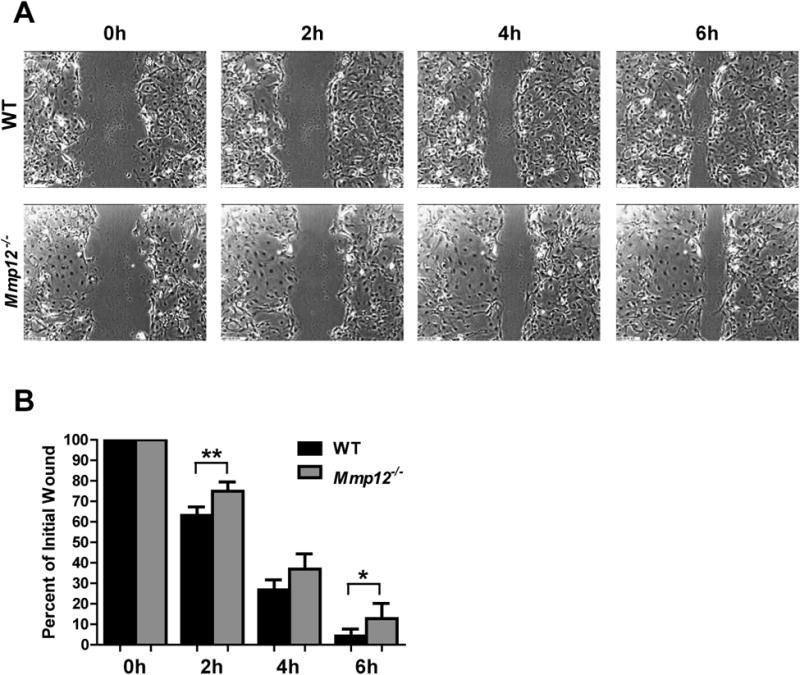
(A) Confluent monolayers of primary epithelial cells from WT and Mmp12-/- mice were wounded by a linear scratch, and wounds were monitored by time-lapse microscopy. Representative phase-contrast images of the initial wound (0h) and 2 hours (2h), 4 hours (4h), and 6 hours (6h) after scratch wound. Results are means ± s.e.m. (B) Quantification of the percent of the initial wound at 2h (63% WT, 75% Mmp12-/-), 4h (27% WT, 37% Mmp12-/-), and 6 hours (4.3% WT, 13% Mmp12-/-) after scratch wound. Results are means ± s.e.m.; ** P <0.05 *and P <0.05., and n = 8 replicate wells per group.
3.4. MMP12 does not affect cell proliferation after injury
To determine if this delay in cell migration seen in the MMP12 deficient cells was due to changes in cell adhesion, the scratched primary cell cultures were stained with antibodies to the cell adhesion markers ZO-1 and E-cadherin. The staining pattern for ZO-1 and E-cadherin was similar between WT and Mmp12-/- cell cultures at both the wound margin and a few cells in from the wound margin (Fig. 4A). These results indicate that although MMP12 has a role in promoting corneal epithelial migration, this effect is not due to a change in the expression of ZO-1 and E-cadherin.
Fig. 4. MMP12 does not affect cell adhesion or cell proliferation following epithelial injury.
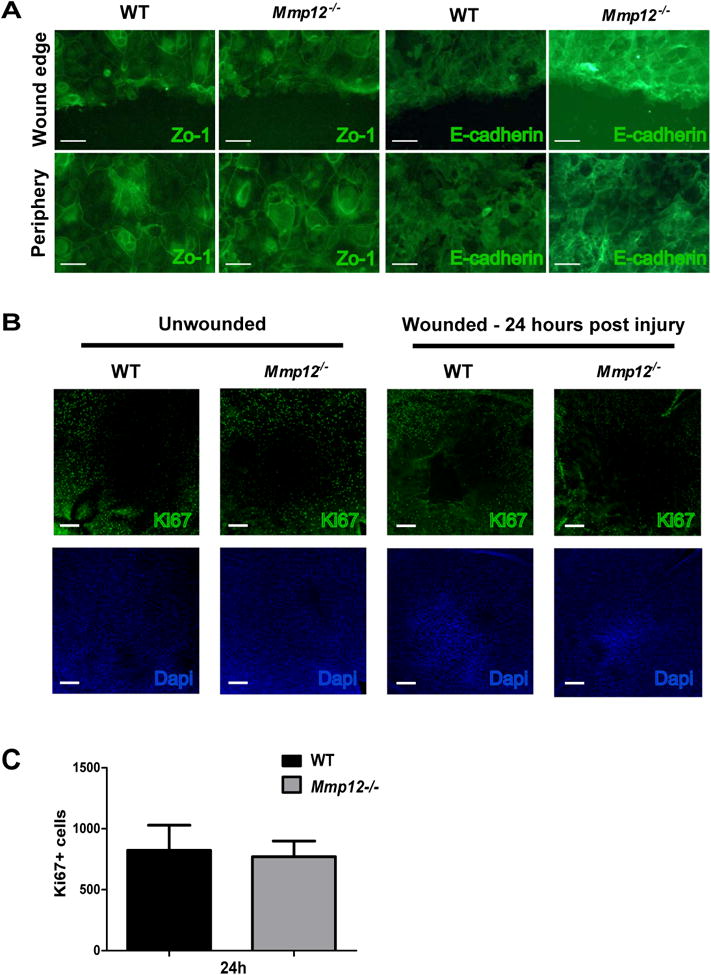
(A) Immunofluorescence of cell adhesion markers Zo-1 and E-cadherin in wounded primary epithelial cell cultures from WT and Mmp12-/- corneas. Images are of cells at the wound edge or peripheral to the wound edge. Scale bars: 50 μm. (B) Micrographs of representative whole-mount preparations of central corneas stained for the proliferation marker Ki-67 in unwounded and wounded (24 hours after injury) corneas. Scale bars: 100 μm. Dapi staining of cell nuclei was used to assess wound closure. (C) Quantification of Ki-67 cell counts in the central corneas of wounded WT and Mmp12-/- mice 24 hours after injury. Mean ± s.e.m. Ki-67 + cell counts are shown and are 823 (WT, n=8) and 770 (Mmp12-/-; n= 16).
Following the migration of epithelial cells to cover the wound, a proliferative phase occurs to aid in re-stratification and restoration of the normal epithelial thickness. Cell division in the wound area is immediately inhibited after wounding to allow for efficient cell migration (Richardson et al., 2016; Suzuki et al., 2003; Terai et al., 2011). MMP12 has been shown to be expressed in migrating mouse corneal epithelial cells (Gordon et al., 2011), the peripheral epithelium of wounded rat corneas (Lyu and Joo, 2005), and primary human corneal epithelial cells treated with Wnt-7a (Lyu and Joo, 2005). To determine if MMP12 deficiency affects cell proliferation following injury, we scratch injured corneas of WT and Mmp12-/- mice, collected the corneas 24 hours later, and stained flat mount preparations with the proliferation marker, Ki-67, and assessed the number of Ki-67 + corneal cells (Fig. 4B). Although we observed fewer Ki-67 + cells in the central cornea of wounded Mmp12-/- corneas compared with WT corneas 24 hours after injury, the result was not significant (P value = 0.060; Fig. 4B). Therefore, an effect of MMP12 on cell proliferation during epithelial wound closure cannot be concluded based on this experiment.
3.5. MMP12 promotes neutrophil infiltration following epithelial injury
Neutrophils are heavily recruited to injured corneas 1 day after injury (Chan et al., 2013). We previously showed that chemically injured Mmp12-/- corneas have decreased neutrophil infiltration and that this is the result of reduced expression of the chemokine CXCL1 (Chan et al., 2013). We next asked if neutrophil infiltration is altered in Mmp12-/- corneas following epithelial injury alone. To visualize the effect of MMP12 on neutrophil infiltration following epithelial injury, we scratch wounded WT and Mmp12-/- mice and examined them by flat mount immunofluorescence staining with primary antibodies against Gr-1. Corneas were collected 8 hours and 24 hours after injury. Eight hours after epithelial injury, significantly fewer neutrophils (22%) accumulated in the anterior stroma of Mmp12-/- corneas compared with WT corneas (P value = 0.017; Fig. 5A,B). This decrease in neutrophil infiltration was even more pronounced 24 hours after injury where 90% fewer neutrophils accumulated in the Mmp12-/- corneas (P value = 0.047; Fig. 5A,B). This result demonstrates the positive recruitment of neutrophils by MMP12 following corneal epithelial injury.
Fig. 5. MMP12 deficiency results in decreased neutrophil infiltration following epithelial injury.

(A) Representative whole-mount micrographs of WT and Mmp12-/- mouse corneas injured 8 hours (8h) and 24 hours (24h) prior. Gr-1+ cells are present at both time points and fewer Gr-1+ cells accumulate in Mmp12-/- corneas. Scale bars: 100 μm. (B) Quantification of Gr-1 levels in wild-type and Mmp12-/- mouse corneas 8 hours (8h) and 24 hours (24h) after injury. Mean pixel levels are shown and at the 8 hour time-point are 612 (WT; n = 5) and 137 (Mmp12-/-; n = 5). At the 24 hour time-point, mean pixel levels are 43658 (WT; n = 3) and 4152 (Mmp12-/-; n = 3), **p<0.05.
4. Discussion
The cellular response to corneal injury depends on the specific cell layers affected (Ljubimov and Saghizadeh, 2015). Corneal injuries can be localized to the epithelium, such as in superficial epithelial abrasions or corneal epithelial erosions, or they may also extend into the deeper corneal stroma or endothelium, as in corneal stromal ulcers. While corneal epithelial, stromal, and endothelial responses to injury share the common repair process of cell migration, they differ in their proliferative, immune, neovascular, regenerative, and signaling pathways (Ljubimov and Saghizadeh, 2015).
Regardless of the cell layer involved, MMPs are the most prominent family of proteinases associated with corneal wound repair (Fini et al., 1998; Gordon et al., 2011; Mulholland et al., 2005). We previously demonstrated that MMP12 has protective roles in the inflammatory, neovascular, and fibrotic responses to chemical injury affecting all layers of the cornea (Chan et al., 2013). In this study, we sought to determine if these protective effects of MMP12 occur following epithelial injury alone, which represents a much more common cause of ocular morbidity. We used a corneal epithelial abrasion model in mice to evaluate MMP12 in this context. We observed early expression of MMP12 in WT mice, with the highest level of expression noted at 8 hours following injury. As epithelial cell migration, proliferation, and inflammation are all early events that are known to occur within the first day after epithelial injury, we studied the role of MMP12 in these specific repair processes. Using in vivo and in vitro analyses, we observed that injured epithelial cells of Mmp12-/- mice were delayed in their ability to migrate. We also observed blunted neutrophil infiltration in injured Mmp12-/- corneas. These findings support a role for MMP12 in promoting early corneal epithelial repair processes.
Different wound types have been used to study the corneal epithelial repair process (Stepp et al., 2014). The use of a dull blade held by a blade breaker to remove the corneal epithelium was first described by Ilene Gipson and her colleagues in the early 1980's (Gipson and Kiorpes, 1982; Gipson et al., 1982; Spurr-Michaud et al., 1988; Zieske et al., 1989). A later study by M. Elizabeth Fini and her colleagues was the first to use the algerbrush as the technique for epithelial debridement (Mohan et al., 2002) and this technique has become a popular method for performing mechanical corneal wounding. While the dull blade technique for epithelial debridement leaves the basement membrane intact, there is debate whether the basement membrane remains intact following algerbrush epithelial removal. Interestingly, these two methods for epithelial removal have been shown to give different results with regard to induction of MMP expression; in particular, MMP9 induction is not observed following blade injury but is induced following algerbrush injury (Gordon et al., 2011; Matsubara et al., 1991b). Using the algerbrush technique, a prior comprehensive expression analysis of all 23 MMP members following corneal epithelial injury in mice showed MMP12 to be one of 6 MMPs significantly up-regulated during epithelial regeneration (Gordon et al., 2011). Our findings confirm the early up-regulation of MMP12 expression following epithelial injury with the highest level of expression noted 8 hours after injury. Early expression of MMP12 was also noted following full-thickness corneal injury, with significantly elevated MMP12 expression seen at 2 days post-injury (Chan et al., 2013). At 6 days following deep corneal injury, MMP12 expression was highest (Chan et al., 2013), while at this time-point MMP12 expression post-epithelial injury alone was low. The pattern of MMP12 expression following corneal injury therefore depends on the corneal cell layers affected. The early peak of MMP12 expression following epithelial injury could be explained by production from a resident cell population, such as local corneal epithelial cells or resident macrophages, while the later MMP12 peak seen following full-thickness injury could depend on production by a recruited cell type, for example macrophages recruited from the peripheral circulation. MMP12 has been shown to be expressed by injured corneal epithelial cells (Gordon et al., 2011; Lyu and Joo, 2005) and macrophages (Chan et al., 2013).
MMPs can regulate cell migration through their effects on cellular adhesive properties. Our in vivo and in vitro data show that MMP12 promotes corneal epithelial migration, but this effect does not appear mediated by changes in the expression of cell adhesive proteins, Zo-1 and E-cadherin. This result contrasts with studies that have found MMPs to delay corneal epithelial healing, notably MMP10 and MMP9. MMP10 is elevated in human diabetic corneal epithelium, and MMP10 over-expression with cathepsin F in human organ-cultured human corneas results in delayed wound healing (Saghizadeh et al., 2010). MMP9 expression is induced in epithelial cells migrating to resurface a corneal wound (Matsubara et al., 1991a), and mice deficient in MMP9 with corneal epithelial injury had faster rates of epithelial resurfacing (Mohan et al., 2002). Interestingly, the role of MMP9 in corneal resurfacing is complex because it was recently shown that galactin-3-induced MMP9 facilitates corneal cell movement by disrupting cell-cell contacts, thereby showing that MMP9 also has the capacity to promote cell migration (Mauris et al., 2014). Taken together, individual MMPs may either promote or inhibit corneal epithelial cell migration and our data demonstrate that MMP12 specifically promotes epithelial migration.
During the process of epithelial repair, mitotic activity is required to replace the cells lost following injury. Corneal epithelial cells both at the leading edge of the wound and at the periphery near the limbus, have particularly high proliferative activity (Nagata et al., 2015). An in vitro study using cultured human corneal epithelial cells found that functional blockade of MMP12 delayed Wnt 7a-induced cell proliferation, suggesting that MMP12 can regulate the proliferation of corneal epithelial cells (Lyu and Joo, 2005). Our results in vivo reveal a non-significant trend towards less proliferative activity in the central cornea of MMP12-deficient mice as compared with wild-type mice.
The early recruitment of neutrophils in response to injury is a key component of the innate immune response (Li et al., 2006). MMPs can regulate innate immunity by cleaving and inactivating chemokines (Dean et al., 2008; Dufour and Overall, 2013). We previously determined that in deep corneal injury, MMP12 has a positive effect on neutrophil infiltration and that it mediates this effect through regulation of chemokine CXCL1 expression (Chan et al., 2013). Deep corneal injuries, as compared to isolated epithelial injuries, induce a distinct cytokine and chemokine response that is more inflammatory and angiogenic (Ljubimov and Saghizadeh, 2015; Torricelli et al., 2016). Nonetheless, our current results using MMP12-deficient mice indicate that MMP12 has a similar stimulatory effect on neutrophil recruitment following corneal epithelial injury alone. Studies have shown that neutrophil emigration is required for efficient corneal re-epithelialization (Li et al., 2006). The inhibition of neutrophil migration into corneas with an epithelial abrasion extends the healing time by 12 to 24 hours (Li et al., 2006). Possible mechanisms by which neutrophils promote re-epithelialization include the delivery of growth factors and the killing of pathogens that cause tissue damage. The promotion of corneal re-epithelialization by MMP12 may be partially mediated by its enhancement of neutrophil recruitment. Our studies were performed in mice in an FVB/n background strain. Prior reports have shown that different inbred mouse strains can yield different cytokine expression profiles and consequently have distinct effects on the healing response to injury (Shi et al., 1997; Walkin et al., 2013). Mouse strain differences have also been noted to have an effect on corneal epithelial healing rates (Pal-Ghosh et al., 2008). Thus, the mouse strain may influence the role of MMPs on epithelial repair through their varying effects on cytokine profiles. Interestingly, the positive effect of MMP12 on neutrophil activities has also been observed in other tissue models of inflammation including lung (Nenan et al., 2007; Warner et al., 2001), cartilage (Bellac et al., 2014), and skin (Dean et al., 2008) where mice of different strain backgrounds were used (A/J, 129SvEv, and B10 strain backgrounds).
Our data demonstrate that MMP12 promotes corneal epithelial repair through its effects on cell migration and inflammation. We have tested two separate corneal injury models, involving full thickness injury (Chan et al., 2013) or a more clinically common injury isolated to the epithelium, and MMP12 appears to have protective effects in both types of injury. Our previous study and this current one represent the first use of MMP12-deficient mice for repair studies in any tissue, an approach that can bring significant understanding of the specific role of MMP12 in tissue repair processes. Our studies were performed in corneal tissue, but the findings may be relevant to lung, skin, intestine, and other tissues lined by a surface epithelium. The intestinal tissue of patients with ischemic and ulcerative colitis has been shown to abundantly express MMP12 in macrophages in inflamed areas of the intestine (Vaalamo et al., 1998) so the reparative effects of MMP12 noted in corneal abrasions may likewise be observed in intestinal ulcers. Several MMPs are expressed following corneal epithelial injury (Gordon et al., 2011) and can have negative roles in corneal healing. Data from this study indicate that MMP12 has a positive effect on the corneal epithelial repair process. Currently, corneal ulcers are treated clinically with oral doxycycline because of its ability to inhibit all MMP activity (Brooks and Ollivier, 2004). Results from this study demonstrate that MMPs do not have a uniformly negative effect on wound healing, and therefore inhibiting all MMP activity may not be beneficial. In fact, stimulating particular MMPs, such as MMP12, may be the more rational therapeutic goal. Guided by a more comprehensive understanding of individual MMPs, we believe improved clinical outcomes can be achieved by targeting the activities of specific MMPs.
Highlights.
Matrix metalloproteinase 12 (MMP12, macrophage metalloelastase) is expressed early after corneal epithelial injury
Loss of MMP12 results in delayed corneal epithelial repair
MMP12 dampens epithelial cell migration and neutrophil infiltration following corneal injury
Acknowledgments
Funding: This study was supported by funds from the National Institutes of Health (R01 EY022739 and K08 EY018858 to MFC; NIH-NEI EY002162 – Core Grant for Vision Research), and an RPB Unrestricted Grant to UCSF Department of Ophthalmology.
The authors thank Zena Werb, PhD (UCSF) for experimental advice and also Jing Li and Ying Yu, MD for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an american ophthalmological society thesis) Transactions of the American Ophthalmological Society. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- Bellac CL, Dufour A, Krisinger MJ, Loonchanta A, Starr AE, Auf dem Keller U, Lange PF, Goebeler V, Kappelhoff R, Butler GS, Burtnick LD, Conway EM, Roberts CR, Overall CM. Macrophage matrix metalloproteinase-12 dampens inflammation and neutrophil influx in arthritis. Cell Rep. 2014;9:618–632. doi: 10.1016/j.celrep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Brooks DE, Ollivier FJ. Matrix metalloproteinase inhibition in corneal ulceration. Vet Clin North Am Small Anim Pract. 2004;34:611–622. doi: 10.1016/j.cvsm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Chan MF, Li J, Bertrand A, Casbon AJ, Lin JH, Maltseva I, Werb Z. Protective effects of matrix metalloproteinase-12 following corneal injury. Journal of cell science. 2013;126:3948–3960. doi: 10.1242/jcs.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MF, Werb Z. Animal Models of Corneal Injury. Bio Protoc. 2015;5:e1516. doi: 10.21769/bioprotoc.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channa R, Zafar SN, Canner JK, Haring RS, Schneider EB, Friedman DS. Epidemiology of Eye-Related Emergency Department Visits. JAMA Ophthalmol. 2016;134:312–319. doi: 10.1001/jamaophthalmol.2015.5778. [DOI] [PubMed] [Google Scholar]
- Chi C, Trinkaus-Randall V. New insights in wound response and repair of epithelium. J Cell Physiol. 2013;228:925–929. doi: 10.1002/jcp.24268. [DOI] [PubMed] [Google Scholar]
- Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- Dufour A, Overall CM. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci. 2013;34:233–242. doi: 10.1016/j.tips.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;(290 Suppl):S12–23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- Fini ME, Parks WC, Rinehart WB, Girard MT, Matsubara M, Cook JR, West-Mays JA, Sadow PM, Burgeson RE, Jeffrey JJ, Raizman MB, Krueger RR, Zieske JD. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996;149:1287–1302. [PMC free article] [PubMed] [Google Scholar]
- Galiacy SD, Fournie P, Massoudi D, Ancele E, Quintyn JC, Erraud A, Raymond-Letron I, Rolling F, Malecaze F. Matrix metalloproteinase 14 overexpression reduces corneal scarring. Gene Ther. 2011;18:462–468. doi: 10.1038/gt.2010.159. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Kiorpes TC. Epithelial sheet movement: protein and glycoprotein synthesis. Dev Biol. 1982;92:259–262. doi: 10.1016/0012-1606(82)90170-1. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Westcott MJ, Brooksby NG. Effects of cytochalasins B and D and colchicine on migration of the corneal epithelium. Investigative ophthalmology & visual science. 1982;22:633–642. [PubMed] [Google Scholar]
- Gordon GM, Austin JS, Sklar AL, Feuer WJ, LaGier AJ, Fini ME. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J Cell Physiol. 2011;226:1461–1470. doi: 10.1002/jcp.22306. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Birk DE. The molecular basis of corneal transparency. Experimental eye research. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami H, Ishizaki M, Fukuda Y, Takahashi H. Expression of matrix metalloproteinases (MMP)-12 by myofibroblasts during alkali-burned corneal wound healing. Curr Eye Res. 2009;34:207–214. doi: 10.1080/02713680802687809. [DOI] [PubMed] [Google Scholar]
- Jeng BH. Abrasions, Planned Defects, and Persistent Epithelial Defects in Corneal Epithelial Wound Healing. JAMA Ophthalmol. 2016;134:1176–1177. doi: 10.1001/jamaophthalmol.2016.3086. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Investigative ophthalmology & visual science. 2004;45:3507–3512. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Investigative ophthalmology & visual science. 2006;47:4365–4372. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Investigative ophthalmology & visual science. 2006;47:1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- Liu CY, Kao WW. Corneal Epithelial Wound Healing. Prog Mol Biol Transl Sci. 2015;134:61–71. doi: 10.1016/bs.pmbts.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Progress in retinal and eye research. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt-7a Up-regulates Matrix Metalloproteinase-12 Expression and Promotes Cell Proliferation in Corneal Epithelial Cells during Wound Healing. 2005:21653–21660. doi: 10.1074/jbc.M500374200. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Girard MT, Kublin CL, Cintron C, Fini ME. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev Biol. 1991a;147:425–439. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Zieske JD, Fini ME. Mechanism of basement membrane dissolution preceding corneal ulceration. Investigative ophthalmology & visual science. 1991b;32:3221–3237. [PubMed] [Google Scholar]
- Mauris J, Woodward AM, Cao Z, Panjwani N, Argueso P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. Journal of cell science. 2014;127:3141–3148. doi: 10.1242/jcs.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwin G, Jr, Owsley C. Incidence of emergency department-treated eye injury in the United States. Arch Ophthalmol. 2005;123:662–666. doi: 10.1001/archopht.123.5.662. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. The Journal of biological chemistry. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Mulholland B, Tuft SJ, Khaw PT. Matrix metalloproteinase distribution during early corneal wound healing. Eye (Lond) 2005;19:584–588. doi: 10.1038/sj.eye.6701557. [DOI] [PubMed] [Google Scholar]
- Nagata M, Nakamura T, Hata Y, Yamaguchi S, Kaku T, Kinoshita S. JBP485 promotes corneal epithelial wound healing. Sci Rep. 2015;5:14776. doi: 10.1038/srep14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenan S, Lagente V, Planquois JM, Hitier S, Berna P, Bertrand CP, Boichot E. Metalloelastase (MMP-12) induced inflammatory response in mice airways: effects of dexamethasone, rolipram and marimastat. Eur J Pharmacol. 2007;559:75–81. doi: 10.1016/j.ejphar.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Pal-Ghosh S, Tadvalkar G, Jurjus RA, Zieske JD, Stepp MA. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Experimental eye research. 2008;87:478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Wakefield D, Di Girolamo N. Fate Mapping Mammalian Corneal Epithelia. Ocul Surf. 2016 doi: 10.1016/j.jtos.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Investigative ophthalmology & visual science. 2010;51:1970–1980. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The Image J ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev. 2015;82:518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. The Journal of biological chemistry. 1993;268:23824–23829. [PubMed] [Google Scholar]
- Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. 1996:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Progress in retinal and eye research. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud SJ, Barza M, Gipson IK. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Investigative ophthalmology & visual science. 1988;29:379–386. [PubMed] [Google Scholar]
- Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Wounding the cornea to learn how it heals. Experimental eye research. 2014;121:178–193. doi: 10.1016/j.exer.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Progress in retinal and eye research. 2003;22:113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Terai K, Call MK, Liu H, Saika S, Liu CY, Hayashi Y, Chikama T, Zhang J, Terai N, Kao CW, Kao WW. Crosstalk between TGF-beta and MAPK signaling during corneal wound healing. Investigative ophthalmology & visual science. 2011;52:8208–8215. doi: 10.1167/iovs.11-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Experimental eye research. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Investigative ophthalmology & visual science. 2013;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- Walkin L, Herrick SE, Summers A, Brenchley PE, Hoff CM, Korstanje R, Margetts PJ. The role of mouse strain differences in the susceptibility to fibrosis: a systematic review. Fibrogenesis Tissue Repair. 2013;6:18. doi: 10.1186/1755-1536-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Lewis CS, Beltran L, Younkin EM, Varani J, Johnson KJ. The role of metalloelastase in immune complex-induced acute lung injury. Am J Pathol. 2001;158:2139–2144. doi: 10.1016/S0002-9440(10)64685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975;142:361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, Gipson IK. Enhancement of vinculin synthesis by migrating stratified squamous epithelium. J Cell Biol. 1989;109:571–576. doi: 10.1083/jcb.109.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]


