Abstract
Metabolic dysfunction accompanying traumatic brain injury (TBI) severely impairs the ability of injured neurons to comply with functional demands. This limits the success of rehabilitative strategies by compromising brain plasticity and function, and highlights the need for early interventions to promote energy homeostasis. We sought to examine whether the TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF) normalizes brain energy deficits and restablishes more normal patterns of functional connectivity, while enhancing the effects of exercise during post-TBI period. Moderate fluid percussion injury (FPI) was performed and 7,8-DHF (5 mg/kg, i.p.) was administered in animals subjected to FPI that either had access to voluntary wheel running for 7 days after injury or were sedentary. Compared to sham-injured controls, TBI resulted in reduced hippocampal activation of the BDNF receptor TrkB and associated CREB, reduced levels of plasticity markers GAP-43 and Syn I, as well as impaired memory as indicated by the Barnes maze task. While 7,8-DHF treatment and exercise individually mitigated TBI-induced effects, administration of 7,8-DHF concurrently with exercise facilitated memory performance and augmented levels of markers of cell energy metabolism viz., PGC-1α, COII and AMPK. In parallel to these findings, resting-state functional MRI (fMRI) acquired at 2 weeks after injury showed that 7,8-DHF with exercise enhanced hippocampal functional connectivity, and suggests 7,8-DHF and exercise to promote increases in functional connectivity. Together, these findings indicate that post-injury 7,8-DHF treatment promotes enhanced levels of cell metabolism, synaptic plasticity in combination with exercise increases in brain circuit function that facilitates greater physical rehabilitation after TBI.
Keywords: 7,8-dihydroxyflavone; exercise; memory; traumatic brain injury; functional connectivity; rehabilitation
1. Introduction
Traumatic brain injury (TBI) is common in sports, domestic, and military environments. It remains unknown how soon after injury that athletes can return to play or patients can be exposed to physical rehabilitation. Given that physical rehabilitation is one of the only established therapies for TBI, it is important to determine how to optimize its efficacy. It appears that the energy demand imposed by activity on neural circuits invalidates the use of exercise during the acute post-injury period when the brain is deficient in energy [1,2]. Therefore, there is a pressing need to normalize brain metabolism during the early post-injury convalescence period in order to support and promote the various mechanisms of functional plasticity to prevent long-term functional deficits. BDNF possesses the unique ability to support molecular events involved with transmission of information across nerve cells and cell metabolism through activation of its TrkB receptor. The flavonoid derivative, 7,8-dihydroxyflavone (7,8-DHF) signals through the same TrkB receptors, and results in activation of BDNF signalling pathways [3]. Many of the pharmacokinetic limitations of BDNF are avoided by the use of 7,8-DHF, which makes it a promising pharmacological agent for supporting activity-based rehabilitation during the acute post-injury after TBI.
Signalling through the BDNF receptor activates the transcription factor cAMP-response-element-binding protein (CREB) which is an important regulator of peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α) [4]. PGC-1α regulates mitochondrial biogenesis in close association with BDNF [5], and these actions lead to changes in cytochrome oxidase II (COII) expression through AMP-activated protein kinase (AMPK) with nuclear-encoded proteins promoting mitochondrial DNA (mtDNA) transcription [6]. We have previously shown that TBI impairs BDNF-TrkB signalling [7] and we hypothesize that this may account for the compromised energy homeostasis that occurs at the earliest stages of brain injury. Therefore in the current study, we investigated whether post-injury administration of the BDNF agonist 7,8-DHF could normalize brain energy homeostasis during the early post-TBI period, boost downstream molecular markers of plasticity, and promote more normal patterns of brain functional connectivity. Recent clinical and experimental studies indicate that functional deficits observed shortly after TBI are associated with significant disruptions in functional connectivity among a wide array of brain circuits [8]. These studies indicate the importance of assessing whole brain functional connectivity in order to provide a more comprehensive understanding of the degree and extent of post-injury derangements in neural circuits that must overcome by treatment, in order to promote functional recovery. Accordingly, we have used resting state functional MRI (rsfMRI) to monitor whole brain functional networks after fluid percussion injury and in response to pharmacologic and exercise interventions in adult rats. The paradigm used here is based on the concept that 7,8-DHF can prime the cellular environment for improved metabolism, plasticity and brain connectivity by facilitating the beneficial effects of post-injury exercise through reinforcement of the TrkB signalling pathway.
2. Materials and methods
2.1. Animals and experimental design
Sprague–Dawley male rats were obtained from Charles River Laboratories (Wilmington, MA) at 8–10 weeks of age and were acclimatized for vivarium 1 week prior to commencement of all experimental procedures. Rats were housed in standard polyacrylic cages maintained on 12-h light/dark cycle in a temperature (22–24 °C) and humidity controlled room with free access to food (Purina, St. Louis, MO, USA) and water. All experimental paradigms were approved by the University of California at Los Angeles (UCLA) Chancellor’s Animal Research Committee (ARC) and were conducted with adherence to the guidelines set out by the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Assessment of 7,8-DHF and exercise following TBI-induced pathology
Rats were trained for 5 days on the Barnes maze to learn the task and then subjected to either sham or fluid percussion injury (FPI) (described below). Rats received intraperitoneal (i.p) treatment with either 7,8-dihydroxyflavone (7,8-DHF; Tokyo Chemical Industry America, OR, USA) at a dose of 5 mg/kg or an equivalent volume of vehicle (VEH; 30% DMSO in PBS) once a day for 7 consecutive days, with or without voluntary wheel running. Treatment was begun the day of surgery until 2 h prior to memory testing. Rats were randomly assigned into five groups: (i) Sham plus Vehicle (Sham/VEH); (ii) Fluid percussion injury plus Vehicle (FPI/VEH); (iii) Fluid percussion injury plus 7,8-DHF (FPI/7,8-DHF); (iv) Fluid percussion injury plus exercise (FPI/Exc) and (v) Fluid percussion injury plus 7,8-dihydroxyflavone plus exercise (FPI/7,8-DHF/Exc). Rats were tested for memory retention by Barnes maze (after the final 7,8-DHF treatment), and were then rapidly decapitated. Brains were excised, frozen in dry ice and stored at −80 °C until use.
2.2. Voluntary running wheel exercise
Rats were individually housed with or without access to a running wheel from post-injury day 0 to 6 [FPI/Exc (n = 6) or FPI/7,8-DHF/Exc (n = 6)] as described previously (Griesbach et al., 2004). Briefly, exercising rats were placed in standard cages equipped with running wheels (31.8 × 10-cm; NalgeNunc International) that rotate against a resistance of 100 g with ad libitum access to food and water. Wheel revolutions were recorded using an automated system (VitalViewer Data Acquisition System; Mini Mitter, Sunriver, OR).
2.3. Behavioral analysis: Barnes maze test
Barnes maze testing was performed before and after experimental TBI with two trials per day with a 5-min test period. In brief, the apparatus comprised a circular acrylic plastic table to form a disk 1.5-cm thick and 115-cm in diameter, with 18, 7-cm holes spaced equidistant around the perimeter. The apparatus was brightly illuminated by overhead halogen lamps. At the start of each trial period, the rats were individually placed in the center of the maze and covered with an opaque ‘start’ cylinder for 10 s before it was raised to allow the animal to explore the maze, to locate and enter an escape box hidden beneath the table. The box remained in the same location throughout the testing period. Their movement was simultaneously tracked with a video camera connected to an automated video tracking system (ANY-maze, Stoelting Co., USA) installed directly overhead at the center of the maze. The training/acquisition phase ended after the animal had entered the escape box or after a maximum test duration, following which the animal was allowed to stay in the box for 30 s and then returned to the home cage. If the animal failed to enter the escape box within 5 min, it was gently lead towards the escape box. Rats were given two trials per day for 5 consecutive days at approximately the same time every day. Subsequently, memory retention was assessed at 7 days of injury. All surfaces were cleaned with dilute ethanol to dissipate odor cues after each trial.
2.4. Western Blot Analysis
The hippocampal tissues from the left hemisphere were homogenized in a lysis buffer containing 20 mM Tris–HCl (pH 8.0), 137 mM NaCl, 1% NP40, 10% glycerol, 1 mM phenylmethylsulfonylfluoride (PMSF), 10 μg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. The homogenates were then centrifuged (12000g at 4 °C) and the supernatants were collected. Total protein was then determined using a BCA Protein Assay kit (Pierce, IL, USA), using bovine serum albumin (BSA) as standard. Protein from the hippocampus was diluted in lysis buffer and 2X loading buffer (100% glycerol, 5% β-mercaptoethanol, 10% SDS, and 2.5% bromophenol blue) and samples were denatured by heated in boiling water for 5-min. Proteins were separated by electrophoresis on a 10% sodiumdocecylsulphate-polyacrylamide gels and subsequently transferred onto PVDF membranes (Millipore, MA, USA). Membranes were then blocked in a Tris-buffered saline [TBS-T: 10 mM Tris-Base (pH 8.0), 150 mM NaCl, 0.5% Tween-20] solution containing 5% (w/v) non-fat dry milk with gentle agitation. Membranes were rinsed in buffer (0.5% Tween-20 in TBS) and then incubated with anti-actin or anti-pTrkB, anti-TrkB, anti-AMPK, anti-cytochrome c oxidase II (COII), anti-GAP-43, anti-synapsin 1 (1:500; Santa Cruz Biotechnology, CA, USA), anti-PGC-1α, anti-pCREB, anti-CREB, (1:1000, Millipore, MA, USA), anti-pAMPK (1:1000; Cell signaling technology, MA, USA). After four successive 10 min washes in TBS-T at room temperature to remove any unbound antibody, membranes were incubated with secondary antibody (anti-rabbit or anti-goat or anti-mouse IgG horseradish peroxidase-conjugate, 1:10,000; Santa Cruz Biotechnology, CA, USA). After another series of TBS-T washes, the complexes were visualized by exposing membranes treated with chemiluminescent substrate (Amersham Pharmacia Biotech Inc., NJ, USA; for 2 min). The film signals were digitally scanned and then quantified using ImageJ. β-actin (anti β-actin; 1:5000) was used as an internal control for normalization western blot such that data were standardized according to actin values.
2.5. rsfMRI
Separate group of rats underwent injury and treatment in an identical manner as described earlier, and rsfMRI data were acquired at 14 days of injury as described before [9]. MRI acquisition was performed using a 7-Tesla MRI scanner (BrukerBioSpin, Oxford Instr, Carteret, NJ, USA) at Ahmanson-Lovelace Brain Mapping Center, UCLA using the S116 gradient system with the maximum strength of 400 mT/m, in combination with a birdcage transmit and an actively decoupled receive-only surface coil, and using Paravision 5.1 (Billerica, MA, USA). Rats were briefly anesthetized with 4% isoflurane (Phoenix, MO, USA) in a mixture of oxygen (30%) flowing at 0.61/min and medetomidine sedation was commenced via the penile vein (0.05 mg/kg in physiological saline) and the isoflurane was discontinued. A continuous infusion of medetomidine was begun via a subcutaneous cannula (0.1 mg/kg/h) to maintain sedation throughout the experiment. Rat was quickly transferred to a purpose-built cradle and secured using three-point immobilization of the head with ear and tooth bars, and was placed in the center of the magnet. Respiration was monitored using a pad placed beneath the animal’s chest and temperature was homoeothermically-controlled by forced air (SA11 Instr, Inc., USA). Acquisition protocol for each animal comprised a multi-slice, gradient echo pilot scan to optimize positioning within the magnet; localized shimming was performed on the head to improve B0 homogeneity. At not less than 30 min following removal of the isoflurane anethsesia, a standard, gradient echo, single-shot, echo planar imaging sequence was used to acquire 600 volumes (20-min) of resting state fMRI data with a 128-read by 64-phase-encoding matrix (X,Y direction respectively) within a 30 mm2 field-of-view (FOV) and 14 × 0.75 mm contiguous, coronal slices, using a repetition/echo time TR/TE) of 2000/35 ms and 10 dummy scans prior to the acquisition. Anatomical, T2-weighted, rapid-relaxation-with-enhancement (RARE) data were acquired at the same coordinates and FOV as the fMRI data and using a 128-read × 128-phase-encoding matrix, a TR/TE 5000/60ms, a RARE factor of 8 and 2 averages. Total duration of each MRI experiment was 1hr.
2.6. Fluid percussion injury
Injury was induced according to previously reported method [10]. In brief, anesthesia was induced in a Mobile Laboratory Animal Anesthesia System (VetEquip Inc., CA, USA). The rats were moved to a stereotaxic frame, stabilized using head ear bars and a mixture of isoflurane (2.5%) and O2 (100%) was delivered through nose cone. After application of eye ointment for corneal protection, a midline, longitudinal incision was made and a 3-mm diameter craniotomy was made over the left parietal cortex (centered at the 3 mm posterior relative to bregma and 6-mm lateral to the midline) using a high-speed drill (Dremel, WI, USA) leaving the dura intact. A rigid plastic injury cap was placed and secured over the craniotomy with dental acrylic cement. When the dental cement hardened, the anesthesia was discontinued. At the first sign of hind-limb withdrawal to a paw pinch, a moderate fluid percussion pulse (2.7 atm) injury was administered into the epidural space by attaching the non-pyrogenic physiological saline-filled cap to the fluid percussion device. Immediately after the injury, each rat was monitored for response to a paw pinch, anesthesia was then resumed and the skin was sutured. Neomycin was applied on the suture and the rats were placed in a heated recovery chamber to be fully ambulatory before being returned to their cages. The sham animals were prepared using the identical surgically procedure but without the fluid pulse.
2.7. Statistical Data Analysis
All results are expressed as mean ± SEM. Protein results are expressed as percentage of Sham/VEH group and statistical analysis was performed by one-way analysis of variance (ANOVA). Post-hoc analyses were conducted using Bonferroni’s analysis to determine the significance of difference among groups. A level of 5% probability was considered as statistically significant. In addition, Pearson’s correlation coefficients analysis was performed on individual samples to assess the degree of association between variables. Data were evaluated using SPSS (version 17.0) statistical software package.
fMRI data were pre-processed as previously described [11] by correcting for slice timing, motion, CSF signal and then band-pass filtered between 0.01–0.25 Hz and smoothing (with a 0.8 mm full width at half maximum Gaussian kernel). The connectivity was explored within the hippocampal-cingulate-prefontal cortex pathways as well as thalamus and sensory-motor cortex. Seed-based analyses were performed from regions of interest ROI’s (3 × 3 × 3 voxels) placed in these regions-cortex, thalamus, subiculum and as well as the additional ones obtained from a segmented rat atlas that was warped into the native anatomical space of each animal (FLIRT, FSL, Oxford, UK) [12]. Following the normalization of Pearson correlation coefficients using Fischer’s z transformation, the seed-based data were warped to standard rat template space for testing of voxel-based, group-level differences by permutation using Randomize (FSL, Oxford, UK) with thresholding using threshold-free cluster enhancement.
3. Results
3.1. Running wheel measures
No significant differences were observed in the wheel revolutions among TBI rats after exercise or 7,8-DHF + exercise (data not shown).
3.2. 7,8-DHF facilitates exercise induced-cognitive recovery after TBI
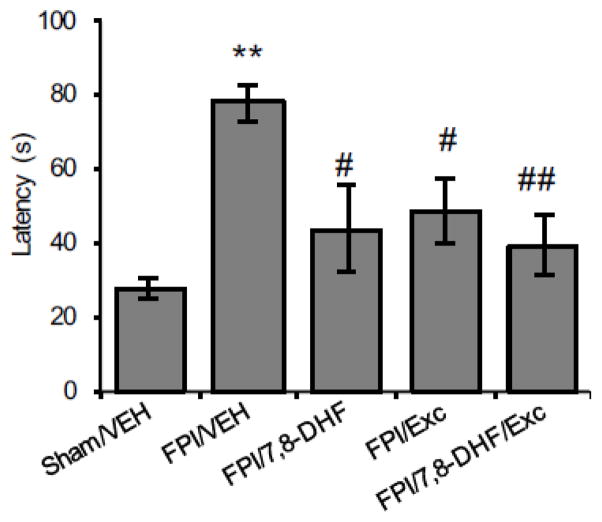
Rats trained in the Barnes maze showed decrease in escape latency time with each training day (days 1–5). All animals learned to locate the escape box at an equivalent rate after completion of training (data not shown). The memory retention test performed at 7 days after surgery showed that TBI significantly increased the escape latency (FPI/VEH vs. Sham/VEH, P < 0.01, Fig. 1). Treatment with either 7,8-DHF or exercise significantly reduced the latency time in TBI animals (FPI/7,8-DHF vs. FPI/VEH, P < 0.05; FPI/Exc vs. FPI/VEH, P < 0.05), while robust memory improvement with much shorter escape latencies was measured with combined treatment with 7,8-DHF and exercise (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01).
Fig. 1.
Effect of 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, i.p) or exercise and their combination on latency times in the Barnes maze test among rats subjected to fluid percussion injury (FPI). Data collected during a 5-min testing session are presented as mean ± SEM. *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH (One-way ANOVA followed by Bonferroni test).
3.3. 7,8-DHF boosts the effects of exercise on TrkB receptor activation after TBI
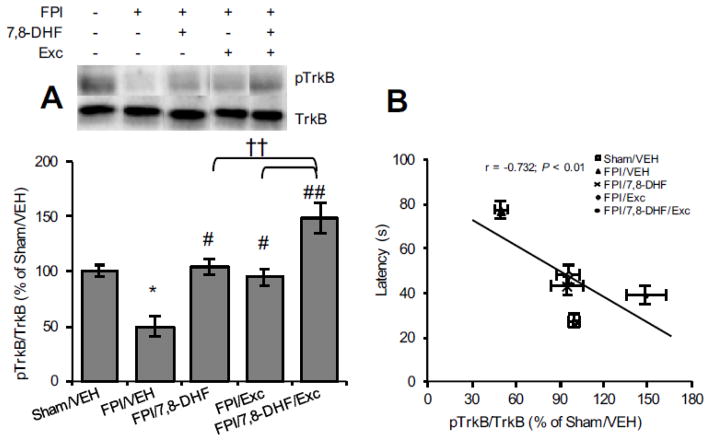
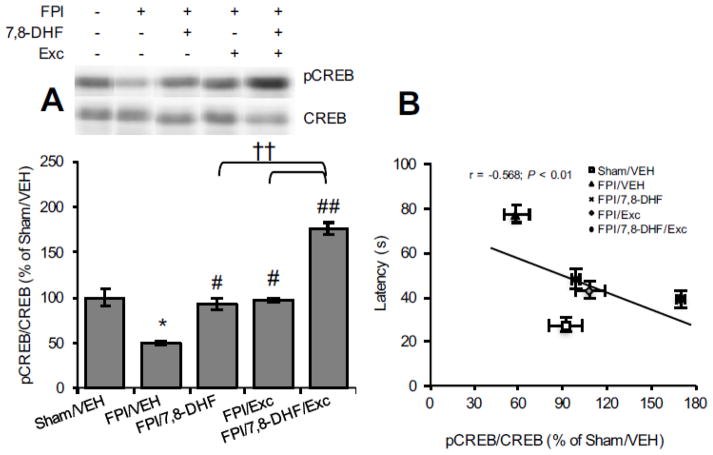
We assessed the separate and combined actions of 7,8-DHF and exercise on TrkB signalling. As shown in Fig. 2A, TBI significantly reduced TrkB phosphorylation levels (FPI/VEH vs. Sham/VEH, P < 0.05) whereas both 7,8-DHF treatment and exercise alone normalized the levels (FPI/7,8-DHF vs. FPI/VEH, P < 0.05; FPI/Exc vs. FPI/VEH, P < 0.05). The combined application of 7,8-DHF and exercise further increased the TrkB phosphorylation (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01), which remained significantly higher compared to either of the interventions (FPI/7,8-DHF/Exc vs. FPI/7,8-DHF, P < 0.05; FPI/7,8-DHF/Exc vs. FPI/Exc, P < 0.01). We found that the level of TrkB signalling was significantly, inversely correlated with latency time in the Barnes maze (r = −0.732, P < 0.01; Fig. 2B). We next looked at CREB levels, a downstream molecule in the TrkB signalling pathway. Similar to the TrkB data, TBI significantly reduced CREB phosphorylation (FPI/VEH vs. Sham/VEH, P < 0.05) and separate treatment with either 7,8-DHF treatment or exercise significantly ameliorated these reductions so that they reached sham levels (FPI/7,8-DHF vs. FPI/VEH, P < 0.05; FPI/Exc vs. FPI/VEH, P < 0.05). Combined application of 7,8-DHF and exercise boosted CREB phosphorylation in injured animals (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01). Given the role of CREB memory function [13], we explored its probable correlation with memory retention. Once again, changes in CREB phosphorylation correlated inversely with the Barnes maze latency time (r = −0.568; P < 0.01; Fig. 3B) indicating an association between CREB phosphorylation and improved memory.
Fig. 2.
Levels of (A) TrkB phosphorylation and (B) correlation analysis of TrkB phosphorylation with latency time (the solid line represents the best fit linear regression line; see accompanying text) in Barnes maze test with 7,8-DHF (5 mg/kg) or exercise their combination among rats subjected to fluid percussion injury (FPI). Data are presented as percentage (%) of Sham/VEH (mean ± SEM). *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH; †P < 0.05, †† P < 0.01 relative to FPI/7,8-DHF or FPI/Exc (one-way ANOVA followed by Bonferroni test).
Fig. 3.
Levels of (A) TrkB phosphorylation and (B) correlation analysis of CREB phosphorylation with memory performance in Barnes maze test with 7,8-DHF (5 mg/kg) or exercise their combination among rats subjected to fluid percussion injury (FPI). Data are presented as percentage (%) of Sham/VEH (mean ± SEM). *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH; †P < 0.05, ††P < 0.01 relative to FPI/7,8-DHF or FPI/Exc (one-way ANOVA followed by Bonferroni test).
3.4. 7,8-DHF and exercise enhances molecules associated with plasticity and synaptogenesis
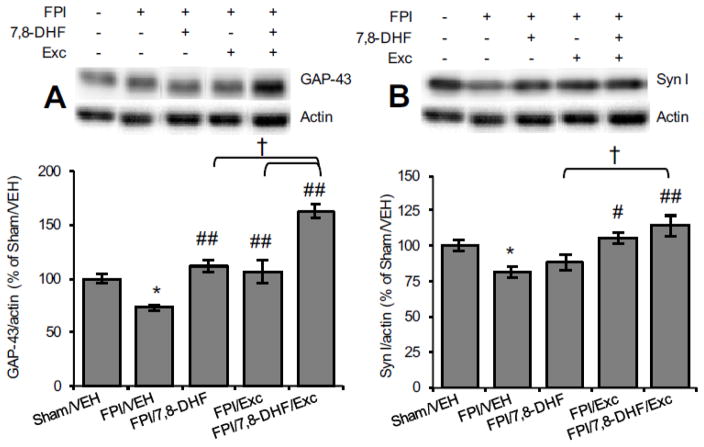
In view of the regulatory action of BDNF on synaptic activity [14], we assessed the influence of 7,8-DHF and exercise on key synaptic plasticity markers. GAP-43 is a marker of neuronal structural plasticity and axonal sprouting with higher expression during functional recovery [15]. As shown in Fig. 4, TBI significantly reduced GAP-43 levels (FPI/VEH vs. Sham/VEH, P < 0.01) while treatment with either 7,8-DHF or exercise restored these levels (FPI/7,8-DHF vs. FPI/VEH, P < 0.01; FPI/Exc vs. FPI/VEH, P < 0.01). The combination of 7,8-DHF and exercise further elevated the GAP-43 levels (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01), being significantly higher compared to 7,8-DHF (FPI/7,8-DHF/Exc vs. FPI/7,8-DHF, P < 0.05) or exercise alone (FPI/7,8-DHF/Exc vs. FPI/Exc, P < 0.05).
Fig. 4.
Levels of (A) GAP-43 and (B) Synapsin-I as a function of 7,8-DHF or exercise and their combination among animals subjected to fluid percussion injury (FPI). Data are presented as percentage (%) of Sham/VEH (mean ± SEM). *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH; †P< 0.05, relative to FPI/7,8-DHF or FPI/Exc (One-way ANOVA followed by Bonferroni test).
Syn I is a vesicle-associated protein involved in neural development and influences memory function [16]. As shown in Fig. 4B, Syn I levels were significantly reduced by TBI (FPI/VEH vs. Sham/VEH, P < 0.05) while exercise counteracted this reduction (FPI/Exc vs. FPI/VEH, P < 0.05). The combined application of 7,8-DHF and exercise robustly elevated Syn I levels (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.05) and the levels remained significantly higher than with 7,8-DHF alone (FPI/7,8-DHF/Exc vs. FPI/7,8-DHF, P < 0.05) after TBI.
3.5. 7,8-DHF enhances the effect of exercise on molecules associated with mitochondrial biogenesis
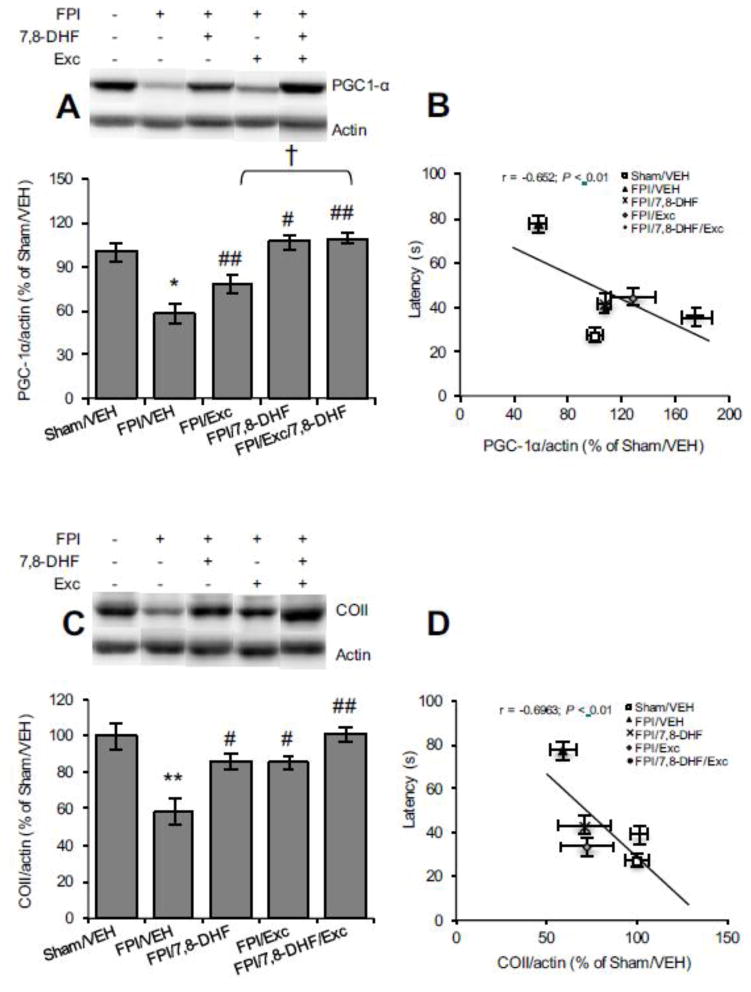
PGC-1α is a key regulator mediating mitochondrial biogenesis and metabolic pathways [17]. As shown in Fig. 5A, TBI significantly reduced PGC-1α levels (FPI/VEH vs. Sham/VEH, P < 0.05). 7,8-DHF treatment (FPI/7,8-DHF vs. FPI/VEH, P < 0.01) or exercise attenuated the TBI effect (FPI/Exc vs. FPI/VEH, P < 0.05). The combined application of 7,8-DHF and exercise restored the PGC-1α levels. The inverse association between latency time in the Barnes maze and PGC-1α levels (r = 0.454, P < 0.05, Fig. 5B) suggests that memory performance may depend on improved mitochondrial biogenesis. In addition, TBI significantly reduced the COII levels (FPI/VEH vs. Sham/VEH, P < 0.01; Fig. 5C) and this effect was ameliorated by 7,8-DHF (FPI/7,8-DHF vs. FPI/VEH, P < 0.05) or exercise (FPI/Exc vs. FPI/VEH, P < 0.05) and even further by their combination (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01). Again, the COII levels changed proportionally to the escape latency in Barnes maze, suggesting an involvement in memory performance (r = 0.693, P < 0.01; Fig. 5D).
Fig. 5.
Protein levels of (A) PGC-1α, (B) correlation analysis of PGC-1α with latency time in Barnes maze test, (C) COII levels and (D) correlation analysis of COII with latency time in Barnes maze test (the solid line represents the best fit linear regression line) with 7,8-DHF (5 mg/kg) or exercise their combination among rats subjected to either fluid percussion injury (FPI). Data are presented as percentage (%) of Sham/VEH (mean ± SEM). *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH; †P < 0.05, relative to FPI/7,8-DHF or FPI/Exc (One-way ANOVA followed by Bonferroni test).
3.6. 7,8-DHF effects on cell metabolic markers
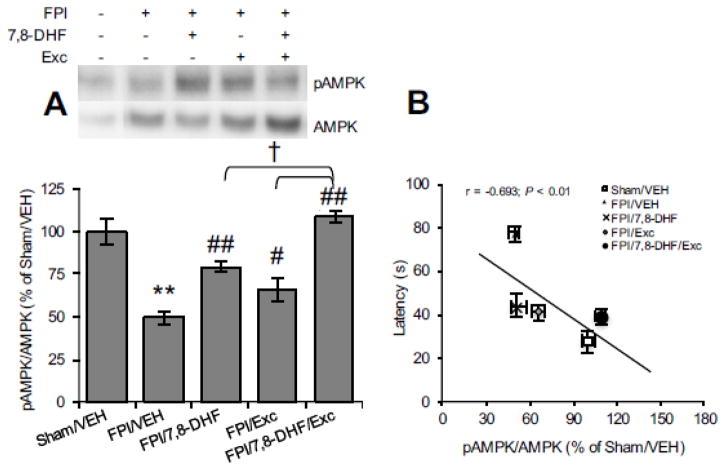
We assessed the AMPK levels, a key ubiquitous metabolic sensor protein involved in bioenergy regulation. The reduced AMPK phosphorylation with TBI (FPI/VEH vs. Sham/VEH, P < 0.05; Fig. 6A) was significantly abrogated by 7,8-DHF (FPI/7,8-DHF vs. FPI/VEH, P < 0.01) or exercise (FPI/Exc vs. FPI/VEH, P < 0.05) whereas the combined 7,8-DHF-exercise robustly mitigated the impact of brain trauma (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.01). Specifically, higher AMPK phosphorylation was observed with 7,8-DHF and exercise compared to either 7,8-DHF (FPI/7,8-DHF/Exc vs. FPI/7,8-DHF, P < 0.05) or exercise per se (FPI/7,8-DHF/Exc vs. FPI/Exc, P < 0.05). As shown in Fig. 6B, AMPK phosphorylation inversely correlated with the latency time (r = −0.693, P < 0.01), suggesting a relationship between energy regulation and memory performance.
Fig. 6.
(A) Phospho-AMPK levels as a function of 7,8-DHF or exercise and their combination treatment and (B) correlation analysis of AMPK with latency time in Barnes maze test among animals subjected to fluid percussion injury (FPI). The results are expressed as percentage (%) of Sham/VEH (mean ± SEM). *P < 0.05, **P < 0.01, relative to Sham/VEH; #P < 0.05, ##P < 0.01, relative to FPI/VEH; †P < 0.05, relative to FPI/7,8-DHF or FPI/Exc (One-way ANOVA followed by Bonferroni test).
3.7. The effects of 7,8-DHF and exercise on functional connectivity
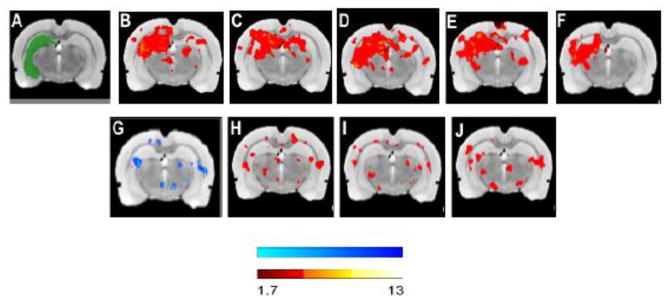
Given that TBI seems to disrupt functional network organization in the brain [18], we examined the effects of 7,8-DHF and exercise on fc after TBI. In group mean data, hippocampal seed analysis showed trends toward reduced intra-hippocampal and cortical-hippocampal fc after injury compared to sham (Fig. 7C vs. 7B), while 7,8-DHF (Fig. 7D) or the combination of 7,8-DHF and exercise (Fig. 7E) increased connectivity. Voxel-based statistical analysis showed that TBI significantly decreased fc (FPI/VEH vs. Sham/VEH, P < 0.05) while increased connectivity was evident with 7,8-DHF treatment (FPI/7,8-DHF vs. FPI/VEH, P < 0.05) or exercise (FPI/Exc vs. FPI/VEH, P < 0.05) and 7,8-DHF and exercise (FPI/7,8-DHF/Exc vs. FPI/VEH, P < 0.05).
Fig. 7.
Functional brain connectivity. Group mean data are normalized r values computed from the correlation between mean BOLD signal in the seeded region (green) and all other brain regions and averaged within each group (top panel) (A) Hippocampus seed; (B) Sham/VEH; (C) FPI/VEH; (D) FPI/Exc; (E) FPI/7,8-DHF and (F) FPI/Exc/7,8-DHF. Voxel-based, statistical contrast (bottom panel) between (G) FPI/VEH vs Sham/VEH; (H) FPI/7,8-DHF vs FPI/VEH; (I) FPI/Exc vs FPI/VEH; (J) FPI/7,8-DHF/Exc vs FPI/VEH (z = 1.7, P < 0.05, unpaired t-test). Scalebar = Z threshold.
4. Discussion
Despite the beneficial effects of exercise as rehabilitative interventions after brain injury [19], on-going dysfunctional brain metabolism, especially in the acute post-injury period following TBI limits the time during which patients can significantly benefit from physical rehabilitation. In light of the fact that brain injury disrupts plasticity and energy metabolism crucial for neuronal function, in this study we sought to assess whether 7,8-DHF abrogates TBI effects and enhances the brain benefits of exercise. The data herein indicate that a combination of 7,8-DHF and exercise enhances hippocampal TrkB activation and further facilitates energy homeostasis coupled with improved memory function after TBI. 7,8-DHF and exercise increased functional connectivity that is important for brain circuit function and for rehabilitative purpose. The overall evidence emphasizes the benefits of 7,8-DHF to facilitate the action of exercise that could be applied to shorten the period of convalescence after TBI.
4.1. Impact on memory, TrkB signalling and plasticity processes
Although the action of TrkB receptor signalling is important for optimal neuronal function [20], the poor pharmacokinetic of BDNF [21] has limited the pharmacological implementation of BDNF to treat neurological disorders. The TrkB signalling cascade possesses the therapeutic potential to limit TBI pathology by critically linking energy metabolism and plasticity. The TrkB receptor agonist 7,8-DHF activates TrkB downstream pathways, thus mimicking the action of BDNF [22] while also enhancing brain metabolism. In the present study, 7,8-DHF and exercise individually rescued TBI-induced memory deficits, and the combined action of both had an added beneficial effect. Moreover, the shorter escape latencies measured among TBI animals receiving 7,8-DHF together with exercise compared to those with just a single intervention suggests that the combined intervention facilitates memory performance in the Barnes maze. Our results are consistent with previous reports indicating that exercise enhances hippocampal BDNF and CREB levels [23]. We previously reported that 7,8-DHF promotes TrkB activation without affecting levels of BDNF [7]. It has been shown that 7,8-DHF robustly binds with the same leucine-rich motif on TrkB domain essential for ‘perpetuate’ receptor activation and phosphorylation compared with BDNF [24,25]. Further, radiotracer-labelled study has demonstrated that 7,8-DHF exerts its strong agonistic activity in a BDNF displaceable manner [26]. Along these lines, repeated 7,8-DHF administration in the present study could possibly preclude the binding of BDNF induced by exercise. In addition, the current results shows that 7,8-DHF facilitates the action of exercise on TrkB and CREB phosphorylation, which builds on the recognized action of TrkB signalling for memory [27]. In this regard, the fact that TrkB receptor specific antagonist, ANA-12 inhibits TrkB phosphorylation reversing the effects of 7,8-DHF [28, 29] and block exercise action on BDNF [30] supports the notion that TrkB signaling to be of fundamental importance mediating 7,8-DHF and exercise action on specific Trk receptor.
Data in the present study indicate a robust effect of combined 7,8-DHF and exercise to ameliorate TBI-induced reductions in hippocampal GAP-43 and Syn I levels. These results are important in the context that reduced synaptic plasticity is a major sequel of TBI [31,32], that could impair memory performance. In addition, substantial evidence supports the action of TrkB activation on synaptic transmission and plasticity in conjunction with the action of Syn I [33,34]. In turn, GAP-43 has also been implicated in synaptic function [35] under the regulatory action of BDNF, as well as in the maintenance and control of neuronal growth [36]. Finally, reduced TrkB receptor activation has been associated with decreased GAP-43 expression in sprouting hippocampal axons [37], further supporting a regulatory action of TrkB on GAP-43.
4.2. Influence on energy homeostasis
Energy homeostasis is essential for cells to function properly particularly under conditions of brain injury. Impairments in brain energy metabolism limits structural and functional damage after injury [38–40] suggesting that treatment strategies that enhance mitochondrial function and biogenesis after TBI will be efficacious. The metabolic regulator PGC-1α is under upstream control of regulators such as SIRT1 and AMPK, that can further influence plasticity by interacting with BDNF [41]. The present study demonstrates that 7,8-DHF greatly complements the action of exercise to enhance PGC-1α levels, which could help to fulfill the energy demands imposed by TBI. A recent report indicates the ability of 7,8-DHF to augment PGC-1α immunoreactivity in the hippocampal cornus ammonis area 1 (CA1), which could be important to regulate BDNF-mediated TrkB signaling [42]. This finding is consistent with the earlier data linking PGC-1α activation with BDNF expression [43].
Our data indicate that, 7,8-DHF and exercise, separately and in combination enhanced COII levels. The increase in COII levels suggests that 7,8-DHF could act as an adjunct to generate higher cellular ATP under TBI conditions. ATP generated by oxidative phosphorylation occurs in the mitochondria and relies on transfer of electrons by a terminal complex (cytochrome c oxidase, CO) of the electron transport chain. The mitochondrial-encoded subunit II (COII) regulates the electrochemical gradient in this complex by translocating protons across the inner membrane to generate ATP [44]. The results showing that levels of PGC-1α and COII change in association with molecules related to the regulation of synaptic plasticity and neural growth, indicate an intimate interaction between cell energy metabolism and brain function. These changes correlated with escape latency in the Barnes maze, in agreement with recent work that showed an association between AMPK activation and memory improvement after TBI [45]. These results emphasize an association between memory performance and cell energy, and are particularly important for the design of strategies to optimize the action of exercise to support physical rehabilitation after TBI. The energy sensor AMPK acts on PGC-1α for transcriptional control of mitochondrial biogenesis [46]. In the present study, TBI reduced AMPK phosphorylation which is consistent with altered cell metabolism, as discussed earlier. The separate applications of 7,8-DHF or exercise were sufficient to counteract the detrimental effects of TBI on AMPK levels, while their combined application produced a more robust AMPK activation. These results strongly suggest that the combined actions of 7,8-DHF and exercise facilitate energy homeostasis during the early phases of TBI. Supporting this, a clinical study has reported a strong dependency between the metabolic state of the brain post-TBI, the timing of a secondary injury, and the ensuing length of the period of metabolic recovery [47]. According to the results of this study, a second injury too close to the primary injury extends the period of metabolic recovery. These results emphasize the importance of interventions that favor early metabolic homeostasis in order to improve the plastic capacity of the brain after TBI.
4.3. Functional brain connectivity
It is becoming understood that TBI results in loss of functional organization of neural circuits underlying brain function [48]. Functional connectivity measures provide insights into information processing including memory [49]. Our results show that TBI reduced intra- and cortical-hippocampal functional connectivity in regions implicated in memory processing, in agreement with previous studies [50,51]). Interestingly, 7,8-DHF, exercise and their combination were able to normalize functional connectivity, in agreement with previous findings that physical activity supports functional network reorganization [52] and BDNF has been implicated in regulation of connectivity of brain networks [53].
5. Conclusion
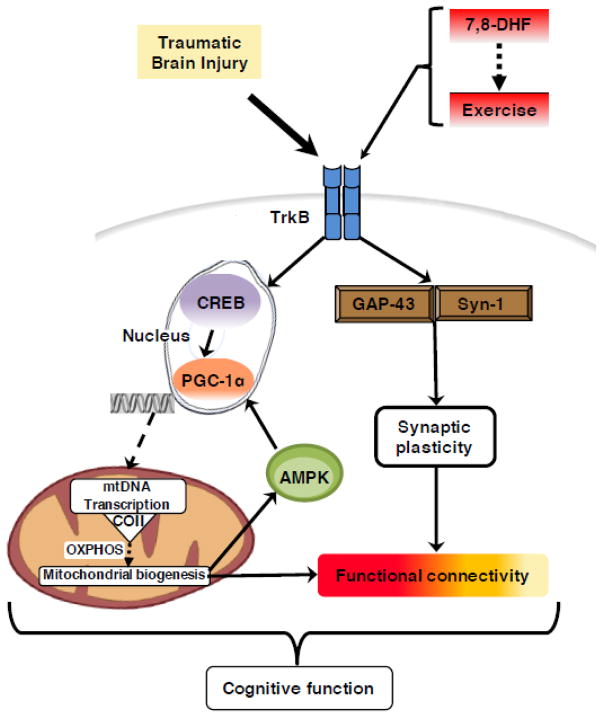
Our findings indicate that 7,8-DHF enhances brain plasticity and memory formation involving cell metabolism. These properties of 7,8-DHF are instrumental for supporting the action of exercise during the acute post-TBI period by normalizing cell metabolism and plasticity (see Fig. 8 for conceptual model). These results have important implications for post-injury rehabilitation therapy because they show that treatment with 7,8-DHF prepares the brain to benefit from earlier rehabilitation after injury by alleviating metabolic dysfunction and enhancing plasticity. This is an important result that should impact the future design of rehabilitation-based therapies by shortening the period of convalescence for athletes to return to play and patients to engage in physical therapy.
Fig. 8.
Proposed mechanism for the action of 7,8-dihydroxyflavone (7,8-DHF) on enhancing the outcome of exercise post-TBI. The flavone derivative 7,8-DHF acts on the BDNF receptor TrkB to increase levels of GAP-43 and Syn I. TrkB phosphorylation upregulates molecular systems involved in cellular energy and metabolic pathways such as PGC-1α and COII -- key component involved in the final step of oxidative metabolism thereby controlling ATP production. The activation of transcriptional machinery can increase mitochondrial function by involving the energy sensor, AMPK. Intervention with 7,8-DHF and exercise increased hippocampal functional connectivity following TBI. The fact that 7,8-DHF affects the BDNF-TrkB pathway is critical for regulation of synaptic plasticity and energy metabolism. The effects of 7,8-DHF and exercise were significant to influence functional connectivity, which provides a reading of functionality of neural circuits. Collectively, the mechanism illustrated can provide basis for using 7,8-DHF to support physical rehabilitation to accelerate functional recovery and heightening cognitive ability after TBI.
Highlights.
Traumatic brain injury (TBI) is associated with a prolonged period of recovery, restricting patients from performing activity.
7,8-DHF supports the action of exercise during the acute post-TBI period by normalizing cell metabolism, plasticity and brain circuit function.
7,8-DHF compensates for the functional demand of rehabilitation, and shortening the convalescence period to promote early recovery after TBI.
Acknowledgments
This work was supported by National Institutes of Health Grant NS050465 and NS091222.
Footnotes
Conflict of interest
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griesbach GS, Tio DL, Nair S, Hovda DA. Recovery of stress response coincides with responsiveness to voluntary exercise after traumatic brain injury. J Neurotrauma. 2014;31:672–682. doi: 10.1089/neu.2013.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2009;11:311–316. doi: 10.1007/s12028-009-9249-1. [DOI] [PubMed] [Google Scholar]
- 3.Sanz-García A, Knafo S, Pereda-Pérez I, Esteban JA, Venero C, Armario A. Administration of the TrkB receptor agonist 7,8-dihydroxyflavone prevents traumatic stress-induced spatial memory deficits and changes in synaptic plasticity. Hippocampus. 2016;26:1179–1188. doi: 10.1002/hipo.22599. [DOI] [PubMed] [Google Scholar]
- 4.Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makela J, Tselykh TV, Kukkonen JP, Eriksson O, Korhonen LT, Lindholm D. Peroxisome proliferator-activated receptor-γ (PPARγ) agonist is neuroprotective and stimulates PGC-1α expression and CREB phosphorylation in human dopaminergic neurons. Neuropharmacology. 2016;102:266–275. doi: 10.1016/j.neuropharm.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23:459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal R, Nobel E, Tyagi E, Zhuang Y, Ying Z, Gomez-Pinilla F. Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochem Biophys Acta. 2015;1852:862–872. doi: 10.1016/j.bbadis.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris NG, Verley DR, Gutman BA, Thompson PM, Yeh HJ, Brown JA. Disconnection and hyper-connectivity underlie reorganization after TBI: A rodent functional connectomic analysis. Exp Neurol. 2016;277:124–138. doi: 10.1016/j.expneurol.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Ying Z, Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp Neurol. 2010;226:191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 12.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 13.Porte Y, Buhot MC, Mons N. Alteration of CREB phosphorylation and spatial memory deficits in aged 129T2/Sv mice. Neurobiol Aging. 2008;29:1533–1546. doi: 10.1016/j.neurobiolaging.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 14.An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall K, Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Res. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John JP, Sunyer B, Hoger H, Pollak A, Lubec G. Hippocampal synapsin isoform levels are linked to spatial memory enhancement by SGS742. Hippocampus. 2009;19:731–738. doi: 10.1002/hipo.20553. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 18.Fagerholm ED, Hellyer PJ, Scott G, Leech R, Sharp DJ. Disconnection of network hubs and cognitive impairment after traumatic brain injury. Brain. 2015;138:1696–1709. doi: 10.1093/brain/awv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaynman S, Ying Z, Gomez-Pinilla F. Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res. 2004;76:356–362. doi: 10.1002/jnr.20077. [DOI] [PubMed] [Google Scholar]
- 21.He J, Xiang Z, Zhu X, Ai Z, Shen J, Huang T, Liu L, Ji W, Li T. Neuroprotective effects of 7, 8-dihydroxyflavone on midbrain dopaminergic neurons in MPP+-treated monkeys. Sci Rep. 2016;6:34339. doi: 10.1038/srep34339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Obianyo O, Chan CB, Huang J, Xue S, et al. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J Biol Chem. 2014;289:27571–27584. doi: 10.1074/jbc.M114.562561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard-Gauthier V, Boudjemeline M, Rosa-Neto P, Thiel A, Schirrmacher R. Towards tropomyosin-related kinase B (TrkB) receptor ligands for brain imaging with PET: radiosynthesis and evaluation of 2-(4-[(18)F]fluorophenyl)-7,8-dihydroxy-4Hchromen-4-one and 2-(4-([N-methyl-(11)C]-dimethylamino)phenyl)-7,8-dihydroxy- 4H-chromen-4-one. Bioorg Med Chem. 2013;21:7816–7829. doi: 10.1016/j.bmc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 28.Ren Q, Zhang JC, Fujita Y, Ma M, Wu J, Hashimoto K. Effects of TrkB agonist 7,8-dihydroxyflavone on sensory gating deficits in mice after administration of methamphetamine. Pharmacol Biochem Behav. 2013;106:124–127. doi: 10.1016/j.pbb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Gao X, Zhao S, Hu W, Chen J. The small-molecule TrkB agonist 7, 8-dihydroxyflavone decreases hippocampal newborn neuron death after traumatic brain injury. J Neuropathol Exp Neurol. 2015;74:557–567. doi: 10.1097/NEN.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbat-Plana A, Cobianchi S, Herrando-Grabulosa M, Navarro X, Udina E. Endogenous modulation of TrkB signaling by treadmill exercise after peripheral nerve injury. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.10.057. [DOI] [PubMed] [Google Scholar]
- 31.Scheff SW, Ansari MA, Roberts KN. Neuroprotective effect of Pycnogenol® following traumatic brain injury. Exp Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz LI, Shohami E. Inosine improves functional recovery after experimental traumatic brain injury. Brain Res. 2014;1555:78–88. doi: 10.1016/j.brainres.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Tarsa L, Goda Y. Synaptophysin regulates activity dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marte A, Messa M, Benfenati F, Onofri F. Synapsins are downstream players of the BDNF-mediated axonal growth. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9659-3. [DOI] [PubMed] [Google Scholar]
- 35.Zou H, Brayer SW, Hurwitz M, Niyonkuru C, Fowler LE, Wagner AK. Neuroprotective, neuroplastic, and neurobehavioral effects of daily treatment with levetiracetam in experimental traumatic brain injury. Neurorehabil Neural Repair. 2013;27:878–888. doi: 10.1177/1545968313491007. [DOI] [PubMed] [Google Scholar]
- 36.Juan WS, Huang SY, Chang CC, Hung YC, Lin YW, Chen TY, Lee AH, Lee AC, Wu TS, Lee EJ. Melatonin improves neuroplasticity by upregulating the growth-associated protein-43 (GAP-43) and NMDAR postsynaptic density-95 (PSD-95) proteins in cultured neurons exposed to glutamate excitotoxicity and in rats subjected to transient focal cerebral ischemia even during a long-term recovery period. J Pineal Res. 2014;56:213–223. doi: 10.1111/jpi.12114. [DOI] [PubMed] [Google Scholar]
- 37.Dinocourt C, Gallagher SE, Thompson SM. Injury-induced axonal sprouting in the hippocampus is initiated by activation of trkB receptors. Eur J Neurosci. 24(20060):1857–1866. doi: 10.1111/j.1460-9568.2006.05067.x. [DOI] [PubMed] [Google Scholar]
- 38.Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro N, Ghavim SS, Harris NG, Hovda DA, Sutton RL. Glucose administration after traumatic brain injury improves cerebral metabolism and reduces secondary neuronal injury. Brain Res. 2013;1525:124–136. doi: 10.1016/j.brainres.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moro N, Ghavim SS, Harris NG, Hovda DA, Sutton RL. Pyruvate treatment attenuates cerebral metabolic depression and neuronal loss after experimental traumatic brain injury. Brain Res. 2016;1642:270–277. doi: 10.1016/j.brainres.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1a in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han M, Zhang JC, Yao W, Yang C, Ishima T, Ren Q, Ma M, Dong C, Huang X, Hashimoto K. Intake of 7,8-dihydroxyflavone during juvenile and adolescent stages prevents onset of psychosis in adult offspring after maternal immune activation. Sci Rep. 2016;6:36087. doi: 10.1038/srep36087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1a/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill JL, Kobori N, Zhao J, Rozas NS, Hylin MJ, Moore AN, Dash PK. Traumatic brain injury decreases AMP-activated protein kinase activity and pharmacological enhancement of its activity improves cognitive outcome. J Neurochem. 2016;139:106–119. doi: 10.1111/jnc.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, Tarascio G, Amorini AM, Di Pietro V, Delfini R, Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- 48.Castellanos NP, Paúl N, Ordóñez VE, Demuynck O, Bajo R, Campo P, Bilbao A, Ortiz T, del-Pozo F, Maestú F. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain. 2013;133:2365–2381. doi: 10.1093/brain/awq174. [DOI] [PubMed] [Google Scholar]
- 49.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marquez de la Plata CD, Garces J, ShokriKojori E, Krishnan K, Pidikiti R, et al. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol. 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mishra AM, Bai X, Sanganahalli BG, Waxman SG, Shatillo O, Grohn O, Hyder F, Pitkanen A, Blumenfeld H. Decreased resting functional connectivity after traumatic brain injury in the rat. PLoS One. 2014;9:e95280. doi: 10.1371/journal.pone.0095280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomason ME, Yoo DJ, Glover GH, Gotlib IH. BDNF genotype modulates resting functional connectivity in children. Front Hum Neurosci. 2009;3:55. doi: 10.3389/neuro.09.055.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]