Abstract
Background
The impact of fewer than 3 doses of HPV vaccine on genital warts is uncertain.
Methods
Using the Truven Health Analytics Marketscan administrative database, we compared rates of genital warts among females receiving 0, 1, 2, or 3 doses of HPV vaccine. Females aged 9–18 on 1/1/2007 who were continuously enrolled in the database through 12/31/2013 were included. Patients were assigned an HPV dose state (0, 1, 2, or 3) based on the last recorded dose. The exposure period began on 1/1/2007 or the date of the final HPV dose, and lasted until the first diagnosis of genital warts or 12/31/2013. Multivariable Poisson regression was performed to determine the risk of genital warts associated with vaccine doses.
Results
Among 387,906 subjects, mean age and exposure period were 14.73 and 5.64 years, respectively. The proportion of doses received were: 52.1%, 7.8%, 9.4%, and 30.7% for 0, 1, 2, and 3 doses respectively. The rate of genital warts was 1.97/1000 person-years. Receipt of 0 or 1 doses was associated with more genital warts than 3 doses. The effectiveness of 2 doses following current CDC guidelines was similar to 3 doses. The risk of genital warts rose with age.
Conclusions
Prevention of genital warts is higher with completion of 3 vaccine doses than with one dose, though 2 dose recommendations appear to provide similar protection. Prospective effectiveness studies of recommended 2-dose schedules against clinical endpoints including persistent infection, genital warts, and cervical dysplasia are necessary to ensure long-term protection of vaccinated cohorts.
Introduction
The effectiveness of a 3-dose series of quadrivalent Human Papillomavirus (HPV) vaccination in reducing cervical, vulvar, vaginal, and anal dysplasia has been demonstrated in clinical trials,[1][2] and post-marketing studies from the U.S. and other countries have indicated lower population levels of genital warts and cervical dysplasia in the years following vaccination.[3][4][5][6][7] Studies indicate that the antibody response of younger adolescents (ages 9–14) who received 2 doses at least 5 months apart was non-inferior to that achieved with 3 doses of vaccination among women aged 15–26 who were participating in clinical efficacy trials.[8][9][10][11] Based primarily on immunogenicity results, the Strategic Advisor Group of Experts (SAGE) of the World Health Organization (WHO) recommended 2 doses at 6–12 month intervals for girls ages 9–14. Several countries in western Europe have subsequently adopted a 2-dose schedule, GAVI countries are required to use a 2-dose schedule to receive financial support, and in the US, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) adopted a 2-dose schedule in October 2016.[12][13]
However, effectiveness data on fewer than 3 doses of HPV vaccination are limited, and concerns have been raised about long-term protection due to waning antibody responses after a few years as well as weaker memory T-cell responses.[14] Data on the relative effectiveness of fewer than 3 doses remain limited, and results are conflicting. This study uses administrative data from a national cohort of privately-insured adolescents to compare the relative protection afforded by 0, 1, 2, and 3 doses of HPV vaccination against genital warts between 2007–2013, an era when 3 doses were routinely recommended for US adolescents.
Materials and Methods
We used data from females in the Truven Health Analytics Marketscan Commercial Claims Database.[15] Marketscan includes claims from over 100 commercial insurance plans for both inpatient and outpatient care for employees and their dependents. Though Marketscan is not a probabilistic sample, it covers all enrollees and dependents from approximately half of provider-sponsored U.S. health insurance plans. The data are statistically de-identified, and the Boston University Institutional Review Board deemed this project exempt.
Inclusion and exclusion criteria
We included girls who were aged 9–18 on 1/1/2007 and were continuously enrolled in the database from 1/1/2007–12/31/2013. The exposure period began 1/1/2007 (for unvaccinated) or the date of the last HPV vaccine injection (for those receiving HPV vaccination). Person-time, defined as the number of months during which a girl was considered to be at risk for genital warts, was assigned according to the girl’s final vaccination status (0, 1, 2, or 3 doses). We felt that allowing girls to contribute person-time during the course of their vaccine series (i.e., time between doses 2 and 3) was problematic as infection acquired prior to completing the series might be counted as a vaccine failure. This methodology reflects that espoused by Gertig et al, who state that including only person-time that reflects a woman’s final vaccination status more closely reflects the efficacy of receipt of only 1 or 2 doses.[16] The exposure period ended when a subject either received a diagnosis of genital warts or at the end of the study period (12/31/2013). Subjects with fewer than 180 days of follow-up were excluded.
Outcome definitions and data analysis
CPT (Current Procedural Terminology coding system) and ICD-9 (International Classification of Diseases, ninth revision) codes were used to identify HPV vaccine injections and genital warts. HPV vaccination codes included CPT (90649, 90650, 90651) and ICD-9 (V04.89). Genital warts were identified as described by Flagg et al (2013).[17] We defined anogenital warts based on the following three scenarios.
-
Scenario a
One or more diagnoses of condyloma acuminata (ICD-9-CM code 078.11) occurring within a given year.
-
Scenario b
One or more less-specific diagnoses of viral warts (ICD-9-CM code: 078.1, 078.10, 078.19) within a given year only if, within 30 days of the diagnosis, there was also a procedure for destruction or excision of an anogenital lesion (CPT code: 45108, 99170) or a diagnosis of a benign anogenital neoplasm (ICD-9-CM code: 211.4, 216.5, 221.8, 222.9).
-
Scenario c
One or more prescriptions for genital wart medications (NCD code: 99207026012, 45802036853, 64380077302, 115147623, 51672414506, 68462053670, 168043224, 45802036862, 64380077319, 99207027675, 99207027028, 99207027175, 52544004513, 52544004613, 591320413, 574061105, 574060115, 10337045003, 10481300801, 38779010005) within a given year, only if within 30 days of the prescription there was also (1) a procedure for destruction or excision of an anogenital lesion (CPT code: 45108, 99170), or (2) a diagnosis of a benign anogenital neoplasm (ICD-9-CM code: 211.4, 216.5, 221.8, 222.9).
Multivariable Poisson regression analyses were conducted to estimate the relative incidence of genital warts associated with receiving 0, 1, 2 or 3 doses, and adjusted for differential observation periods, age, region, area-level socioeconomic indicators, and calendar year. To avoid including genital warts representing prevalent infections rather than new infections, we performed a sensitivity analysis using a 1-year washout period from the first dose. To better understand the role of factors other than age, we estimated two models, one adjusting only for age (base model), and another also adjusting for geographic region, income, proportion of minorities in county of residence, and calendar year (full model); from each we obtained estimates of estimated incidence rate ratios of genital warts comparing fully vaccinated girls to those receiving < 3 doses, and among girls who initiated vaccination prior to age 15, comparing girls receiving 3 doses with those receiving 2 doses with a >5-month inter-dose interval.
Results
A total of 387,906 adolescent females were included in the study (Table 1). The mean age was 14.73 years, and average length of follow-up was 5.64 years. In regard to geographic location, 10% of girls lived in the northeastern U.S., 24% in the north central region, 38% in the southern region, and 28% in the western region. Approximately one third of girls lived in counties with <20% or >35% racial/ethnic minority residents as defined by the U.S. census bureau.
Table 1.
Demographic characteristics of study sample (females aged 9–18 as of 01/01/2007)
| N | 387,906 |
| Age*, mean (std. dev.) | 14.73 (3.13) |
| Average of Exposure (years), mean (std. dev.) | 5.64 (1.85) |
| Ever had genital warts during 2007–2013 (%) | 1.11 |
| Region (%) | |
| Northeast | 10.08 |
| North Central | 23.65 |
| South | 38.48 |
| West | 27.78 |
| Medium Household Income, 2007 (%) | |
| <47,000 | 34.87 |
| 47,000–57,000 | 33.33 |
| >57,000 | 31.80 |
| Percent of County-Level Minority Population, 2010 (%) | |
| <20% | 33.71 |
| 20%–35% | 30.86 |
| >35% | 35.42 |
Patient age at start of exposure period.
Fifty-two percent of girls (n=201,933) remained unvaccinated throughout the study, 7.8% (n=30,428) received 1 dose, 9.4% (n=36,583) received 2 doses, and 30.7% (n=118,962) received 3 doses (Table 2). Among girls receiving more than 1 dose of HPV vaccine, 60.4% (93,957/155,545) received their second dose within three months of their first dose, and 47.1% (73,210/155,545) received their third dose within 5 months of their second dose. Furthermore, 52.5% (81,587/155,545) received their subsequent doses more than 1 month off schedule, with 34.4% (53,508/155,545) receiving doses at 12-month intervals, likely corresponding to annual preventive care visits. Different inter-dose intervals were observed between girls who completed 3 doses and those who completed only 2 doses: 70% of girls who ultimately received 3 doses completed their second dose on time (within 3 months of the first dose), compared with 26% of girls who received only 2 doses during the study period (Figure 1; p<0.001).
Table 2.
Incidence rates of genital warts by dosage HPV vaccine received
| Number of HPV doses | Number of girls | Percent (%) | Average age* | Person- years of exposure | Number of genital warts cases** | Genital warts cases per 1,000 person- years (95% CI) |
|---|---|---|---|---|---|---|
| 0 | 201,933 | 52.1 | 13.2 | 1,404,215 | 3,053 | 2.17 (2.10, 2.25) |
| 1 | 30,428 | 7.8 | 16.7 | 126,714 | 241 | 1.90 (1.68, 2.16) |
| 2 | 36,583 | 9.4 | 16.7 | 151,862 | 268 | 1.76 (1.57, 1.99) |
|
2*** <5 month interval |
18,757 | 4.8 | 16.4 | 86,581 | 148 | 1.71 (1.46, 2.01) |
|
2*** ≥5 interval |
17,826 | 4.6 | 16.9 | 65,282 | 120 | 1.84 (1.54, 2.20) |
| 3 | 118,962 | 30.7 | 16.3 | 504,413 | 756 | 1.50 (1.40, 1.61) |
| Total | 387,906 | 14.7 | 2,187,205 | 4,318 | 1.97 (1.92, 2.03) |
Patient age at start of exposure period.
Multiple cases that occurred to individual girls were counted only once.
Data include all girls, regardless of age at vaccine series initiation.
Figure 1.
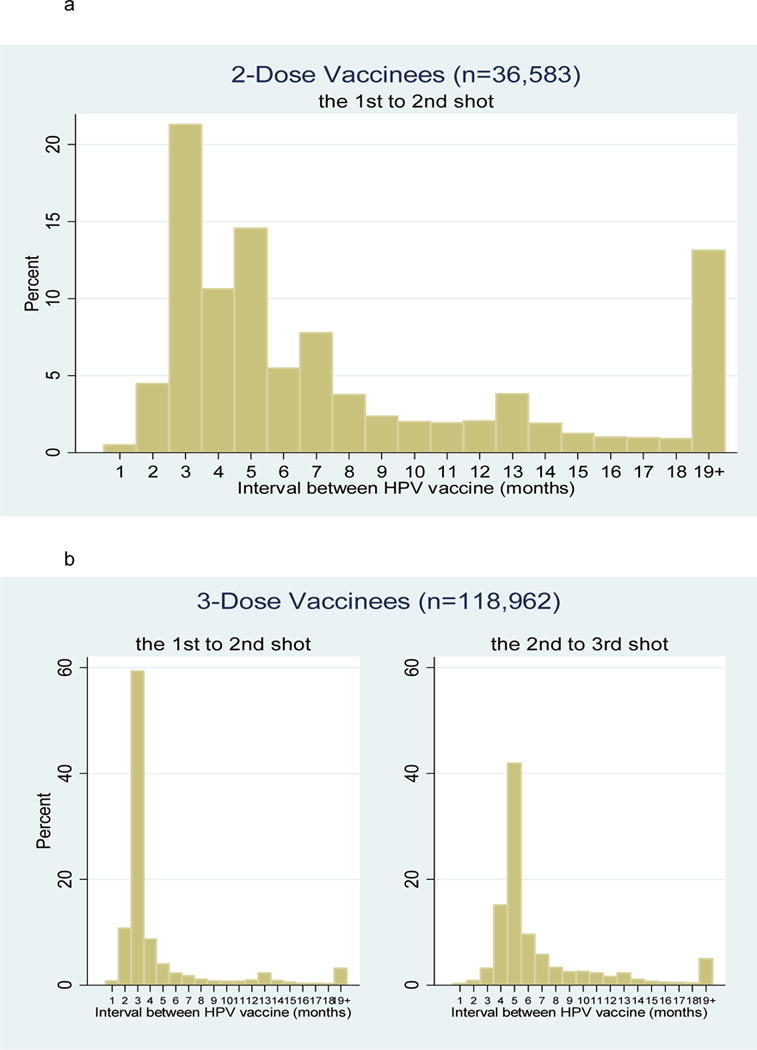
(a). Time (months) between HPV vaccine doses among girls who completed only 2 doses
(b). Time (months) between HPV vaccine doses among girls who completed all 3 doses
The overall rate of genital warts was 1.97/1000 person years (Table 2). The rate of genital warts decreased with additional HPV vaccine doses, from 2.17 cases/1000 person-years for unvaccinated girls, to 1.90 cases/1000 person-years for girls receiving 1 dose, to 1.76 cases/1000 person-years for girls receiving 2 doses, to 1.5 cases/1000 person-years for girls receiving 3 doses. Receipt of 3 doses was significantly more effective than 0 or 1 dose; the difference between 2 and 3 doses did not reach statistical significance. Incidence rates of genital warts were similar for girls who completed 2 doses at < or ≥5 month intervals. Poisson regression analyses (Table 3) indicated that unvaccinated girls had nearly double the risk of genital warts compared with girls who completed the series [IRR 1.90 (95% CI 1.66–2.18)]. Girls who received 1 dose had fewer genital warts than unvaccinated girls, but more than girls who completed the series [IRR 1.22 95%CI (1.05, 1.41)].
Table 3.
Incidence Rate Ratios of genital warts comparing fully vaccinated individuals with those receiving <3 Doses, IRR (95%CI)
| Base Model (N=387,906) | Full Model (N=387,906) | |
|---|---|---|
| Doses received | ||
| 0 | 2.27 (2.08, 2.48) | 1.90 (1.66, 2.18) |
| 1 | 1.22 (1.06, 1.43) | 1.22 (1.05, 1.41) |
| 2 | 1.13 (0.98, 1.30) | 1.12 (0.97, 1.29) |
| Age* (ref=9–10) | ||
| 11–12 | 1.46 (1.26, 1.70) | 1.46 (1.26, 1.70) |
| 13–14 | 2.51 (2.19, 2.87) | 2.52 (2.20, 2.89) |
| 15–16 | 3.44 (3.01, 3.93) | 3.50 (3.07, 4.00) |
| 17–18 | 4.49 (3.94, 5.14) | 4.65 (4.07, 5.31) |
| 19–20 | 6.04 (5.02, 7.26) | 6.36 (5.28, 7.65) |
| 21–22 | 8.35 (6.35, 10.99) | 10.51 (7.88, 14.02) |
| 23–25 | 6.73 (3.84, 11.81) | 9.86 (5.44, 17.87) |
| Region (ref=South) | ||
| Northeast | 0.84 (0.74, 0.94) | |
| North Central | 0.95 (0.88, 1.03) | |
| West | 0.71 (0.65, 0.77) | |
| Income (ref= >57,000) | ||
| <4,7000 | 0.92 (0.85, 1.00) | |
| 4,7000–5,7000 | 0.91 (0.84, 0.98) | |
| % of minority population (ref= >35%) | ||
| <20% | 1.02 (0.95, 1.11) | |
| 20%–35% | 1.06 (0.97, 1.14) | |
| Year (beginning of exposure period) | ||
| 2008 | 0.86 (0.74, 0.99) | |
| 2009 | 0.75 (0.63, 0.90) | |
| 2010 | 0.74 (0.60, 0.92) | |
| 2011 | 0.64 (0.49, 0.83) | |
| 2012–2013 | 0.59 (0.44, 0.79) |
Patient age at start of exposure period.
Note: In the base model, the reference group is those aged 9–10 years. In the full model the reference group is those aged 9–10, living in South region, with high household income (>$57,000), in high minority areas (>35%) and year 2007.
To study the potential impact of recent guideline changes, we examined girls who began their HPV vaccine series before their 15th birthday, and compared rates of genital warts among those who completed 3 doses (n=30,527) and those who completed 2 doses given at least 5 months apart (n=3,154) (Table 4). During an average of 4.6 years of follow-up after completing their final vaccine doses, similar proportions of girls in both groups developed genital warts: 156 girls (0.51%) in the 3-dose group and 14 (0.44%) in the 2-dose group. Results were similar for analyses in Tables 3 and 4 when a one-year washout period was included (data not shown).
Table 4.
Incidence Rate Ratios of genital warts among girls who initiated vaccination prior to age 15, comparing girls receiving 3 doses with those receiving 2 doses with a >5-month inter-dose interval, IRR (95%CI)
| Base Model (N=33,851*) | Full Model (N=33,851) | |
|---|---|---|
| 2 doses with a 5-month interval | 0.91 (0.53, 1.58) | 0.97 (0.56, 1.68) |
| Age** (ref= 9–11) | ||
| 12 | 0.43 (0.23, 0.82) | 0.50 (0.26, 0.97) |
| 13 | 0.68 (0.39, 1.20) | 0.83 (0.46, 1.48) |
| 14 | 0.60 (0.34, 1.05) | 0.72 (0.41, 1.29) |
| Region (ref=South) | ||
| Northeast | 0.74 (0.44, 1.23) | |
| North Central | 0.97 (0.65, 1.44) | |
| West | 0.68 (0.44, 1.05) | |
| Income (ref= >57,000) | ||
| <4,7000 | 0.65 (0.44, 0.96) | |
| 4,7000–5,7000 | 0.69 (0.48, 1.00) | |
| % of minority population (ref= >35%) | ||
| <20% | 1.02 (0.67, 1.56) | |
| 20%–35% | 1.12 (0.75, 1.67) | |
| Year (beginning of exposure period) | ||
| 2008 | 0.85 (0.59, 1.24) | |
| 2009 | 0.55 (0.34, 0.91) | |
| 2010 | 0.62 (0.34, 1.13) | |
| 2011–2012 | 0.69 (0.29, 1.67) |
3 dose group: n=30,527 total girls, 156 cases of genital warts (0.51%); 2 dose group: n=3,154 total girls, 14 genital warts (0.44%)
Patient age at start of exposure period. Sample limited to females aged 9–14 at start of exposure periods.
Additional factors associated with genital wart risk are described in Table 3. The risk of genital warts increased substantially with age from IRR 1.46 (95%CI 1.26–1.70) for ages 11–12 to 10.51 (95%CI 7.88–14.02) for ages 21–22 compared to ages 9–10. Genital wart rates were lower in the northeast and western regions compared with the south, and the west was lower than the north central region. Genital wart rates were inversely correlated with vaccination rates in all regions except the west, which had both low vaccination and genital warts rates (data not shown). No differences were noted by proportion of minorities in the counties in which the girls resided. Overall rates of genital warts decreased significantly after 2007 from 2.13 to 1.63 per 1000 person years between 2007 and 2013 (data not shown), with a trend toward more substantial decreases over time [2008 IRR 0.86 (95%CI 0.74–0.99), 2012–2013 IRR 0.59 (95%CI 0.44–0.79) compared to 2007].
Discussion
This study of a national sample of adolescent girls indicates that completion of at least 2 doses of HPV vaccine are necessary to provide protection against genital warts. Targeted analyses examining 2 doses at ≥ 5 month intervals indicate that this regimen appears to provide similar protection against genital warts as 3 doses among girls initiating the series prior to age 15, supporting WHO and CDC guidelines. Receipt of only 1 dose, however, provides inferior protection.
While studies with the bivalent vaccine have shown high effectiveness with fewer than 3 doses,[18][11] existing ecological studies with the quadrivalent vaccine have tended to indicate a dose-response effect, with 3 doses required for maximum protection. A study of genital warts in Sweden among girls vaccinated prior to age 17 found that the IRR for genital warts among the recipients of 3 doses was 0.18 (95% CI, 0.15–0.22), for 2 doses was 0.29 (95% CI, 0.21–0.40), and for 1 dose was 0.31 (95% CI, 0.20–0.49).[19] This study also examined the contribution of prevalent infections by using washout periods of different lengths, and found that differences between 2 and 3 doses were attenuated by buffer periods longer than 4 months after each dose. In our study, including a washout period of 12 months after the first dose did not affect the results. A study in Belgium found a vaccine efficacy against genital warts of 89.2% (95% CI: 81.5; 93.7) for 3 doses, 69.7% (95% CI: −26.6; 87.5) for 2 doses, and 36.9% (95% CI: −15.5; 65.5) after 1 dose.[20] Another study from Denmark was designed to assess the difference in effectiveness against genital warts between 2 and 3 doses, specifically looking at time between doses 1 and 2.[21] These authors found that genital warts occurred significantly less frequently with each additional dose, and demonstrated a twofold increase in the risk of genital warts for those receiving 2 doses compared to 3 doses (IRR 2.19 95%CI 1.86–2.54). When only girls less than age 16 receiving doses at least 6 months apart were considered, the effectiveness approached that of 3 doses, but confidence intervals were wide. Our study provides additional evidence of protection against genital warts among girls adhering to recommended 2-dose schedules, though results should be interpreted cautiously due to the small number of total cases.
While reductions in genital warts are an important early marker of vaccine effectiveness, reductions in cervical dysplasia and cancers are far more important vaccine-related outcomes. The only study examining the relationship of the number of quadrivalent HPV vaccine doses to the development of cervical dysplasia was conducted by Gertig and colleagues [16]. Using the final vaccination state as the start time for dysplasia risk (i.e., cervical dysplasia was only counted after the final vaccination had been received, rather than counting disease that occurred in the time between doses), complete vaccination was associated with a 38% reduction in the risk of any high-grade dysplasia (RR 0.62, 95%CI 0.48–0.79) but receipt of 1 or 2 doses was not associated with any reduction (RR 1 dose 1.26 95% CI 0.78–2.04, RR 2 doses 1.02 95% CI 0.66–1.58). Although these results were not stratified by age at vaccination, time between doses, or other behavioral risk factors for HPV acquisition, and small numbers of girls with incomplete dosing limited statistical power to detect differences, they do raise concerns. Thus, collecting additional long term follow-up data on 2-dose regimens is paramount.
The only randomized controlled trial addressing the question of dosing schedules compares immunologic responses, virus prevalence, and disease outcomes among girls aged 10–18 years who received either 2 doses at 0 and 6 months or 3 doses at 0, 2, and 6 months [22]. Of note, the randomized trial design was interrupted, therefore the data are analyzed and reported as an observational study, but still provide important information. The antibody responses were found to be equivalent between the 2- and 3-dose group for HPV 6, 11, and 16, but inferior for HPV 18. The incidence of transient HPV 16/18 infections at 4–6 years of follow-up were 0.4% (2/543) in the 3-dose group and 0.8% (4/519) in the 2-dose group, though no persistent HPV 16/18 infections were seen in either group. A randomized controlled trial in Canada with the quadrivalent vaccine, and a non-randomized study in Denmark with the bivalent vaccine also noted inferior antibody titers of HPV 18 in girls receiving 2 doses compared to 3 doses, though antibody avidity was equivalent in the Danish study. The clinical impact of these findings remains to be seen.[10][23]
While most immunogenicity data report relatively similar values between 2- and 3-dose regimens, clinical effectiveness data are sparse and subject to a number of limitations, including short intervals between doses and preponderance of subjects age over 15 at vaccination. Girls who received fewer than three doses may also differ from those who completed the series in their risk of HPV exposure, which is not captured in this database. All published ecological studies suffer from these limitations, including ours, although we did perform additional analyses comparing different dosing intervals to begin to address this issue. Additional limitations to our study include the use of administrative data, which are subject to coding errors. However, the validity of the Truven Marketscan database has been previously validated,[15] and we would not expect rates of coding errors to differ between vaccination states. In addition, relatively small numbers of girls with genital warts receiving 2 doses may limit the ability to detect statistically significant differences between groups.
Conclusions
Although these early data supporting 2-dose schedules are encouraging, they report on genital warts only, not on cervical dysplasia or cancer outcomes. HPV vaccine protection must last many years to provide adequate cancer protection, therefore ongoing studies are paramount. To ensure that HPV vaccination will continue to be effective on a population level, ongoing prospective studies using linked cohort data with a minimum of 15 years of follow-up to ensure the longterm effectiveness of 2-dose regimens at 0 and 6–12 months should be conducted with the endpoints of persistent viral infection, genital warts, and cervical dysplasia.
Short summary.
A national study of U.S. adolescents found that 3 doses of HPV vaccination may provide similar genital wart protection to 2 doses given at >5 month intervals at age ≥15.
Acknowledgments
Funding: American Cancer Society Mentored Research Scholar Grant (MRSG-09-151-01). The funders had no role in design of the intervention, design of the study, or writing of the manuscript.
Footnotes
Conflicts of interest: none
References
- 1.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 2.ACIP. Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 3.Markowitz LE, Hariri S, Lin C, et al. Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 4.Leval A, Herweijer E, Arnheim-Dahlstrom L, et al. Incidence of Genital Warts in Sweden Before and After Quadrivalent Human Papillomavirus Vaccine Availability. J Infect Dis. doi: 10.1093/infdis/jis405. Published Online First: 18 July 2012. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22815381. [DOI] [PubMed]
- 5.Read TR, Hocking JS, Chen MY, et al. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2011;87:544–7. doi: 10.1136/sextrans-2011-050234. [DOI] [PubMed] [Google Scholar]
- 6.Brotherton JM, Fridman M, May CL, et al. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 7.Powell SE, Hariri S, Steinau M, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31:109–13. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 8.Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum Vaccin. 2011;7:161–9. doi: 10.4161/hv.7.2.13690. doi:13690 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Krajden M, Cook D, Yu A, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol CVI. 2011;18:418–23. doi: 10.1128/CVI.00489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. Jama. 2013;309:1793–802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 11.Lazcano-Ponce E, Stanley M, Muñoz N, et al. Overcoming barriers to HPV vaccination: non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months Vaccine. 2014;32:725–32. doi: 10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Meites Elissa, Kempe Allison, Markowitz Lauri E. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 65:1405–8. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Evidence based recommendations on Human Papilloma Virus (HPV) Vaccines Schedules Background paper for SAGE discussions. 2014 http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf.
- 14.Donken R, Knol MJ, Bogaards JA, et al. Inconclusive evidence for non-inferior immunogenicity of two- compared with three-dose HPV immunization schedules in preadolescent girls: A systematic review and meta-analysis. J Infect. 2015;71:61–73. doi: 10.1016/j.jinf.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Danielson E. Health research data for the real world: the MarketScan databases. 2014 http://truvenhealth.com/Portals/0/Users/031/31/31/PH_13434%200314_MarketScan_WP_web.pdf.
- 16.Gertig DM, Brotherton JM, Budd AC, et al. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227. doi: 10.1186/1741-7015-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health. 2013;103:1428–35. doi: 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romanowski B, Schwarz TF, Ferguson LM, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011;7:1374–86. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herweijer E, Leval A, Ploner A, et al. Association of varying number of doses of quadrivalent human papillomavirus vaccine with incidence of condyloma. Jama. 2014;311:597–603. doi: 10.1001/jama.2014.95. [DOI] [PubMed] [Google Scholar]
- 20.Dominiak-Felden G, Gobbo C, Simondon F. Evaluating the Early Benefit of Quadrivalent HPV Vaccine on Genital Warts in Belgium: A Cohort Study. PLoS One. 2015;10:e0132404. doi: 10.1371/journal.pone.0132404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomberg M, Dehlendorff C, Sand C, et al. Dose-Related Differences in Effectiveness of Human Papillomavirus Vaccination Against Genital Warts: A Nationwide Study of 550,000 Young Girls. Clin Infect Dis. 2015;61:676–82. doi: 10.1093/cid/civ364. [DOI] [PubMed] [Google Scholar]
- 22.Sankaranarayanan R, Prabhu PR, Pawlita M, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donken R, Schurink-van ’t Klooster TM, Schepp RM, et al. Immune responses after two- versus three-doses of HPV vaccination up to 4½ years post vaccination: an observational study among Dutch routinely vaccinated girls (HPV2D) J Infect Dis. doi: 10.1093/infdis/jiw588. Published Online First: 23 December 2016. [DOI] [PubMed] [Google Scholar]


