Abstract
Objectives
In patients receiving concurrent chemoradiation for locally advanced non-small cell lung cancer (NSCLC), consolidation chemotherapy is frequently given even though several randomized trials have failed to show a benefit. We explored the potential benefits of consolidation chemotherapy using a population-based comparative effectiveness approach.
Materials and Methods
Surveillance, Epidemiology, and End Results-Medicare was used to identify patients with Stage III NSCLC aged ≥65 and diagnosed 2002–2009. We selected patients who received concurrent chemoradiotherapy and determined whether they were (concurrent-consolidation) or were not (concurrent-alone) treated with consolidation chemotherapy. Outcomes were overall and cancer specific survival using a conditional landmark analysis approach.
Results
1,688 patients treated with concurrent-alone or concurrent-consolidation were identified with a median follow up of 29 months. Choice of chemotherapy agents did not correlate with outcome. For concurrent-consolidation versus concurrent-alone, the median overall survival was 21 months versus 18 months, respectively (log-rank p = 0.008) and the median cancer specific survival was 23 months versus 19 months, respectively (log-rank p = 0.03). On multivariate analysis, concurrent-consolidation remained associated with improved overall survival (HR 0.85, p = 0.04), and there was a trend for improved cancer specific survival (HR 0.87, p = 0.12). Inverse probability of treatment weighting using propensity scores demonstrated similar findings. Importantly, the benefit of concurrent-consolidation held only for patients treated with carboplatin-taxane but not with cisplatin-etoposide.
Conclusion
Survival outcomes were similar among the five most commonly employed platinum-based doublets. We found that patients receiving cisplatin during radiation do not appear to benefit from additional chemotherapy. However, for patients receiving carboplatin, consolidation chemotherapy was associated with improved overall and cancer specific survival.
Keywords: Non-Small Cell Lung Cancer, Chemoradiation, Comparative Effectiveness Research, Platinum-Doublet Chemotherapy, SEER-Medicare
1. Introduction
For locally advanced non-small-cell lung cancer (NSCLC) patients (i.e. stage IIIA/B), combined modality therapy (chemoradiation) is generally recommended [1]. Studies repeatedly demonstrated the benefit of chemotherapy over radiation alone, as well as the benefit of using a platinum-based agent, typically with a second agent, termed “platinum-based doublet therapy” [1–3]. Chemotherapy can be given in various sequences: before radiation (sequential), during radiation (concurrent-alone), before and during radiation (induction-concurrent), or during and after radiation (concurrent-consolidation). As for radiation therapy, generally treatment is 60–66 Gy in 2 Gy fractions, although hyperfractionated or accelerated courses are also being studied [4].
Controversies remain regarding the optimal choice for the sequence of chemotherapy [1, 5, 6]. Although there are randomized trials showing a lack of efficacy with consolidation after cisplatin-based chemotherapy [7–9], there are no randomized trials studying consolidation after carboplatin-based chemotherapy. Rather, evidence for consolidation after carboplatin-based chemotherapy has been limited to single-arm trials [10]. Using SEER-Medicare, we studied the use of platinum-based doublet therapies as well as chemoradiation sequences among elderly patients in the US.
2. Material and Methods
2.1 Patient Selection
Patients diagnosed with NSCLC from January 2002 to December 2009 were identified using Surveillance, Epidemiology, and End Results (SEER)-Medicare. SEER-Medicare is a linked dataset maintained by the National Cancer Institute and contains data from 17 registries accounting for approximately 28% of the US population [11]. The dataset contains demographic, clinical, pathological, outcomes, and Medicare insurance claims data [12]. Follow up was through December 2010.
The cohort included patients aged ≥65 with pathologically confirmed stage IIIA/B NSCLC. Staging was according to the 3rd edition of the AJCC, as only patients diagnosed since 2004 had documented TNM data [13]. Patients with a malignant pleural effusion were excluded, as they are now classified as stage IV. Patients must have been enrolled in Medicare Parts A and B for 12 months prior to diagnosis until death or censoring, and were excluded for enrollment in a health maintenance organization to ensure Medicare claims completeness and characterize pre-diagnosis comorbidities. Patients with an invalid diagnosis date or who were diagnosed at death were excluded.
2.2 Chemoradiation Definition and Associated Variables
Medicare billing claims were used to determine treatment with chemoradiation within 3 months of diagnosis and to exclude patients with prior resection. Radiation therapy (RT) was categorized as treatment with either intensity modulated (IMRT) or 3D-conformal (3D-CRT) radiation therapy, and required 30–40 daily treatment claims (Supplemental Table 1) [14]. RT facility was categorized as a freestanding center, hospital-based NCI center, or hospital-based non-NCI center. Radiation oncologist density was categorized by quartile, and was determined from the Area Health Resources Files (AHRF) [15]. In the AHRF, regions are divided into health service areas, which are defined as one or more counties with self-contained resources for routine hospital care [16].
Chemotherapy was restricted to platinum-based doublet therapy (carboplatin or cisplatin). The second chemotherapy agent that made up the doublet therapy must have started no more than 1 week from the start of the platinum agent (Supplemental Table 1). Sequential was defined as radiation starting 8–45 days after the end of chemotherapy. Concurrent-alone was chemotherapy and radiation starting and ending within 2 weeks of each other. Induction-concurrent was chemotherapy starting more than 2 weeks prior to radiation (but not more than 3 months). Concurrent-consolidation was chemotherapy continuing for more than 2 weeks after radiation, but the next cycle after radiation must have been within 45 days of completion of radiation, and could include starting a new regimen. Similar methods have previously been used to define chemoradiation sequences [17–19].
2.3 Patient Demographic, Clinical, and Diagnostic Variables
Using SEER data, patient demographic data were classified by age, sex, race, marital status, urban setting, area educational attainment (≥4 years of college), and area median income. Geographic area was categorized into West, Midwest, South, and Northeast based on SEER registry. Clinical data were classified by histology, tumor size, and nodal involvement. Using Medicare claims from 12 months prior to diagnosis, a modified Charlson-Deyo comorbidity index and COPD status were determined [20, 21]. Oxygen use was determined from home oxygen supply claims. A proxy performance score (PS) was determined to indicate overall health [14, 22]. PS included hospitalization, skilled nursing or long-term care stay, home health use, and claims for ambulation assistance equipment, bedside commode, or hospital bed.
Diagnostic workup for 3 months before treatment was determined, and included performance of PET, brain imaging, and invasive mediastinal staging. Brain imaging included magnetic resonance (MRI) and computed tomography (CT). Invasive mediastinal staging included video-assisted thoracoscopic surgery (VATS) mediastinal biopsy, bronchoscopy with nodal biopsy, mediastinoscopy, and mediastinotomy.
2.4 Statistical Analysis
The cohort consisted of the five most commonly used platinum-based doublet agents. Patient treatment was grouped according to 1) chemotherapy agents used (chemoradiation regimen) and 2) chemoradiation sequence. Differences between chemoradiation sequences were assessed using χ2 tests and Kruskal-Wallis tests. To compare outcomes among patients treated with concurrent-alone or concurrent consolidation, the Kaplan-Meier (KM) method was used to estimate overall survival (OS) and cancer specific survival (CSS). For OS, censoring was at last follow-up, and for CSS non-cancer associated deaths were also censored. Differences in OS and CSS between chemoradiation regimens and sequences were compared with log-rank tests. Multivariate Cox models were adjusted for demographic, clinical, and treatment confounders. Carboplatin-paclitaxel and concurrent-alone were used as references. To account for cases with missing marital status, tumor size, nodal status, or radiation oncologist density, we used multiple imputations with fully conditional specification (20 imputations). Multivariate logistic regressions were used for imputation conditional on all other clinical, demographic, and treatment-related variables in addition to outcome (OS). A secondary complete case analysis was performed.
All patients in the concurrent-consolidation group must have survived long enough to receive additional chemotherapy. To account for this guarantee-time bias, a conditional landmark analysis was used. Only patients surviving more than 45 days after completion of radiation were included. A sensitivity analysis was done using an extended multivariate Cox regression model comparing concurrent-alone to concurrent-consolidation. For this analysis, the chemoradiation sequence was considered a time-varying covariate where patients could enter the concurrent-consolidation group only after completion of radiation. The proportional hazards assumption was evaluated using log-log plots and a time-interaction variable. When this assumption was violated, we used Royston-Parmar flexible parametric models [23]. Model fit was determined using the likelihood ratio.
To adjust for selection bias between patients receiving concurrent-alone and concurrent-consolidation, an inverse probability of treatment weighting (IPTW) analysis was done using propensity scores. A multivariate logistic regression was used to determine the probability of treatment with concurrent-consolidation, conditional on all demographic, clinical, and treatment characteristics. Then a multivariate Cox model was performed with weighting by the inverse of the probability of the treatment received. Subgroup analyses were done with the IPTW method for patients receiving 1) carboplatin-taxane and 2) cisplatin-etoposide. A benefit to concurrent-consolidation was observed with the carboplatin-taxane group only, and so we calculated the power to show the same benefit for the cisplatin-etoposide group [24]. To account for the impact of PET staging, a sensitivity analysis using the IPTW method was performed limited to patients whose workup included PET.
Statistical significance was set at 0.05, and all tests were two-tailed. To adjust for multiple hypotheses comparisons, the method of Benjamini-Hochberg was used [25]. Statistical tests were performed using SAS (version 9.3, SAS, Cary, North Carolina) and R (version 3.0.2, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
We identified patients with stage III NSCLC diagnosed 2002–2009 who were treated with a platinum-based doublet therapy and radiation (Figure 1). The five most common chemoradiation regimens were: carboplatin-paclitaxel (1,423 patients), cisplatin-etoposide (242 patients), carboplatin-docetaxel (186 patients), carboplatin-etoposide (59 patients), and carboplatin-gemcitabine (33 patients). From 2002 to 2009 cisplatin-etoposide increased from 8% to 17% (Figure 2). The chemoradiation sequences were: concurrent-consolidation (896 patients), concurrent-alone (792 patients), induction-concurrent (140 patients), and sequential (115 patients). During the study period, sequential decreased from 14% to 3% and concurrent-consolidation increased from 35% to 45% (Figure 2). When concurrent-consolidation was given, 176 patients (20%) started a new chemotherapy regimen. The most common new regimen was docetaxel (129 patients) (Supplemental Table 2).
Figure 1. Cohort of patients treated with chemoradiation for stage IIIA/B non-small-cell lung cancer identified from SEER-Medicare diagnosed 2002–2009.
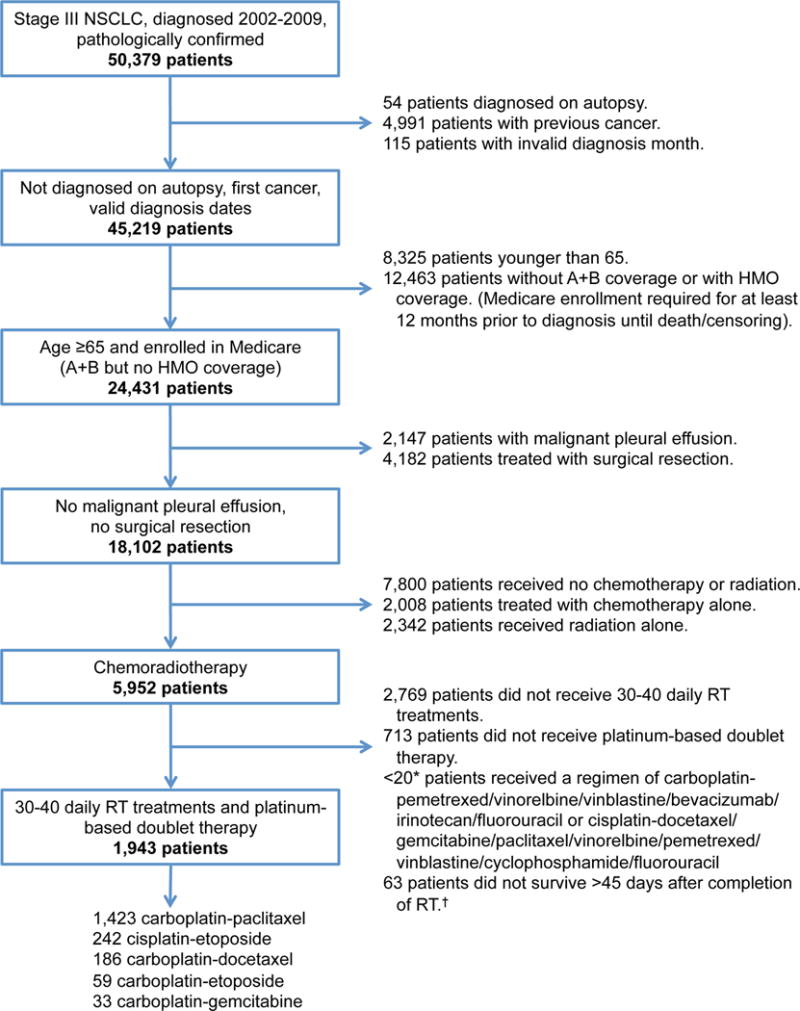
The cohort selection of patients identified using SEER-Medicare data. * Exact figures <11 not specified to protect patient identity. † These patients were included in the extended Cox regression model.
Figure 2. The use of chemoradiation regimens and sequences for stage III non-small-cell lung cancer over time.
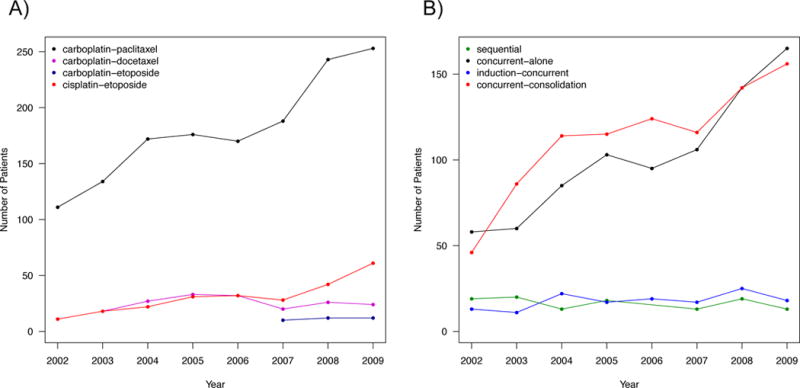
Prevalence of: a) the most commonly employed platinum-based chemoradiation regimens and b) chemoradiation sequences used from 2002–2009 for stage III NSCLC. Data points representing <11 patients not shown to protect patient identity.
Since they were the most commonly employed regimens, we subsequently focused our analyses on patients treated with concurrent-consolidation or concurrent-alone (n = 1,688). Patients receiving concurrent-consolidation were more likely to be younger, married, from an area with more radiation oncologists, diagnosed as N3, staged with a mediastinal procedure, and treated with carboplatin (Table 1). Median follow-up for these patients was 29 months (415 patients). Median OS and CSS did not vary on the basis of chemotherapy regimen (log-rank p = 0.36 and p = 0.63, respectively) (Figure 3). Median OS was 18 months with concurrent-alone treatment and 21 months with concurrent-consolidation (log-rank p = 0.008), and median CSS was 23 months versus 19 months (log-rank p = 0.03).
Table 1.
Differences of demographic, clinical, and treatment characteristics of patients with stage III NSCLC by chemoradiation sequence.
| Concurrent-alone | Concurrent-consolidation | Induction-Concurrent | Sequential | P | P* | |
|---|---|---|---|---|---|---|
| Characteristic | n=792 | n=896 | n=140 | n=115 | ||
| Chemoradiation regimen | ||||||
| Carboplatin-paclitaxel | 595 | 654 | 99 | 75 | <.0001 | 0.0005 |
| Carboplatin-docetaxel | 48 | 100 | 21 | 17 | ||
| Carboplatin-gemcitabine | <11 | <11 | <20 | <20 | ||
| Carboplatin-etoposide | 19 | >22 | <11 | <11 | ||
| Cisplatin-etoposide | 128 | >99 | <11 | <11 | ||
| Age | ||||||
| Median | 74 | 72 | 73 | 74 | <.0001 | <.0001 |
| Gender | ||||||
| Male | 427 | 504 | 75 | 59 | 0.64 | 0.35 |
| Female | 365 | 392 | 65 | 56 | ||
| Race | ||||||
| White | 685 | 764 | 129 | 104 | 0.16 | 0.15 |
| Black | 57 | 76 | <11 | <11 | ||
| Hispanic | 28 | 20 | <11 | <11 | ||
| Other | 22 | 36 | <11 | <11 | ||
| Marital status | ||||||
| Unmarried | 324 | 315 | 53 | 35 | 0.03 | 0.003 |
| Married | 456 | 549 | >73 | >66 | ||
| Unknown | 12 | 32 | <11 | <11 | ||
| Year of diagnosis | ||||||
| 2002 | 54 | 46 | 13 | 18 | 0.0009 | 0.18 |
| 2003 | 58 | 85 | 11 | 18 | ||
| 2004 | 82 | 113 | 21 | 13 | ||
| 2005 | 99 | 115 | 17 | 17 | ||
| 2006 | 94 | 124 | 19 | <11 | ||
| 2007 | 106 | 116 | 17 | 11 | ||
| 2008 | 138 | 141 | 25 | >9 | ||
| 2009 | 161 | 156 | 17 | 12 | ||
| Geographic region | ||||||
| West | 258 | 280 | 51 | 34 | 0.10 | 0.80 |
| Midwest | 151 | 170 | 20 | 12 | ||
| South | 212 | 235 | 36 | 29 | ||
| Northeast | 171 | 211 | 33 | 40 | ||
| Urban region | ||||||
| Urban | 701 | 793 | 128 | >97 | 0.36 | 1.00 |
| Rural | 91 | 103 | 12 | <11 | ||
| Educational attainment of area (≥4 years college) | ||||||
| 1st quartile | 199 | 231 | 28 | 22 | 0.26 | 0.92 |
| 2nd quartile | 199 | 230 | 34 | 27 | ||
| 3rd quartile | 197 | 225 | 41 | 25 | ||
| 4th quartile | 197 | 210 | 37 | 41 | ||
| Median income of area | ||||||
| 1st quartile | 198 | 237 | 29 | 21 | 0.26 | 0.66 |
| 2nd quartile | 210 | 217 | 34 | 24 | ||
| 3rd quartile | 197 | 218 | 41 | 31 | ||
| 4th quartile | 187 | 224 | 36 | 39 | ||
| Modified Charlson comorbidity | ||||||
| 0 | 511 | 563 | 98 | 66 | 0.69 | 0.85 |
| 1 | 196 | 231 | 29 | 35 | ||
| 2 | 51 | 65 | <11 | <11 | ||
| ≥3 | 34 | 37 | <11 | <11 | ||
| Performance score proxy | ||||||
| 0 | 723 | 827 | >121 | >98 | 0.47 | 0.14 |
| 1 | 42 | 52 | <11 | <11 | ||
| ≥2 | 27 | 17 | <11 | <11 | ||
| COPD | ||||||
| No | 512 | 590 | 106 | 70 | 0.05 | 0.61 |
| Yes | 280 | 306 | 34 | 45 | ||
| Home oxygen use | ||||||
| No | 727 | 838 | >122 | 103 | 0.26 | 0.19 |
| Yes | 65 | 58 | <11 | 12 | ||
| Tumor size | ||||||
| ≤2 cm | 66 | 77 | 15 | 11 | 0.33 | 0.16 |
| 2.1–3 cm | 114 | 119 | 20 | 12 | ||
| 3.1–5 cm | 247 | 269 | 38 | 32 | ||
| 5.1–7 cm | 153 | 151 | 28 | 25 | ||
| ≥7 cm | 75 | 121 | 22 | 11 | ||
| Unknown | 137 | 159 | 17 | 24 | ||
| Nodal status | ||||||
| N0 | 115 | 107 | 16 | 11 | 0.14 | 0.02 |
| N1 | 37 | >20 | <11 | <11 | ||
| N2 | 511 | 570 | 88 | >69 | ||
| N3 | 117 | 180 | >20 | 18 | ||
| Unknown | 12 | <11 | <11 | <11 | ||
| Histology | ||||||
| Adenocarcinoma | 214 | 265 | 51 | 35 | 0.35 | 0.51 |
| Squamous cell | 303 | 330 | 51 | 39 | ||
| Other | 275 | 301 | 38 | 41 | ||
| Diagnostic PET done | ||||||
| No | 425 | 503 | 93 | 89 | <.0001 | 0.33 |
| Yes | 367 | 393 | 47 | 26 | ||
| Brain CT/MR done | ||||||
| No | 295 | 313 | 61 | 72 | <.0001 | 0.33 |
| Yes | 497 | 583 | 79 | 43 | ||
| Invasive mediastinal imaging performed | ||||||
| No | 629 | 671 | 116 | 89 | 0.06 | 0.03 |
| Yes | 163 | 225 | 24 | 26 | ||
| Radiation type | ||||||
| IMRT | 110 | 97 | 17 | 15 | 0.29 | 0.06 |
| 3D-CRT | 682 | 799 | 123 | 100 | ||
| Radiation facility type | ||||||
| Freestanding center | 198 | 206 | 32 | 36 | 0.12 | 0.48 |
| Hospital-based center | 219 | 268 | 47 | 21 | ||
| Hospital-based NCI center | 375 | 422 | 61 | 58 | ||
| Area radiation oncologist density | 179 | 222 | 36 | 22 | ||
| 1st quartile | 0.006 | 0.0009 | ||||
| 2nd quartile | 180 | 162 | 27 | 22 | ||
| 3rd quartile | 164 | 177 | 35 | 34 | ||
| 4th quartile | 162 | 246 | 28 | >17 | ||
| Unknown | 107 | 89 | 14 | <11 |
P value represents concurrent-alone versus concurrent-consolidation. Exact figures not specified in some cells to protect patient identity.
Figure 3. Survival analysis of patients with stage III non-small-cell lung cancer treated with concurrent-alone or concurrent-consolidation chemoradiation by regimen and sequence.
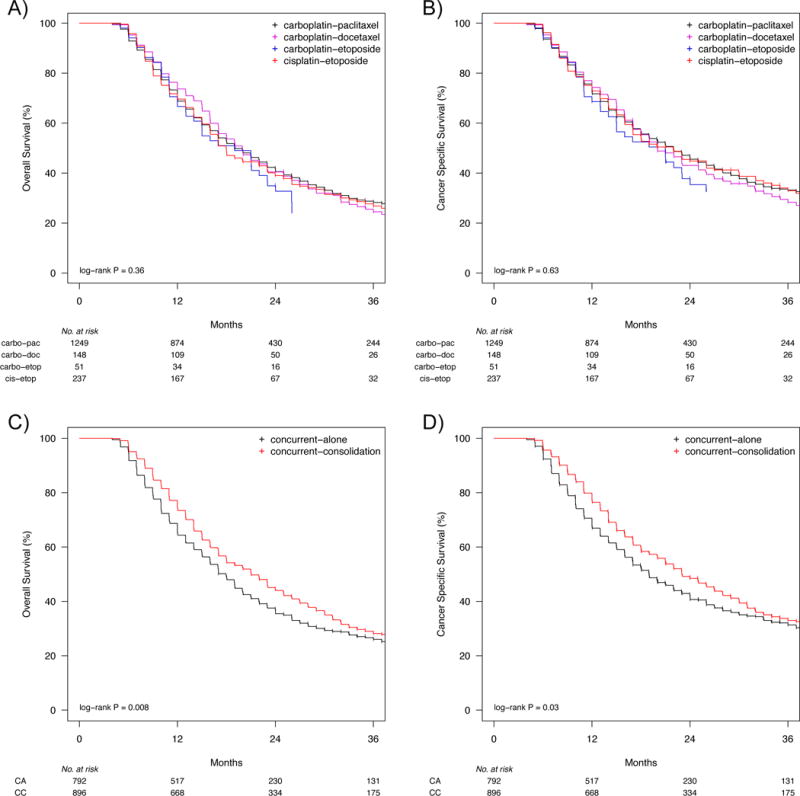
a) OS and b) CSS did not vary by chemotherapy regimen for patients treated with chemoradiation. Concurrent-consolidation resulted in improved outcomes compared to concurrent-alone, with c) a median OS of 21 months versus 18 months, and d) a median CSS of 23 months versus 19 months. Median follow up was 29 months, and curves are truncated at 36 months. Data is not shown where n < 11 to protect patient identity.
Multivariate regression models for OS and CSS were performed using parametric Royston-Parmar models, because the proportional hazards assumption was violated. OS did not significantly vary with chemotherapy regimen. With regards to chemoradiation sequence, there was a significant benefit to concurrent-consolidation compared to concurrent-alone (HR 0.85, 95% confidence interval [CI] 0.76–0.95, adjusted p = 0.04) (Table 2). Concurrent-consolidation was also associated with a trend for improved CSS (HR 0.87, 95% CI 0.77–0.98, adjusted p = 0.12). All models demonstrated goodness of fit. Sensitivity analyses using extended Cox regression models similarly showed improved outcomes with concurrent-consolidation compared to concurrent-alone (Supplemental Table 3). A complete case analysis was performed to validate the multiple imputations methods and resulted in similar findings (Supplemental Table 4).
Table 2.
Comparison of overall survival (OS) and cancer specific survival (CSS) by treatment, clinical, and demographic covariates for patients with stage III NSCLC treated with chemoradiation. Hazard ratios are determined from multivariate Royston-Parmar flexible parametric models.
| OS | CSS | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | P | Adjusted P* | Hazard Ratio | P | Adjusted P* | |
| Chemoradiation sequence | ||||||
| Concurrent-alone | Reference | Reference | ||||
| Concurrent-consolidation | 0.85 | 0.006 | 0.04 | 0.87 | 0.03 | 0.12 |
| Chemoradiation regimen | ||||||
| Carboplatin-paclitaxel | Reference | Reference | ||||
| Cisplatin-etoposide | 1.07 | 0.40 | 0.63 | 1.04 | 0.68 | 0.88 |
| Carboplatin-docetaxel | 1.02 | 0.88 | 0.94 | 1.04 | 0.75 | 0.88 |
| Carboplatin-etoposide | 1.28 | 0.12 | 0.36 | 1.29 | 0.13 | 0.36 |
| Carboplatin-gemcitabine | 1.92 | 0.27 | 0.48 | 1.36 | 0.67 | 0.88 |
| Age | 1.01 | 0.06 | 0.24 | 1.01 | 0.03 | 0.12 |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.92 | 0.17 | 0.38 | 0.96 | 0.59 | 0.87 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 0.91 | 0.45 | 0.64 | 0.88 | 0.32 | 0.61 |
| Hispanic | 0.82 | 0.27 | 0.48 | 0.93 | 0.69 | 0.88 |
| Other | 0.96 | 0.78 | 0.94 | 0.98 | 0.89 | 0.94 |
| Marital status | ||||||
| Unmarried | Reference | Reference | ||||
| Married | 1.09 | 0.19 | 0.40 | 1.15 | 0.05 | 0.16 |
| Educational attainment of area (≥4 years college) | ||||||
| 1st quartile | Reference | Reference | ||||
| 2nd quartile | 0.96 | 0.67 | 0.90 | 0.97 | 0.76 | 0.88 |
| 3rd quartile | 0.95 | 0.58 | 0.81 | 0.96 | 0.71 | 0.88 |
| 4th quartile | 1.05 | 0.69 | 0.90 | 1.03 | 0.78 | 0.88 |
| Median income of area | ||||||
| 1st quartile | Reference | Reference | ||||
| 2nd quartile | 1.11 | 0.20 | 0.41 | 1.13 | 0.19 | 0.43 |
| 3rd quartile | 1.21 | 0.05 | 0.22 | 1.27 | 0.020 | 0.11 |
| 4th quartile | 1.03 | 0.82 | 0.94 | 1.07 | 0.59 | 0.87 |
| Modified Charlson comorbidity | ||||||
| 0 | Reference | Reference | ||||
| 1 | 1.10 | 0.16 | 0.38 | 1.06 | 0.43 | 0.75 |
| 2 | 1.17 | 0.17 | 0.38 | 1.08 | 0.53 | 0.87 |
| ≥3 | 1.17 | 0.29 | 0.49 | 1.21 | 0.22 | 0.48 |
| Performance score proxy | ||||||
| 0 | Reference | Reference | ||||
| 1 | 0.98 | 0.90 | 0.94 | 0.98 | 0.91 | 0.94 |
| ≥2 | 1.04 | 0.84 | 0.94 | 1.13 | 0.56 | 0.87 |
| COPD | ||||||
| No | Reference | Reference | ||||
| Yes | 1.00 | 0.95 | 0.97 | 1.04 | 0.62 | 0.88 |
| Home oxygen use | ||||||
| No | Reference | Reference | ||||
| Yes | 1.17 | 0.17 | 0.38 | 1.01 | 0.92 | 0.94 |
| Tumor size | ||||||
| ≤2 cm | Reference | Reference | ||||
| 2.1–3 cm | 1.02 | 0.85 | 0.94 | 1.05 | 0.70 | 0.88 |
| 3.1–5 cm | 1.26 | 0.040 | 0.22 | 1.33 | 0.020 | 0.11 |
| 5.1–7 cm | 1.17 | 0.21 | 0.42 | 1.20 | 0.16 | 0.39 |
| ≥7 cm | 1.57 | 0.0006 | 0.007 | 1.66 | 0.0004 | 0.005 |
| Nodal status | ||||||
| N0 | Reference | Reference | ||||
| N1 | 1.30 | 0.11 | 0.36 | 1.37 | 0.06 | 0.20 |
| N2 | 1.18 | 0.06 | 0.24 | 1.14 | 0.17 | 0.39 |
| N3 | 1.35 | 0.005 | 0.04 | 1.37 | 0.007 | 0.05 |
| Histology | ||||||
| Adenocarcinoma | Reference | Reference | ||||
| Squamous cell | 1.02 | 0.76 | 0.94 | 0.98 | 0.77 | 0.88 |
| Other | 0.99 | 0.88 | 0.94 | 1.00 | 0.99 | 0.99 |
| Diagnostic PET done | ||||||
| No | Reference | Reference | ||||
| Yes | 0.90 | 0.08 | 0.30 | 0.85 | 0.02 | 0.11 |
| Brain CT/MR done | ||||||
| No | Reference | Reference | ||||
| Yes | 1.09 | 0.15 | 0.38 | 1.05 | 0.45 | 0.76 |
| Invasive mediastinal staging performed | ||||||
| No | Reference | Reference | ||||
| Yes | 0.84 | 0.01 | 0.08 | 0.85 | 0.03 | 0.12 |
| Radiation type | ||||||
| IMRT | Reference | Reference | ||||
| 3D-CRT | 0.93 | 0.42 | 0.91 | 0.91 | 0.33 | 0.61 |
| Radiation facility type | ||||||
| Freestanding center | Reference | Reference | ||||
| Hospital-based center | 1.06 | 0.44 | 0.64 | 1.09 | 0.29 | 0.60 |
| Hospital-based NCI center | 0.94 | 0.36 | 0.59 | 0.99 | 0.91 | 0.94 |
| Area radiation oncologist density | ||||||
| 1st quartile | Reference | Reference | ||||
| 2nd quartile | 1.00 | 0.97 | 0.97 | 0.91 | 0.34 | 0.61 |
| 3rd quartile | 0.90 | 0.26 | 0.48 | 0.86 | 0.12 | 0.35 |
| 4th quartile | 0.88 | 0.16 | 0.38 | 0.81 | 0.03 | 0.12 |
Benjamini-Hochberg adjusted p values.
Abbreviations: HR = hazard ratio; 3D-CRT = 3-dimensional conformal radiation therapy; IMRT = intensity-modulated radiation therapy; ET = positron emission tomography; NCI = National Cancer Institute.
In an IPTW analysis, concurrent-consolidation remained associated with improved OS (HR 0.87, p = 0.01) and a trend for improved CSS (HR 0.89, p = 0.06) compared to concurrent-alone. A subgroup analysis was performed for patients receiving 1) carboplatin-paclitaxel/docetaxel, and 2) cisplatin-etoposide. When a carboplatin-based regimen was used, concurrent-consolidation was significantly associated with improved OS and CSS (Table 3). When cisplatin-etoposide was used, concurrent-consolidation was not associated with a significant difference in OS or CSS. However, there was only a 34% power to detect the same OS benefit seen with the carboplatin-taxane cohort. Patients receiving cisplatin-etoposide were much more likely than those receiving carboplatin-paclitaxel/docetaxel to switch chemotherapy regimens for consolidation (78% vs 11%, p < 0.0001). A sensitivity analysis including only patients who had a diagnostic PET scan demonstrated similar findings with the subgroups (Supplemental Table 5).
Table 3.
Subgroup analysis comparing concurrent-consolidation to concurrent-alone for patients treated with a carboplatin- or cisplatin-based chemotherapy regimen during the concurrent phase of chemoradiation. Hazard ratios for overall survival (OS) and cancer-specific survival (CSS) were determined from inverse probability of treatment weighting analysis, with a hazard ratio <1 representing a benefit with concurrent-consolidation. Propensity scores were used to represent probability of treatment.
| OS | CSS | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Carboplatin-paclitaxel/docetaxel | 0.83 | (0.73–0.95) | 0.006 | 0.85 | (0.74–0.98) | 0.02 |
| Cisplatin-etoposide | 0.91 | (0.60–1.40) | 0.67 | 0.89 | (0.57–1.41) | 0.63 |
4. Discussion
For stage III NSCLC, chemoradiation is the standard treatment for the majority of patients with multi-station or bulky adenopathy. However, no standard chemoradiation regimen or sequence strategy has emerged despite decades of research. We analyzed patients diagnosed 2002–2009 using SEER-Medicare, allowing us to determine the variations in chemoradiation regimens and sequences and perform comparative effectiveness analyses. The most commonly utilized chemotherapy regimens consisted of platinum-based doublet therapies, of which we found carboplatin-paclitaxel to be the most commonly employed, and the most common chemoradiation sequences were concurrent-alone and concurrent-consolidation. Focusing on concurrent-alone and concurrent-consolidation demonstrated there was no significant variation in outcomes with regards to the choice of chemotherapy regimen. As for chemoradiation sequence, we found that concurrent-consolidation treatment was associated with improved OS and a trend for improved CSS compared to concurrent-alone. A significant OS and CSS advantage with concurrent-consolidation was demonstrated for patients treated with carboplatin-based regimens but not cisplatin-etoposide.
With regards to platinum-based chemotherapy in combination with radiation, its use is supported by a large meta-analysis from 52 trials showing a benefit with cisplatin [26]. However, few randomized trials have directly compared chemotherapy regimens [5], and most have not shown a significant benefit of one platinum-based combination over another, including the CALGB 9431 and PROCLAIM studies [27, 28].
A retrospective study of the Veterans Health Administrative Data showed that compared to carboplatin-paclitaxel, there was no advantage with cisplatin-etoposide, although there was increased toxicity [29]. This was also shown in a prior SEER-Medicare study limited to patients receiving concurrent-alone or sequential chemoradiation [30]. Similarly, we did not find any significant outcome difference between the most commonly used platinum-doublet agents compared to carboplatin-paclitaxel when concurrent-alone or concurrent-consolidation are used, suggesting that choice of doublet in this setting does not have a major impact on patient outcome.
As for the importance of consolidation therapy, prior studies have found conflicting results. A single-arm study of concurrent-consolidation using cisplatin-etoposide during RT and docetaxel consolidation resulted in a promising median survival of 26 months (compared to 15 months of a historical comparison of concurrent-alone) [31]. However, a subsequent randomized trial by Hanna was prematurely terminated for futility [7]. Additionally, there was no benefit to consolidation chemotherapy found in the GILT study using cisplatin-vinorelbine or a South Korean trial using cisplatin-docetaxel [8, 9]. Finally, this lack of benefit of consolidation therapy was shown in a meta-analysis of 41 trials by Tsujino [10]. On the contrary, our study demonstrated improved outcomes with concurrent-consolidation.
Notably, our results were dominated by carboplatin-containing regimens, as 83% of patients received carboplatin-paclitaxel/docetaxel. On subgroup analysis, we found a benefit of concurrent-consolidation for patients treated with carboplatin-paclitaxel/docetaxel, but not with cisplatin-etoposide. Although this study was not adequately powered to detect a similar benefit to concurrent-consolidation in the cisplatin-etoposide cohort, finding no significant difference to concurrent-alone is consistent with the cisplatin-based trials reported by Hanna, Flentje, and Ahn. As for the meta-analysis by Tsujino, all 3 of the randomized trials employed cisplatin, and there was not a separate analysis limited to the carboplatin trials. The benefit we found with concurrent-consolidation using carboplatin may be explained by the fact that carboplatin is typically employed at lower doses during radiation due to toxicity concerns and only given at higher “systemic” doses during consolidation [29, 32, 33]. In contrast, cisplatin is used at “systemic” doses during radiotherapy [7–9, 27–29, 31, 32, 34, 35]. Thus, consolidation chemotherapy in patients receiving carboplatin regimens is likely needed to achieve similar sterilization of micrometastatic disease as can be attained when cisplatin-containing regimens are used with concurrent-alone.
In addition to the strengths compared to prior studies outlined above, our study also had several limitations, including that it is retrospective and relies on Medicare claims and SEER reporting. It is not possible to determine the selection criteria physicians used for a particular chemoradiation regimen or sequence. Thus there is a potential concern for selection bias, which could affect outcomes. To minimize this bias, we controlled for a modified Charlson comorbidity score as well as a proxy for performance status [19]. We are also unable to determine exact radiation dosimetric, targeting, and motion management techniques. However, to mitigate radiotherapy variation, treatment was limited to IMRT or 3D-CRT and models were adjusted for treatment year. Furthermore, we included only patients with 30–40 daily radiation treatment claims, as prior studies have shown that treatment with at least ~60 Gy results in improved survival [36, 37].
5. Conclusions
In summary, using SEER-Medicare we found that for patients with locally advanced NSCLC undergoing definitive chemoradiation survival outcomes are similar for carboplatin- or cisplatin-containing regimens, as long as consolidation chemotherapy is given for patients receiving carboplatin. Our data therefore support a personalized approach to use of consolidation chemotherapy based on the choice of drugs given during radiation.
Supplementary Material
Highlights.
Chemoradiation is used to treat many patients with stage III NSCLC.
Carboplatin- and cisplatin-containing regimens resulted in similar outcomes.
Consolidation chemotherapy after concurrent chemoradiation improved survival.
Consolidation chemotherapy only benefited patients treated with carboplatin.
When using carboplatin, consolidation chemotherapy should be considered.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Funding
This work was supported by grants from Varian and the Stanford Society of Physician Scholars. The supporting institutions had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- IMRT
intensity-modulated radiation therapy
- 3D-CRT
3-dimensional conformal radiation therapy
- PET
positron emission tomography
- NCI
National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
BWL and MD have received research support from Varian Medical Systems. BWL has received research support from RaySearch Laboratories, and speaking honoraria from Varian Medical Systems. HAW has received research support from Novartis and Eli Lilly. JPH and MIP have no disclosures.
References
- 1.Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S–340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.O’Rourke N, Roque IFM, Farre Bernado N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010:CD002140. doi: 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Mauguen A, Le Pechoux C, Saunders MI, Schild SE, Turrisi AT, Baumann M, Sause WT, Ball D, Belani CP, Bonner JA, Zajusz A, Dahlberg SE, Nankivell M, Mandrekar SJ, Paulus R, Behrendt K, Koch R, Bishop JF, Dische S, Arriagada R, De Ruysscher D, Pignon JP. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price A. Emerging developments of chemoradiotherapy in stage III NSCLC. Nat Rev Clin Oncol. 2012;9:591–598. doi: 10.1038/nrclinonc.2012.135. [DOI] [PubMed] [Google Scholar]
- 6.Bayman N, Blackhall F, McCloskey P, Taylor P, Faivre-Finn C. How can we optimise concurrent chemoradiotherapy for inoperable stage III non-small cell lung cancer? Lung Cancer. 2014;83:117–125. doi: 10.1016/j.lungcan.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Hanna N, Neubauer M, Yiannoutsos C, McGarry R, Arseneau J, Ansari R, Reynolds C, Govindan R, Melnyk A, Fisher W, Richards D, Bruetman D, Anderson T, Chowhan N, Nattam S, Mantravadi P, Johnson C, Breen T, White A, Einhorn L, Hoosier Oncology G, Oncology US. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 8.Flentje M, Huber RM, Engel-Riedel W, Andreas S, Kollmeier J, Staar S, Dickgreber N, Vaissiere N, De Almeida C, Edlich B, Fietkau R. GILT–A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther Onkol. 2016;192:216–222. doi: 10.1007/s00066-016-0941-8. [DOI] [PubMed] [Google Scholar]
- 9.Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, Chen M, Kim DW, Kim HK, Min YJ, Kang JH, Choi JH, Kim SW, Zhu G, Wu YL, Kim SR, Lee KH, Song HS, Choi YL, Sun JM, Jung SH, Ahn MJ, Park K. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
- 10.Tsujino K, Kurata T, Yamamoto S, Kawaguchi T, Kubo A, Isa S, Hasegawa Y, Ou SH, Takada M, Ando M. Is consolidation chemotherapy after concurrent chemo-radiotherapy beneficial for patients with locally advanced non-small-cell lung cancer? A pooled analysis of the literature. J Thorac Oncol. 2013;8:1181–1189. doi: 10.1097/JTO.0b013e3182988348. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Surveillance, Epidemiology, and End Results. In. [Google Scholar]
- 12.National Cancer Institute. SEER-Medicare Linked Database. In. [Google Scholar]
- 13.Beahrs OH, Henson DE, Hutter RVP, Kennedy BJ. Manual for staging of cancer. 3rd. Philadelphia: Lippincott; 1988. [Google Scholar]
- 14.Harris JP, Murphy JD, Hanlon AL, Le QT, Loo BW, Jr, Diehn M. A population-based comparative effectiveness study of radiation therapy techniques in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:872–884. doi: 10.1016/j.ijrobp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services, Health Resources and Services Administration. Bureau of Health Workforce Area Health Resources Files (AHRF) Rockville, MD: [Google Scholar]
- 16.US Department of Health and Human Services. Area Health Resources Files. In. [Google Scholar]
- 17.Zhu J, Sharma DB, Gray SW, Chen AB, Weeks JC, Schrag D. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA. 2012;307:1593–1601. doi: 10.1001/jama.2012.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Sharma DB, Chen AB, Johnson BE, Weeks JC, Schrag D. Comparative effectiveness of three platinum-doublet chemotherapy regimens in elderly patients with advanced non-small cell lung cancer. Cancer. 2013;119:2048–2060. doi: 10.1002/cncr.28022. [DOI] [PubMed] [Google Scholar]
- 19.Davidoff AJ, Gardner JF, Seal B, Edelman MJ. Population-based estimates of survival benefit associated with combined modality therapy in elderly patients with locally advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:934–941. doi: 10.1097/JTO.0b013e31820eed00. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 22.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 23.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21:552–560. doi: 10.1016/s0197-2456(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 26.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 27.Vokes EE, Herndon JE, 2nd, Crawford J, Leopold KA, Perry MC, Miller AA, Green MR. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 28.Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, Martinez Aguillo M, Aerts J, Govindan R, Rubio-Viqueira B, Lewanski C, Gandara D, Choy H, Mok T, Hossain A, Iscoe N, Treat J, Koustenis A, San Antonio B, Chouaki N, Vokes E. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 29.Santana-Davila R, Devisetty K, Szabo A, Sparapani R, Arce-Lara C, Gore EM, Moran A, Williams CD, Kelley MJ, Whittle J. Cisplatin and Etoposide Versus Carboplatin and Paclitaxel With Concurrent Radiotherapy for Stage III Non-Small-Cell Lung Cancer: An Analysis of Veterans Health Administration Data. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.56.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezer N, Smith CB, Galsky MD, Mhango G, Gu F, Gomez J, Strauss GM, Wisnivesky J. Cisplatin vs. carboplatin-based chemoradiotherapy in patients >65 years of age with stage III non-small cell lung cancer. Radiother Oncol. 2014;112:272–278. doi: 10.1016/j.radonc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN, Jr, Burris H, Gumerlock P, Kuebler JP, Bearden JD, 3rd, Crowley J, Livingston R, Southwest Oncology G Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–2010. doi: 10.1200/JCO.2003.04.197. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Wu S, Ou G, Bi N, Li W, Ren H, Cao J, Liang J, Li J, Zhou Z, Lv J, Zhang X. Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2012;77:89–96. doi: 10.1016/j.lungcan.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, Curran WJ., Jr Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 34.Curran WJ, Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E, Machtay M, Sause W, Cox JD. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 36.Perez CA, Stanley K, Rubin P, Kramer S, Brady LW, Marks JE, Perez-Tamayo R, Brown GS, Concannon JP, Rotman M. Patterns of tumor recurrence after definitive irradiation for inoperable non-oat cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1980;6:987–994. doi: 10.1016/0360-3016(80)90106-6. [DOI] [PubMed] [Google Scholar]
- 37.Koshy M, Malik R, Sher DJ, Spiotto M, Mahmood U, Aydogan B, Weichselbaum RR. The effect of radiotherapy dose on survival in stage III non-small-cell lung cancer patients undergoing definitive chemoradiotherapy. Clin Lung Cancer. 2014;15:365–371. doi: 10.1016/j.cllc.2014.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


