Abstract
Patterns of myeloid growth factor (GF) usage and febrile neutropenia (FN) were examined in patients >60 years of age with diffuse large B-cell non-Hodgkin lymphoma (DLBCL) enrolled on CALGB 9793/ECOG-SWOG 4494, receiving initial therapy with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or rituximab+CHOP (R-CHOP). Myeloid GFs were administered to 256/520 (49%) patients. Indications for use were: prevent dose reduction/dose delay (81%, 207/256); treat FN or nonfebrile neutropenia (19%, 48/256). One or more FN episodes occurred in 41% (212/520) of patients, with FN most often in cycle 1 (38% of episodes). In multivariate analysis, risk factors for FN included age >65 years (OR=2.6, 95% CI:[1.4,4.9]) and anemia (hemoglobin <12 g/dl) (OR=2.2, 95% CI:[1.4,3.5]. Myeloid GF use was common in this older DLBCL population receiving CHOP-based therapy, as was FN, especially during cycle one. Risk factors predictive for FN should be used prospectively to identify patients for whom myeloid GFs are best utilized.
Introduction
The use of anthracycline-based combination chemotherapy to treat diffuse aggressive non-Hodgkin lymphoma (NHL) in older patients is complicated by the frequent occurrence of neutropenia, febrile neutropenia (FN), and serious infections.1–3 In addition, resulting dose reductions or delays could potentially impact treatment outcome. The frequency of and specific indications for myeloid growth factor (GF) usage had not been well-studied systematically in the era following adoption of the American Society of Clinical Oncology (ASCO) guidelines.4,5 In an effort to balance optimal outcome with economic considerations, it would be valuable to identify patient subsets in which these agents may be best utilized. A US Intergroup Trial of patients ≥60 years of age with diffuse aggressive NHL (predominantly diffuse large B-cell non-Hodgkin lymphoma [DLBCL]) provides the opportunity for an analysis of GF utilization and FN, both of which are common in this population. Risk factors predictive of FN may also be identified, and used to identify high risk patients for whom GF support is best utilized.
Patients and Methods
The United States Intergroup Trial (CALGB 9793/ECOG-SWOG 4494) for therapy of previously untreated patients ≥60 years of age with diffuse aggressive B-cell non-Hodgkin’s lymphoma was open for accrual from December 1997 through July 2001. Additional eligibility criteria included: demonstration of CD20 expression, Ann Arbor stage I–IV measurable disease, ECOG performance status (PS) 0–3, left ventricular ejection fraction ≥45%, adequate renal and hepatic function, absolute neutrophil count (ANC) >1500/mm3, and platelet count >100,000/mm3. After signed informed consent, patients were randomized to therapy with either: cyclophosphamide, 750 mg/m2 intravenously (IV), doxorubicin 50 mg/m2 IV, vincristine 1.4 mg/m2 (maximum, 2.0 mg) IV, all Day 1, and prednisone, 100 mg/m2/day orally Days 1–5 (CHOP), or CHOP plus rituximab, 375mg/m2 IV, administered seven and three days prior to cycle 1, and two days prior to cycles 3, 5 and 7 (R-CHOP). Patients were restaged after four, six, and eight cycles of therapy. Patients responding to induction therapy (CR, PR) underwent a second randomization to observation or maintenance rituximab, 375mg/m2 IV weekly X4, repeated every six months for two years. The primary outcomes of this study have been previously reported.6,7
Protocol guidelines were provided for therapy-related myelosuppression. If Day 1 ANC was <1500 cells/mm3, treatment was delayed a week. If FN occurred in the prior treatment cycle, cyclophosphamide and doxorubicin doses were reduced by 50% in the next cycle. These doses could be increased by 25% if the subsequent cycle was well tolerated, with no grade 3/4 hematologic toxicities. Myeloid GFs (granulocyte colony stimulating factor [G-CSF], granulocyte-macrophage CSF [GM-CSF]) were not allowed with the first treatment cycle. ASCO GF guidelines were to be followed thereafter, including GF use to maintain dose intensity in event of neutropenic fever or dose reduction/delay.5 Prophylactic antimicrobial therapy usage was at the discretion of the treating physician.
Case report forms of all enrolled study patients were reviewed, with data compiled in a collection tool and added to the study database. Data from the maintenance treatment period were not assessed for this analysis. A separate data collection form for each cycle of induction chemotherapy included body surface area (BSA), delivered doses of each agent, and date of Day 1. The timeliness of cycle administration (i.e., every 21 days) was recorded, or if treatment was delayed, the number of days. Any use of myeloid GF (G-CSF, GM-CSF) was reported, and if so, which cycle days and duration of administration. Reasons for usage were: 1) treatment for FN, 2) treatment for non-febrile neutropenia (NFN) (nadir ANC ≤1000/mm3 with no fever), 3) hospitalization for FN in a prior cycle, or 4) to prevent dose reduction/dose delay. For purpose of the primary study analysis, myeloid GF usage was defined as use of either G-CSF or GM-CSF to prevent dose reduction/delay, or as prophylaxis for a prior FN hospitalization, given within Days 1–6 of the treatment cycle.
Data were also collected on the occurrence of neutropenia (defined as <1000/mm3 neutrophils plus bands), and characterized by the Common Toxicity Criteria grading scale. It was assessed by cycle if the patient had FN (defined as fever of ≥38.30 C, with ANC ≤1000/mm3) or NFN. Also collected was the nadir cycle day, number of days ANC<1000/mm3, hospitalization and length of stay for FN, and the use, if any, of oral prophylactic antimicrobial agents.
Objectives
The study was designed to assess myeloid GF usage and occurrence of FN in an older patient DLBCL population receiving initial CHOP or R-CHOP therapy. Frequency of and indications for GF usage, and incidence and risk factors for FN, were retrospectively determined.
Statistical Considerations
Descriptive statistics were provided for baseline characteristics. Two-sided Fisher’s exact tests were used to assess associations between each risk factor and GF usage, as well as FN incidence.8 To evaluate the correlation of GF usage with patient characteristics, the primary endpoint for analysis is the proportion of patients whose indication for GF use was either to prevent dose reduction/dose delay, or as prophylaxis in patients with a prior FN hospitalization, and was given within cycle Days 1–6. Estimated proportions are reported as well as 95% confidence intervals (CI). The time to first FN was also evaluated by baseline characteristics, and differences between risk groups were tested using the log-rank method.9 The logistic regression model was used to examine associations of risk factors with GF usage or FN incidence,10 and the Cox proportional hazards model was used to examine associations of risk factors with time to first FN.11 Adjusted odds ratio (OR), hazard ratio estimates and their corresponding 95% confidence intervals are provided. Hazard rate (HR) of first FN incidence was also plotted using an in-house S-Plus function hazex.
Results
A total of 632 patients were enrolled on this trial, of which 267 R-CHOP and 279 CHOP patients were eligible6. Among these 546 patients, GF usage data were available for 528 patients, eight of whom received first cycle GF and were excluded from analysis. Baseline demographics of the 520 eligible patients are detailed (Table 1). The majority of patients were ≥65 years of age, with a favorable PS (0/1). Approximately 60% of patients had a high-intermediate or high International Prognostic Index (IPI) risk score. Oral prophylactic antibiotics were utilized in 7% of cycles.
Table 1.
Patient characteristics
| Patient Characteristic | Induction Treatment | All Patients | |
|---|---|---|---|
|
| |||
| R-CHOP (n=250) | CHOP (n=270) | ||
|
| |||
| N (%) | N (%) | N (%) | |
|
| |||
| Age (yrs) | 61(24) | 69(26) | 130(25) |
| 60–64 | |||
| 65–69 | 71(28) | 68(25) | 139(27) |
| 70–74 | 58(23) | 67(25) | 125(24) |
| 75–79 | 40(16) | 47(17) | 87(17) |
| ≥ 80 | 20(8) | 19(7) | 39(8) |
| Male gender | 130(52) | 132(49) | 262(50) |
| ECOG performance status 0–1 | 216(86) | 231(86) | 447(86) |
| Hemoglobin <12 g/dl | 96(38) | 115(43) | 211(41) |
| Elevated LDH | 146(58) | 156(58) | 302(58) |
| Marrow involvement | 46(18) | 52(19) | 98(19) |
| Bulky disease (≥10 cm) | 59(24) | 53(20) | 112(22) |
| Ann Arbor stage III–IV disease | 184(74) | 197(73) | 381(73) |
| International Prognostic Index (IPI) group | |||
| Low/Low Intermediate Risk | 105(42) | 109(41) | 214(41) |
| High Intermediate/High Risk | 145(58) | 160(59) | 305(59) |
| Age-adjusted IPI group | |||
| Low/Low Intermediate Risk | 124(50) | 133(49) | 257(49) |
| High Intermediate/High Risk | 126(50) | 136(50) | 262(50) |
LDH, IPI score, and age-adjusted IPI score are unknown for 1 CHOP patient
Myeloid GF Usage
Of the 520 evaluable patients, 256 (49%) received a myeloid GF during therapy. GF used was G-CSF (93%), GM-CSF (5%), or both GF (2%) over various cycles. The median number of cycles for which GF was used was three (range, 1–7). Median duration of GF usage in a cycle was nine days. Overall, GF were used in 520/3216 (16%) cycles of administered chemotherapy. GFs were used to prevent dose reduction/dose delay or because of a prior FN hospitalization in 82%, or for the treatment of either FN or NFN in 18%. The primary study analysis definition of GF usage (GF administration within Days 1–6 of the treatment cycle either to prevent dose reduction/dose delay or as prophylaxis for a prior FN hospitalization) was met in 173/520 patients (33%, 95% C.I. [29,38%]). Among the other 83 patients, the indication for GF was treatment of FN/NFN in 48 (9%), prevent dose reduction/dose delay or as prophylaxis but begun after cycle day 6 in 34 (7%), and unknown for 1 (<1%). Significantly more patients used GF with later cycles of therapy (Figure 1). In cycle 6, 97/431 patients (23%) received GF compared with only 59/506 patients (13%) in cycle 2 (p <0.001). In summary, GF were utilized to prevent chemotherapy dose reduction/dose delay in approximately 60% of patients, and were used for secondary prophylaxis in cycle(s) following FN hospitalization in one third of patients.
Figure 1.
Myeloid growth factor usage by cycle of therapy.
GF use was evaluated by patient characteristics and induction therapy. Age ≥65 years and baseline hemoglobin <12 g/dl were identified as significant risk factors in univariate analysis (p=0.003 and p<0.0001, respectively) (Table 2) and multivariate logistic regression analysis after adjusting for induction therapy, gender, bulky disease, and IPI score (p=0.003 and p<0.0001, respectively) (Table 3). The estimated OR for GF use is 2.0 (95% CI [1.3,3.3]) for patients ≥65 years of age compared to those <65 years. The estimated OR for GF use is 2.2 (95% CI [1.5,3.3]) for those patients with baseline hemoglobin <12 g/dl compared to those with hemoglobin ≥ 12 g/dl. GF use did not differ by induction regimen (p>0.8).
Table 2.
| Factor | Level | Number of Patients | Growth Factor | Febrile Neutropenia | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Use at any cycle N (%) |
p-value | Observed in first cycle of therapy N (%) |
p-value | |||
|
| ||||||
| Induction treatment | R-CHOP | 250 | 83(33) | 0.99 | 40(16) | 0.08 |
| CHOP | 270 | 90(33) | 60(22) | |||
|
| ||||||
| Age (yrs) | <65 | 130 | 29(22) | 0.003 | 13(10) | 0.002 |
| ≥65 | 390 | 144(37) | 87(22) | |||
|
| ||||||
| Gender | Male | 262 | 79(30) | 0.14 | 42(16) | 0.07 |
| Female | 258 | 94(36) | 58(23) | |||
|
| ||||||
| ECOG performance status | 0–1 | 447 | 151(34) | 0.6 | 78(17) | 0.02 |
| 2–3 | 73 | 22(30) | 22(30) | |||
|
| ||||||
| Hemoglobin (g/dl) | <12 | 211 | 92(44) | <0.0001 | 58(27) | 0.0001 |
| ≥12 | 309 | 81(26) | 42(14) | |||
|
| ||||||
| Lactic dehydrogenase | Normal | 217 | 64(29) | 0.13 | 31(14) | 0.02 |
| Elevated | 302 | 109(36) | 69(23) | |||
|
| ||||||
| Marrow involvement | No | 422 | 137(32) | 0.48 | 78(18) | 0.39 |
| Yes | 98 | 36(37) | 22(22) | |||
|
| ||||||
| Bulky disease (≥10 cm) | No | 408 | 140(34) | 0.37 | 78(19) | 0.89 |
| Yes | 112 | 33 (29) | 22(20) | |||
|
| ||||||
| Ann Arbor stage | I–II | 139 | 44(32) | 0.67 | 29(21) | 0.62 |
| III–IV | 381 | 129(34) | 71(19) | |||
|
| ||||||
| International Prognostic*** | Low/LI | 305 | 66(31) | 0.34 | 31(14) | 0.02 |
| Index (IPI) group | HI/High | 214 | 107(35) | 69(23) | ||
|
| ||||||
| Age-adjusted*** | Low/LI | 257 | 78(30) | 0.16 | 42(16) | 0.10 |
| IPI group | HI/High | 262 | 95(36) | 58(22) | ||
GF use is defined as yes if GF was used either to prevent dose reduction/dose delay, or as prophylaxis in patients with a prior FN hospitalization, and was given within Days 1–6 of the treatment cycle
Fisher’s exact p-value is reported
LI=Low intermediate
Table 3.
Multivariate logistic regression model for growth factor (GF) use * at any cycle and first cycle febrile neutropenia (FN)**
| Factor | Reference Group | GF use at any cycle | First cycle FN | ||
|---|---|---|---|---|---|
|
| |||||
| Estimated odds ratio, 95% CI | p-value | Estimated odds ratio, 95% CI | p-value | ||
|
| |||||
| Induction treatment | R-CHOP | 1.0 (0.7, 1.4) | 0.9 | 1.5 (0.96, 2.4) | 0.08 |
| Gender | Male | 1.1 (0.7, 1.5) | 0.7 | 1.2 (0.8, 1.9) | 0.44 |
| Age (yrs) | <65 | 2.0 (1.3, 3.3) | 0.003 | 2.6 (1.4, 4.9) | 0.004 |
| Hemoglobin (g/dl) | ≥12 | 2.2 (1.5, 3.3) | <0.0001 | 2.2 (1.4, 3.5) | 0.0009 |
| Bulky disease | No | 0.8 (0.5, 1.3) | 0.3 | 1.0 (0.6, 1.8) | 0.90 |
| IPI score | Low/LI** | 1.0 (0.7, 1.5) | 0.9 | 1.4 (0.8, 2.2) | 0.21 |
GF use is defined as yes if GF was used either to prevent dose reduction/dose delay, or as prophylaxis in patients with a prior FN hospitalization, and was given within Days 1–6 of the treatment cycle
Wald chi-square p-value is reported
LI=Low intermediate
Febrile Neutropenia
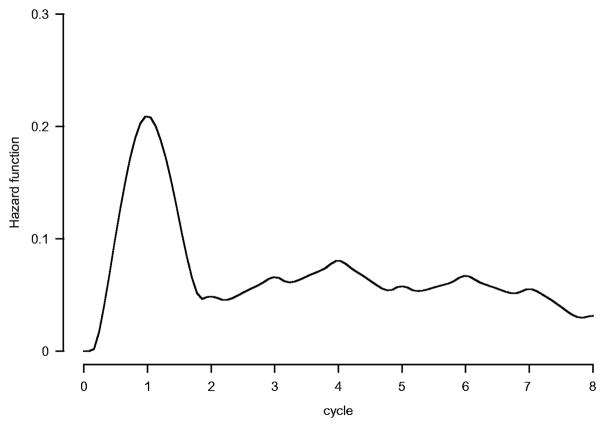
Among the 520 patients, 212 (41%) had ≥1 episode of FN with a 95% CI (37,45%); 141 (27%) had ≥1 FN hospitalization, with a median 5 (range, 1–121) day length of stay. Overall, FN occurred in 261/3216 (8%) delivered cycles of therapy. Median time to FN was 11 days; 38% of all FN episodes occurred in cycle one, when GF usage was not allowed per protocol. The hazard for the first FN occurrence was highest during cycle one (p<0.0001) (Figure 2).
Figure 2.
Hazard of first febrile neutropenia.
Patient study entry characteristics were analyzed to identify predictive factors for FN during cycle one, to minimize the impact of subsequent dose reduction/dose delay and GF use. A univariate analysis of cycle one FN suggested that patients of age ≥65 years (p=0.002), baseline hemoglobin <12 g/dl (p=0.0001), PS 2–3 (p=0.02), and elevated lactic dehydrogenase (LDH) (p=0.02) were risk factors, with a trend for type of induction therapy (16% vs. 22%, p=0.08) and gender (16% vs. 23%, p=0.07) (Table 2). After adjusting for other factors in a multivariate logistic regression model, an increased risk for first cycle FN was observed for patients ≥65 years of age (OR= 2.6, 95% CI [1.4,4.9], p=0.004) and those with baseline hemoglobin <12 g/dl (OR= 2.2, 95% CI [1.4,3.5], p=0.0009) (Table 3). A marginal difference was observed by induction therapy, indicating that CHOP-treated patients may have a higher risk of cycle one FN (OR= 1.5, 95% CI [0.96,2.4], p=0.08). Although significant in univariate analysis, gender and PS were not selected in the logistic model because of their high correlation with IPI score.
Data regarding NFN, as well as duration of any therapy-related neutropenia, are reported (Table 4). In general, any neutropenia was of brief duration. There was no trend in duration of either NFN or FN by cycle of therapy. Older age (≥65 years) was not associated with first cycle NFN incidence (p>0.9).
Table 4.
Incidence and duration of febrile neutropenia (FN) and non-febrile neutropenia (NFN)
| Cycle: | Number of patients | Grade 3 or higher Non-Febrile Neutropenia (NFN) | Grade 3 or higher Febrile Neutropenia (FN) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Percent of patients | Median (range) duration (days) | Median (range) time to occurrence (days) | Percent of patients | Median (range) duration (days) | Median (range) time to occurrence (days) | ||
|
| |||||||
| 1 | 518 | 39 | 2(1–10) | 14(8–22) | 18 | 4(1–8) | 12(7–18) |
| 2 | 505 | 32 | 1(1–9) | 14(2–23) | 4 | 3(1–5) | 11(8–16) |
| 3 | 491 | 28 | 1(1–16) | 13(2–22) | 5 | 4(1–6) | 11(7–22) |
| 4 | 472 | 32 | 1(1–15) | 14(1–36) | 7 | 3(1–6) | 12(7–16) |
| 5 | 447 | 29 | 1(1–10) | 13(1–24) | 6 | 3(1–6) | 11(8–17) |
| 6 | 427 | 31 | 2(1–12) | 14(7–32) | 6 | 3(1–9) | 12(8–16) |
| 7 | 181 | 33 | 1(1–11) | 13(1–22) | 4 | 5(1–8) | 13(9–15) |
| 8 | 167 | 29 | 1(1–8) | 12(1–18) | 4 | 2(1–6) | 9(4–15) |
Discussion
The U.S. Intergroup trial facilitated a large prospective collection of GF usage data in NHL patients ≥60 years of age receiving CHOP-based therapy, such that patterns of GF utilization and FN occurrence could be examined. Overall, GF were used in 16% of treatment cycles, being administered to approximately half the study patients. Prophylactic myeloid GF support has been shown to reduce grade 3/4 neutropenia and infectious complications as FN in older DLBCL patients receiving anthracycline-based regimens.2,3,12–18 In our trial, GF were used in most patients to deliver full dose therapy on time, with or without a prior FN episode.
Relative delivered dose intensity (RDI) has been reported to have an impact on survival with R-CHOP therapy in multiple series.19–22 In several retrospective reports, DLBCL patients who received ≤90% average RDI had decreased OS, with progressively increasing RDI being associated with increased OS.19,20 Others have reported that a lower RDI threshold (i.e., RDI <70) negatively impacts PFS and OS.23 Maintaining RDI is more challenging with advancing age, due to excessive hematologic toxicities and the occurrence of FN, resulting in dose reductions.21,22,24–26 Having PS >1 is also associated with decreased RDI, with the use of primary myeloid growth factor prophylaxis having a protective effect.27
In the U.S. Intergroup study, we found that age >65 years and hemoglobin <12 g/dl were significantly associated with GF use. Once initiated, GF support continued for a median three cycles of therapy, with median duration of nine days within a cycle. Data on GF usage from the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trial of CHOP or R-CHOP therapy in elderly DLBCL patients are more limited.28,29 For patients with grade 4 neutropenia or FN, G-CSF was administered during all subsequent treatment cycles. If grade 4 neutropenia occurred despite GF support, cyclophosphamide and doxorubicin doses were decreased by 50% in subsequent cycles. Similar to our findings, GF support was more common with later cycles of therapy, with 37% of patients requiring GF support for cycle four, and 43% for cycle eight.
A second objective of our study was to determine the incidence of FN and ascertain prognostic factors. FN occurred in 41% of our patients, with 38% of all FN episodes occurring during cycle one, and 47% during cycles one and two. This is consistent with prior reports, in which 58% of FN events occurred in cycle one and 74% in the first two cycles of comparable therapy in a primarily community-based population.30 Variable rates of FN occurrence have been reported since our study.19,21,27,31–37 In the German trials including younger and older patients, FN rates were up to 50%.34,35 Choi et al reported a 42% FN incidence with R-CHOP therapy, with 48% of the episodes occurring in cycle 1, similar to our findings.37 However, FN rates of 22–27% were reported in other series of CHOP/R-CHOP-treated patients, many of whom were over age 60.19,21,27,33 Interestingly, in a recent report of adults ≥18 years (51% ≥65 years) receiving R-CHOP, FN occurrence rate was 19%, with 9% FN occurrence in cycle 1.31 However, in both the low (<20%) and high (≥20%) risk FN subgroups, the use of primary myeloid growth factor prophylaxis had little impact on FN occurrence. Lastly in a large series of patients receiving R-CHOP21 and R-CHOP14 therapies, FN rates were 19% and 20%, respectably, with corresponding cycle 1 FN rates of 47% and 30%.20 Primary myeloid growth factor prophylaxis was used for 36% of R-CHOP21-treated patients, and 84% of those receiving R-CHOP14.
We found that age >65 years and hemoglobin <12 g/dl were independent risk factors of FN during the first cycle. Age >65 years had been previously reported as a risk factor for FN in multiple series, as well as co-morbidities, marrow involvement, baseline neutropenia, hypoalbuminemia, lymphopenia, and planned average relative dose intensity >80%.30,38–41 In more recent studies, it was (comment in age grp…) found that female, gender, presence of co-morbidities, and marrow involvement were predictive for FN occurrence in multivariate analyses, but age ≥65 yrs and albumin ≤3.5 g/dl were significant only in univariate analysis.37 In another series, older age, poor PS, baseline hemoglobin <12 g/dl, and lack of myeloid growth factor prophylaxis were associated with FN occurrence in any cycle of therapy.31 Co-morbidities were not a risk factor, possibly because of correlation with age and PS. Additional risk factors identified in other series include albumin ≤3.5 g/dl, older age, poor PS, advanced stage disease, presence of co-morbidities, low baseline blood counts, and low BSA/BMI.5,42,43 A trend toward a lower risk of first cycle FN with R-CHOP was observed in our study (OR 1.5, 95% CI [0.95, 2.4]). Theoretically, the greater efficacy of R-CHOP in reducing tumor burden may be responsible for this observation. The relationship of FN to baseline anemia in our study is not readily explained. Anemia was not a surrogate marker for marrow involvement in our series, as the presence of marrow involvement at diagnosis did not predict for FN. However, anemia may be related to reduced marrow reserve or chronic disease in older patients.
R-CHOP therapy has been identified as having an intermediate probability of FN occurrence by the NCCN and EORTC guidelines.44,45 In these, as well as the ASCO guidelines, primary myeloid GF prophylaxis has been recommended for older DLBCL patients receiving such therapy.44–47 The use of such primary prophylaxis varies substantially among countries.31 However, despite decreasing the relative risk of severe neutropenia and FN occurrence, primary GF prophylaxis has no impact on parameters as infection-related mortality, quality of life, and response parameters.48,49 Even more importantly in recent reports, primary GF prophylaxis has been found not to be cost-effective compared to secondary prophylaxis in the treatment of older DLBCL patients.50,51 Primary prophylaxis would be considered favorable only if FN hospitalization costs increased 2.5-fold from the present, the cost of GF were substantially less, and/or first cycle FN risk was >47%.
Since the publication of the initial R-CHOP trials, it has been recognized that not all elderly patients will tolerate this regimen. The use of pre-phase vincristine and prednisone therapy has been advocated, with reduction in induction therapy toxicities.52 In a recent retrospective report in DLBCL patients at least 80 yrs of age, delivery of standard dose R-CHOP was felt to be unrealistic, and although rituximab use was associated with decreased mortality, one-yr OS was better when anthracycline dose intensity was <85%, versus ≥85%, perhaps related to baseline PS.53 In contrast, others have reported that therapy without an anthracycline results in shorter DFS as well as less FN.54 Non-anthracycline regimens including etoposide, as R-CEOP and R-CEPP, may be utilized. Series of dose-reduced R-CHOP therapy have been reported, with better tolerability, fewer adverse reactions, and reasonable outcome parameters.55–58 Regardless, identification of subgroups at enhanced risk for FN occurrence remains important for optimal myeloid GF utilization. Minimization of myelosuppression and subsequent infectious complications, especially in older patients, not only reduces morbidity and mortality, but also allows delivery of full dose therapy, which impacts disease outcome.
Acknowledgments
Research support for data retrieval provided in part by a grant from Amgen
References
- 1.Gómez H, Mas L, Casanova L, et al. Elderly patients with aggressive non-Hodgkin’s lymphoma treated with CHOP chemotherapy plus granulocyte-macrophage colony-stimulating factor: Identification of two age subgroups with differing hematologic toxicity. J Clin Oncol. 1998;16:2352–2358. doi: 10.1200/JCO.1998.16.7.2352. [DOI] [PubMed] [Google Scholar]
- 2.Doorduijn JK, van der Holt B, van Imhoff GW, et al. CHOP compared with CHOP plus granulocyte colony-stimulating factor in elderly patients with aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:3041–3050. doi: 10.1200/JCO.2003.01.076. [DOI] [PubMed] [Google Scholar]
- 3.Gómez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: Results of a multivariate analysis. J Clin Oncol. 1998;16:2065–2069. doi: 10.1200/JCO.1998.16.6.2065. [DOI] [PubMed] [Google Scholar]
- 4.Update of recommendations for the use of hematopoietic colony-stimulating factors. Evidence-based clinical practice guidelines. J Clin Oncol. 1996;14:1957–1960. doi: 10.1200/JCO.1996.14.6.1957. [DOI] [PubMed] [Google Scholar]
- 5.Ozer H, Armitage JO, Bennett CL, et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: Evidence-based, clinical practice guidelines. J Clin Oncol. 2000;18:3558–3585. doi: 10.1200/JCO.2000.18.20.3558. [DOI] [PubMed] [Google Scholar]
- 6.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 7.Morrison VA, Weller EA, Habermann TM, et al. Maintenance rituximab (MR) compared to observation (OBS) after R-CHOP or CHOP in older patients (pts) with diffuse large B-cell lymphoma (DLBCL) J Clin Oncol. 2007;225:443s. (Abstract 8011) [Google Scholar]
- 8.Cox DR. Analysis of Binary Data. London: Methuen and Co; 1970. [Google Scholar]
- 9.Peto R, Pike MC, Armitage NE, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox DR, Snell EJ. Analysis of Binary Data. London: Methuen and Co; 1970. [Google Scholar]
- 11.Cox DR. Regression models and life tables. J Royal Stat Soc B. 1972;34:181–220. [Google Scholar]
- 12.Zinzani PL, Pavone E, Storti S, et al. Randomized trial with or without granulocyte colony-stimulating factor as adjunct to induction VNCOP-B treatment of elderly high-grade non-Hodgkin’s lymphoma. Blood. 1997;89:3974–3979. [PubMed] [Google Scholar]
- 13.Björkholm M, Ösby E, Hagberg H, et al. Randomized trial of r-metHu granulocyte colony-stimulating factor (G-CSF) as adjunct to CHOP or CNOP treatment of elderly patients with aggressive non-Hodgkin’s lymphoma. Blood. 1999;94:599a. (Abstract 2655) [Google Scholar]
- 14.Zagonel V, Babare R, Merola MC, et al. Cost-benefit of granulocyte colony-stimulating factor administration in older patients with non-Hodgkin’s lymphoma treated with combination chemotherapy. Ann Oncol. 1994;5(suppl 2):127–132. doi: 10.1093/annonc/5.suppl_2.s127. [DOI] [PubMed] [Google Scholar]
- 15.Bertini M, Freilone R, Vitolo U, et al. P-VEBEC: a new 8-weekly schedule with or without rG-CSF for elderly patients with aggressive non-Hodgkin’s lymphoma (NHL) Ann Oncol. 1994;5:895–900. doi: 10.1093/oxfordjournals.annonc.a058727. [DOI] [PubMed] [Google Scholar]
- 16.Zelenetz AD. Risk models for chemotherapy-induced neutropenia in non-Hodgkin’s lymphoma. Oncology. 2003;17:21–26. [PubMed] [Google Scholar]
- 17.Ösby E, Hagberg H, Kvaløy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood. 2003;101:3840–3848. doi: 10.1182/blood-2002-10-3238. [DOI] [PubMed] [Google Scholar]
- 18.Burton C, Linch D, Hoskin P, et al. A phase II trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin’s lymphoma. Br J Cancer. 2006;94:806–813. doi: 10.1038/sj.bjc.6602975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87:277–283. doi: 10.1007/s00277-007-0399-y. [DOI] [PubMed] [Google Scholar]
- 20.Pettengell R, Schwenkglenks M, Bosly A, et al. Association of reduced relative dose intensity and survival in lymphoma patients reeiving CHOP-21 chemotherapy. Ann Hematol. 2008;87:429–430. doi: 10.1007/s00277-008-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wieringa A, Boslooper K, Hoogendoorn M, et al. Comorbidity is an independent prognostic factor in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP: a population-based cohort study. Br J Haematol. 2014;165:489–496. doi: 10.1111/bjh.12765. [DOI] [PubMed] [Google Scholar]
- 22.Advani RH, Chen H, Habermann TM, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup study (ECOG 4494, CALGB 9793): Consideration of age greater than 70 years in an elderly prognostic index (E-IPI) Br J Haematol. 2010;151:143–151. doi: 10.1111/j.1365-2141.2010.08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirakawa T, Yamaguchi H, Yokose N, Gomi S, Inokuchi K, Dan K. Importance of maintaining the relative dose intensity of CHOP-like regimens combined with rituximab in patients with diffuse large B-cell lymphoma. Ann Hematol. 2010;89:897–904. doi: 10.1007/s00277-010-0956-7. [DOI] [PubMed] [Google Scholar]
- 24.Dixon DO, Neilan B, Jones SE, et al. Effect of age on therapeutic outcome in advanced diffuse histiocytic lymphoma: the Southwest Oncology group experience. J Clin Oncol. 1986;4:295–305. doi: 10.1200/JCO.1986.4.3.295. [DOI] [PubMed] [Google Scholar]
- 25.Lugtenburg P, Silvestre AS, Rossi FG, et al. Impact of age group o febrile neutropenia risk assessment and management in patients with diffuse large B-cell lymphoma treated with R-CHOP regimens. Clin Lymph Myel Leuk. 2012;5:297–305. doi: 10.1016/j.clml.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Pettengell R, Johnson HE, Lugtenburg PJ, et al. Impact of febrile neutropenia on R-CHOP chemotherapy delivery and hospitalizations among patient with diffuse large B-cell lymphoma. Support Care Cancer. 2012;20:647–652. doi: 10.1007/s00520-011-1306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettengell R, Schwenkglenks M, Leonard R, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16:1299–1309. doi: 10.1007/s00520-008-0430-4. [DOI] [PubMed] [Google Scholar]
- 28.Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 29.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 30.Lyman GH, Morrison VA, Dale DC, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44:2069–2076. doi: 10.1080/1042819031000119262. [DOI] [PubMed] [Google Scholar]
- 31.Salar A, Haioun C, Rossi FG, et al. The need for improved neutropenia risk assessment in DLBCL patients receiving R-CHOP-21: Findings from clinical practice. Leuk Res. 2012;36:548–553. doi: 10.1016/j.leukres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:187–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 33.Lee L, Crump M, Khor S, et al. Impact of rituximab on treatment outcome of patients with diffuse large b-cell lymphoma: a population-based analysis. J Haematol. 2012;158:481–488. doi: 10.1111/j.1365-2141.2012.09177.x. [DOI] [PubMed] [Google Scholar]
- 34.Pfreundschuh M, Trumper L, Osterberg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large B-cell lymphoma: a randomized controlled trial by the MabThera international trial (MInT) group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 35.Pfreundschuh M, Schubert J, Ziepert M, et al. German High-Grade Non-Hodgkin Study Group (DSHNHL) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomized controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Kang CI, Chung DR, Peck KR, Kim WS, Kim SJ. Clinical significance of non-neutropenic fever in the management of diffuse large B-cell lymphoma patients treated with rituximab-CHOP: Comparison with febrile neutropenia and risk factor analysis. Cancer Res Treat. 2015;47:448–457. doi: 10.4143/crt.2014.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi YW, Jeong SH, Ahn MS, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patient treated with rituximab-CHOP. J Koren Med Sci. 2014;29:1493–1500. doi: 10.3346/jkms.2014.29.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison VA, Picozzi V, Scott S, et al. The impact of age on delivered dose intensity and hospitalizations for febrile neutropenia in patients with intermediate-grade non-Hodgkin’s lymphoma receiving initial CHOP chemotherapy: A risk factor analysis. Clin Lymph. 2001;2:47–56. doi: 10.3816/clm.2001.n.011. [DOI] [PubMed] [Google Scholar]
- 39.Intragumtornchai T, Sutheesophon J, Sutcharitchan P, et al. A predictive model for life-threatening neutropenia and febrile neutropenia after the first course of CHOP chemotherapy in patients with aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2000;37:351–360. doi: 10.3109/10428190009089435. [DOI] [PubMed] [Google Scholar]
- 40.Ray-Coquard I, Borg C, Bachelot T, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003;88:181–186. doi: 10.1038/sj.bjc.6600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 42.Pettengell R, Bosly A, Szucs TD, et al. Impact of Neutropenia in Chemotherapy-European Study Group. Multivariate analysis of febrile neutropenia occurrence in patients with non-Hodgkin lymphoma: data from the INC-EU Prospective Observational European Neutropenia Study. Br J Haematol. 2009;144:677–685. doi: 10.1111/j.1365-2141.2008.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy. Crit Rev Oncol Hematol. 2014;90:190–199. doi: 10.1016/j.critrevonc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network. NCCN Clinical Practiv=ce Guidelines in Oncology. http://www.nccn.org/index.asp.
- 45.Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of the EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. (Chan refs 19,21 – see above) [DOI] [PubMed] [Google Scholar]
- 46.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 47.Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte-colony stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24:2475–2484. doi: 10.1093/annonc/mdt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma: The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. (No authors listed) [PubMed] [Google Scholar]
- 49.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 50.Chan KKW, Siu E, Krahn MD, Imrie K, Alibhai SMH. Cost-utility analysis of primary prophylaxis versus secondary prophylaxis with granulocyte colony-stimulating factor in elderly patients with diffuse aggressive lymphoma receiving curative-intent chemotherapy. J Clin Oncol. 2012;30:1064–1071. doi: 10.1200/JCO.2011.36.8647. [DOI] [PubMed] [Google Scholar]
- 51.Lathia N, Isogai PK, De Angelis C, et al. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against febrile neutropenia in lymphoma patients. J Nat Cancer Inst. 2013;105:1078–1085. doi: 10.1093/jnci/djt182. [DOI] [PubMed] [Google Scholar]
- 52.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116:5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 53.Carson KR, Riedell P, Lynch R, et al. Comparative effectiveness of anthracycline-containing chemotherapy in United States veterans age 80 or older with diffuse large B-cell lymphoma. J Geriatr Oncol. 2015;6:211–218. doi: 10.1016/j.jgo.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nolasco-Medina D, Reynoso-Noveron N, Mohar-Betancourt A, Aviles-Salas A, Garcia-Perez O, Candelaria M. Comparison of three chemotherapy regimens in eldery patients with diffuse large B-cell lymphoma: Experience at a single national reference center in Mexico. Biomed Res Internat. 2016 doi: 10.1155/2016/9817606. http://dx.doi.org/10.1155/2016/9817606. [DOI] [PMC free article] [PubMed]
- 55.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicenter, single-arm, phase 2 trial. Lancet Oncol. 2011;12:460–468. doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 56.Shin HJ, Chung JS, Song MK, Kim SK, Choe S, Cho GJ. Addition of rituximab to reduced-dose CHOP chemotherapy is feasible for elderly patients with diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2012;69:1165–1172. doi: 10.1007/s00280-011-1814-6. [DOI] [PubMed] [Google Scholar]
- 57.Kikuchi M, Nakasone H, Akahoshi Y, et al. Reduced-dose (two-thirds) R-CHOP chemotherapy for elderly patient with non-Hodgkin lymphoma. J Chemother. 2015;27:99–105. doi: 10.1179/1973947814Y.0000000219. [DOI] [PubMed] [Google Scholar]
- 58.Meguro A, Ozaki K, Sato K, et al. Rituximab plus 70% cyclophosphamide, doxorubicin, vincristine and prednisone for Japanese patients with diffuse large B-cell lymphoma aged 70 years or older. Leuk Lymphoma. 2012;53:43–49. doi: 10.3109/10428194.2011.600486. [DOI] [PubMed] [Google Scholar]