Abstract
Cardiometabolic disease, comprising cardiovascular diseases, type 2 diabetes, and their associated risk factors including metabolic syndrome and obesity, is the leading cause of death worldwide. Plant foods are rich sources of different groups of bioactive compounds, which might not be essential throughout life but promote health and well-being by reducing the risk of age-related chronic diseases. However, heterogeneity in the responsiveness to bioactive compounds can obscure associations between their intakes and health outcomes, resulting in the hiding of health benefits for specific population groups and thereby limiting our knowledge of the exact role of the different bioactive compounds for health. The heterogeneity in response suggests that some individuals may benefit more than others from the health effects of these bioactive compounds. However, to date, this interindividual variation after habitual intake of plant bioactive compounds has been little explored. The aim of this review is to provide an overview of the existing research that has revealed interindividual variability in the responsiveness to plant-food bioactive compound consumption regarding cardiometabolic outcomes, focusing on polyphenols, caffeine and plant sterols, and the identified potential determinants involved.
Keywords: plant-food bioactives, interindividual variability, cardiometabolic health, determinants of interindividual variability, biological responsiveness
Introduction: Plant-Food Bioactive Compounds and Cardiometabolic Health
Cardiometabolic diseases encompass a cluster of cardiovascular, metabolic, prothrombotic, and inflammatory abnormalities that are recognized as disease states by the American Society of Endocrinology, the National Cholesterol Education Program, and the WHO (1). Food intake plays a key role in reducing the risk of cardiometabolic diseases, with data suggesting that >30% of all deaths could be prevented through dietary changes, particularly by increased consumption of plant-based foods (2). Plant foods are rich sources of fiber and essential micronutrients, such as vitamins and minerals. They are also sources of a large group of bioactive compounds, which might not be essential throughout life or cause clinically manifested deficiencies, but when consumed with the diet, these phytochemicals may promote health and well-being in adulthood and the elderly population by reducing the risk of age-related chronic diseases (3). The major categories of dietary phytochemicals include polyphenols, such as flavonoids or phenolic acids, carotenoids, or plant sterols. Growing evidence from mechanistic studies, clinical trials, and prospective cohort studies suggest that these bioactive compounds may help in promoting health when consumed as part of the habitual diet. Polyphenols are secondary metabolites of plants and are found in fruits, vegetables, and their products (4). Carotenoids, including β-carotene, lycopene, lutein, and zeaxanthin, are phytochemicals found in many fruits and vegetables and account for the brilliant colors of these foods (5). Phytosterols are cholesterol-like molecules found in all plant foods, with the highest concentrations occurring in vegetable oils, but they can also be found in nuts, breads, or whole vegetables (6). Furthermore, caffeine ranks as one of the most commonly consumed dietary micronutrients; it is found in coffee beans, cacao beans, kola nuts, guarana berries, and tea leaves including yerba mate (7).
Accumulating evidence from cohort studies suggests that an increased intake of polyphenols, which are the most abundant category of phytochemicals present in our foods, may reduce the risk of cardiovascular diseases (CVDs) (8). This evidence is supported by animal and clinical studies that have reported beneficial effects of the intake of polyphenol-rich foods or purified compounds on intermediate risk factors for CVD, including LDL cholesterol, blood pressure, and endothelial function (9–12). The most convincing clinical evidence for the cardioprotective benefits of the consumption of dietary polyphenols relates to their observed beneficial effect on endothelial function (13, 14). Health-protective effects have also been described for other phytochemicals. For example, the consumption of suitable doses of plant sterols has repeatedly been shown in randomized controlled trials (RCTs) to reduce LDL cholesterol concentrations and thus reduce risk of subsequent CVD (15). Another phytochemical-rich source is coffee, one of the most widely consumed beverages worldwide. Coffee consumption may reduce the risk of type 2 diabetes mellitus and hypertension, as well as other conditions associated with cardiovascular risk (16), and epidemiological studies suggest that regular coffee drinkers have reduced mortality, predominantly as a result of their reduced risk of developing CVD (17).
The bioavailability and tissue distribution of phytochemicals in humans are key factors that need to be clearly established and associated with their biological effects. The fate of phytochemicals in the body, including absorption, metabolism, and distribution, may vary according to the categories of phytochemicals. Ingested polyphenols can be absorbed from the stomach or the small intestine and can undergo conjugation in the intestine and liver to give methyl, glucuronide, and sulfate derivatives (phase II metabolites) (11). Native polyphenols can also break down, producing smaller phenolic acid derivatives, such as protocatechuic, vanillic, or ferulic acid. These phenolic acids can also undergo phase I and phase II metabolism in the liver (18). The bioavailability of plant-food bioactive compounds is complex and presents interindividual variation (19, 20); however, the extent of such variability and the major determinants involved are currently not established. An example of interindividual variation in the metabolism of plant bioactive compounds is the conversion by the gut microbiota of the soy isoflavone precursors, daidzin and daidzein, to the microbial-derived metabolite equol. After a soy challenge, 20–30% of Western (21) and 50–60% of Asian populations (22) produce equol. The bacteria involved in the conversion have been identified, but the determinants that govern the daidzein-metabolizing phenotype still have not been fully elucidated. The gut microbiota has also a key role in the metabolism of other plant-food bioactive compounds, such as lignans and ellagitannins (23, 24). Genetic polymorphisms can also contribute to the interindividual variation in bioavailability. For example, the role of genetic polymorphisms in the interindividual variability in bioavailability of caffeine was demonstrated. Caffeine is mainly metabolized by cytochrome P450 1A2 (CYP1A2) in the liver, and subjects with the CYP1A2*1F allele variant (associated with a low enzyme inducibility) are considered slow caffeine metabolizers compared with the rapid caffeine metabolizers carrying the wild-type allele (25). Other factors such as age, sex, and dietary habits may affect the bioavailability of plant-food bioactive compounds. For example, sex differences in the glucuronidation of resveratrol, a polyphenol present in grapes and wine, have recently been observed, which may be explained by sex-specific uridine 5′-diphospho–glucuronosyltransferase isoenzyme expression profiles regulated by sex hormones (26).
The existence of an interindividual variability in the bioavailability of plant-food bioactive compounds suggests that there could also exist an interindividual variability in biological response to the consumption of these compounds. Heterogeneity in the responsiveness to plant bioactive compounds can obscure associations between habitual intakes and health outcomes, resulting in a potential masking of health benefits for specific population groups and thereby limiting our knowledge of the role of the different bioactives for health. Improving our knowledge of the factors, both genetic and nongenetic [such as age, sex, or (epi)genotype], that influence whether plant-food bioactive compounds are more or less effective in individuals will be invaluable to progress in the development of effective and innovative solutions leading to health improvements (27). However, to date, this interindividual variation in efficacy of plant-food bioactive compounds to modulate physiological outcomes has been little explored. The aim of this review is to provide an overview of the existing studies, both prospective and clinical trials, that has revealed interindividual variability in the responsiveness to the consumption of major plant-food bioactive compounds present in our diet: polyphenols, caffeine, and plant sterols. This review focuses on interindividual variability regarding cardiometabolic outcomes and discloses the potential determinants involved.
Interindividual Variability in Biomarkers of Cardiometabolic Health and Underlying Determinants after the Consumption of Plant-Food Bioactive Compounds Identified from Prospective Studies
We identified 6 prospective studies addressing the impact of interindividual variability in biomarkers of cardiometabolic health after habitual intake of a range of different plant bioactive compounds, including coffee and soy (Table 1). One area of particular interest relates to the microbially derived soy isoflavone metabolite, equol. In one prospective study, which examined associations between urinary equol excretion, serum lipids, and carotid intima thickness (IMT) in 572 Chinese participants, 25% were equol excreters on their usual diet. In relation to other characteristics, the number of equol producers was similar between men and women, and there was no significant difference between equol-producer phenotype and age, dietary intakes, blood pressure, or BMI (in kg/m2). Equol excreters had significantly lower TG and IMT levels compared to non-equol excreters (46). Although there was no association between soy isoflavone intake and serum lipids or IMT in the non–equol excreters, equol excreters within the highest quartile of intake (>5.4 mg/d) had significantly lower IMT and higher HDL cholesterol concentrations than those in the lowest quartile of soy intake. Although this was an Asian population, habitual intakes of isoflavones were low, with a mean intake of 13 mg/d in both the equol- and non–equol-producer groups (46). The findings are therefore intriguing because data from the extensive literature on soy-intervention studies suggest that an isoflavone intake >25 mg/d is required for any biological or clinical effect (47). The lack of an effect of isoflavone intake on CVD risk in women from the EPIC (European Prospective Investigation into Cancer and Nutrition) population was therefore not surprising, given that the median intake of isoflavones was only 0.4 mg/d. This study did not assess equol-producer status, and there was no difference in the association between habitual isoflavone intake and CVD risk when stratified by smoking (ever compared with never), BMI, hormone replacement therapy use, age at intake, and hypercholesterolemia (48). This prospective study also examined associations between habitual lignan intakes and CVD risk in women and observed no association with intake (median intake was low, 1 mg/d), although the authors suggested a decreased risk of developing CVD in participants who were past smokers and had a higher habitual lignan intake. Therefore, available data on soy and the microbially derived metabolite equol are very limited. The impact of the equol-producer phenotype requires further investigation in population groups in which there is a wide variability in intakes in order to more carefully examine the magnitude of interindividual variability in response to biomarkers of cardiometabolic health and particularly the importance of the microbially derived metabolite equol.
TABLE 1.
Summary of studies that revealed interindividual variability in biomarkers of cardiometabolic health after the consumption of plant-food bioactive compounds1
| Bioactive family, reference | Bioactive compound or food | Dose, unit/d | Study design | Duration | Participants, n | Participant characteristics | Variable biomarker | Determinant of variability |
| Polyphenols | ||||||||
| Franklin et al. (28) | Cocoa extract | 1.4 g | Parallel | 4 wk | 50 | Healthy obese men and women | oxLDL | Sex |
| Wilson et al. (29) | Cocoa flavanols | 450 mg | Parallel study | 14 d | 42 | Young and elderly healthy men | Systolic blood pressure and arterial stiffness | Age |
| Egert et al. (30) | Cocoa flavanols | 814 mg | Crossover | 4 wk | 30 | Overweight adults | Arterial stiffness | Sex |
| Avolio et al. (31) | Cocoa flavanols | 821 mg | Longitudinal study | 4–6 d | 34 | Healthy young and elderly subjects | Blood pressure and endothelial function | Age |
| McLean and LeCouter (32) | Cocoa flavanols | 907 mg | Crossover | Acute | 42 | Healthy subjects | Platelet function | Sex |
| Thijssen et al. (33) | Green tea catechin | 836 mg | Acute study | Acute | 20 | Healthy obese men and women | Blood pressure, pulse stiffness index, insulin | Genetic polymorphism in COMT gene |
| Fisher and Hollenberg (34) | Green tea catechin | 1.06 g | Randomized, double-blind, crossover study | Acute | 50 | Healthy male subjects | Blood pressure, vascular function (digital volume pulse) | Genetic polymorphism in COMT gene |
| Mubarak et al. (35) | Quercetin | 150 mg | Crossover | 6 wk | 93 | Overweight or obese young and elderly subjects | Systolic blood pressure | Age and disease state (hypertension) |
| Sachse et al. (25) | Isoflavones | 99 mg | Parallel study | 1 y | 202 | Postmenopausal women | Blood pressure and endothelial function | Gut microbiota (equol producers) |
| Mackay et al. (36) | Isoflavones | 30–52 g | Parallel | 1 mo | 85 | Hypercholesterolemic men and postmenopausal, hypercholesterolemic women | HDL-C | Gut microbiota (equol producers) |
| Nagao et al. (37) | S-equol | 10 mg | Crossover | 12 wk | 54 | Overweight or obese volunteers | Hba1c and LDL-C–CAV1 | Gut microbiota (equol producers) and sex |
| Coffee | ||||||||
| Rodriguez-Mateos et al. (38) | Coffee | 600 mL | Parallel | 4 wk | 121 | Healthy men and women | TC | Genetic polymorphism in apoE gene |
| Weber et al. (39) | Coffee | 600 mL | Parallel | 4 wk | 120 | Healthy men and women | Blood homocysteine | Genetic polymorphism in MTHFR gene |
| Corretti et al. (40) | Caffeine | 3 mg/kg | Crossover | Acute | 110 | Healthy men | Blood pressure | Genetic polymorphism in ADORA2A and ADORA2B genes |
| Plant sterols | ||||||||
| Hollman et al. (41) | Plant sterol | 2 g | Crossover | 28 d | 63 | Mildly hypercholesterolemic adults | TC and LDL-C | Basal cholesterol metabolism |
| Ostertag et al. (42) | Plant sterol | 1.1 and 2.2 g | Parallel | 5 wk | 217 | Hypercholesterolemic adults | TC, LDL-C, apoB, and TC:LDL-C and LDL:HDL ratios | Genetic polymorphism in apoE gene |
| West et al. (43) | Plant sterol | 2 g | Crossover | 4 wk | 82 | Hypercholesterolemic men | LDL-C | Genetic polymorphism in ABCG8 and NPC1L1 genes |
| Kaya et al. (44) | Plant sterol | 2 g | Crossover | 4 wk | 113 | Hypercholesterolemic subjects | LDL-C | Basal cholesterol metabolism |
| Ibero-Baraibar et al. (45) | Plant sterol | 3.2 g | Parallel | 4 wk | 67 | Men and women with normal or increased blood cholesterol concentration | TC | Genetic polymorphism in CYP7A1 gene |
ABCG8, ATP-binding cassette heterodimeric transporters G8; ADORA2A, adenosine A2a receptor; ADORA2B, α-2B adrenergic receptor; CAV1, cardio-ankle vascular index; COMT, catechol-O-methyltransferase; CYP7A1, cholesterol 7 α-hydroxylase; Hba1c, glycated hemoglobin; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MTHFR, methylenetetrahydrofolate reductase; NPC1L1, Niemann-Pick C1 Like 1; oxLDL, oxidized LDL; TC, total cholesterol.
Four prospective studies have examined the impact of several factors in explaining the association between coffee intake and CVD risk (19, 49, 50). Whether polymorphism in the CYP1A2 gene, coding for the main enzyme responsible for the metabolism of caffeine, modulates the association between coffee intake and risk of CVD and related biomarkers was addressed in 3 studies. In one study, the risk of hypertension associated with coffee intake was shown to vary according to CYP1A2 genotype, with carriers of the slow-metabolism *1F allele (59% of the 323 young, hypertensive participants, aged 18–45 y) at increased risk with higher coffee intake but not participants with the fast-metabolism *1A/*1A genotype (50). In a more recent study from this same hypertensive cohort, the association between coffee intake and impaired fasting glucose was stronger in carriers of the *1F variant, with the highest risk in heavy drinkers [≥4 cups/d (400 mL/d)] (51). In relation to myocardial infarction, in a case-control study coffee intake was only associated with an increased risk of nonfatal myocardial infarction among participants with slow-caffeine metabolism (*1F variant) (19). Only one study examined whether the relation between coffee intake and incident of coronary artery disease (CAD) is dependent on the metabolism of catecholamines, specifically polymorphisms of the catechol-O-methyltransferase (COMT) gene. In a cohort of 773 men, the relation between consumption of caffeinated coffee and the incidence of fatal and nonfatal CAD was dependent on COMT genotype. In men who were either homozygous for the high-activity COMT allele or heterozygous, substantial coffee intake did not increase the incidence of acute coronary events. However, for those who were homozygous for the low-activity COMT allele, heavy coffee consumption (median intake, 0.94 L/d) was associated with a higher incidence of acute coronary events, and the relative CAD incidence was >200% higher among drinkers of >6.5 cups of coffee/d (815.5 mL coffee/d) after multivariable adjustment (49). Taken together, these few prospective studies have shown that there is interindividual variability in response to the consumption of plant-food bioactive compounds and that individuals do not equally benefit from the consumption of these phytochemicals. Different determinants, such as gut microbiota, genetic polymorphism, or smoking, have been suggested to be involved in these between-subject variations. It should also be noted that coffee is a source of not only caffeine, the amount of which can vary depending on brewing (52), but also of other micronutrients, such as chlorogenic acid, which has been shown to mediate the blood pressure rise caused by coffee intake (35).
Determinants of Interindividual Variability in Biomarkers of Cardiometabolic Health after the Consumption of Plant-Food Bioactive Compounds Identified from Clinical Trials
Impact of age on effects of plant-food bioactive compounds
Age is the strongest independent cardiovascular risk factor for CVD, as indicated in most methods of risk scoring, such as the Framingham risk score or the European Society of Cardiology SCORE (Systematic Coronary Risk Evaluation) system (29, 53). Aging is also associated with increased vascular stiffness, endothelial dysfunction, and isolated systolic hypertension (28, 31, 54). All these age-associated changes in the vascular system are known to have an effect on the bioactivity of some drugs, such as verapamil, albuterol, or benzodiazepines (32), and potentially could also have an effect on the bioactivity of plant-food bioactives, which undergo the same conjugation pathways when absorbed.
To date, few studies have examined the effects of age on the cardiometabolic effects of food bioactive compounds (30, 55, 56) (Table 1). Three studies have investigated age-dependent effects of cocoa flavanols (CFs) on vascular function (30, 34, 56), with conflicting results. However, only one of them was a controlled study specifically designed to investigate the effects of flavanols in the context of the aged cardiovascular system. A double-blind RCT (56) demonstrated that consumption of a flavanol-rich drink (450 mg CF) 2 times/d for 2 wk reversed age-related increases in blood pressure together with vascular stiffness in healthy elderly men. CF-intake–associated improvements in the compliance of large arteries were complemented by a decrease in pulse wave velocity (PWV) and aortic augmentation of systolic blood pressure (SBP). Endothelial function in large conduit arteries was also significantly improved in healthy young and elderly individuals. These beneficial effects were associated with an improved dilatory capacity of resistance arteries, lower diastolic blood pressure (DBP), and increases in microcirculatory perfusion and RBC deformability. Cardiac output was not affected by CFs. Importantly, despite age-dependent differences in baseline flow-mediated dilatation (FMD), PWV, and DBP, the magnitude of the changes in the vascular response to CFs was not significantly different between the young and the elderly. In contrast, flavanol consumption improved only SBP and the augmentation index (AIX) in the elderly group (changes of SBP of −6 mm Hg and AIX of −7%). This is probably because SBP is slightly higher in the elderly, mainly caused by stiffer arteries. Plasma concentrations of flavanol metabolites were not significantly different between young and elderly individuals, suggesting that differences in bioavailability could not explain the differences observed in biological responses. Of note, endothelial dysfunction is a well-established response to cardiovascular risk factors and precedes the development of atherosclerosis. The measurement of ultrasound-based endothelium-dependent FMD in the brachial artery is the more widely used noninvasive measure of endothelial function and constitutes a clinical surrogate marker of vascular health (33). This technique consists of assessing the change in the diameter of the brachial artery after the increase in shear stress induced by a reactive hyperemia, with the degree of dilatation reflecting arterial endothelial NO release (40). The aortic AIX is closely related to wave reflections and constitutes a surrogate marker of arterial stiffness (39). A heightened aortic AIX is associated with an elevated risk of cardiovascular events.
In agreement with previous data, a recent study showed that the absorption, distribution, metabolism, and excretion (ADME) of CFs was not significantly different between young and elderly healthy subjects (mean ± SD age, 26 ± 6 and 70 ± 4 y, respectively; n = 40) after consumption of a similar amount of CFs (400 mg CFs, 5.3 mg CFs/kg body weight) (38). However, small but significant differences in metabolism were reported at a higher intake amount of CFs (800 mg CFs, 10.7 mg CFs/kg body weight), with higher glucuronidation, lower methylsulfation, and lower urinary excretion of gut microbial γ-valerolactone metabolites observed in the elderly. This observation suggests that dose-response studies covering the amounts of bioactive intake that can be achievable through a normal diet are necessary when investigating the interindividual variability in the ADME of plant-food bioactive compounds.
A study also investigated whether the consumption of a flavanol-rich cocoa drink (821 mg CFs) could improve blood pressure and endothelial function in healthy young and elderly men (age 31 ± 2.7 and 61 ± 1.9 y, respectively) (55). No changes in blood pressure or endothelial function (measured by peripheral arterial tonometry) were observed in any group after 4–6 d of daily CF consumption. However, an effect on the last day of the study was seen in both groups after 90–180 min of CF consumption, and when compared with baseline values of day 1, the effect was higher for the elderly volunteers. Pulse wave analysis showed a similar pattern, with higher vascular responses in the elderly after acute consumption. The authors attributed these effects to an increase in NO production because responses to the endothelial NO synthase inhibitor l-nitroarginine-methyl-ester were also greater in the elderly. Nevertheless, the relevance of comparing changes in vascular function after acute consumption on days 4–6 with baseline levels on day 1 remains to be established.
An additional RCT reported age-dependent effects of quercetin on blood pressure (30). Supplementation of 150 mg quercetin/d for 6 wk resulted in a decrease in SBP by 2.6 mm Hg in the entire study group, by 2.9 mm Hg in the subgroup of hypertensive subjects, and by 3.7 mm Hg in the subgroup of younger adults aged 25–50 y. These observations suggest that the blood pressure-lowering effects of quercetin may be greater in younger than in older people. The authors hypothesized that improved endothelial function may be affecting younger and middle-aged individuals, and because with increasing age the arteries become stiffer, the potential to improve vascular function by nutrients and bioactive compounds decreased. However, differences between young and elderly subjects were not reported in this work, in which only differences between the total number of subjects and a younger subgroup were given, so these findings need to be confirmed in a study specifically designed to investigate those age groups.
In summary, there is currently very limited evidence to suggest that the cardiometabolic response to food bioactive compounds is age dependent. From the 3 studies that have been identified, only 2 were specifically designed to test age-dependent effects of food bioactive compounds, with only one being controlled and showing significant effects on CVD risk biomarkers both acutely and after short-term supplementation (56). These authors concluded that some of the beneficial effects of flavanols are age dependent and others not, with FMD, PWV, and DBP showing similar effects in both young and elderly subjects, whereas SBP and AIX improved only in the elderly. Taken together, it could be suggested that age is one factor affecting the variability in the vascular response to plant-food bioactive compounds.
Impact of sex on the effects of plant-food bioactive compounds
We identified 3 studies looking at differences between men and women in their response to CF intake (42, 43, 45) (Table 1). Ostertag et al. (42) reported differences in the responses to the flavanol-containing chocolate compared with white chocolate between men and women. Platelet aggregation was significantly decreased in men but not in women 2 h after consumption of dark or flavanol-enriched dark chocolate and 6 h after flavanol-enriched dark chocolate compared with white chocolate. Sex differences in the effects of the chocolate on markers of platelet activation were also observed: platelet P-selectin expression was decreased only in men 2 h after consumption of flavanol-rich dark and white chocolate compared with standard dark chocolate; fibrinogen-binding was increased only in women 2 h after consumption of flavanol-enriched dark chocolate compared with white chocolate. The sex differences highlighted in this study are interesting, but their interpretation is somewhat problematic because of the absence of an appropriate control group matched for flavanols. For example, a significant effect of white chocolate compared with standard dark chocolate was observed on platelet activation and ex vivo bleeding time in men only. Because white chocolate does not contain flavanols, the observed effect must be due to other components in the white chocolate that are probably also found in dark chocolate, somewhat compromising its use as a suitable control for determining the effects of CFs. Cocoa itself is complex and contains many other potentially bioactive compounds in addition to flavanols, again compromising interpretation of the effects and differences between sex.
West et al. (43) reported the effects of dark chocolate and cocoa-beverage consumption on markers of endothelial function on 30 middle-aged overweight adults. The CF intervention caused significant increases in basal diameter and peak diameter of the brachial artery and basal blood flow volume in both men and women. However, significant reductions in peripheral arterial stiffness in response to the cocoa treatment were observed only in women, evidenced as substantial decreases in the AIX (83%). The effects of cocoa treatment in men were small and nonsignificant. However, it should be noted that the women had substantially higher AIX values at baseline, and this probably related to the differences in response between men and women.
Finally, Ibero-Baraibar et al. (45) reported that men derived a greater benefit from consuming a cocoa-supplemented diet than did women, although this effect was observed only for changes in oxidized LDL (oxLDL). Improvements in many of the variables assessed were demonstrated between baseline and the end of the study, such as blood pressure and anthropometric or body composition variables, which was to be expected in response to the 15% caloric restriction provided by both diets. oxLDL in the cocoa group was significantly lower than in the control group. After adjusting the data for weight and total and LDL cholesterol, it was shown that cocoa consumption significantly affected the change in oxLDL in men but not in women. To explain this difference, the authors refer to a previously reported difference in the antioxidant status between men and women, with men exhibiting poorer antioxidant status (i.e., men have a higher oxidation status than women), making them more susceptible than women to an antioxidant effect of the flavanols (44). However, the physiological relevance of a change in oxLDL is unknown because this variable is not an established surrogate marker of CVD risk (41).
In summary, a sex effect in response to plant-food bioactive compounds has been reported in very few studies, and all of those focused on flavanols. From the 3 articles identified to date, differences in the response between men and women were observed in the AIX and antioxidant status.
Impact of genetic polymorphism on the effects of plant-food bioactive compounds
Eight studies have reported the impact of genetic polymorphisms on the cardiometabolic health effects of green tea (57, 58), coffee or caffeine (59–61), and plant sterols (62–64) (Table 1). The beneficial effects of green tea catechins may be predisposed by polymorphisms in genes encoding phase II metabolism enzymes during and after the consumption. The missense mutation rs4680 (G to A) in the COMT gene, coding for methylation enzyme, results in a 40% decrease in enzyme activity. In a pilot study performed by Miller et al. (58), 20 subjects (10 homozygous COMT GG or 10 AA genotype) were given green tea extract capsules (836 mg green tea catechins) in a fasted state and with a high-carbohydrate breakfast. The modification in digital volume pulse (DVP) stiffness index from baseline was observed to be different between genotype groups at 120 and 240 min, with a lower stiffness index in the GG individuals. The alteration in blood pressure from baseline was also observed to be different between genotype groups, with a bigger increase in SBP and DBP at 120 min in the GG group. It was observed that the AA group had a greater increase in plasma insulin concentrations at 120 and 180 min compared with baseline, although the glucose profiles were similar. No differences were observed in vascular reactivity evaluated by using laser-Doppler iontophoresis, total nitrite, plasma lipids, total antioxidant capacity, or markers of inflammation.
The same investigators assessed the effect of the COMT genotype on the heterogeneity in response to green tea catechins regarding vascular reactivity and blood pressure in a study with 50 volunteers instead of 20 as in previous study (57). These subjects (25 with AA and 25 with GG COMT rs4680 genotype) completed a randomized, double-blind, crossover study. Peripheral arterial tonometry, DVP, and blood pressure were evaluated at baseline and 90 min after intake of 1.06 g green tea extract or placebo. A genotype-treatment interaction was shown for the DVP reflection index with green tea extract in the AA COMT group. A genotypic effect was described for urinary methylated epigallocatechin during the first 5.5 h, with the GG COMT group having higher concentrations (57). Taken together, these 2 studies suggest that differences in small-vessel tone according to COMT genotype are evident after the acute administration of green tea extract.
The response in serum cholesterol to diet may be modulated by the APOE, ε2/ε3/ε4 alleles, which is also a predictor of variation in the risk of CAD and CAD death. Strandhagen et al. (61) tested the hypothesis that the APOE polymorphism may affect the cholesterol-raising effect of coffee. One hundred twenty-one healthy, nonsmoking men (22%) and women (78%) aged 29–65 y were provided with 0.6 L filter-brewed coffee/d for 4 wk. APOE ε2-positive volunteers presented significantly lower total cholesterol (TC) concentrations at baseline, but the cholesterol-raising effect of coffee was not significantly influenced by APOE allele carrier status. These results suggest that the APOE ε2 allele is associated with a lower serum cholesterol concentration, but it does not seem to affect the cholesterol-raising effect of coffee.
In a study with a similar design and similar study population, the hypothesis that methylenetetrahydrofolate reductase gene polymorphism, known to influence plasma total homocysteine (tHcy), is associated with the effect of coffee on plasma homocysteine-raising effect has been investigated (60). The authors examined the impact of consumption of 0.6 L coffee/d supplemented or not with 200 μg folic acid on tHcy with respect to the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms. tHcy, at baseline, was higher in the 677TT genotype group than in the 677CC genotype group, and this group had a higher increase in tHcy on coffee exposure than did the 677CC and 677CT genotype groups. Supplementation with 200 μg folic acid, when compared with the placebo, decreased the tHcy-increasing effect of coffee in the 677TT genotype group. The A1298C polymorphism did not modulate tHcy concentration. Therefore, it was suggested that the homocysteine-increasing impact of coffee is especially obvious in individuals with the homozygous 677TT genotype (60).
Renda et al. (59) evaluated acute blood pressure responses to caffeine and evaluated whether they are affected by candidate gene variants affecting caffeine metabolism: CYP1A2, adenosine metabolism (adenosine A2a receptor and α-2B adrenergic receptor), or catecholamine receptors. In this study, 110 healthy male subjects with moderate coffee consumption underwent ambulatory blood pressure monitoring at 6-min intervals for 2 h. Each volunteer was given, in a double-blind design, 0.04 L of either a decaffeinated coffee preparation plus 3 mg caffeine/kg or the corresponding vehicle (decaffeinated). Compared with decaffeinated coffee, caffeine significantly increased both SBP and DBP. Plasma caffeine and adrenaline increased after caffeine but not after decaffeinated coffee. Of the 11 gene polymorphisms analyzed, an association was detected between the adenosine A2a receptor TT variant and the α-2B adrenergic receptor I variant and the change in SBP in responses to caffeine (59). This study suggests that the variability in the acute blood pressure response to coffee may be due to the genetic polymorphisms of the adenosine A2A receptors and the α2-adrenergic receptors.
The impact of plant sterol consumption on plasma cholesterol is highly variable. The ATP-binding cassette heterodimeric transporters G5 and G8 (ABCG5 and ABCG8) were assumed to mediate intestinal cholesterol efflux, whereas Niemann-Pick C1 Like 1 (NPC1L1) protein is believed to be important for intestinal cholesterol influx. Individual or combined genetic polymorphism of these genes could explain interindividual variations in plasma cholesterol response after the consumption of plant sterols. Zhao et al. (64) investigated the association between ABCG5/ABCG8 and NPC1L1 single nuclear polymorphisms and sterol absorption and corresponding plasma concentrations. The trial was a 4-wk crossover study with 82 hypercholesterolemic men presenting high compared with low basal plasma plant sterol concentrations who consumed spreads with or without 2 g plant sterols/d. For the ABCG8 1289 C>A (T400 K) polymorphism, the carriers of the A allele with high basal plasma plant sterol concentrations presented a 390% higher reduction in serum LDL cholesterol than did their low basal plasma counterparts. For the NPC1L1 haplotype of 872 C>G (L272L) and 3929 G>A (Y1291Y), volunteers carrying mutant alleles presented a 240% decrease in LDL cholesterol concentrations compared with the volunteers with the wild-type allele (64). The results demonstrate that genetic and metabolic biomarkers may predict interindividual lipid concentration responsiveness to plant sterol intervention and might be important in developing individualized cholesterol-lowering approaches.
Plant sterol esters decrease serum TC and LDL cholesterol but with important interindividual variability. In a randomized, double-blind, controlled study, hypercholesterolemic subjects consumed a reduced saturated-fat and cholesterol diet for 4 wk followed by a 5-wk intervention during which they consumed a control spread (n = 87) or a spread with plant sterol esters (1.1 or 2.2 g plant sterols/d; n = 120) (63). During sterol consumption, TC, LDL cholesterol, and apoB concentrations and the ratios of TC to LDL cholesterol and LDL to HDL were observed to be lower only in subjects carrying ɛ2 or ɛ3 allele of apoE gene, and serum TG decreased only in subjects carrying ɛ2 allele. Thus, responses to plant sterols diverge depending on apoE genotype and might be of small important in apoE4 carriers.
Plant sterols may disrupt the micellar solubilization of cholesterol by the bile acid pool, thus influencing intestinal cholesterol absorption. Plasma lipid variation relates to the promoter variant −204A>C (rs3808607) of the cholesterol 7 α-hydroxylase (CYP7A1) gene encoding for α-hydroxylase, an enzyme for bile acid synthesis. De Castro-Orós et al. (62) hypothesized that this polymorphism could be linked with the interindividual variation in responses to plant sterol consumption. They investigated 67 volunteers (31 AA and 36 AC+CC) with lipid responses to plant sterols documented in 2 studies. Compared with AA subjects, C-allele carriers presented higher decreases in TC and increases in ratios of lathosterol to cholesterol. These studies suggest that the −204A>C variant is associated with greater CYP7A1 activity. Increased intestinal bile acids and the resulting enhanced efficient of cholesterol absorption could elucidate why C-allele carriers present enhanced cholesterol-lowering and increased feedback cholesterol synthesis to plant sterol intervention.
Impact of pathophysiological status on the effects of plant-food bioactives
The metabolic status of subjects has been proposed as the factor that predicts the response of plasma cholesterol to an intervention with plant sterols (Table 1). Rideout et al. (65) highlighted between-subject differences in LDL responsiveness to plant sterols that could compromise the overall conclusion on their efficacy. They observed that, in a pooled study population of 113 subjects (from 3 different RCTs), 4 wk of a controlled diet supplemented with 2 g plant sterols/d led to a mean decrease in LDL cholesterol concentrations of 7.3%. After this observation, the authors stratified the population cohort into responders (mean reduction of 15.2%) and nonresponders (mean reduction of 3.7%) and proposed the basal cholesterol fractional synthesis rate (FSR) as the determinant of the response based on 1) a correlation of FSR with the percentage of change in LDL cholesterol (r = 0.22, P = 0.02), 2) 23% higher basal FSR values in nonresponders compared with the values of the responders group, 3) after stratification based on basal FSR, subjects in the first quartile showed the best response, with a mean decrease of 12.4% compared with the control period, whereas for those in the last quartile, the decrease was only 3.17%.
The same authors have proposed the ratio of lathosterol to cholesterol (L:C), a surrogate marker of endogenous cholesterol synthesis, as the predictor of the effects of plant sterols on cholesterol concentrations (36). In this randomized, single-blind, crossover, placebo-controlled trial, mildly hypercholesterolemic adults were preselected based on their baseline L:C. The participant cohort of 63 individuals consisted of 24 subjects with high endogenous cholesterol synthesis (HECS) and 39 subjects with low endogenous cholesterol synthesis (LECS). In addition to the L:C values, the 2 subgroups were significantly different in terms of body weight, BMI, and HDL cholesterol and TG concentrations, as well as the ratios of plasma phytosterols (sitosterol, cholestanol, and desmosterol) to cholesterol. After daily consumption of a diet enriched with 2 g plant sterols for 28 d, significant decreases in TG concentrations of 0.40 ± 0.07 and 0.09 ± 0.09 mmol/L were found in the HECS and LECS groups, respectively, compared with the placebo group, while the decreases in LDL concentrations were 0.29 ± 0.05 and 0.05 ± 0.07 mmol/L in the HECS and LECS groups, respectively. This work suggests that participants in the HECS group were 3 times more likely to respond to plant sterol supplementation than those in the LECS group. Finally, there was a positive correlation between the L:S and the overall decrease in TGs but not in LDL cholesterol (r = 0.24, P < 0.05). From these observations the authors suggested that the link between high L:S and the metabolic syndrome indicates that subjects with the metabolic syndrome are responsive to plant sterols. Neither of these 2 articles reported genetic polymorphism as a putative determinant of variation, although it is known that it can influence basal cholesterol synthesis as discussed above.
The effects of an 8-wk consumption of soy nuts (providing 110 mg isoflavones/d) on anthropometric variables, blood pressure, lipid concentrations, and inflammatory markers in postmenopausal women were evaluated based on the stratification by metabolic syndrome status (66). Participants included 60 women, 49 without metabolic syndrome and 11 with metabolic syndrome. In healthy volunteers, the isoflavone-enriched diet, compared with the placebo diet, resulted in a significant decrease in both SBP and DPB, and C-reactive protein (CRP). In contrast, in the group of women with the metabolic syndrome the supplemented diet significantly decreased DBP and CRP, as well as the circulating concentrations of TGs and intercellular adhesion molecule 1. Further stratification of the subjects on the ability to produce equol indicated that in women without the metabolic syndrome the changes in SBP were more pronounced in equol producers. Similarly, the DBP decrease was significant only in the equol-producer group. In response to the isoflavone-enriched diet, a significant decrease of CRP was observed only in equol producers. In women with the metabolic syndrome, the observed effects were significant only in the equol-producer group (7 of 11 subjects). As a final conclusion, the authors commented that soy consumption induced more pronounced beneficial effects on biomarkers of CVD in women with metabolic syndrome than in healthy women and that the magnitude of the responsiveness was tightly dependent on their ability to produce equol and thereby on the composition of their gut microbiota. However, the low number of subjects, especially after the stratification to equol producers and nonproducers with the metabolic syndrome, with 7 and 4 subjects, respectively, makes the conclusion strictly exploratory.
The interindividual variation in response to dietary polyphenol intake depending on the pathophysiological status was also reported. In a crossover, randomized, placebo-controlled study, Egert et al. (30) evaluated the effects of a 6-wk quercetin supplementation (150 mg/d) on blood pressure, lipid concentrations, and inflammatory markers in 93 subjects with central obesity and elevated plasma concentrations of TGs and CRP. In the entire study population, SBP and pulse pressure were significantly lower after quercetin supplementation compared with the baseline values. After subgroup analysis, no effects on either of these variables were observed in the group of normotensive subjects, whereas in the group of (pre)hypertensive subjects quercetin supplementation resulted in a significant decrease in SBP. Similarly, the pulse pressure was significantly lower after quercetin treatment compared with baseline without significant effects on both variables after the placebo period.
The influence of medication used on the response to catechins has also been reported (37). A double-blind, parallel-design, randomized controlled study investigated the effects of a 12-wk consumption of catechin-enriched green tea, providing 582.8 mg catechin/d, or green tea, providing 96.3 mg catechin/d, in 43 subjects with type 2 diabetes (not on insulin therapy). In comparison with the effects of standard green tea, catechin-enriched green tea induced a significant decrease in waist circumference and TC concentration and an increase in insulin concentration in the entire population. Further stratification of both groups based on the use of insulinotropic agents as antidiabetic therapy, including either oral sulfonylureas or glinidines, showed that the effect of catechin-enriched green tea on insulin concentrations and glycated hemoglobin was significant only in a subgroup of patients receiving insulinotropic therapy and not in subjects with another type of medication. Both results indicate the potential of catechins to act synergistically with the therapeutics regarding their insulinotropic action, with the ultimate beneficial effects on protein glycation. It should be noted that the number of patients receiving insulinotropic therapy was 17 of 23 in the test group and 16 of 20 in the control group, thus the lack of the effect in the group with noninsulinotropic therapeutics could be because of the low number of participants.
Taken together, these few studies suggest that metabolic or disease-related traits of volunteers enrolled in clinical trials can lead to interindividual variation in response to plant-food bioactive intake.
Impact of bioavailability on the effects of plant-food bioactive compounds
Besides the factors discussed above, the bioavailability of plant-food bioactive compounds could be an important factor in determining the interindividual variability in response to their consumption. It could be expected that individuals presenting a higher capacity of absorption and metabolism of these compounds, thus achieving higher concentration of bioactive metabolites in the circulation, would gain greater benefits compared with low absorbers and metabolizers. However, this assumption has been poorly documented so far. Indeed, some studies highlighted in the present review suggest that this is not always the case; for example, age-dependent differences in vascular response were seen despite no age-dependent differences in plasma concentrations of metabolites (56).
Another important issue in this context is that it is not currently known which of the main bioactive metabolites present in circulation after plant-food consumption are those responsible for the observed effects and which concentrations are required to induce optimal benefits for different subgroups. A few studies have shown correlations and temporal associations between vascular outcomes and specific circulating polyphenol metabolites (67–70), which is one step further in establishing a relation between certain bioactive metabolites and health outcomes. Hence, future clinical trials with plant foods should measure circulating concentrations of individual metabolites, demonstrate the existence of a correlation between them and their biological impacts, and report the variabilities in both ADME and biological responses.
Gut microbiota metabolism of food phytochemicals, and particularly polyphenols, has been identified as a relevant source of interindividual variability in ADME (71), and this can be the result of differences in the microbial ecosystems that colonize the human gut (72) and to the gut microbiome richness (73). These differences in gut microbial metabolism may also affect the health effects of dietary phytochemicals and be responsible for variability in biological response.
Six clinical studies have examined the role of gut microbiota metabolism in explaining interindividual variation in response to the consumption of polyphenols on cardiometabolic disease biomarkers (blood pressure, endothelial function, and TGs CRP, soluble intercellular adhesion molecule, or cholesterol concentrations). These studies are all related to soy isoflavones, daidzein, the precursor of the microbial-derived metabolite, and equol, which is known to have a higher bioactivity than daidzein itself. In a clinical study assessing the effect of soy intake on cardiovascular health in postmenopausal women (60–75 y old), it was demonstrated that changes in endothelial function and blood pressure were not significantly different between the soy and the placebo groups. In the soy group, however, stratification by equol-producer status suggested that endothelial function and blood pressure were improved only in equol producers (48). Another study on hypercholesterolemic men and postmenopausal women (n = 55) showed that the beneficial effects of soy intake on plasma LDL cholesterol, HDL, and apo1 concentrations occurred only in equol producers (n = 30) (74). In women with the metabolic syndrome, soy intake only reduced blood pressure, TGs, CRP, and soluble intercellular adhesion molecules in equol producers (72). Thus, these studies suggest that the microbiota profile responsible for the production of equol is a determinant of the variability in response to the consumption of soy protein–containing isoflavones.
Several studies have also explored whether equol is the active metabolite after soy isoflavone intake by supplying S-equol orally. Some controversial results were found. Consumption of S-equol decreased LDL cholesterol and the cardio-ankle vascular index in overweight or obese Japanese subjects, with the effects being more prominent in the subgroup of female nonproducers (75). In contrast, a recent study evaluated the effect of S-equol on prospectively recruited equol and non–equol producers, and although soy intake improved carotid-femoral PWV in equol producers, a single dose of S-equol had no cardiovascular benefits in non–equol producers (76). This study suggests that the equol-producer phenotype is critical in explaining the vascular benefits of equol, but more studies are necessary to confirm these findings.
There is growing evidence that gut microbiota speciation correlates with the risk of CVD, obesity, and type 2 diabetes (73, 77–79), and some studies also suggest a relation between gut microbiota, diet, obesity (80), and atherosclerosis (81). Interestingly, the equol-producing bacteria identified so far include species of the genera Adlercreutzia, Eggerthella, Paraeggerthella, and Slackia, all of them belonging to Coriobacteriaceae, a family that has been associated with beneficial metabolic processes in obesity and diabetes (82). Therefore, the occurrence of these bacterial species responsible for equol production can be an indication of a potential beneficial response to the consumption of plant-food bioactive compounds regarding cardiometabolic diseases.
It is well established that ellagic acid is converted to urolithins by the colon microbiota, and 3 different and consistent urolithin phenotypes (0, A, and B) were observed in various clinical intervention studies with ellagitannins, urolithin B being more frequently observed in subjects with metabolic syndrome (83). Supporting these findings, urolithin A was inversely correlated with the severity of metabolic syndrome and obesity (84, 85). Thus, the correlation of these phenotypes with cardiovascular health deserves further investigation.
Conclusions
In this review, we have highlighted the limited existing data and summarized the available clinical and prospective studies that have investigated, to some extent, the interindividual variability in the biological response to the consumption of plant-food bioactive compounds. These studies suggest that some individuals may benefit more from the health effects of these bioactives than others, and genetic and nongenetic factors may be important ones contributing to the variability in biological effects observed between individuals (Figure 1). Nevertheless, there are still very few studies robustly assessing determinants of this between-subject variability, with <25 clinical studies identified to date (Table 1). Each of them examined different biomarkers and different bioactive compounds, which precludes the pooling of data from available studies. With such wide heterogeneity in studied food bioactive compounds, cardiometabolic biomarkers, study populations, and study designs, it is not yet possible to draw conclusions based on sound scientific results.
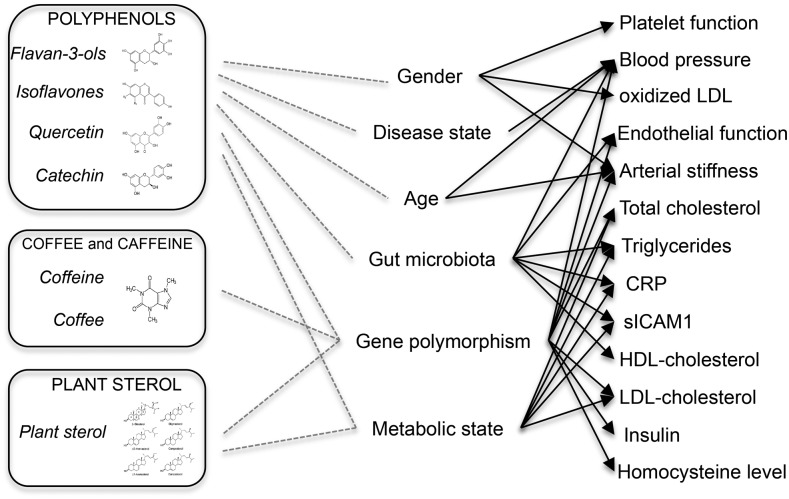
FIGURE 1.
Schematic presentation of factors involved in the interindividual variability in biomarkers of cardiometabolic health in response to the consumption of plant-food bioactive compounds. CRP, C-reactive protein; sICAM1, soluble intercellular adhesion molecule 1.
Nevertheless, the limited evidence suggest that genetic factors may be important for the interindividual variability, in particular, genetic polymorphisms of genes involved in phase I and phase II metabolism, such as COMT or CYP7A1, and others, such as the APOE genotype or cholesterol transporters. The gut microbiota is an emerging key player explaining variability, as evidenced by the differences in biological response observed between equol and non–equol producers, but also in the differential effects observed in relation to ellagitannin metabolism. Finally, health and metabolic status seem to be other factors playing a role, with some evidence suggesting that “at-risk” participants or patients may be more likely to gain benefits from increased plant bioactive compound intake than healthy individuals may be. Although some variability according to age and sex has been shown, the current evidence is not strong enough to make any conclusion.
From this review, it clearly appeared that current published studies reporting interindividual variability were not initially designed to study between-subject variation in the response. In most of these studies, the interindividual variability was observed post hoc and without adequate a priori definition of subgroups, planning, and power calculation that result in low numbers of subjects in subgroups and inadequate study power for statistical analysis. Therefore, there is a need for additional controlled-intervention studies specifically designed to identify the factors affecting the variability in the response to plant-food bioactive compounds. Future intervention studies should be suitably powered and randomized based on the factor of variability of interest (for example, young and elderly, male and female volunteers). Furthermore, it would be important to avoid as much as possible the use of complex foods as sources of bioactive compounds; indeed, because of the difficulty of having well-matched controls, the attribution of the observed effects to the bioactive compounds of interest is questionable. In these studies, it will also be crucial to systematically measure both biomarkers of effects and bioavailability variables, including the concentration and nature of circulating metabolites whose biological potential may be variable. In the long run, this knowledge will guide the provision of evidence-based, targeted dietary recommendations.
Acknowledgments
We acknowledge networking support by the COST Action FA 1403 POSITIVe (interindividual variation in response to consumption of plant food bioactives and determinants involved), supported by COST (European Cooperation in Science and Technology). All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ABC, ATP-binding cassette heterodimeric transporter; ADME, absorption, distribution, metabolism, and excretion; AIX, augmentation index; CF, cocoa flavanol; CAD, coronary artery disease; COMT, catechol-O-methyltransferase; CRP, C-reactive protein; CVD, cardiovascular disease; CYP1A2, cytochrome P450 1A2; CYP7A1, cholesterol 7 α-hydroxylase; DBP, diastolic blood pressure; DVP, digital volume pulse; FMD, flow-mediated dilatation; FSR, fractional synthesis rate; HECS, high endogenous cholesterol synthesis; IMT, carotid intima thickness; L:C, ratio of lathosterol to cholesterol; LECS, low endogenous cholesterol synthesis; NPC1L1, Niemann-Pick C1 Like 1; oxLDL, oxidized LDL; PWV, pulse wave velocity; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; tHcy, plasma total homocysteine.
References
- 1.Castro JP, El-Atat FA, McFarlane SI, Aneja A, Sowers JR. Cardiometabolic syndrome: pathophysiology and treatment. Curr Hypertens Rep 2003;5:393–401. [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med 2013;369:954–64. [DOI] [PubMed] [Google Scholar]
- 3.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol 2008;19:73–82. [DOI] [PubMed] [Google Scholar]
- 4.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004;79:727–47. [DOI] [PubMed] [Google Scholar]
- 5.Khoo HE, Prasad KN, Kong KW, Jiang Y, Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules 2011;16:1710–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr 2002;22:533–49. [DOI] [PubMed] [Google Scholar]
- 7.Heckman MA, Weil J, Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 2010;75:R77–87. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auclair S, Milenkovic D, Besson C, Chauvet S, Gueux E, Morand C, Mazur A, Scalbert A. Catechin reduces atherosclerotic lesion development in apo E-deficient mice: a transcriptomic study. Atherosclerosis 2009;204:e21–7. [DOI] [PubMed] [Google Scholar]
- 10.Chanet A, Milenkovic D, Deval C, Potier M, Constans J, Mazur A, Bennetau-Pelissero C, Morand C, Berard AM. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J Nutr Biochem 2012;23:469–77. [DOI] [PubMed] [Google Scholar]
- 11.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habauzit V, Morand C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Ther Adv Chronic Dis 2012;3:87–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 14.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50. [DOI] [PubMed] [Google Scholar]
- 15.AbuMweis SS, Marinangeli CP, Frohlich J, Jones PJ. Implementing phytosterols into medical practice as a cholesterol-lowering strategy: overview of efficacy, effectiveness, and safety. Can J Cardiol 2014;30:1225–32. [DOI] [PubMed] [Google Scholar]
- 16.O’Keefe JH, Bhatti SK, Patil HR, DiNicolantonio JJ, Lucan SC, Lavie CJ. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J Am Coll Cardiol 2013;62:1043–51. [DOI] [PubMed] [Google Scholar]
- 17.Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ, Wang CL, Chen YM. Coffee consumption and risk of coronary heart diseases: a meta-analysis of 21 prospective cohort studies. Int J Cardiol 2009;137:216–25. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 2014;88:1803–53. [DOI] [PubMed] [Google Scholar]
- 19.Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006;295:1135–41. [DOI] [PubMed] [Google Scholar]
- 20.Guadamuro L, Delgado S, Redruello B, Florez AB, Suarez A, Martinez-Camblor P, Mayo B. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front Microbiol 2015;6:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr 2010;140:1355S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Espin JC, Larrosa M, Garcia-Conesa MT, Tomas-Barberan F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med 2013;2013:270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr 2012;52:936–48. Retraction of: Crit Rev Food Sci Nutr. 2015;55:1792. [DOI] [PubMed] [Google Scholar]
- 25.Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C→A polymorphism in intron 1 of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol 1999;47:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellinger RW, Garcia AM, Meyskens FL Jr. Differences in the glucuronidation of resveratrol and pterostilbene: altered enzyme specificity and potential gender differences. Drug Metab Pharmacokinet 2014;29:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manach C, Milenkovic D, Van de Wiele T, Rodriguez-Mateos A, de Roos B, Garcia-Conesa MT, Landberg R, Gibney ER, Heinonen M, Tomas-Barberan F, et al. . Addressing the inter-individual variation in response to consumption of plant food bioactives - towards a better understanding of their role in healthy ageing and cardiometabolic risk reduction. Mol Nutr Food Res 2016. Sep 30 (Epub ahead of print; DOI: 10.1002/mnfr.201600557). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001;37:869–74. [DOI] [PubMed] [Google Scholar]
- 29.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- 30.Egert S, Bosy-Westphal A, Seiberl J, Kurbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, et al. . Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 2009;102:1065–74. [DOI] [PubMed] [Google Scholar]
- 31.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 1985;71:202–10. [DOI] [PubMed] [Google Scholar]
- 32.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004;56:163–84. [DOI] [PubMed] [Google Scholar]
- 33.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011;300:H2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher ND, Hollenberg NK. Aging and vascular responses to flavanol-rich cocoa. J Hypertens 2006;24:1575–80. [DOI] [PubMed] [Google Scholar]
- 35.Mubarak A, Bondonno CP, Liu AH, Considine MJ, Rich L, Mas E, Croft KD, Hodgson JM. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: a randomized trial. J Agric Food Chem 2012;60:9130–6. [DOI] [PubMed] [Google Scholar]
- 36.Mackay DS, Gebauer SK, Eck PK, Baer DJ, Jones PJ. Lathosterol-to-cholesterol ratio in serum predicts cholesterol-lowering response to plant sterol consumption in a dual-center, randomized, single-blind placebo-controlled trial. Am J Clin Nutr 2015;101:432–9. [DOI] [PubMed] [Google Scholar]
- 37.Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, Yamamoto T, Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17:310–7. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Mateos A, Cifuentes-Gomez T, Gonzalez-Salvador I, Ottaviani JI, Schroeter H, Kelm M, Heiss C, Spencer JP. Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects. Mol Nutr Food Res 2015;59:1504–12. [DOI] [PubMed] [Google Scholar]
- 39.Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184–9. [DOI] [PubMed] [Google Scholar]
- 40.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–65. [DOI] [PubMed] [Google Scholar]
- 41.Hollman PC, Cassidy A, Comte B, Heinonen M, Richelle M, Richling E, Serafini M, Scalbert A, Sies H, Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr 2011;141:989S–1009S. [DOI] [PubMed] [Google Scholar]
- 42.Ostertag LM, Kroon PA, Wood S, Horgan GW, Cienfuegos-Jovellanos E, Saha S, Duthie GG, de Roos B. Flavan-3-ol-enriched dark chocolate and white chocolate improve acute measures of platelet function in a gender-specific way–a randomized-controlled human intervention trial. Mol Nutr Food Res 2013;57:191–202. [DOI] [PubMed] [Google Scholar]
- 43.West SG, McIntyre MD, Piotrowski MJ, Poupin N, Miller DL, Preston AG, Wagner P, Groves LF, Skulas-Ray AC. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr 2014;111:653–61. [DOI] [PubMed] [Google Scholar]
- 44.Kaya A, Uzunhasan I, Baskurt M, Ozkan A, Ataoglu E, Okcun B, Yigit Z. Oxidative status and lipid profile in metabolic syndrome: gender differences. Metab Syndr Relat Disord 2010;8:53–8. [DOI] [PubMed] [Google Scholar]
- 45.Ibero-Baraibar I, Abete I, Navas-Carretero S, Massis-Zaid A, Martinez JA, Zulet MA. Oxidised LDL levels decreases after the consumption of ready-to-eat meals supplemented with cocoa extract within a hypocaloric diet. Nutrition, metabolism, and cardiovascular diseases. Nutr Metab Cardiovasc Dis 2014;24:416–22. [DOI] [PubMed] [Google Scholar]
- 46.Cai Y, Guo K, Chen C, Wang P, Zhang B, Zhou Q, Mei F, Su Y. Soya isoflavone consumption in relation to carotid intima-media thickness in Chinese equol excretors aged 40–65 years. Br J Nutr 2012;108:1698–704. [DOI] [PubMed] [Google Scholar]
- 47.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr 1999;129:758S–67S. [DOI] [PubMed] [Google Scholar]
- 48.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, van der Schouw YT. Dietary phytoestrogens and vascular function in postmenopausal women: a cross-sectional study. J Hypertens 2004;22:1381–8. [DOI] [PubMed] [Google Scholar]
- 49.Happonen P, Voutilainen S, Tuomainen TP, Salonen JT. Catechol-o-methyltransferase gene polymorphism modifies the effect of coffee intake on incidence of acute coronary events. PLoS One 2006;1:e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palatini P, Ceolotto G, Ragazzo F, Dorigatti F, Saladini F, Papparella I, Mos L, Zanata G, Santonastaso M. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens 2009;27:1594–601. [DOI] [PubMed] [Google Scholar]
- 51.Palatini P, Benetti E, Mos L, Garavelli G, Mazzer A, Cozzio S, Fania C, Casiglia E. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur J Epidemiol 2015;30:209–17. [DOI] [PubMed] [Google Scholar]
- 52.Fox GP, Wu A, Yiran L, Force L. Variation in caffeine concentration in single coffee beans. J Agric Food Chem 2013;61:10772–8. [DOI] [PubMed] [Google Scholar]
- 53.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, et al. . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 54.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 1994;24:471–6. [DOI] [PubMed] [Google Scholar]
- 55.Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L, Resuehr H, George B, Jeansonne MM, Allison DB. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One 2015;10:e0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez-Mateos A, Kraemer T, Cortese-Krott MM, Kuhnle GG, Spencer JP, et al. . Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial. Age (Dordr) 2015;37:9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Lovegrove JA, Minihane AM. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol Nutr Food Res 2012;56:966–75. [DOI] [PubMed] [Google Scholar]
- 58.Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Minihane AM. The impact of the catechol-O-methyltransferase genotype on the acute responsiveness of vascular reactivity to a green tea extract. Br J Nutr 2011;105:1138–44. [DOI] [PubMed] [Google Scholar]
- 59.Renda G, Zimarino M, Antonucci I, Tatasciore A, Ruggieri B, Bucciarelli T, Prontera T, Stuppia L, De Caterina R. Genetic determinants of blood pressure responses to caffeine drinking. Am J Clin Nutr 2012;95:241–8. [DOI] [PubMed] [Google Scholar]
- 60.Strandhagen E, Zetterberg H, Aires N, Palmer M, Rymo L, Blennow K, Landaas S, Thelle DS. The methylenetetrahydrofolate reductase C677T polymorphism is a major determinant of coffee-induced increase of plasma homocysteine: a randomized placebo controlled study. Int J Mol Med 2004;13:811–5. [PubMed] [Google Scholar]
- 61.Strandhagen E, Zetterberg H, Aires N, Palmer M, Rymo L, Blennow K, Thelle DS. The apolipoprotein E polymorphism and the cholesterol-raising effect of coffee. Lipids Health Dis 2004;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Castro-Orós I, Pampín S, Cofán M, Mozas P, Pintó X, Salas-Salvadó J, Rodríguez-Rey JC, Ros E, Civeira F, Pocoví M. Promoter variant -204A>C of the cholesterol 7alpha-hydroxylase gene: association with response to plant sterols in humans and increased transcriptional activity in transfected HepG2 cells. Clin Nutr 2011;30:239–46. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Muniz FJ, Maki KC, Schaefer EJ, Ordovas JM. Serum lipid and antioxidant responses in hypercholesterolemic men and women receiving plant sterol esters vary by apolipoprotein E genotype. J Nutr 2009;139:13–9. [DOI] [PubMed] [Google Scholar]
- 64.Zhao HL, Houweling AH, Vanstone CA, Jew S, Trautwein EA, Duchateau GS, Jones PJ. Genetic variation in ABC G5/G8 and NPC1L1 impact cholesterol response to plant sterols in hypercholesterolemic men. Lipids 2008;43:1155–64. [DOI] [PubMed] [Google Scholar]
- 65.Rideout TC, Harding SV, Mackay D, Abumweis SS, Jones PJ. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am J Clin Nutr 2010;92:41–6. [DOI] [PubMed] [Google Scholar]
- 66.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med 2007;167:1060–7. [DOI] [PubMed] [Google Scholar]
- 67.Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol 2007;49:74–80. [DOI] [PubMed] [Google Scholar]
- 68.Morand C, Dubray C, Milenkovic D, Lioger D, Martin JF, Scalbert A, Mazur A. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr 2011;93:73–80. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, Spencer JP. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr 2013;98:1179–91. [DOI] [PubMed] [Google Scholar]
- 70.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103:1024–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selma MV, Espin JC, Tomas-Barberan FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem 2009;57:6485–501. [DOI] [PubMed] [Google Scholar]
- 72.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. . Enterotypes of the human gut microbiome. Nature 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, et al. . Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500:541–6. [DOI] [PubMed] [Google Scholar]
- 74.Wong JM, Kendall CW, Marchie A, Liu Z, Vidgen E, Holmes C, Jackson CJ, Josse RG, Pencharz PB, Rao AV, et al. . Equol status and blood lipid profile in hyperlipidemia after consumption of diets containing soy foods. Am J Clin Nutr 2012;95:564–71. [DOI] [PubMed] [Google Scholar]
- 75.Usui T, Tochiya M, Sasaki Y, Muranaka K, Yamakage H, Himeno A, Shimatsu A, Inaguma A, Ueno T, Uchiyama S, et al. . Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin Endocrinol (Oxf) 2013;78:365–72. [DOI] [PubMed] [Google Scholar]
- 76.Hazim S, Curtis PJ, Schar MY, Ostertag LM, Kay CD, Minihane AM, Cassidy A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial. Am J Clin Nutr 2016;103:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. . Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. . A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. . Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. . Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. . Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clavel T, Desmarchelier C, Haller D, Gerard P, Rohn S, Lepage P, Daniel H. Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes 2014;5:544–51. [DOI] [PubMed] [Google Scholar]
- 83.Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma MV, Espín JC. Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 2014;62:6535–8. [DOI] [PubMed] [Google Scholar]
- 84.Mora-Cubillos X, Tulipani S, Garcia-Aloy M, Bullo M, Tinahones FJ, Andres-Lacueva C. Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome. Mol Nutr Food Res 2015;59:2480–90. [DOI] [PubMed] [Google Scholar]
- 85.Selma MV, Romo-Vaquero M, Garcia-Villalba R, Gonzalez-Sarrias A, Tomas-Barberan FA, Espin JC. The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism. Food Funct 2016;7:1769–74. [DOI] [PubMed] [Google Scholar]