Abstract
A large body of evidence supports the notion that incorrect or insufficient nutrition contributes to disease development. A pivotal goal is thus to understand what exactly is appropriate and what is inappropriate in food ingestion and the consequent nutritional status and health. The effective application of these concepts requires the translation of scientific information into practical approaches that have a tangible and measurable impact at both individual and population levels. The agenda for the future is expected to support available methodology in nutrition research to personalize guideline recommendations, properly grading the quality of the available evidence, promoting adherence to the well-established evidence hierarchy in nutrition, and enhancing strategies for appropriate vetting and transparent reporting that will solidify the recommendations for health promotion. The final goal is to build a constructive coalition among scientists, policy makers, and communication professionals for sustainable health and nutritional policies. Currently, a strong rationale and available data support a personalized dietary approach according to personal variables, including sex and age, circulating metabolic biomarkers, food quality and intake frequency, lifestyle variables such as physical activity, and environmental variables including one’s microbiome profile. There is a strong and urgent need to develop a successful commitment among all the stakeholders to define novel and sustainable approaches toward the management of the health value of nutrition at individual and population levels. Moving forward requires adherence to well-established principles of evidence evaluation as well as identification of effective tools to obtain better quality evidence. Much remains to be done in the near future.
Keywords: food, genetics, microbiome, nutritional status, personalized nutrition
Introduction
Nutritional guidelines: a historical perspective
The general concept that appropriate nutrition is a very powerful agent capable of promoting human health is strongly shared worldwide and has been supported by a large set of epidemiologic, observational, and experimental studies and clinical trials over the last century (1–3). Likewise, a consistent body of evidence supports the thesis that incorrect or insufficient nutrition contributes to disease development (4). However, understanding what exactly is appropriate and what is incorrect or insufficient nutrition has been a challenge. Moreover, the effective application of these concepts requires the translation of scientific information into practical approaches that have a tangible, measurable impact at both individual and population levels.
Over time, translational challenges have brought scientists together to try to provide guidance to people through the definition of nutritional recommendations and guidelines that may be successfully implemented in different geographic, cultural, ethnic, and socioeconomic contexts. Numerical recommendations for nutrients or foods are not independent of each other, and therefore the review process in setting guidelines should consider studies that have used both approaches. Moreover, some coherence between the different approaches is also expected to be more confident in the trust that can be placed in a guideline. The question about how much of a given nutrient is needed to meet various biological requirements was initially answered by setting reference values for energy and nutrients [dietary reference values (DRVs); RDA], mainly intended to address malnutrition due to nutrient deficiency. It must be noted that the early use of terms such as “recommended” in such guidance documents (e.g., Recommended Dietary Intakes) generated the idea of an intrinsic benefit in meeting these levels of nutrients at the individual level. The more appropriate term “reference” is now commonly used as well as the validity of referring such values mainly to populations. Examples of DRVs exist at international [e.g., European Food Safety Authority European Dietary Reference Values (5)] and national levels. It must be noted that DRVs, generalized to a reference average adult, represent the basis of nutrition labeling of foods, with evident limits when used to provide guidance to individuals. Conversely, with the current status of the evidence, where there is often large residual uncertainty even about big, high-level questions in nutrition arriving at specific numerical recommendations about an exact desirable threshold for specific nutrients (e.g., no more than a particular percentage of added sugars) or foods (e.g., less than a particular amount of red meat weekly) is often an unreliable exercise with outcomes that are debatable. In this perspective, it seems meaningless to simply recommend reducing saturated fat intake to <8% energy intake given the current knowledge that individual FAs behave biologically very differently and depend on the specific food matrix. Thus, in this context, recommendations to reduce dark chocolate (stearic acid) and cheese intakes may conceivably be counterproductive for health.
Nutritional guidelines have been developed over the last decades to meet different requirements, such as public health needs, and to promote a healthy lifestyle aimed at reducing the prevalence of some noncommunicable diseases (NCDs) (6). Currently, nutritional guidelines produced by national and international institutions and scientific organizations represent a large body of documents, including, for example, the widely used WHO guidelines on nutrition (7), the Dietary Guidelines for Americans 2015–2020 Eighth Edition (8, 9), the European dietary reference values for nutrient intakes, updated by the European Food Safety Authority (5), and the Nordic Nutrition Recommendations (2012) (10). In particular, WHO guidelines are intended as global, evidence-based recommendations directed to a wide audience, including policy makers, their expert advisers, and technical and program staff at organizations involved in the design, implementation, and scaling-up of nutrition actions for public health. The Dietary Guidelines for Americans are designed for professionals to help individuals consume a healthy, nutritionally adequate diet. By US law, the information in these Dietary Guidelines forms the foundation for developing federal food, nutrition, and health policies and programs in the United States. Beyond the mentioned activities, many countries worldwide refer their food policies to national guideline approaches. Furthermore, the UN Development Program is promoting the Sustainable Development Goals, which is a universal call to action to end poverty, protect the planet, and ensure that all people enjoy peace and prosperity; it also includes gender issues (11, 12).
Over the years, nutritional guidelines have been developed with the aid of expert panels and systematic reviews and have been distributed for expert and community comment in an effort to link scholarship and policy (13). Although the evidence basis in nutrition has increased in volume in recent years, its robustness is still often uncertain, and the derived nutritional guidelines may not have been significantly improved. This situation leads not only to limitations of interpretation that are not always clear-cut in the guidelines themselves but also to criticism of the validity of the process by which the guidelines were developed. Thus, there is a need to move forward to improve the quality and efficacy of nutritional guidelines, following international standards (14) and a process that requires validation with regard to various pivotal elements, such as full transparency in scientific data collection and analysis, documented evidence-based justification, grading, and evaluation of effectiveness. Interaction with policy makers and authoritative communication among all stakeholders, including citizens, is desirable (15), but one wants to guard also against biases that various stakeholders may have. Of particular relevance to the public is the need to understand the individual- and sex-related features of proposed nutritional recommendations. Real-life studies should also be encouraged to overcome the possible bias between population groups selected in trials and actual population composition and profile.
Therefore, the agenda for the development of nutritional guidelines should advance through evaluation of methodology in nutrition research, evidence hierarchy in nutrition, and strategies for appropriate vetting and reporting aimed at empowering recommendations, including specific implications for the future such as personalized nutrition in health promotion (Text Box 1).
TEXT BOX 1 NUTRITIONAL GUIDELINES: A HISTORICAL PERSPECTIVE
Appropriate nutrition is a powerful factor preventing multiple age-related chronic diseases and promoting human health.
Excessive unbalanced—but also insufficient—nutrition contributes to disease development.
Current nutritional guidelines based on observational epidemiological studies and some clinical trials have provided guidance to health professionals, policy makers, and the public for decades.
Nutritional recommendations and guidelines are most effective when implemented within appropriate geographic, cultural, ethnic, and socioeconomic contexts without forgetting age and sex differences.
Nutritional guidelines should evolve through the incorporation of insights into the methodology of guideline methods, better evidence, adherence to grading data within established hierarchy of available evidence, avoiding conflicts of interest and aiming at a constructive coalition among all stakeholders.
Current and Developing Status of Knowledge
Methodology in nutrition research
Historically, nutritional guidelines have been based on all the evidence available, including not only human clinical studies but also data available from experimental animal work and physiological studies. This evidence also includes information from population-based epidemiologic studies that have identified food patterns, nutrient intakes, and lifestyles associated with health promotion or with increased risk or progression of NCDs (4, 16). Moreover, additional insights have been generated by some randomized clinical trials (RCTs) and innovative new study designs, such as Mendelian Randomization and environment-wide association studies.
To evaluate the evidence useful for both population and individual decisions requires that ≥3 steps be taken into consideration: 1) what are the uncertainty limits of the available evidence, 2) how can we improve credibility by reducing uncertainty and variability, and 3) does the increased evidence credibility lead to improved usefulness in dietary guidance (17)? Interestingly, although 96% of the biomedical literature claims significant, positive results, the validity of these claims is often questionable (18). The reasons are multiple and generally well known although not widely appreciated or acknowledged. Among these are the problems of nonrandomized designs, post hoc data “cherry-picking” and “P hacking” to support desired hypotheses, lack of a priori data analysis plans or post hoc transparency in data analyses, selective reporting of results, lack of study registration on public databases, lack of a replication culture, and limited data sharing (19). Moreover, in some instances, publication quality in the field of nutrition shows a lack of consistency, especially for observational evidence, in which analytical approaches to newer data suggest that effects of soft outcomes (e.g., surrogate endpoints) may well be overestimated (20). Conclusions drawn from these newer insights support the very high rate of refutation observed in the most-cited claims of observational studies that were not validated in RCTs (21, 22). Nonetheless, among the scientific community, an inherent resistance to refutation is frequently observed so that unreliable and contradictory papers often have long lives as supporting references (23). Interestingly, it has recently been demonstrated that 68.5% of studies reporting routinely collected data did so for research questions already addressed by RCTs (24), suggesting that observational data may not be as informative as often claimed. Most likely, some inherent obstacles must be overcome in making sure that expert committees review draft guidelines and evidence provided in the format of a consensus conference, with the participation of all stakeholders. Moreover, because a substantial part of the evidence in nutrition is based on observational data, credibility on the causal pathway is often questionable (25), which is a problem compounded by weak conclusions drawn from diverse subgroups, stratified analyses, and data dredging in the absence of any preregistration. Conclusions drawn from nutrition research studies are sometimes based on statistically significant but small or tiny effect sizes (26). Tiny effects may still be credible, but they are highly susceptible to even minimal bias. Although larger-scale data and new measurement platforms offer novel opportunities, they also provide the potential for even higher error and misleading claims.
RCTs are an important pillar in evidence-based medicine and require improvement when done in the nutrition field. There is a need for improved transparency and improved, quality evidence of nonregulated interventions, especially compared with the rate of registration and publication of nondrug trials (27), because a large number of nutritional trials have never been registered (19).
Additionally, RCT designs in nutrition require attention to pragmatic issues to reduce the user burden of dietary assessment and long-term compliance, personnel and funding mechanisms to accommodate large sample sizes, and focus on important, patient-relevant outcomes (28). Additional strategies to improve nutritional research include the analysis of subgroups with stratified effects, pooling studies, and the use of biomarkers when available, although the effect could be diluted in the case of large RCTs (19, 29, 30).
A relevant observation when evaluating the health impact of nutrition is that people actually eat intact foods and not isolated nutrients. Most generalized nutritional guidelines are couched in terms of daily nutrient intakes. Although this is clearly important, it appears obvious that bioavailability of nutrients incorporated into a food matrix may be affected by their effects on digestion and absorption, which are also modulated by the matrix effect or by the actions of the gut microbiota. Moreover, the composition of many foods is not completely known, food composition tables are often incomplete or out of date, and some compounds are impossible to measure or are unknown. Not surprisingly, even for the most extensively studied questions, discrepancies may occur when nutrients or foods are evaluated (e.g., substitution of saturated fat with polyunsaturated fat in substitution studies compared with assessing the association between saturated fat intake in dairy products and health outcomes). These factors add to the uncertainty of conclusions about nutrients drawn from studies using foods (Text Box 2).
TEXT BOX 2 METHODOLOGY IN NUTRITION RESEARCH
Most evidence on nutrition is based on observational data.
Clinical outcomes with nominally statistically significant results are often arguable and of debatable clinical significance.
Randomized trials can confirm causality but have inherent design constraints for nutritional interventions.
Most secondary subgroup analyses and stratified effects are weak at best.
Large-scale data and new measurement platforms offer improved opportunities but have the potential for even higher error rates.
Clinical nutrition research designs and implementation studies require reforms focused on improved credibility and utility.
Taking advantage of big data.
In recent decades, major improvements in measurement capacity and computerized data analysis have led to fast high-throughput analyses at much lower costs. These have allowed different and heterogeneous sources of data to be integrated in novel ways that provide reliable new insights. Big data may be derived from epidemiological cohorts or related biorepositories with the power to elucidate millions of genetic variants and thousands of environmental and nutritional factors in their study participants. Although this is crucial, it is rather difficult to study and understand because it is inherently individual, with several variables based on genome and the individual interaction with the surrounding environment (starting from parents’ experience and fetal interferences). Electronic medical records of millions of patients, containing clinical, pharmacological, and laboratory data, are currently being repurposed for research and discovery. These research practices generate new concepts for discovery, which in turn raise new questions concerning what to measure and how in health research, whether and how to use and interpret these big data for discovery, and what roles they will eventually play in developing guidelines (31). Human health recommendations may benefit from large-scale data when noise is minimized, because false alarms due to confounding variables or other biases are possible even with very-large-scale studies (32–34).
Enhancement of the validity of guideline precision may emerge from big data analysis if accompanied by systematic testing, addressing multiplicity (29, 35–38), and replicating experiments, as well as considering the vibration of effects (shifts in the effect-size distribution due to selected adjustments or other analytical choices) in shaping the empirical distribution of effect sizes due to model choice (20). Furthermore, nutritional exposures and behavior are highly correlated with one another (33, 34, 39, 40), posing challenges in evaluating possible associations. Therefore, it is an imperative, along with systematically testing associations with clinical outcomes, to estimate how large (or small) an association is with respect to all other possible correlations (33).
There is also a need to assess associations between not just single nutritional factors and outcomes but an entire system of correlated nutritional factors and outcomes to accurately capture the complex and correlated dietary behavior of humans. There is the further need to document analytical approaches and provide both accessible analytical tools and computer infrastructure to enable reproducible research. There are various aspects of reproducible research, ranging from the ability to recompute data analytic results given an observed dataset and knowledge of the pipeline (41) to reproducibility across different datasets (reproducibility of results) and reproducibility of inferences from the same datasets and analyses (42) (Text Box 3).
TEXT BOX 3 TAKING ADVANTAGE OF BIG DATA
Big data analysis may provide answers based on a multitude of new ways to interrogate datasets and uncover insights into generating improved guidelines.
Big data may be derived from large epidemiological cohorts and/or related biorepositories and have the potential power to elucidate relations among millions of genetic variants and thousands of environmental and nutritional factors, but their utility is still to be proven.
The huge number of analytic scenarios can multiply the analytical challenges and magnify potential biases.
To enhance the validity of conclusions from big data there is a need for:
systematic testing procedures to address multiple hypotheses testing and results replication to enhance the validity of the results
consideration of the dense correlative nature of both clinical outcomes and nutritional factors
modeling scenarios that are fully detailed and explicitly transparent
increased education for literacy in understanding and interpreting information at the big-data level
Human health could benefit from large-scale data only if large-scale bias is likewise minimized.
Toward personalized nutrition.
When approaching novel methodologies in nutrition research, it is important to focus on the associations among individual genotypes and phenotypes, aiming at personalized nutritional strategies that will effectively promote the health of individuals. Ideally, the complex gene-gene and gene-environment interactions and epigenetic modulation should be taken into consideration when assessing nutritional and other environmental links with NCDs such as obesity, dyslipidemia, cardiovascular diseases, and cancer (43–46).
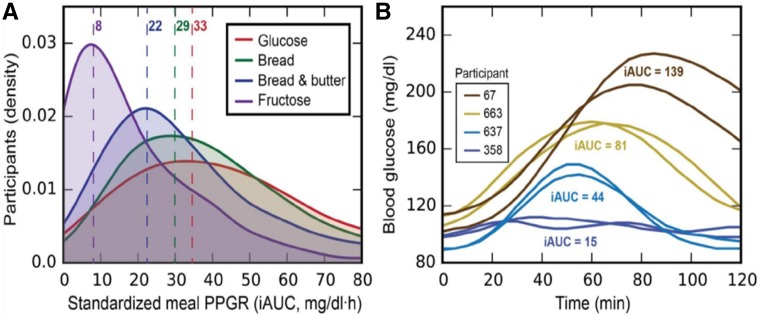
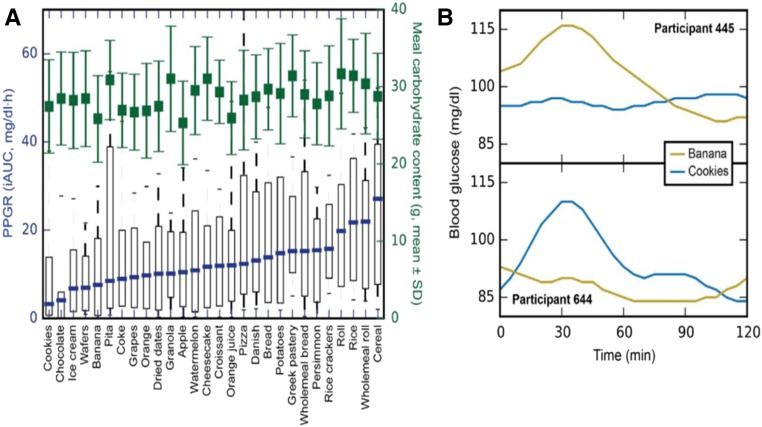
In the context of the current population epidemics of metabolic diseases, related to the interaction between the genome and nutritional changes and environmental factors, there are well-appreciated differences in how individuals within the population respond to the same environmental stimuli. For example, people have largely different glucose responses to the same food (47), and recent data suggest that integrating individual information into a multidimensional algorithm that predicts specific responses to food may allow definition of personalized diets (48). It has been proposed that one should individualize the diet according to personal variables, including sex, age, and microbiome profile (49). Major determinants of the variability in an individual’s glucose response to food may include food quality, intake frequency, and lifestyle, including physical activity, circulating metabolic biomarkers, and the gut microbiota. Data based on continuous postprandial glucose measurements have demonstrated that whether a food is nominally good or bad, regarding its effect on the postprandial glycemic response, is largely dependent on the individual consuming the specific food in relation to his or her personalized variables (48). Thus, individual people can have very different responses to the same food (Fig. 1). For instance, in response to white bread consumption, some people have the expected postprandial glucose spikes, whereas others do not (47, 50) (Fig. 2). Moreover, dietary interventions targeting postmeal glucose responses induce consistent changes in the gut microbiota, with relevant variations according to the type of diet (high-glucose response compared with low-glucose response diets) (51). Therefore, diets designed to maintain normal postprandial blood glucose concentrations must be personally tailored. If so, population-based guidelines may have limited generalizability when the prevalence of specific genetic, lifestyle, and other factors able to have a large impact in modifying the effect of the diet consumed is large in the population addressed. In any case, predictive diets for individuals are quite complex, and population-based clinical trials that test the value of the intervention of personalized recommendations on health outcomes, including time to cardiovascular disease, cancer, and death, must occur. To date, few observational investigations have shown the utility of integration of high-dimension information, including (prevalent) genetic variants, microbiome, and environmental exposures. These studies, already partially ongoing, will hopefully demonstrate feasibility for large-scale clinical trials for personalized interventions. Therefore, at this stage such predictors can assist in devising a dietary plan but cannot replace the general nutrition recommendations (Text Box 4).
FIGURE 1.
PPGRs to identical standardized meals can be highly variable among different people. (A) Population responses to standardized meals. Kernel density estimation histogram of PPGRs of healthy individuals (n = 800) to 4 selected meals. (B) Four individual responses to bread, showing the high interpersonal variability in PPGRs to bread across participants. iAUC, incremental AUC; PPGR, postprandial glucose response. Reprinted from reference 48 with permission from Elsevier.
FIGURE 2.
PPGRs to real-life meals can be highly variable among different people. (A) IQRs (10th–90th percentiles) of the PPGRs of healthy individuals (n = 800) to different meals along with the amounts of carbohydrates consumed (green; means ± SDs). (B) An example of inverse PPGRs to a set of 2 isocaloric real-life meals. iAUC, incremental AUC; PPGR, postprandial glucose response. Reprinted from reference 48 with permission from Elsevier.
TEXT BOX 4 TOWARD PERSONALIZED NUTRITION
People have highly variable postmeal glucose responses to identical meals.
Following current dietary guidelines may result in high glycemic responses in some subjects, accelerating metabolic disease development, which the guidelines were intended to prevent.
An individual’s microbiome is a driver of interpersonal variability in postmeal responses.
Integrating personal parameters and microbiome features into an algorithm may allow more accurate predictions of personalized postmeal glucose response to defined meals.
Personalized diets normalize postmeal glucose responses and increase compliance.
A personalized nutritional approach based on validated algorithms may be relevant for effectively promoting individual health.
The implementation of such novel approaches (e.g., big-data analysis, personalized nutrition algorithms) needs to be evaluated against conclusions based on traditional methods. Moreover, although the inclusion of these methods into new guidelines will surely improve the knowledge base, it remains to be shown whether the problems these methods create in more complex interpretations will lead to demonstrable improvements in better health and disease outcome in the long-term and in various sociocultural and economic conditions.
Evidence hierarchy in science with a focus on nutrition
The evidence hierarchy, built on the principles of the scientific method, is a construct widely shared among all sciences. Nutrition, as a science, must comply with and be judged by the same scientific principles as far as the grading the quality of its evidence is concerned. In practice, difficulties associated with designing and conducting human studies using real foods may limit compliance with these principles at the highest levels of the evidence hierarchy. Nonetheless, limitations of this kind do not serve as reasons to elevate the level of evidence obtained but rather to limit the certainty of conclusions drawn from the evidence available. Moreover, within this context, properly grading the caliber of available evidence is pivotal because trustworthy guidelines must systematically weigh both the amount of evidence and its quality. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, adopted by >100 organizations worldwide, has become the standard for rating the quality of evidence (52). The GRADE Working Group (53) has provided tools that indicate the reasons for a recommendation (direction, strength, and certainty) and allow adoption, adaptation, and new development of recommendations globally. Key criteria include the following: how big is the problem locally; how direct is the evidence; how does it impact on resources, equity, acceptability, and feasibility (54, 55).
In the GRADE system, randomized trials are initially graded as high-quality evidence, but their grade can be rated down to moderate or low/very low based on limitations in 5 categories: risk of bias, inconsistency, indirectness, imprecision, and publication bias (54, 56). On the other hand, observational studies are initially graded as low-quality evidence but can be rated up to a higher grade, primarily on the basis of large effect sizes. GRADE also provides guidance for grading recommendations as strong or weak. A panel makes strong recommendations when the net benefits clearly favor one option. A panel makes weak recommendations in the face of uncertainty, either because the evidence is of low or very-low quality or because the desirable and undesirable consequences (54, 57) are closely balanced. In making decisions regarding direction and strength of recommendations, guideline panels should always consider the magnitude of the desirable and undesirable consequences, the certainty of the evidence regarding those consequences, and the values and preferences of the population to whom the recommendation applies, the last being crucial in ensuring compliance. Panels may also consider resource use, acceptability, feasibility, and equity in making their recommendations. If guidelines are not adapted to real life, it is unlikely they will be used.
Evidence in nutrition: strategies for appropriate vetting and reporting, aimed at empowering recommendations
To produce trustworthy and optimal guidelines mandates a well-constructed panel of discussants, including scientific experts in the specific nutritional areas, methodologists including statisticians, practicing clinicians and patients, and policy makers, needs to become involved when necessary if medical and clinical care guidelines are under consideration. Expert translators are also of paramount importance when considering the wide practical use of these guidelines and the subsequent impact on clinical practice as well as on the population.
Standards for trustworthy guidelines are well established (e.g., Institute of Medicine recommendations) (58). Several authoritative international organizations (WHO, Institute of Medicine, the Guideline International Network, and the GRADE Working Group) agree on the key principles for the development of high-quality guidelines (59). International standards exist that will also ensure trustworthiness for nutritional guidelines based on progression to higher levels as bias in the quality of evidence declines (60).
Recommendations should be based on an explicit and transparent process that maximizes the use of the highest-quality graded evidence; minimizes distortions, biases, and conflicts of interest; provides a clear explanation of the logical relations between alternative care options and health outcomes; and provides ratings of both the quality of evidence and the strength of recommendations (61). More realistically, to provide high-quality systematic reviews of today’s expansive literature will require more than the voluntary spare time of already-pressed scientists. Governments should be obliged to appropriate the funds necessary for producing timely, high-quality, and evidence-based dietary reference intakes (62).
Because foods are so intimately related to lifestyles and food cultures in humans, instruments to assess the quality of life in relation to nutrition and nutrition-related lifestyle changes are also needed (63). Moreover, there is a need to assess sustainability, (e.g., environmental impact or economic impact) with regard to future recommendations. As it is obviously appropriate, trustworthy guidelines should be reconsidered and revised when important, new evidence warrants modifications of recommendations.
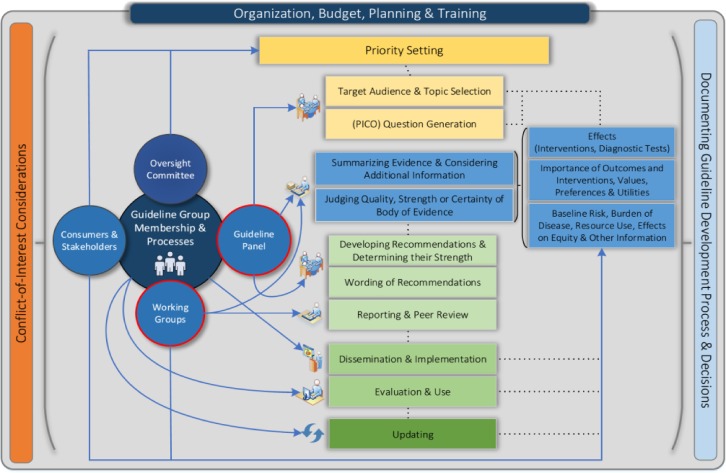
The presence of conflicts of interest can lead to biased and potentially incorrect recommendations (64). International principles for disclosure of interests and management of conflicts in guidelines have been developed to address this issue. However, declaration of conflict alone seems a poor strategy overall. More acceptable options for managing conflicts are to exclude altogether those with major conflicts or to allow input by conflicted individuals to participate in the discussion but excluding them from the decision-making process. Several tools for the development of trustworthy guidelines are available. In particular, a comprehensive checklist of items and related resources can help guideline developers in their enterprise (14) (Fig. 3). Additional tools include the Essential Reporting Items for Practice Guidelines in Healthcare (65) statement, which helps those producing guidelines report them properly and in a certain format for the lay audience (Text Box 5).
FIGURE 3.
Diagram of the guideline development process. The steps and involvement of various members of the guideline development group are interrelated and not necessarily sequential. The guideline panel and supporting groups work collaboratively, informed through consumer and stakeholder involvement, and report to an oversight committee or board overseeing the process. Considerations for organization, planning, and training encompass the entire guideline development project and steps, such as documenting the methodology used, the decisions made, and considering conflicts of interest, occur throughout the process. PICO, patient/problem, intervention, comparison, outcome. Reprinted from reference 14 with permission from Access Copyright.
TEXT BOX 5 EVIDENCE IN NUTRITION: STRATEGIES FOR APPROPRIATE VETTING AND REPORTING, AIMED AT EMPOWERING RECOMMENDATIONS
Trustworthy guidelines should:
be based on a systematic review of the existing evidence
be developed by a knowledgeable, multidisciplinary panel of experts and representatives from key affected groups
consider important patient subgroups and patient preferences as appropriate
be based on an explicit and transparent process that minimizes distortions, biases, and conflicts of interest
provide a clear explanation of the logical relations between alternative care options and health outcomes
provide ratings of the quality of the evidence and strength of the recommendations
be reconsidered and revised as appropriate when important new evidence arises
Implications for the future
Based on the concepts developed above, one might envision a series of implications for the future aimed at improving nutritional guidelines and effectively applying them to people worldwide. These could also consider personalized nutrition, ethnic and geographic preferences, more effective translation of nutrition guidelines for the public, and promotion of sustainability and cooperation among all nutrition stakeholders.
Among them, the food industry plays a central role when food industry interventions in industrial food production are taken into consideration. Very rarely it is possible to access foods that have not been treated industrially or have not undergone a treatment (i.e., pesticide treatment or genetic manipulation) at any level of the production chain. How is it possible to manage this artificial input into the food chain? What is the impact on individuals?
Ethnic and geographic issues.
Lessons learned from employing the experimental principles discussed immediately above might also be extended to individualizing guidelines based on ethnic and national food preferences. The selection of specific local foods included in a diet represents a critical issue in the translation of guidelines as well as likely health promotion outcomes, because dietary compliance is intimately related to local and ethnic food preferences. It is now also well appreciated that nutrient-based recommendations should be focused on foods as the source of nutrients. Moreover, recommendations should not be based on indirect evidence, such as a prediction from nutrient composition listed in the label, but on solid scientific evidence, accumu-lated from actual subject responses to the particular foods themselves (48). These refinements can lead to improvement in dietary approaches based on traditional or regional habits that have already been validated and translated into recommendations for health promotion, e.g., starting with the Mediterranean Diet (66) and translating it into corresponding regional geographic variants, including the recently developed New Nordic Diet in Denmark (67, 68).
Effectively translating nutritional guidelines for the public.
A particularly relevant issue in effective guideline development is how to properly communicate the information to the general public in the current era of widespread, largely uncontrolled dissemination of information via an almost limitless variety of media outlets. The revolution in online media has drastically altered the pressure on journalists to reach readers, changing the ways that complicated stories, such as nutritional topics, are written and presented. Indeed, nutritional issues, which are often intrinsically complex, are difficult to report comprehensively and, even when truly balanced, frequently fail online. Because ambiguity does not sell, there is pressure to oversimplify.
The traditional fact-checking stringency of legitimate print media outlets has largely been bypassed by many of the newer electronic “information” sites online. The result has been an abundance of often-conflicting information that both generates public confusion and produces issues of credibility (69, 70). Problems often begin with the reliability of the media translation of the original research reports. Recently, 18 kinds of media spin were identified, and ≥1 spin was found in 88% of media research reports: 25% failed to report adverse events mentioned in the scientific article, 49% claimed a causal effect despite a nonrandomized study design, and 21% extrapolated a beneficial effect from an animal study to humans (71). For many people, the media are the main provider of the information that individuals use to make decisions about their health. Thus inaccurate, incomplete, or imprecise reporting of the research reports themselves is a major impediment in conveying solid nutrition evidence from scientists to citizens. However, the scientists themselves are not blameless in this context. Lazarus et al. (72) reported finding ≥1 example of spin in 84% of scientific reports studied, most commonly the improper implication of causality and a high degree of overselling the research findings in approximately half of the publication abstracts. Furthermore, although peer reviewers identified an example of spin in about half of the research manuscripts they reviewed, resulting in author removal of two-thirds of these items, the peer reviewers failed to identify spin in three-quarters of the abstracts of the manuscripts reviewed (73). Surprisingly, for 15% of the reviewed articles, the referees themselves suggested adding some spin, and in 9% of the reviewed articles the authors themselves added additional spin (73).
Promoting sustainability and cooperation among all nutrition stakeholders.
For guidelines to be maximally effective there is a need for cooperation among all nutrition stakeholders (individuals, citizens of any age and sex, scientists, clinicians, policy makers, the food industry, the communications industry, etc.).
Furthermore, reshaping food systems around sustainable diets is one of the world’s biggest challenges for the 21st century. Sustainability is a complex concept, and sustainable development was first introduced in Europe in the 1980s. In the ensuing years, there has been a growing concern for sustainability, including the food and nutrition field, which has gained the attention of researchers, academics, and practitioners and has become a focus for governments, private organizations, and other stakeholders (74). Countries vary in their conceptual understanding of sustainability and in its practical implementation determined by their own health agencies in the complex local policy environment. Nevertheless, the nature of global interconnectivity today poses sustainability problems that must be solved at the international level. Different approaches (evidence briefs, policy dialogues, and benchmarking) mandate international information and debate on policymaking.
Conclusions
In this article, the most important issues relevant to improving nutritional guidelines are discussed and the proposed concepts and actions are the result of the merged efforts of a qualified panel of experts in the related areas. The following conclusions of such joint work are proposed.
Nutritional guidelines: a historical perspective
There is a need to move forward to improve the quality and efficacy of nutritional guidelines, starting from an unbiased assessment of the currently consolidated information. The future agenda should advance through evaluation of newly available methodology in nutrition research to personalize guideline recommendations, properly grade the evidence quality, adhere to evidence hierarchy in nutrition, and enhance strategies for appropriate vetting and transparent reporting to solidify the recommendations for health promotion. The final goal is to build a constructive coalition among scientists, policy makers, and communications professionals to develop and implement sustainable health and nutritional policies. Constructive integration that facilitates harmonization among institutions is necessary for the formulation of nutritional recommendations, guidelines, and policies, because they must be implemented in different geographical, cultural, ethnic, and socioeconomic contexts to produce a relevant public health impact.
Methodology in nutrition research
Nutritional trials require an improvement in the design, collection, analysis, transparency, and quality of evidence at all levels of research. To improve nutritional research, it is important to increase study registration in public databases and to include predeclaration of endpoints and analytical approaches and open access for data. Nutritional guidelines need to be periodically reexamined and revised accordingly as new data become available. Moreover, there is a need to ensure that dietary essential nutrient and food recommendations apply to all subjects present in the society. Innovative scientific research generates new concepts for discovery, raising new questions concerning what and how to use the novel findings. The pervasive expansion of big data in the health research field has opened new horizons for their use for discovery or to develop guidelines (31), generating many challenges, especially in the context of causal pathway interpretation. Human health could benefit from large-scale data analysis, if large-scale noise is minimized and confounding variables or other biases are evaluated (32–34). Proper use of big data may help in designing nutritional guidelines for individual intervention and improve their effectiveness and relevance over the limitations of the generalized approach available today (48).
Evidence hierarchy in science, with a focus on nutrition
The principles of the scientific method apply to nutrition as they do to all disciplines classified as scientific. Trustworthy guidelines should be based on systematic summaries of the best available, properly graded evidence addressing each recommendation that is part of the guidelines. In making decisions regarding direction and strength of recommendations, guideline panels should consider the totality of evidence and the magnitude of the desirable and undesirable health effects, the domains of evidence certainty or uncertainty both with respect to the desired goals and potential undesirable effects. To support sustainability, guideline panels should also consider all desirable and undesirable consequences, including resource use, environmental and ecological consequences, acceptability, feasibility, and equity, in making their recommendations (54–57, 75, 76).
Evidence in nutrition: strategies for appropriate vetting and reporting, aimed at empowering recommendations
To produce trustworthy and optimal guidelines, it is mandatory to have a well-constructed, well-balanced panel of discussants, including experts in specific areas, methodologists, and practicing clinicians and patients if medical and clinical care guidelines are under consideration (77). Guidelines should be based on an explicit and transparent process that minimizes distortions, biases, and conflicts of interest; provides a clear explanation of the logical relations between alternative care options and health outcomes; and provides ratings of the quality of the evidence and the strength of the recommendations (61). The GRADE recommendation classifies systematic reviews of RCTs with an initial score of high and classifies systematic reviews of cohort studies with a score of low. As the studies are evaluated, the individual RCTs can be rated lower and the individual cohort studies can be rated higher depending on prespecified limitations of the former and the effect sizes of the latter. To complement this methodologic gap, improved measures and tools that also take into account nutrition research–specific requirements (e.g., dietary assessment methods and their validation or funding bias) for assessing the meta-evidence (quality of the evidence of the meta-analyses) need to be developed. Recently, an attempt to adapt the GRADE approach to specifically address peculiarities of nutrition research has been proposed [NutriGRADE from Schwingshackl et al. (78)]. For optimal implementation, this approach is best conducted with interaction with the GRADE working group, which we encourage and welcome strongly.
Implications for the future
Novel approaches may lead to the development of nutritional, exercise, and pharmacological interventions targeting the metabolic and molecular causes of human ageing and health promotion, inhibiting pro-aging pathways that control the accumulation of molecular damage in multiple tissues or minimizing the risks of diseases that contribute to or accelerate those pathways (48, 79). Accurate predictions of the individual metabolic response integrating different approaches may lead to personalized nutrition able to combine health promotion and the possible use of locally available foods (48). The transfer of this information to novel nutritional guidelines to improve the effectiveness of current generalized guidelines, however, still appears complex.
Although most guidelines have historically focused on the essential nutrient components of foods, future nutritional recommendations must evaluate evidence derived from ingestion of whole foods or diets.
A crucial issue is the communication of the fundamental nutritional information in the current electronic media environment, where traditional factual evidence verification is often lacking. Improved communications and effectiveness require cooperation among all nutrition stakeholders (the lay public, basic scientists, practicing clinicians, policy makers, industry, education, communication, etc.). The specific issue of sustainability requires the additional communication among governments, nations, and international regulatory agencies.
In conclusion, there is a strong and urgent need to develop a successful commitment among all the stakeholders to define novel approaches to the management of the health value of nutrition at the individual and population levels. Moving forward requires adherence to well-established principles of evidence evaluation and the identification of effective tools to obtain better-quality evidence. Much remains to be done in the near future. A starting step is to identify common acceptable definitions (Text Box 6).
TEXT BOX 6 COMMON ACCEPTABLE DEFINITIONS
Biomarkers
A biomarker is a natural molecule, gene, or functional characteristic by which a specific physiological or pathological process can be identified. They are commonly used to diagnose conditions and to assess how advanced an individual’s illness is.
Conflict of interest
An interest that may affect an individual’s ability to impartially assess the evidence or provide a perspective on a particular topic. Conflicts can be financial, where the person is in direct or indirect receipt of financial support, or intellectual, where the person may have a reputation built on a particular stance on an issue.
Diet
Diet is the sum of food and drink consumed by an individual and often implies its quality, composition, and effects on health.
Dietary guidelines
Dietary guidelines translate nutritional guidelines into food intake recommendations by using nontechnical language, enabling individual consumers to compose their daily diet in a way that provides the appropriate nutrition.
Feasibility/implementation
Feasibility and implementation consider how health policy will be implemented, including assessing and mitigating any individual, social, cultural, economic, and practical barriers to implementation; for example, not recommending food sources of nutrition that the majority of the population may not be able to access because of financial constraints or availability.
Food
Food consists of essential body nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals, which are ingested and assimilated by an individual to produce energy, stimulate growth, and maintain life.
Guidelines
Guidelines are a series of recommendations on a particular topic (e.g., health condition or aspect of health, such as nutrition), developed by a multidisciplinary panel based on an independent systematic review of the best available evidence. Guideline panels can include health professionals and academics specializing in that area, as well as representatives of other groups such as the general public, the policy makers, and the industry.
Nutrition
Nutrition interprets the interaction of nutrients and other substances in food in relation to the linked metabolic effects within the body. It includes food intake, absorption, assimilation, metabolism, and excretion.
Nutritional guidelines
Nutritional guidelines focus on the quantities of individual nutrients and quality and quantity of whole foods that people should consume to achieve a healthy nutritional state. Nutritional guidelines may include estimates such as DRVs, reference intake, and daily intake. These guidelines usually apply to the entire healthy population by using broad groups, such as different age ranges, but can also be tailored to more focused population groups. The general public often come into contact with these when examining food packaging, which may have DRVs on the front, etc.
Nutritional status
Nutritional status includes the condition of the body, influenced by the actions and interactions generated from the food intake through metabolism and absorption in the gut (exercised by microbiome, genetic, and food component interactions), and the consequent metabolism and handling within the body (due to genetic and organ—not only liver and kidney—functions) toward to the nutritional status differences on health effects.
Policy makers
Policy makers are professionals working within local and national government who are responsible for translating research findings into actionable health policy to promote health in their population, for example, creating food-based guidelines based on nutritional guidelines, the best available evidence, and stakeholder input.
RCT
An RCT is a clinical study with a specific design aimed to reduce bias when testing a new treatment. Subjects participating in the trial are randomly allocated to either the group receiving the treatment under investigation or to a group receiving standard treatment (or placebo treatment) as the control.
Substitution effect
When advised to eat less of one nutrient (e.g., carbohydrate) or individual food, the public will substitute that item with another. Substitution advice should be provided to ensure healthy substitutions that do not have unintended harms.
Surrogate disease biomarker
In some research areas, it may be challenging to conduct studies that are sufficiently long term to wait for disease outcomes (such as heart attack) or answers that may be required in the meantime. In such cases, biomarkers of that disease (e.g., blood pressure) can be measured to predict the likely risk of later developing the disease. However, these results indicate a possible risk rather than providing direct causal proof.
Weak/qualified/conditional recommendations
Where evidence is limited in terms of its quality or quantity, this affects the level of certainty in any conclusions based on that evidence. Describing recommendations as weak, qualified, or conditional communicates this level of uncertainty.
Acknowledgments
Panel of experts invited to the meeting in Venice, Italy—Carlo Agostoni: Pediatric Medium Intensity Care Unit, Department of Clinical Sciences and Community Health, Università degli Studi di Milano, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; Arne Astrup: Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark; Dennis M Bier: Children’s Nutrition Research Center, Baylor College of Medicine, Houston, TX; Furio Brighenti: Department of Food Sciences, University of Parma, Italy; Paolo Cavallo Perin: Department of Medical Sciences, University of Turin, Italy; Elena Colombo: Giovanni Lorenzini Medical Science Foundation, Milan, Italy; Rob Cook: Bazian, Economist Intelligence Unit Healthcare, London, United Kingdom; Lorenzo Maria Donini: Food Science and Human Nutrition Research Unit, Sapienza University, Rome, Italy; Christopher Emsden: Policy Sonar, Rome, Italy; Emanuela Folco: Giovanni Lorenzini Medical Science Foundation, Milan, Italy, and Houston, TX; Luigi Fontana: Department of Clinical and Experimental Sciences, University of Brescia, Italy, and Department of Medicine, Washington University, St. Louis, MO; Robert A. Gibson: School of Agriculture, Food and Wine, FOODplus Research Centre, University of Adelaide, Australia; Maria Giovanna Graziani: Gastroenterology and Digestive Endoscopy Unit, San Giovanni Addolorata Hospital, Rome, Italy; Ranieri Guerra, Department of Preventive Health, Ministry of Health, Rome, Italy; Gordon H Guyatt: Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada; John PA Ioannidis: C.F. Rehnborg Chair in Disease Prevention, Department of Health Policy and Research, Stanford University, Stanford, CA; Ann S. Jackson: Giovanni Lorenzini Medical Foundation, Houston, TX; David M. Klurfeld: Human Nutrition Program, USDA Agricultural Research Service, Beltsville, MD; Paolo Magni: Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano, Milan, Italy; Carlos Daniel Magnoni: Department of Nutrition and Nutritional Therapy, HCor Heart Hospital (SP), Department of Clinical Nutrition, Dante Pazzanese Cardiovascular Institute, Sao Paulo, Brazil; Maria Makrides: Healthy Mothers, Babies and Children, South Australian Health and Medical Research Institute, Adelaide, Australia; Basil Mathioudakis: Consulting sprl, Food Legislation and Nutrition, Brussels, Belgium; Alessandro Monaco: Giovanni Lorenzini Medical Science Foundation, Milan, Italy; Elvira Naselli: La Repubblica, Rome, Italy; Elly O’Brien: Bazian, Economist Intelligence Unit, London, United Kingdom; Chirag J. Patel: Department of Biomedical Informatics, Harvard Medical School, Boston, MA; Sergio Pecorelli: Giovanni Lorenzini Medical Foundation, Houston, TX; Andrea Peracino: Giovanni Lorenzini Medical Science Foundation, Milan, Italy; Giorgio Racagni: Department of Pharmacology and Biomolecular Sciences, Faculty of Pharmaceutical Sciences, Università di Milano, Milan, Italy; Holger J Schünemann: Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada; Raanan Shamir: Institute Gastroenterology, Nutrition and Liver Diseases, Schneider Children’s Medical Center of Israel - Sackler Faculty of Medicine, University of Tel Aviv, Israel; Katherine L Tucker: Department of Clinical Laboratory and Nutritional Sciences, University of Massachusetts, Lowell, MA; Peter Whoriskey: The Washington Post, Washington, DC; Niv Zmora: Department of Immunology, Weizmann Institute of Science, Rehovot, Israel. All authors read and approved the final version of the paper.
Footnotes
Abbreviations used: DRV, dietary reference value; GRADE, The Grading of Recommendations Assessment, Development and Evaluation; NCD, noncommunicable disease; RCT, randomized clinical trial.
References
- 1.Watts ML, Hager MH, Toner CD, Weber JA. The art of translating nutritional science into dietary guidance: history and evolution of the Dietary Guidelines for Americans. Nutr Rev 2011;69:404–12. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell 2015;161:106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy SP, Yates AA, Atkinson SA, Barr SI, Dwyer J. History of nutrition: the long road leading to the dietary reference intakes for the United States and Canada. Adv Nutr 2016;7:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onvani S, Haghighatdoost F, Surkan PJ, Larijani B, Azadbakht L. Adherence to the healthy eating index and alternative healthy eating index dietary patterns and mortality from all causes, cardiovascular disease and cancer: a meta-analysis of observational studies. J Hum Nutr Diet 2017;30:216–26. [DOI] [PubMed] [Google Scholar]
- 5.European Food Safety Authority. Dietary reference values and dietary guidelines [Internet]. c2017 [cited 2017 Mar 20]. Available from: https://www.efsa.europa.eu/en/topics/topic/drv.
- 6.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. WHO guidelines on nutrition[Internet]. c2017 [cited 2017 Mar 20]. Available from: http://www.who.int/publications/guidelines/nutrition/en/.
- 8.Office of Disease Prevention and Health Promotion. Dietary guidelines for Americans 2015–2020 [Internet]. c2017 [cited 2017 Mar 20]. Available from: https://health.gov/dietaryguidelines/2015/guidelines/.
- 9.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: development and major conclusions. Adv Nutr 2016;7:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordic co-operation. Nordic nutrition recommendations 2012 [Internet]. c2017 [cited 2017 Mar 20]. Available from: https://www.norden.org/en/theme/nordic-nutrition-recommendation.
- 11.Sustainable Development. Sustainable Development Goals [Internet]. c2017 [cited 2017 Mar 20]. Available from: https://sustainabledevelopment.un.org/sdgs.
- 12.Taukobong HF, Kincaid MM, Levy JK, Bloom SS, Platt JL, Henry SK, Darmstadt GL. Does addressing gender inequalities and empowering women and girls improve health and development programme outcomes? Health Policy Plan 2016;31:1492–514. [DOI] [PubMed] [Google Scholar]
- 13.Morgan PJ. Back to the future: the changing frontiers of nutrition research and its relationship to policy. Proc Nutr Soc 2012;71:190–7. [DOI] [PubMed] [Google Scholar]
- 14.Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar KT, Kowalski S, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brownell KD, Roberto CA. Strategic science with policy impact. Lancet 2015;385:2445–6. [DOI] [PubMed] [Google Scholar]
- 16.LaRocca TJ, Martens CR, Seals DR. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev 2016. Sep 28 (Epub ahead of print; DOI: 10.1016/j.arr.2016.09.002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohlhorst SD, Russell R, Bier D, Klurfeld DM, Li Z, Mein JR, Milner J, Ross AC, Stover P, Konopka E. Nutrition research to affect food and a healthy life span. Am J Clin Nutr 2013;98:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016;315:1141–8. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis JP. We need more randomized trials in nutrition-preferably large, long-term, and with negative results. Am J Clin Nutr 2016;103:1385–6. [DOI] [PubMed] [Google Scholar]
- 20.Patel CJ, Burford B, Ioannidis JP. Assessment of vibration of effects due to model specification can demonstrate the instability of observational associations. J Clin Epidemiol 2015;68:1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA 2005;294:218–28. [DOI] [PubMed] [Google Scholar]
- 22.Young SS, Karr A. Deming, data and observational studies. Significance 2011;8:116–20. [Google Scholar]
- 23.Brown AW, Ioannidis JP, Cope MB, Bier DM, Allison DB. Unscientific beliefs about scientific topics in nutrition. Adv Nutr 2014;5:563–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JP. Routinely collected data and comparative effectiveness evidence: promises and limitations. CMAJ 2016;188:E158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JP. Implausible results in human nutrition research. BMJ 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 26.Siontis GC, Ioannidis JP. Risk factors and interventions with statistically significant tiny effects. Int J Epidemiol 2011;40:1292–307. [DOI] [PubMed] [Google Scholar]
- 27.Dal-Ré R, Bracken MB, Ioannidis JP. Call to improve transparency of trials of non-regulated interventions. BMJ 2015;350:h1323. [DOI] [PubMed] [Google Scholar]
- 28.Hébert JR, Frongillo EA, Adams SA, Turner-McGrievy GM, Hurley TG, Miller DR, Ockene IS. Perspective: randomized controlled trials are not a panacea for diet-related research. Adv Nutr 2016;7:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van Horn L, Daviglus ML, et al. A nutrient-wide association study on blood pressure. Circulation 2012;126:2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium(FORCe). Omega-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 2016;176:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoury MJ, Ioannidis JP. Medicine. Big data meets public health. Science 2014;346:1054–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel CJ, Chen R, Kodama K, Ioannidis JP, Butte AJ. Systematic identification of interaction effects between genome- and environment-wide associations in type 2 diabetes mellitus. Hum Genet 2013;132:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel CJ, Ioannidis JP. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J Epidemiol Community Health 2014;68:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel CJ, Ioannidis JP. Studying the elusive environment in large scale. JAMA 2014;311:2173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel CJ, Cullen MR, Ioannidis JP, Butte AJ. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol 2012;41:828–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel CJ, Rehkopf DH, Leppert JT, Bortz WM, Cullen MR, Chertow GM, Ioannidis JP. Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States National Health and Nutrition Examination Survey. Int J Epidemiol 2013;42:1795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merritt MA, Tzoulaki I, Tworoger SS, De Vivo I, Hankinson SE, Fernandes J, Tsilidis KK, Weiderpass E, Tjønneland A, Petersen KE, et al. Investigation of dietary factors and endometrial cancer risk using a nutrient-wide association study approach in the EPIC and Nurses’ Health Study (NHS) and NHSII. Cancer Epidemiol Biomarkers Prev 2015;24:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merritt MA, Tzoulaki I, van den Brandt PA, Schouten LJ, Tsilidis KK, Weiderpass E, Patel CJ, Tjønneland A, Hansen L, Overvad K, et al. Nutrient-wide association study of 57 foods/nutrients and epithelial ovarian cancer in the European Prospective Investigation into Cancer and Nutrition study and the Netherlands Cohort Study. Am J Clin Nutr 2016;103:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ioannidis JP. Exposure-wide epidemiology: revisiting Bradford Hill. Stat Med 2016;35:1749–62. [DOI] [PubMed] [Google Scholar]
- 40.Ioannidis JP, Loy EY, Poulton R, Chia KS. Researching genetic versus nongenetic determinants of disease: a comparison and proposed unification. Sci Transl Med 2009;1:7ps8. [DOI] [PubMed] [Google Scholar]
- 41.Leek JT, Peng RD. Opinion: reproducible research can still be wrong: adopting a prevention approach. Proc Natl Acad Sci USA 2015;112:1645–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman SN, Fanelli D, Ioannidis JP. What does research reproducibility mean? Sci Transl Med 2016;1:341ps12. [DOI] [PubMed] [Google Scholar]
- 43.Parnell LD, Lee YC, Lai CQ. Adaptive genetic variation and heart disease risk. Curr Opin Lipidol 2010;21:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett BJ, Hall KD, Hu FB, McCartney AL, Roberto C. Nutrition and the science of disease prevention: a systems approach to support metabolic health. Ann N Y Acad Sci 2015;1352:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 2016;130:943–86. [DOI] [PubMed] [Google Scholar]
- 46.Reddon H, Gueant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci (Lond) 2016;130:1571–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vega-López S, Ausman LM, Griffith JL, Lichtenstein AH. Interindividual variability and intra-individual reproducibility of glycemic index values for commercial white bread. Diabetes Care 2007;30:1412–7. [DOI] [PubMed] [Google Scholar]
- 48.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 49.Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe 2016;19:12–20. [DOI] [PubMed] [Google Scholar]
- 50.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials 2010;31:5–11. [DOI] [PubMed] [Google Scholar]
- 51.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature 2016;535:65–74. [DOI] [PubMed] [Google Scholar]
- 52.GRADE Working Group. The GRADE working group [Internet]. c2017 [cited 2017 Mar 20]. Available from: http://www.gradeworkinggroup.org/.
- 53.GRADEpro GDT. GRADE’s software for summary of findings tables, health technology assessment and guidelines [Internet]. c2017 [cited 2017 Mar 20]. Available from: www.GRADEpro.org.
- 54.Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, et al. ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: introduction. BMJ 2016;353:i2016. [DOI] [PubMed] [Google Scholar]
- 55.Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, Scholten R, Langendam M, Leeflang MM, Akl EA, et al. ; GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 2016;76:89–98. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt GH, Alonso-Coello P, Schunemann HJ, Djulbegovic B, Nothacker M, Lange S, Murad MH, Akl EA. Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol 2016;80:3–7. [DOI] [PubMed] [Google Scholar]
- 57.Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, et al. ; the GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. BMJ 2016;353:i2089. [DOI] [PubMed] [Google Scholar]
- 58.The National Academies of Sciences, Engineering, and Medicine. Health and medicine division [Internet]. c2017 [cited 2017 Mar 20]. Available from: https://www.nationalacademies.org/hmd.
- 59.Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 9. Grading evidence and recommendations. Health Res Policy Syst 2006;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schünemann HJ, Fretheim A, Oxman AD; WHO Advisory Committee on Health Research. Improving the use of research evidence in guideline development: 1. Guidelines for guidelines. Health Res Policy Syst 2006;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fretheim A, Schunemann HJ, Oxman AD. Improving the use of research evidence in guideline development: 3. Group composition and consultation process. Health Res Policy Syst 2006;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bier DM, Willett WC. Dietary Reference Intakes: resuscitate or let die? Am J Clin Nutr 2016;104:1195–6. [DOI] [PubMed] [Google Scholar]
- 63.Schünemann HJ, Sperati F, Barba M, Santesso N, Melegari C, Akl EA, Guyatt G, Muti P. An instrument to assess quality of life in relation to nutrition: item generation, item reduction and initial validation. Health Qual Life Outcomes 2010;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schünemann HJ, Al-Ansary LA, Forland F, Kersten S, Komulainen J, Kopp IB, Macbeth F, Phillips SM, Robbins C, van der Wees P, et al. ; Board of Trustees of the Guidelines International Network. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med 2015;163:548–53. [DOI] [PubMed] [Google Scholar]
- 65.The RIGHT Working Group. A proposal of essential reporting items for practice guidelines in health systems (RIGHT) [Internet]. c2017 [cited 2017 Mar 20]. Available from: http://www.equator-network.org/wp-content/uploads/2009/02/RIGHT-Guideline.pdf.
- 66.Medina-Remón A, Casas R, Tresserra-Rimbau A, Ros E, Martínez-González MA, Fitó M, Corella D, Salas-Salvadó J, Lamuela-Raventos RM, Estruch R; PREDIMED Study Investigators Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A sub-study of The PREDIMED trial. Br J Clin Pharmacol 2017;83:114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mithril C, Dragsted LO, Meyer C, Blauert E, Holt MK, Astrup A. Guidelines for the New Nordic diet. Public Health Nutr 2012;15:1941–7. [DOI] [PubMed] [Google Scholar]
- 68.Mithril C, Dragsted LO, Meyer C, Tetens I, Biltoft-Jensen A, Astrup A. Dietary composition and nutrient content of the New Nordic Diet. Public Health Nutr 2013;16:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yavchitz A, Boutron I, Bafeta A, Marroun I, Charles P, Mantz J, Ravaud P. Misrepresentation of randomized controlled trials in press releases and news coverage: a cohort study. PLoS Med 2012;9:e1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vinkers CH, Tijdink JK, Otte WM. Use of positive and negative words in scientific PubMed abstracts between 1974 and 2014: retrospective analysis. BMJ 2015;351:h6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haneef R, Lazarus C, Ravaud P, Yavchitz A, Boutron I. Interpretation of results of studies evaluating an intervention highlighted in Google health news: a cross-sectional study of news. PLoS One 2015;10:e0140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lazarus C, Haneef R, Ravaud P, Boutron I. Classification and prevalence of spin in abstracts of non-randomized studies evaluating an intervention. BMC Med Res Methodol 2015;15:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazarus C, Haneef R, Ravaud P, Hopewell S, Altman DG, Boutron I. Peer reviewers identified spin in manuscripts of nonrandomized studies assessing therapeutic interventions, but their impact on spin in abstract conclusions was limited. J Clin Epidemiol 2016;77:44–51. [DOI] [PubMed] [Google Scholar]
- 74.Johnston JL, Fanzo JC, Cogill B. Understanding sustainable diets: a descriptive analysis of the determinants and processes that influence diets and their impact on health, food security, and environmental sustainability. Adv Nutr 2014;5:418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 76.Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris S, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 2013;66:726–35. [DOI] [PubMed] [Google Scholar]
- 77.Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 10. Integrating values and consumer involvement. Health Res Policy Syst 2006;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopanteils E, Iqbal K, et al. Perspective: nutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 2016;7:994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature 2014;511:405–7. [DOI] [PubMed] [Google Scholar]