Abstract
The guidelines for nutritional support in critically ill adult patients differ in various aspects. The optimal amount of energy and nutritional substrates supplied is important for reducing morbidity and mortality, but unfortunately this is not well known, because the topic is complex and every patient is individual. The aim of this review was to gather recent pertinent information concerning the nutritional support of critically ill patients in the intensive care unit (ICU) with respect to the energy, protein, carbohydrate, and lipid intakes and the effect of their specific utilization on morbidity and mortality. Enteral nutrition (EN) is generally recommended over parenteral nutrition (PN) and is beneficial when administered within 24–48 h after ICU admission. In contrast, early PN does not provide substantial advantages in terms of morbidity and mortality, and the time when it is safe and beneficial remains unclear. The most advantageous recommendation seems to be administration of a hypocaloric (<20 kcal · kg–1 · d–1), high-protein diet (amino acids at doses of ≥2 g · kg–1 · d–1), at least during the first week of critical illness. Another important factor for reducing morbidity is the maintenance of blood glucose concentrations at 120–150 mg/dL, which is accomplished with the use of insulin and lower doses of glucose of 1–2 g · kg–1 · d–1, because this prevents the risk of hypoglycemia and is associated with a better prognosis according to recent studies. A fat emulsion is used as a source of required calories because of insulin resistance in the majority of patients. In addition, lipid oxidation in these patients is ∼25% higher than in healthy subjects.
Keywords: energy expenditure, energy metabolism, metabolism, indirect calorimetry, parenteral nutrition, enteral nutrition, timing for nutrition commencement, glucose control
Introduction
Critically ill patients are those who need continuous monitoring and artificial support of ≥1 vital organ (1). Critical illness is commonly associated with a state of catabolic stress, during which the patient shows signs of a systemic inflammatory response. This response is associated with complications, such as increased infectious morbidity, multiorgan dysfunction, prolonged length of hospitalization, and disproportionate mortality (2). These complications are usually caused by physiologic instability, which often leads to disability or death within hours or even minutes. Neurologic or cardiopulmonary system damage is the most frequent life-threatening damage (3).
Cells and organs in the human body need nutrients to generate ATP and synthesize molecules. Thus, an adequate supply of nutrients is necessary for the functioning of organs and systems and ultimately for survival. Because intensive care unit (ICU) patients are often unable to eat, the use of enteral nutrition (EN) and parenteral nutrition (PN) is necessary (1). Optimal nutritional support for ICU patients is currently the subject of many professional debates (4–6). Many patients receive either too many or too few calories, and thus, their intake is not based on their metabolic needs (7, 8). Internationally, the prevalence of malnutrition in ICU patients has been estimated to be ≤43% (9). Kvåle et al. (10) reported that 40% of patients lost >10 kg of body weight (BW) during the period directly after ICU admission. This happens because the metabolic rate of these patients increases, and the utilization of nutritional substrates is impaired (8). Caloric intake requirements vary significantly throughout the critical state; thus, it becomes more difficult to administer appropriate nutritional support (7). It is also very important that the substrate metabolism is interlinked (e.g., amino acids and glycerol can be used for gluconeogenesis), and there may be a different tolerance for deficiencies of each substrate (11).
The aim of this review was to collect useful information from clinical studies and to describe the specifics of adult ICU patient metabolism in relation to nutritional requirements. In particular, we aimed to determine the effect of nutritional support on morbidity and mortality in these patients, because it has not been well characterized to date.
For this review, we conducted literature searches through Embase and Medline (journal coverage dates back to 1947), PubMed (journal coverage dates back to the 1950s), and Google Scholar (date coverage is not specified). The searches in the databases were carried out up to February 2017. During the literature searches, the following Medical Subject Headings terms were used: “multiple trauma,” “critical illness,” “enteral nutrition,” “parenteral nutrition,” “early nutrition,” “late nutrition,” “nutrition,” “timing,” “metabolism,” “energy expenditure,” “energy intake,” “hypocaloric nutrition,” “normocaloric nutrition,” “protein,” “hyperproteic nutrition,” “high protein nutrition,” “carbohydrate,“ “glucose control,” “insulin,” “lipid,” and “phytosterols.” Meta-analyses, randomized controlled trials, and reviews were included. All of the discussion is based on our own clinical experience together with recommendations from practice guidelines [mainly the American Society of Parenteral and Enteral Nutrition (ASPEN) and the European Society for Clinical Nutrition and Metabolism (ESPEN)]. This review combines and discusses the views of various professional societies together with the latest scientific findings, resulting in a coherent and objective opinion.
Current Status of Knowledge
Timing for commencement of nutrition administration
EN is frequently recommended over PN because it may preserve the function of the gut mucosal barrier and have beneficial effects on gut immunity (1, 2, 12, 13). EN also has a vital trophic effect on the gastrointestinal mucosa and enhances its neuroendocrine functions (1, 2). In addition, EN is associated with decreased morbidity (lower infection rates, better wound healing, and decreased durations of mechanical ventilation, ICU and hospital lengths of stay, and recovery) and mortality. In the current literature, there is evidence that early EN administered within 24–48 h after ICU admission is beneficial (2, 12, 13). The disadvantage of EN is that it may be contraindicated in some patients, such as those with anatomic intestinal discontinuity or splanchnic ischemia. These patients have to receive PN (14).
Appropriate timing for the commencement of PN administration remains a controversial topic (12–16). Whereas European guidelines (17) recommend initiation of administration of total PN as soon as possible (within 2 d if EN fails), the American guidelines (2) recommend waiting until the seventh day in the absence of previous malnutrition. However, in severely malnourished patients or patients at high nutritional risk, PN should be initiated as soon as possible in the case of EN failure.
The reason that waiting is recommended is that PN could suppress autophagy, which is necessary for the recycling of intracellular nutrients and for maintaining energy homeostasis during nutrient deprivation. According to some studies (18–20), autophagy is essential for the immune response and for the so-called housekeeping functions, such as the removal of the toxic aggregates of damaged proteins and organelles. Therefore, autophagy could be necessary for recovery from organ failure (15). There are also views that aggressive nutrient supply, especially via the parenteral route, exacerbates the inflammatory response in the patient’s body (21). This occurs through increased immune dysfunction and reduced resistance to infections, which results in increased morbidity (22).
In the Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients trial, Casaer et al. (16) compared the early initiation of PN (within 48 h after ICU admission) with late initiation (on day 8) for adults in the ICU to supplement insufficient EN (a protocol for the early initiation of EN was used in both groups). Tight glycemic control was used in both groups. They found no significant difference in mortality between the late and early initiation of PN among patients who were at risk of malnutrition. However, the delayed initiation of PN until day 8 was associated with fewer ICU infections (22.8% compared with 26.2% for early-initiation PN patients; P = 0.008), but a higher degree of acute inflammation. Delayed initiation was also related to a shorter duration of mechanical ventilation (P = 0.006), a shorter course of renal replacement therapy (P = 0.008), a shorter ICU stay (HR: 1.06; 95% CI: 1.00, 1.13; P = 0.04) despite a slight increase in hypoglycemic episodes, a shorter hospital stay (HR: 1.06; 95% CI: 1.00, 1.13; P = 0.04), and reduced health care costs of €1110 (∼$1600)/patient (P = 0.04) (13, 14, 16). The Early Parenteral Nutrition Completing Enteral Nutrition in Adult Critically Ill Patients trial (16) used PN in unselected patients (patients with various diagnoses, mostly after cardiac surgery, who rarely need nutritional support, especially PN), which has been described as one of the major pitfalls of the study (14). However, a retrospective cohort study by Sena et al. (23) basically confirmed the fact that early PN (during the first postinjury week) may contribute to increased infectious morbidity (RR: 2.1; 95% CI: 1.6, 2.6; P < 0.001) and a worse clinical outcome (increased risk of death before and after adjustment, more common late acute respiratory distress syndrome, and longer duration of mechanical ventilation and ICU length of stay). A systematic review by Bost et al. (12) also showed that the early administration (<48 h after admission) of supplemental PN compared with late administration (at the end of the first week after ICU admission) does not confer major benefits with respect to morbidity and mortality (percentage of patients discharged from the ICU, in-ICU and in-hospital mortality, ICU length of stay, hospital length of stay, infection rates, nutrition targets, duration of mechanical ventilation, glucose control, duration of renal replacement therapy, muscle wasting and fat loss). Similarly, another recent meta-analysis (24) concluded that early PN (within 24−48 h) has no benefits on the survival rate for critically ill patients.
In contrast, in the study by Doig et al. (25), an early (first day of ICU stay) PN strategy (EN was relatively contraindicated) resulted in significantly fewer days of invasive ventilation (7.73 compared with 7.26 d/10 patient × ICU days; risk difference: −0.47; 95% CI: −0.82, −0.11; P = 0.01), less muscle wasting (0.43 compared with 0.27 score increase/wk; mean difference: −0.16; 95% CI: −0.28, −0.038; P = 0.01), and fat loss (0.44 compared with 0.31 score increase/wk; mean difference: −0.13; 95% CI: −0.25, −0.01; P = 0.04), but it did not significantly shorten ICU or hospital stays or improve the 60-d mortality rate (22.8% for standard care compared with 21.5% for early PN; risk difference: −1.26%; 95% CI: −6.6, 4.1; P = 0.60). It is worth noting that Doig et al. (25) did not observe the deleterious effects in the early PN group observed by Casaer et al. (16). Nevertheless, early PN was compared with the commencement of nutritional support administration on day 3 or later according to the current practice in Australia and New Zealand, thus both early and late PN were administered earlier than in other studies (25). However, the need for early PN is also explained by the fact that high amounts of stress hormones and inflammatory cytokines in critically ill patients prevent the synthesis of ketones. Thus, the mechanisms of adaptation to starvation in these individuals are weakened (26).
In conclusion, according to the majority of available studies and the latest review by Gunst and Van den Berghe (27), current knowledge does not support early administration of PN (although ESPEN does) and indicates that low caloric intake during the acute phase of trauma is preferable for patients (EN could be sufficient, although it does not completely meet energy needs). However, the time when the initiation of PN is safe and effective remains unclear.
Metabolism
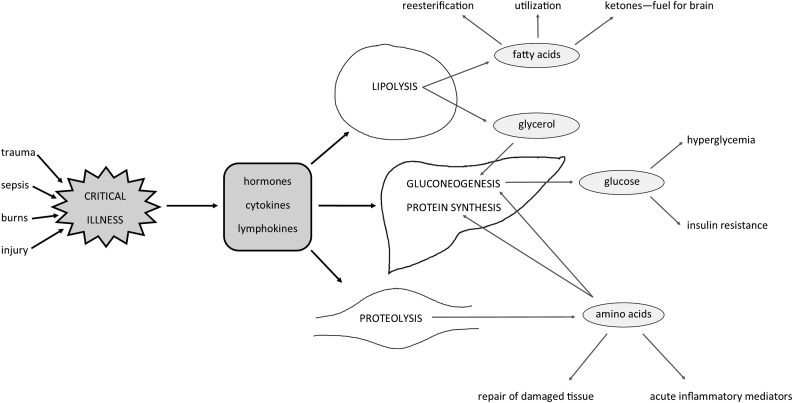
The acute stress response elicited by accidental or surgical injury, sepsis, burns, or other serious diseases (e.g., myocardial infarction) results in the release of regulatory endocrine hormones (cortisol, catecholamines, and glucagon), cytokines, and lymphokines, leading to a change of substrate utilization, catabolism, and hypermetabolism (Figure 1) (28). These mediators counteract insulin action in the liver and adipose tissue. This leads to insulin resistance and hyperglycemia by increasing peripheral glycogenolysis and lipolysis and by increasing hepatic gluconeogenesis and glycogenolysis (29, 30). Consequently, glucose oxidation is decreased in these patients, although serum insulin concentrations are increased (31). This may be considered a response to the need to provide a ready energy source for the “fight or flight” reaction that characterizes the physiologic response to stress (30). For this reaction, it is necessary to ensure the availability of limited carbohydrate stores for glucose-dependent and insulin-dependent vital organ systems (31). This is done by preventing the use of glucose in muscle and adipose tissue (low-priority pathways). Thus, the glucose is spared for less dispensable purposes and is located in the injured tissues or vital organs. Interestingly, during trauma, noninjured muscle is insulin resistant, whereas injured muscle is usually not insulin resistant (17).
FIGURE 1.
Metabolic response in critical illness. All mediators released during the stress response counteract insulin action. This leads to protein catabolism, lipolysis, and gluconeogenesis. In general, protein catabolism predominates. Amino acids released from muscles are deaminated in the liver and used for gluconeogenesis or converted into inflammatory proteins. During lipolysis, TGs are hydrolyzed to FFAs and glycerol in the adipose tissue. Glycerol is additionally used for gluconeogenesis in the liver, and FAs may be used in the liver and muscle, converted to ketone bodies, or re-esterified. Because of gluconeogenesis, hyperglycemia and insulin resistance occurs.
Whereas carbohydrate oxidation is reduced, the oxidation of lipids is increased. During a systemic inflammatory reaction, adipose tissue TGs are hydrolyzed to FFAs and glycerol. Glycerol is additionally used for gluconeogenesis in the liver and FAs may be used in the liver and muscle, converted to ketone bodies, or re-esterified (32, 33).
During this catabolic stress response, protein stores in the muscles and organs are also broken down. This happens when the body proteins are used for conversion into more essentials proteins (e.g., repair of damaged tissue or manufacturing of inflammatory mediators) (34) or released from the periphery and transported to the liver, where they are deaminated by the formation of new glucose (35). Moreover, normal anabolic activity, which is necessary for maintaining the synthesis of new proteins, is reduced (26). After the initial mobilization of amino acids during prolonged starvation in healthy people, protein degradation decreases. This occurs as soon as the energy generation from fat metabolism is increased. However, during sepsis and inflammation, this important control fails, and the production of endogenous glucose continues (36).
In addition, although plasma concentrations of substrates may increase due to catabolism, their availability for utilization by the peripheral tissues may be reduced (this phenomenon is caused by insulin resistance and the inhibition of lipoprotein lipase) (37). This clearly problematic situation has been called autocannibalism (28).
Energy expenditure
The gold standard for the determination of energy expenditure (EE) in hospitalized patients is indirect calorimetry (38–40). Unfortunately, this method is not currently available in many medical centers (7, 41). For this reason, doctors use equations to determine the caloric requirements of individual patients, together with their own judgment (7). The problem is that no equation correctly predicts the EE of every single patient because there are many determinants of EE (e.g., severity of trauma, sepsis, fever, age, restless physical activity, pharmacotherapy, and duration and evolutionary phase of the critical illness) that have unknown dose responses, and they overlap with, add to, or subtract from each other in very complicated ways (42).
It is commonly believed that the daily EE of critically ill patients exceeds normal resting EE (REE) by 50%. This equates to 36 kcal · kg–1 · d–1 (42–44). However, it has been proven that the REE of many critically ill patients is normal (22–25 kcal · kg–1 · d–1) (42, 45–47). This clearly indicates that critically ill patients who are inactive, moderately stressed, and continuously fed have an average REE close to their total daily EE (42).
Energy intake
According to ESPEN guidelines (17), the aim of nutritional support should be to provide an appropriate energy supply, which should be as close as possible to the measured energy expenditure so that it can decrease negative energy balance. When indirect calorimetry is not available, ESPEN (48) recommends delivering ≤25 kcal · kg actual BW−1 · d–1 during the acute phase (48 h after ICU admission), and ≤30 kcal · kg actual BW−1 · d–1 during the postacute phase (≥4 d after admission). Severely malnourished patients on PN should initially receive 10 kcal · kg actual BW−1 · d–1, and this target should be progressively increased to reach 25–30 kcal · kg actual BW−1 · d−1 over 3–4 d. In obese or overweight patients, the energy requirements should be estimated as 15 kcal · kg actual BW−1 · d–1 or 20 kcal· kg ideal BW−1 · d–1 (49). Similarly, ASPEN (2) also recommends the use of indirect calorimetry. In the absence of indirect calorimetry, predictive equations or simplistic weight-based equations (25–30 kcal · kg–1 · d–1) should be used to determine energy requirements. When EN is tolerated, >80% of the estimated or calculated energy goals should be administered in severely malnourished patients or patients at high nutritional risk during the first week of hospitalization. When PN is needed (EN is not feasible, and patients are at high risk of severe malnutrition), hypocaloric PN dosing (≤20 kcal· kg–1 · d–1 or 80% of estimated energy needs) with adequate protein doses (≥1.2 g · kg–1 · d–1) is recommended over the first week of ICU hospitalization. In obese patients with a BMI (in kg/m2) >30, permissive underfeeding or hypocaloric feeding with EN is recommended. Thus, the amount of administered calories should not be >65–70% of energy requirements or 11–14 kcal · kg actual BW−1 · d−1 (or 22–25 kcal · kg–1 ideal BW · d–1 with BMI >50).
It is commonly believed that increasing energy and protein intake in critically ill patients to the prescribed amounts decreases mortality. Despite this, bloodstream infections are more common in ICU patients who do not receive ≥25% of their recommended kilocalories (50). Further, a prolonged negative energy balance is associated with an increased number of infections, as well as an increased length of mechanical ventilation, length of ICU stay, total number of complications, and duration of antibiotic use (51). An international multicenter observational study (52) of 2772 mechanically ventilated patients confirmed that the use of higher caloric intake (an increase of 1000 cal/d to 1034 kcal/d) during the first 12 d of ICU stay is associated with decreased mortality (OR for 60-d mortality: 0.76; 95% CI: 0.61, 0.95; P = 0.014) and an increased number of ventilator-free days (3.5 ventilator-free days; 95% CI: 1.2, 5.9; P = 0.003). However, the association of increased calories with lower mortality was observed only in patients with a BMI <25 or ≥35, and there was no benefit for patients with a BMI from 25 to <35. Similarly, Petros et al. (53) showed that hypocaloric feeding (values are expressed as mean ± SD; 11.3 ± 3.1 kcal · kg–1 · d–1 = 50% of daily EE) during the first week of ICU stay was associated with more nosocomial infections (26.1% compared with 11.1%, respectively) and a higher hospital mortality rate (P = 0.67), but less insulin demand and gastrointestinal intolerance than normocaloric feeding (mean ± SD; 19.7 ± 5.7 kcal · kg–1 · d–1 = 100% of daily EE). It is important to mention that daily protein intake in both mentioned studies was <1 g · kg ideal BW−1 · d–1, and that is probably the major reason the prognosis of these patients was poor. This statement can be supported by a study in rats (54) that showed that protein refeeding, unlike glucose refeeding, improves mitochondrial function. Thus, it is supposed that hypocaloric nutrition may be beneficial only when an adequate amount of protein is administered (55). This view was supported by a randomized controlled trial by Rugeles et al. (56). In this trial, 80 patients were allocated into 2 groups. The intervention group received 1.4 g protein · kg–1 · d–1, whereas the control group received 0.76 g protein · kg–1 · d–1. The caloric intake of both groups was similar (12 kcal · kg–1 · d–1 in the intervention group compared with 14 kcal · kg–1 · d–1 in the control group) and lower than the caloric goals recommended by current clinical guidelines. This means that in the intervention group, 40% of the total calories were from protein intake (30% from carbohydrates), whereas in the control group, 20% of the calories came from protein (55% from carbohydrates). The final conclusion of Rugeles et al. (56) was that hyperproteic hypocaloric (defined as 15 kcal · kg–1 · d–1 with >1.5 g protein · kg–1 · d–1, however, the planned caloric and protein goals were not finally administered) EN administered during the first 7 d of ICU stay is associated with a reduced risk of multiple organ failure and with fewer hyperglycemic events. Nevertheless, when this research group (57) repeated the study with the same amount of administered proteins in both groups (1.7 g · kg–1 · d–1) and different caloric intakes (15 compared with 25 kcal · kg–1 · d–1) during the first 7 d of ICU stay, there was suddenly no difference in outcomes (change in sequential organ failure assessment at 96 h, hyperglycemic episodes, 28-d mortality rate, length of ICU stay, or mechanical ventilation) except for lower insulin requirements in the hypocaloric nutrition group. Similarly, permissive underfeeding (40–60% of caloric requirements) with full protein intake (1.2–1.5 g · kg–1 · d–1) did not differ from standard nutrition (70–100% of caloric requirements) in either mortality or other outcomes (serial sequential organ failure assessment scores, nitrogen balance, BW, concentrations of C-reactive protein, prealbumin, creatinine, bilirubin, partial pressure of arterial carbon dioxide, hemoglobin, lipids, potassium, magnesium, phosphate, transferrin, urinary nitrogen excretion, number of days free from mechanical ventilation, and number of ICU-free days) in the study by Arabi et al. (55). The only exceptions were lower glycaemia and thus insulin requirements. The intervention lasted for 14 d or until patient discharge. Charles et al. (58) basically had the same results: caloric provision (25–30 kcal · kg–1 · d–1 compared with 50% of this value with 1.5 g · kg–1 · d–1 of protein) was not associated with major outcomes (infection rate, ICU length of stay, hospital length of stay, glucose concentration, or mortality). Finally, a review by Van Zanten (59) also concluded that, according to the majority of randomized clinical trials, hypocaloric feeding has not shown a major benefit on outcomes for critically ill patients. Another systematic review (60) showed very similar results. These results also demonstrated no significant difference in outcomes (risk of acquired infections, hospital mortality, ICU length of stay or ventilator-free days) between patients receiving hypocaloric compared with normocaloric nutritional support.
In contrast, in the study by Krishnan et al. (61), patients who received moderate caloric intake (33–65% of the prescribed target by the American College of Chest Physicians) had better clinical outcomes (OR: 1.22; 95% CI: 1.15, 1.29) than patients who received higher intake (OR: 0.82; 95% CI: 0.70–0.94). Similar results were also found in a retrospective study of 121 patients (62). These patients were divided into 4 quartiles according to daily caloric intake [10.1 ± 2.5 (quartile 1), 15.8 ± 1.4 (quartile 2), 19.6 ± 1.0 (quartile 3), and 26.2 ± 4.4 (quartile 4) kcal · kg–1 · d–1] and protein intake [0.5 ± 0.1 (quartile 1), 0.8 ± 0.1 (quartile 2), 1.0 ± 0.1 (quartile 3), 1.4 ± 0.2 (quartile 4) g · kg–1 · d–1]. Patients receiving the greatest caloric (26.2 ± 4.4 kcal · kg–1 · d–1) or protein (1.4 ± 0.2 g · kg–1 · d–1) intake during the first 7 d after injury had poorer outcomes (increased length of hospital stay and increased ventilator days) than those receiving 25%–61% fewer calories or 29–64% less protein. These results correspond with the fact that hypocaloric feeding helps to avoid the consequences of overfeeding, such as hypercapnia, hyperglycemia, uremia, and hypertriglyceridemia (63). What is more, studies have shown that hypocaloric nutrition achieves a similar nitrogen balance to traditional regimens (64). There is no standardized definition of hypocaloric feeding, but it usually involves meeting the energy needs with <100% of the recommended nonprotein calories (typically ≤20 kcal · kg–1 · d–1) and normal amounts of protein calories (1.5–1.75 g · kg–1 · d–1) (63).
Although some studies have shown improved outcomes in hypocaloric feeding patients, not all studies agree. This is in part because the efficacy of hypocaloric nutrition depends on the amount of administered proteins. Although they are not conclusive, the currently available data suggest that 10–20 kcal · kg ideal or adjusted BW−1 · d–1 may be beneficial in these patients, but must be administered with a sufficient amount of protein (≥1.5–2 g · kg ideal BW−1 · d–1) (64). Although the latest studies do not confirm all of the mentioned benefits, hypocaloric hyperproteic nutrition is also more physiologic for the patient, so the metabolic impact should be lowered. In addition, the tolerance of hypocaloric nutrition in the gastrointestinal tract is better (when EN is administered) and costs are lower (57, 65). Thus, high-protein hypocaloric nutritional support seems to be the best option, at least during the first week of critical illness.
Protein intake
According to ESPEN guidelines (17), the recommended protein intake is 1.3–1.5 g · kg ideal BW−1 · d–1 (17). The limitation of these guidelines is that they are based on the results of 2 small studies, which showed that protein synthesis was maximally stimulated by a protein intake of 1.5 g · kg–1 · d–1. However, it is not clear whether these results from small studies can be applied to a large population of patients with various diseases, energy expenditures, and nitrogen losses (66). The ASPEN guidelines (2) recommend 1.2–2.0 g · kg ideal BW−1 · d–1 for patients with BMI <30 and 2.0–2.5 g · kg ideal BW−1 · d–1 for patients with BMI >30. The common problem with both guidelines is the lack of studies specifically matching intake to expenditure (66). In addition to ASPEN, the clinical care guidelines of the Society of Critical Care Medicine (67) also recommend protein or amino acid intake as high as 2.0 g · kg–1 · d–1. The Society of Critical Care Medicine and ASPEN guidelines specifically recommend 2.0 g protein · kg ideal BW−1 · d–1 as the minimum amount provided to patients with severe burns or multiple traumas and to permissively underfed obese patients, along with a minimum of 2.5 g · kg ideal BW−1 · d–1 for critically ill and permissively underfed morbidly obese patients (68).
Wolfe et al. (69) and Shaw et al. (70) showed that when the necessary number of calories is administered to patients with severe trauma, approximately one-third of the administered protein will be used for protein synthesis (anabolism), the second third will be used directly (catabolism), and the last third will become part of the plasma reserves. Thus, roughly two-thirds of the total body protein is ready for immediate use if needed, regardless of how much protein is provided by nutritional support. This finding thus serves as a basic argument against the hypothesis that excessive provision of protein can preserve lean body mass (71). However, a large number of underfed patients who are less critically ill may benefit from higher doses of ≤2 g amino acids · kg–1 · d–1. Moreover, currently available data do not justify limiting amino acids to 1.5 g · kg–1 · d–1(42). A systematic review by Hoffer and Bistrian (68) even confirmed that doses ≤2.5 g · kg–1 · d–1 are safe in critically ill patients, and, for most, this may be optimal. The main conclusion of this systematic review is that nitrogen balance improves with increasing protein provision up to the highest studied dose of 2.5 g · kg–1 · d–1. These findings correspond with a study by Dickerson et al. (72), which showed that higher protein intake (≥2 g · kg–1 · d–1) was associated with improved nitrogen balance. In addition, Allingstrup et al. (73) demonstrated that the provision of protein was related to mortality hazard. They suggested that survival time depended on the provision of protein and amino acids (0.79, 1.06, and 1.46 g protein · kg–1 · d–1 correlated with a 10-d survival rate of 50%, 78%, and 87% of patients, respectively). Weijs et al. (5) also showed that the early intake of high amounts of protein (defined as intake of ≥1.2 g · kg–1 · d–1 at day 4) was associated with lower hospital mortality rates (OR: 0.42; 95% CI: 0.21, 0.83; P = 0.013; 843 patients were included). However, the benefit of the early intake of high amounts of protein was found only in nonseptic and nonoverfed patients.
Conversely, a higher amount of protein intake (1.9 g · kg–1 · d–1) did not improve the protein sparing effect in the study of Ishibashi et al. (74), in which they found 1.2 g protein · kg–1 · d–1 to be optimal. The disadvantage of this study is the fact that it was retrospective (11). Other surprising results have been published (5). First, in postmortem muscle biopsies (12 patients), impaired autophagy was associated with the amount of infused amino acid calories (75). Second, the cumulative amount of protein and amino acids administered early during the ICU stay corresponded to delayed recovery (76). And finally, a small observational study showed a correlation between more pronounced muscle wasting and higher protein intake (77).
Although there is evidence that does not support the administration of higher doses of amino acids in critical illness and opinions on this matter vary, our review of the worldwide literature favors high-protein nutritional support (≥2 g · kg–1 · d–1), because the higher doses of protein seem to improve nitrogen balance (even though not all of the protein is used for anabolism) and contribute to better prognosis together with the hypocaloric nutrition discussed above. It is also important to mention that protein is essential for survival and recovery for the maintaining of muscle mass and many essential functions.
Carbohydrate intake
The recommended dose of carbohydrates in PN is currently 3–3.5 g · kg–1 · d–1. However, lower doses are recommended for patients at high risk of hyperglycemia (critically ill patients, diabetics, septic patients, or patients treated with steroids). These patients should receive ∼1–2 g carbohydrates · kg–1 · d–1 (78), and glucose delivery should not be >6 g · kg–1 · d–1 at a rate <5 mg · kg–1 · min–1 (49).
It has been shown that the exogenous supply of glucose or carbohydrates does not reduce the rate of gluconeogenesis or is minimally affected in injured or septic patients (28, 79). Moreover, glucose utilization is reduced due to the increased activity of the sympathetic nervous system, which increases lipolysis and, consequently, the availability of FFAs in critically ill patients. In this case, glucose infusion further increases the sympathetic activity, leading to a disorder of its own usage and a state of insulin resistance. Under these conditions, a high intake of carbohydrates therefore creates physiologic stress rather than serving as nutritional support (80, 81). Thus, the hyperglycemia induced by exogenous glucose uptake can amplify the inflammatory response, making it inappropriate (82). It is also important to consider that there is no persuasive evidence that carbohydrates are essential nutrients for humans because they can be synthesized from lactate, glycerol, and amino acids in the liver and kidneys and perhaps in other tissues, such as muscle and the gut. However, glucose is a convenient and safe source of calories used in PN (17). Its administration is also essential for stimulation of the secretion of insulin and other anabolic hormones, which promote protein synthesis and counteract lipolysis (28).
Because of insulin resistance and hyperglycemia, lower doses of carbohydrates (1–2 g · kg–1 · d–1) are recommended.
Glucose control
Evidence indicates that a simple metabolic intervention with insulin can lead to normoglycemia and thus to an improvement in survival and a reduction of morbidity among critically ill patients. The mechanism by which insulin decreases blood glucose concentrations in critically ill patients is not currently clear. Analysis of liver and skeletal muscle biopsies obtained immediately after the death of critically ill patients has shown that insulin lowers blood glucose predominantly by increasing skeletal muscle glucose uptake. Based on these results, it could be that adipose tissue and skeletal muscle remain relatively responsive to insulin, whereas the liver is much more resistant (83).
Although long-term hyperglycemia leads to the development of diabetes, this short-term endogenous hyperglycemia, which develops during acute conditions, has not yet been properly explained. However, it is probably involved in maintaining an adequate inflammatory response; therefore, it should not be aggressively reduced (82). Conversely, intervention studies, in which glucose was lowered with insulin showed better outcomes, suggesting that an increased glucose concentration is a cause rather than just a marker of illness (83). Krinsley (84) published a study of 1826 patients, in which they found a relation between even mildly elevated blood glucose concentrations during the ICU stay and increased hospital mortality among a heterogeneous population of critically ill patients (P < 0.001). The hospital mortality rate was lowest (9.6%) in patients with mean serum glucose values between 80 and 99 mg/dL and increased significantly and progressively (42.5%) as the mean serum glucose concentrations exceeded 300 mg/dL. Blood glucose concentration was also an independent predictor of postoperative infection and hospital and ICU length of stay (r = 0.870; P < 0.001). Similar results were obtained in 3 studies by Van den Berghe et al. (85–87), in which intensive insulin therapy and maintenance of glucose concentrations between 80 and 110 mg/dL produced the best clinical outcomes. However, such therapy also posed the greatest risk of hypoglycemia. In one of the largest observational databases, Badawi et al. (88) also demonstrated that, among ∼200,000 critically ill patients, mortality was the lowest in patients with a blood glucose concentration of 80–110 mg/dL. This mortality progressively increased with the severity and duration of hyperglycemia and hypoglycemia, as well as with higher fluctuations of glycemia.
Several potential mechanisms may explain the benefit of tight glycemic control. These mechanisms are prevention of immune dysfunction, reduction of systemic inflammation, and protection of the endothelium and mitochondrial ultrastructure and function (87). This is caused by reducing the concentrations of C-reactive protein and adhesion molecules and by the antiapoptotic properties of insulin (83). According to another hypothesis, this benefit could also be due to the elimination of glucose-induced osmotic diuresis, the maintenance of macrophage and neutrophil function, the enhancement of erythropoiesis, the reduction of cholestasis, the direct anabolic effect of insulin on respiratory muscles, and the reduction of hyperglycemic injury to neuronal axons (89).
Other studies (90–92), however, did not confirm the benefit of tight glycemic control. A cohort study by Treggiari et al. (92) conversely concluded that this therapy increases mortality in some critically ill patients. One of the main reasons why clinical trials and meta-analyses showed negative results for tight glycemic control was the high incidence of hypoglycemia (93). For ICU patients, this condition is dangerous primarily because of the lack of specific warning signals. When the patient is under deep sedation, it is not possible to assess changes in his neurologic condition. Thus, it is necessary to continuously monitor the patient’s blood glucose concentration (30, 94).
Hsu et al. (95) compared 2 different insulin regimens in their study. In the first group of 55 patients, blood glucose concentrations were maintained between 120 and 140 mg/dL, and in the second group of 57 patients, the concentrations were allowed to climb to values between 180 and 200 mg/dL. Thus, in both cases, the blood glucose concentrations were higher than those that occur with intensive insulin therapy, and this ensured a lower risk of severe hypoglycemia. Patients with lower blood glucose concentrations (120–140 mg/dL) had a significantly higher nitrogen balance (P = 0.070), lower nitrogen loss in urine (P = 0.027), and higher concentrations of serum albumin (P = 0.047) and prealbumin (P = 0.001). Krinsley (89) also showed that in a heterogeneous population of critically ill patients, the maintenance of blood glucose concentrations at <140 mg/dL (the mean glucose value was 130.7 mg/dL) was associated with a 29.3% decrease in mortality (P = 0.002), a 10.8% decrease in length of stay (P = 0.01), a 75% decrease in the development of new renal insufficiency (P = 0.03), and an 18.7% decrease in the number of patients undergoing a transfusion of packed RBCs (P = 0.04). Several other studies have confirmed that maintaining blood glucose concentrations of 120–150 mg/dL may yield a better prognosis and reduce morbidity caused by hypoglycemia (96). In addition, ASPEN (2) even recommends maintaining blood glucose concentrations of 140–180 mg/dL.
According to the mentioned studies, a lower blood glucose concentration makes for a better prognosis. However, due to a high risk of hypoglycemia, which may be fatal for these patients, it is rational to be careful and maintain blood glucose concentrations of 120–150 mg/dL.
Lipid intake
The maximal recommended dose of lipid emulsion is ∼1.0–1.2 g · kg–1 · d–1 (17, 37). Wichmann et al. (97) compared the safety of lipid emulsions in patients after major abdominal surgery and showed that a rate of administration of ≤1.5 g · kg–1 · d–1 was safe. In Australia, it is current practice to administer lipid emulsions at a rate of ≤2 g · kg–1 · d–1 (17, 98). However, the lipid supply must not be >23 mg · kg–1 · min–1 or >60% of the total energy input. Additionally, “hidden” fat from the propofol should be included (49).
Exogenous lipid administration is necessary to prevent FFA exhaustion, and it also serves as an energy substrate (28, 32). Because the majority of patients receiving PN have an inadequate response to glucose, a fat emulsion is used as a source of needed calories (99). Studies on nitrogen balance (100, 101) have even shown that intravenous administration of a fat emulsion has a protein-sparing effect in malnourished patients and in patients undergoing surgery. By using radioactively labeled fat emulsions, Nordenström et al. (99) found that lipid oxidation is ∼25% greater in patients after trauma or during infection than in healthy subjects. These labeled emulsions also showed a very similar rate of oxidation to that of the total body fat. This therefore indicates that exogenous fat emulsions are used in the same way as endogenous fat stores and chylomicrons. Additionally, Tappy et al. (102) demonstrated that the administration of lipids decreased lipogenesis more than the administration of glucose-based PN did. Lipid administration induced a smaller increase (7% compared with 26%; P < 0.05) in plasma glucose and insulin concentrations (40% compared with 284%; P < 0.01) and did not increase CO2 production (glucose-based PN increased CO2 by 15%; P < 0.01). However, administered lipids did not inhibit endogenous glucose production or net protein oxidation.
Lipids (∼9 kcal/g) generally provide more calories than dextrose (3.4 kcal/g) or amino acids (4 kcal/g). Thus, the volume of PN required to achieve adequate caloric intake may be substantially reduced. The low volume and high osmolarity of PN enable its safe administration through peripheral and central routes (51). Probably the most important benefit of using lipid emulsions in PN is the reduction of metabolic complications (hyperglycemia and high CO2 production resulting from the stimulation of the de novo lipogenesis and hepatic steatosis), which occurs after excessive hypertonic glucose infusion (32, 51).
Finally, FAs can influence inflammatory and immune processes through effects on the structure and function of cell membranes, modification of the inflammatory mediator profile, and alterations in gene expression. Specifically, ω-3 PUFAs (EPA and DHA) can counter the actions of ω-6 FAs, which may promote inflammatory processes (17). In addition, ω-3 PUFAs displace arachidonic acid from cell membranes, antagonize proinflammatory eicosanoids (e.g., prostaglandin E2 and leukotriene B4), promote the production of less inflammatory eicosanoids (e.g., thromboxane and prostaglandin E3), and inhibit inflammatory mediators (e.g., inducible NO synthase) (63).
Conversely, the high amount of ω-6 FAs in soybean oil may negatively influence systemic inflammation, immune status, and clinical outcomes. In sepsis and trauma, soybean oil may even promote the production of proinflammatory eicosanoids and increase oxidative stress (103). TGs and other components of lipid emulsions also create chylomicrons, which are then hydrolyzed in the body into FFAs and other remnant particles that are taken up by the liver. When the liver is unable to process all the FFAs, hyperlipidemia and steatosis may occur (in critically ill patients, the plasma concentrations of FFAs are primary increased because of metabolic stress) (51). At the least, it should be noted that lipid emulsions contain phytosterols, which in large amounts can cause cholestasis and PN-associated liver disease. Thus, parenteral lipid emulsions should be administered with caution in these patients (51, 104). In contrast, many newly developed fat emulsions contain lower amounts of ω-6 FAs and phytosterols that may help prevent these complications (e.g., fish oil lipid emulsions contain negligible amounts of phytosterols) (105).
When lipid emulsions with lower amounts of phytosterols and ω-6 FAs are used, they are an important source of energy, because unlike glucose, they are fully utilized in the bodies of critically ill patients, where they help to prevent metabolic complications occurring after glucose infusion.
Conclusions
Currently, nearly half of critically ill patients are malnourished. The inflammatory response typical for this metabolic state causes hyperglycemia, the loss of lean body mass, and the inability to properly utilize all nutritional substrates. The provision of an adequate amount of each nutritional substrate may help to reduce morbidity and mortality in these patients. However, the appropriate amounts of proteins, carbohydrates, and lipids vary for each patient. The gold standard for determining energy and nutritional requirements is indirect calorimetry. Unfortunately, indirect calorimetry is not currently available in many medical centers. Generally, EN is recommended over PN, and its administration should start as soon as possible. The time of commencement of PN administration remains a controversial topic. Doses of protein between 2.0 and 2.5 g · kg–1 · d–1 should be recommended because they contribute to the reduction of catabolism and, in combination with hypocaloric nutrition (10–20 kcal · kg–1 · d–1), seem to be the most beneficial. Important factors for reducing morbidity also include the maintenance of blood glucose concentrations of 120–150 mg/dL with insulin and the administration of lower glucose doses of 1–2 g · kg–1 · d–1, because this prevents the risk of hypoglycemia and results in a better prognosis. Because of the impaired response to glucose, lipid emulsions should be used as the source of caloric needs. Lipid emulsions achieve the energy requirements with lower volume, prevent FFA exhaustion, and prevent metabolic complications that may occur after high glucose infusion rates. Conclusions regarding energy and nutritional substrate intakes are summarized in Table 1, where they are compared with the statements of 2 major professional societies.
TABLE 1.
Energy, protein, carbohydrate, and lipid intakes according to review conclusions in comparison to statements of professional societies1
| Subject | ASPEN recommendation | ESPEN recommendation | Conclusion of the review |
| Energy intake | >80% (25–30 kcal · kg–1 · d–1) of energy requirements in EN or hypocaloric PN dosing (≤20 kcal · kg–1 · d–1 or 80% of estimated energy needs) with adequate protein doses (≥1.2 g · kg–1 · d–1) in severely malnourished patients or patients at high nutritional risk during the first week of hospitalization (2) | ≤25 kcal · kg actual BW−1 · d–1 during the acute phase (48 h after ICU admission), and ≤30 kcal · kg actual BW−1 · d–1 during the postacute phase (≥4 d postadmission) (48) | Hypocaloric nutrition (10–20 kcal · kg · ideal or adjusted BW−1 · d–1) with a sufficient amount of protein (≥1.5–2 g · kg ideal BW−1 · d–1) at least during the first week of ICU stay (63, 64) |
| Protein intake | 1.2–2.0 g · kg actual BW−1 · d–1 for patients with BMI (in kg/m2) <30 and 2.0–2.5 g · kg ideal BW−1 · d–1 for patients with BMI >30 (2) | 1.3–1.5 g · kg ideal BW−1 · d–1 (17) | ≥2 g · kg–1 · d–1 (maximum; 2.5 g · kg–1 · d–1) (42, 68, 72) |
| Carbohydrate intake | Not defined | ≥2 g · kg–1 · d–1 (17) | 1–2 g · kg–1 · d–1 (78) |
| Maintenance of blood glucose concentrations of 140–180 mg/dL (2) | Maintenance of blood glucose concentrations <180 mg/dL (17) | Maintenance of blood glucose concentrations of 120–150 mg/dL (89, 95, 96) | |
| Lipid intake | Doses not defined | 0.7–1.5 g · kg–1 · d–1 (17) | >1–1.2 g · kg–1 · d–1 (maximum; 2 g · kg–1 · d–1) (97, 98) |
| Avoid soy-based lipids in the first week of hospitalization (2) | Avoid phytosterols and ω-6 FAs (51, 103–105) |
ASPEN, American Society of Parenteral and Enteral Nutrition; BW, body weight; EN, enteral nutrition; ESPEN, European Society of Parenteral and Enteral Nutrition; ICU, intensive care unit; PN, parenteral nutrition.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ASPEN, American Society of Parenteral and Enteral Nutrition; BW, body weight; EE, energy expenditure; EN, enteral nutrition; ESPEN, European Society for Clinical Nutrition and Metabolism; ICU, intensive care unit; PN, parenteral nutrition; REE, resting energy expenditure.
References
- 1.Singer P, Shapiro H, Bendavid I. Behind the ESPEN Guidelines on parenteral nutrition in the ICU. Minerva Anestesiol 2011;77:1115–20. [PubMed] [Google Scholar]
- 2.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159–211. [DOI] [PubMed] [Google Scholar]
- 3.Frost P, Wise MP. Recognition and early management of the critically ill ward patient. Br J Hosp Med (Lond) 2007;68:M180–3. [DOI] [PubMed] [Google Scholar]
- 4.Casaer MP, Mesotten D, Schetz MR. Bench-to-bedside review: metabolism and nutrition. Crit Care 2008;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weijs PJ, Looijaard WG, Beishuizen A, Girbes AR, Oudemans-van Straaten HM. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit Care 2014;18:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg A, Rooyackers O, Bellander BM, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Crit Care 2013;17:R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker RN, Heuberger RA. Predictive equations for energy needs for the critically ill. Respir Care 2009;54:509–21. [PubMed] [Google Scholar]
- 8.Wandrag L, Gordon F, O’Flynn J, Siddiqui B, Hickson M. Identifying the factors that influence energy deficit in the adult intensive care unit: a mixed linear model analysis. J Hum Nutr Diet 2011;24:215–22. [DOI] [PubMed] [Google Scholar]
- 9.Turner P. Providing optimal nutritional support on the intensive care unit: key challenges and practical solutions. Proc Nutr Soc 2010;69:574–81. [DOI] [PubMed] [Google Scholar]
- 10.Kvåle R, Ulvik A, Flaatten H. Follow-up after intensive care: a single center study. Intensive Care Med 2003;29:2149–56. [DOI] [PubMed] [Google Scholar]
- 11.Singer P, Hiesmayr M, Biolo G, Felbinger TW, Berger MM, Goeters C, Kondrup J, Wunder C, Pichard C. Pragmatic approach to nutrition in the ICU: expert opinion regarding which calorie protein target. Clin Nutr 2014;33:246–51. [DOI] [PubMed] [Google Scholar]
- 12.Bost RB, Tjan DH, van Zanten AR. Timing of (supplemental) parenteral nutrition in critically ill patients: a systematic review. Ann Intensive Care 2014;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Aguilar-Nascimento JE, Bicudo-Salomao A, Portari-Filho PE. Optimal timing for the initiation of enteral and parenteral nutrition in critical medical and surgical conditions. Nutrition 2012;28:840–3. [DOI] [PubMed] [Google Scholar]
- 14.Berger MM, Pichard C. Development and current use of parenteral nutrition in critical care - an opinion paper. Crit Care 2014;18:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schetz M, Casaer MP, Van den Berghe G. Does artificial nutrition improve outcome of critical illness? Crit Care 2013;17:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506–17. [DOI] [PubMed] [Google Scholar]
- 17.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr 2009;28:387–400. [DOI] [PubMed] [Google Scholar]
- 18.Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr Opin Cell Biol 2010;17:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell 2011;17:728–41. [DOI] [PubMed] [Google Scholar]
- 20.Cuervo AM, Macian F. Autophagy, nutrition and immunology. Mol Aspects Med 2012;17:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bengmark S. Nutrition of the critically ill - emphasis on liver and pancreas. Hepatobiliary Surg Nutr 2012;1:25–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bengmark S. Nutrition of the critically ill–a 21st-century perspective. Nutrients 2013;5:162–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sena MJ, Utter GH, Cuschieri J, Maier RV, Tompkins RG, Harbrecht BG, Moore EE, O’Keefe GE. Early supplemental parenteral nutrition is associated with increased infectious complications in critically ill trauma patients. J Am Coll Surg 2008;207:459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Gao X, Tian F, Wu C, Wang X. Early parenteral nutrition alone or accompanying enteral nutrition in critically ill patients: a systematic review and meta-analysis. Asia Pac J Clin Nutr 2015;24:227–33. [DOI] [PubMed] [Google Scholar]
- 25.Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Davies AR, O’Leary M, Solano T, Peake S, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA 2013;309:2130–8. [DOI] [PubMed] [Google Scholar]
- 26.Hashemian SM, Cahill N, Murch L, Wang M, Jamaati HR, Malekmohammad M, Farzanegan B, Tabarsi P, Marjani M, Sadr M, et al. Improving the practice of nutrition therapy in the NRITLD critically Ill patients: an international quality improvement project. Tanaffos 2011;10:31–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Gunst J, Van den Berghe G. Parenteral nutrition in the critically ill. Curr Opin Crit Care 2017;23:149–58. [DOI] [PubMed] [Google Scholar]
- 28.Weissman C. Nutrition in the intensive care unit. Crit Care 1999;3:R67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilias I, Vassiliadi DA, Theodorakopoulou M, Boutati E, Maratou E, Mitrou P, Nikitas N, Apollonatou S, Dimitriadis G, Armaganidis A, et al. Adipose tissue lipolysis and circulating lipids in acute and subacute critical illness: effects of shock and treatment. J Crit Care 2014;29:1130 e5–9. [DOI] [PubMed] [Google Scholar]
- 30.Harper J. Glucose control in the intensive care unit: how it is done. Proc Nutr Soc 2007;66:362–6. [DOI] [PubMed] [Google Scholar]
- 31.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, Schneeweiss B, Zauner C. Severity of insulin resistance in critically ill medical patients. Metabolism 2007;56:1–5. [DOI] [PubMed] [Google Scholar]
- 32.Adolph M, Heller AR, Koch T, Koletzko B, Kreymann KG, Krohn K, Pscheidl E, Senkal M; Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Lipid emulsions - guidelines on parenteral nutrition, chapter 6. Ger Med Sci 2009;7:Doc22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109–17. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher J. Giving nutrition support to critically ill adults. Nurs Times 2015;111:12–6. [PubMed] [Google Scholar]
- 35.Wilmore DW, Kinney JM. Panel report on nutritional support of patients with trauma or infection. Am J Clin Nutr 1981;34(6 Suppl):1213–22. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths RD, Bongers T. Nutrition support for patients in the intensive care unit. Postgrad Med J 2005;81:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler TR. Nutrition support in critical illness–bridging the evidence gap. N Engl J Med 2011;365:562–4. [DOI] [PubMed] [Google Scholar]
- 38.Kross EK, Sena M, Schmidt K, Stapleton RD. A comparison of predictive equations of energy expenditure and measured energy expenditure in critically ill patients. J Crit Care 2012;27:321 e5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta NM, Smallwood CD, Joosten KF, Hulst JM, Tasker RC, Duggan CP. Accuracy of a simplified equation for energy expenditure based on bedside volumetric carbon dioxide elimination measurement–a two-center study. Clin Nutr 2015;34:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boullata J, Williams J, Cottrell F, Hudson L, Compher C. Accurate determination of energy needs in hospitalized patients. J Am Diet Assoc 2007;107:393–401. [DOI] [PubMed] [Google Scholar]
- 41.Frankenfield D, Hise M, Malone A, Russell M, Gradwell E, Compher C; Evidence Analysis Working Group. Prediction of resting metabolic rate in critically ill adult patients: results of a systematic review of the evidence. J Am Diet Assoc 2007;107:1552–61. [DOI] [PubMed] [Google Scholar]
- 42.Hoffer LJ. Protein and energy provision in critical illness. Am J Clin Nutr 2003;78:906–11. [DOI] [PubMed] [Google Scholar]
- 43.Elwyn DH, Kinney JM, Askanazi J. Energy expenditure in surgical patients. Surg Clin North Am 1981;61:545–56. [DOI] [PubMed] [Google Scholar]
- 44.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med 1999;27:1295–302. [DOI] [PubMed] [Google Scholar]
- 45.Baker JP, Detsky AS, Stewart S, Whitwell J, Marliss EB, Jeejeebhoy KN. Randomized trial of total parenteral nutrition in critically ill patients: metabolic effects of varying glucose-lipid ratios as the energy source. Gastroenterology 1984;87:53–9. [PubMed] [Google Scholar]
- 46.Hunter DC, Jaksic T, Lewis D, Benotti PN, Blackburn GL, Bistrian BR. Resting energy expenditure in the critically ill: estimations versus measurement. Br J Surg 1988;75:875–8. [DOI] [PubMed] [Google Scholar]
- 47.Paauw JD, McCamish MA, Dean RE, Ouellette TR. Assessment of caloric needs in stressed patients. J Am Coll Nutr 1984;3:51–9. [DOI] [PubMed] [Google Scholar]
- 48.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C, et al. ; DGEM (German Society for Nutritional Medicine). ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 2006;25:210–23. [DOI] [PubMed] [Google Scholar]
- 49.Thibault R, Heidegger CP, Berger MM, Pichard C. Parenteral nutrition in the intensive care unit: cautious use improves outcome. Swiss Med Wkly 2014;144:w13997. [DOI] [PubMed] [Google Scholar]
- 50.Stewart ML. Interruptions in enteral nutrition delivery in critically ill patients and recommendations for clinical practice. Crit Care Nurse 2014;34:14–21, quiz 2. [DOI] [PubMed] [Google Scholar]
- 51.Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJ. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med 2010;36:735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, Heyland DK. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 2009;35:1728–37. [DOI] [PubMed] [Google Scholar]
- 53.Petros S, Horbach M, Seidel F, Weidhase L. Hypocaloric vs normocaloric nutrition in critically Ill patients: a Prospective Randomized Pilot Trial. JPEN J Parenter Enteral Nutr 2016;40:242–9. [DOI] [PubMed] [Google Scholar]
- 54.Briet F, Jeejeebhoy KN. Effect of hypoenergetic feeding and refeeding on muscle and mononuclear cell activities of mitochondrial complexes I–IV in enterally fed rats. Am J Clin Nutr 2001;73:975–83. [DOI] [PubMed] [Google Scholar]
- 55.Arabi YM, Aldawood AS, Solaiman O. Permissive underfeeding or standard enteral feeding in critical illness. N Engl J Med 2015;373:1175–6. [DOI] [PubMed] [Google Scholar]
- 56.Rugeles SJ, Rueda JD, Diaz CE, Rosselli D. Hyperproteic hypocaloric enteral nutrition in the critically ill patient: a randomized controlled clinical trial. Indian J Crit Care Med 2013;17:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rugeles S, Villarraga-Angulo LG, Ariza-Gutierrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D. High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care 2016;35:110–4. [DOI] [PubMed] [Google Scholar]
- 58.Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, McLeod MD, Guidry CA, Stukenborg GJ, Swenson BR, et al. Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr 2014;100:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Zanten AR. Full or hypocaloric nutritional support for the critically ill patient: is less really more? J Thorac Dis 2015;7:1086–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marik PE, Hooper MH. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: a systematic review and meta-analysis. Intensive Care Med 2016;42:316–23. [DOI] [PubMed] [Google Scholar]
- 61.Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest 2003;124:297–305. [DOI] [PubMed] [Google Scholar]
- 62.Ash JL, Gervasio JM, Zaloga GP, Rodman GH. Does the quantity of enteral nutrition affect outcomes in critically ill trauma patients? J Parenter Enteral Nutr 2005;29:S10. [Google Scholar]
- 63.Todd SR, Gonzalez EA, Turner K, Kozar RA. Update on postinjury nutrition. Curr Opin Crit Care 2008;14:690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boitano M. Hypocaloric feeding of the critically ill. Nutr Clin Pract 2006;21:617–22. [DOI] [PubMed] [Google Scholar]
- 65.Patiño JF, de Pimiento SE, Vergara A, Savino P, Rodriguez M, Escallón J. Hypocaloric support in the critically ill. World J Surg 1999;23:553–9. [DOI] [PubMed] [Google Scholar]
- 66.Kreymann G, DeLegge MH, Luft G, Hise ME, Zaloga GP. The ratio of energy expenditure to nitrogen loss in diverse patient groups–a systematic review. Clin Nutr 2012;31:168–75. [DOI] [PubMed] [Google Scholar]
- 67.Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med 2016;44:390–438. [DOI] [PubMed] [Google Scholar]
- 68.Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012;96:591–600. [DOI] [PubMed] [Google Scholar]
- 69.Wolfe RR, Goodenough RD, Burke JF, Wolfe MH. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg 1983;197:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw JH, Wildbore M, Wolfe RR. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 1987;205:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunha HF, Rocha EE, Hissa M. Protein requirements, morbidity and mortality in critically ill patients: fundamentals and applications. Rev Bras Ter Intensiva 2013;25:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dickerson RN, Pitts SL, Maish GO III, Schroeppel TJ, Magnotti LJ, Croce MA, Minard G, Brown RO. A reappraisal of nitrogen requirements for patients with critical illness and trauma. J Trauma Acute Care Surg 2012;73:549–57. [DOI] [PubMed] [Google Scholar]
- 73.Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, Espersen K, Hartvig Jensen T, Wiis J, Perner A, Kondrup J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr 2012;31:462–8. [DOI] [PubMed] [Google Scholar]
- 74.Ishibashi N, Plank LD, Sando K, Hill GL. Optimal protein requirements during the first 2 weeks after the onset of critical illness. Crit Care Med 1998;26:1529–35. [DOI] [PubMed] [Google Scholar]
- 75.Vanhorebeek I, Gunst J, Derde S, Derese I, Boussemaere M, Guiza F, Martinet W, Timmermans JP, D’Hoore A, Wouters PJ, et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab 2011;96:E633–45. [DOI] [PubMed] [Google Scholar]
- 76.Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 2013;187:247–55. [DOI] [PubMed] [Google Scholar]
- 77.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–600. Erratum in: JAMA 2014;311:625. [DOI] [PubMed] [Google Scholar]
- 78.Bolder U, Ebener C, Hauner H, Jauch KW, Kreymann G, Ockenga J, Traeger K; Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine. Carbohydrates - guidelines on parenteral nutrition, chapter 5. Ger Med Sci 2009;7:Doc23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hartl WH, Jauch KW. Metabolic self-destruction in critically ill patients: origins, mechanisms and therapeutic principles. Nutrition 2014;30:261–7. [DOI] [PubMed] [Google Scholar]
- 80.Carlson GL. Insulin resistance and glucose-induced thermogenesis in critical illness. Proc Nutr Soc 2001;60:381–8. [DOI] [PubMed] [Google Scholar]
- 81.Askanazi J, Carpentier YA, Elwyn DH, Nordenstrom J, Jeevanandam M, Rosenbaum SH, Gump FE, Kinney JM. Influence of total parenteral nutrition on fuel utilization in injury and sepsis. Ann Surg 1980;191:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Losser MR, Damoisel C, Payen D. Bench-to-bedside review: glucose and stress conditions in the intensive care unit. Crit Care 2010;14:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazzeri C, Tarquini R, Giunta F, Gensini GF. Glucose dysmetabolism and prognosis in critical illness. Intern Emerg Med 2009;4:147–56. [DOI] [PubMed] [Google Scholar]
- 84.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–8. [DOI] [PubMed] [Google Scholar]
- 85.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–67. [DOI] [PubMed] [Google Scholar]
- 86.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, Bouillon R, Schetz M. Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm. Diabetes 2006;55:3151–9. [DOI] [PubMed] [Google Scholar]
- 87.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–61. [DOI] [PubMed] [Google Scholar]
- 88.Badawi O, Waite MD, Fuhrman SA, Zuckerman IH. Association between intensive care unit-acquired dysglycemia and in-hospital mortality. Crit Care Med 2012;40:3180–8. [DOI] [PubMed] [Google Scholar]
- 89.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc 2004;79:992–1000. [DOI] [PubMed] [Google Scholar]
- 90.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ 2009;180:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 2008;300:933–44. [DOI] [PubMed] [Google Scholar]
- 92.Treggiari MM, Karir V, Yanez ND, Weiss NS, Daniel S, Deem SA. Intensive insulin therapy and mortality in critically ill patients. Crit Care 2008;12:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amrein K, Kachel N, Fries H, Hovorka R, Pieber TR, Plank J, Wenger U, Lienhardt B, Maggiorini M. Glucose control in intensive care: usability, efficacy and safety of Space GlucoseControl in two medical European intensive care units. BMC Endocr Disord 2014;14:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lacherade JC, Jacqueminet S, Preiser JC. An overview of hypoglycemia in the critically ill. J Diabetes Sci Technol 2009;3:1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu CW, Sun SF, Lin SL, Huang HH, Wong KF. Moderate glucose control results in less negative nitrogen balances in medical intensive care unit patients: a randomized, controlled study. Crit Care 2012;16:R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eakins J. Blood glucose control in the trauma patient. J Diabetes Sci Technol 2009;3:1373–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 2007;35:700–6. [DOI] [PubMed] [Google Scholar]
- 98.Ali AB, Chapman-Kiddell C, Reeves MM. Current practices in the delivery of parenteral nutrition in Australia. Eur J Clin Nutr 2007;61:554–60. [DOI] [PubMed] [Google Scholar]
- 99.Nordenström J, Carpentier YA, Askanazi J, Robin AP, Elwyn DH, Hensle TW, Kinney JM. Metabolic utilization of intravenous fat emulsion during total parenteral nutrition. Ann Surg 1982;196:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elwyn DH, Kinney JM, Gump FE, Askanazi J, Rosenbaum SH, Carpentier YA. Some metabolic effects of fat infusions in depleted patients. Metabolism 1980;29:125–32. [DOI] [PubMed] [Google Scholar]
- 101.Jeejee hoy KN, Anderson GH, Nakhooda AF, Greenberg GR, Sanderson I, Marliss EB. Metabolic studies in total parenteral nutrition with lipid in man. Comparison with glucose. J Clin Invest 1976;57:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tappy L, Schwarz JM, Schneiter P, Cayeux C, Revelly JP, Fagerquist CK, Jequier E, Chiolero R. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit Care Med 1998;26:860–7. [DOI] [PubMed] [Google Scholar]
- 103.Manzanares W, Dhaliwal R, Jurewitsch B, Stapleton RD, Jeejeebhoy KN, Heyland DK. Parenteral fish oil lipid emulsions in the critically ill: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014;38:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaloga GP. Phytosterols, lipid administration, and liver disease during parenteral nutrition. JPEN J Parenter Enteral Nutr 2015;39(1 Suppl):39S–60S. [DOI] [PubMed] [Google Scholar]
- 105.Fell GL, Nandivada P, Gura KM, Puder M. Intravenous lipid emulsions in parenteral nutrition. Adv Nutr 2015;6:600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]