Abstract
Background
It has been demonstrated that upregulation of CXCL12 and CXCR4 in spinal cord involves in the pathogenesis of neuropathic, inflammatory, and cancer pain. However, whether CXCL12/CXCR4 signaling contributes to postsurgical pain remains unknown. The aim of the present study is to investigate the role of CXCL12/CXCR4 signaling in the genesis of postsurgical pain and the underlying mechanism.
Results
Plantar incision in rat hind paw resulted in increased expressions of CXCL12 and CXCR4 in spinal dorsal horn. Double immunofluorescence staining revealed that CXCL12 expressed in neurons and astrocytes, and CXCR4 exclusively co-localized with neuronal cells. Prior administration of AMD3100, a specific antagonist of CXCR4, or CXCL12 neutralizing antibody, intrathecally attenuated plantar incision-induced mechanical allodynia and thermal hyperalgesia. Plantar incision also augmented the phosphorylation of NF-κB p65 in spinal cord. Pre intrathecal (i.t.) injection of PDTC, a specific NF-κB activation inhibitor, alleviated plantar incision-induced postsurgical pain and reduced the expression of CXCL12 in spinal cord. Correlated with the upregulation of CXCL12 and CXCR4, plantar incision also resulted in an increased phosphorylation of extracellular signal-regulated kinase 1/2 and Akt in spinal cord. Prior i.t. administration of AMD3100 prevented extracellular signal-regulated kinase, but not Akt, activation in spinal cord. Rats when given a repetitive i.t. PD98059, a specific extracellular signal-regulated kinase inhibitor, started 30 min before surgery also ameliorate plantar incision-induced mechanical and thermal pain hypersensitivity.
Conclusion
Our results suggests that plantar incision-induced activation of NF-κB signaling may mediate upregulation of CXCL12 in spinal cord, and CXCL12/CXCR4 signaling via extracellular signal-regulated kinase activation contributes to the genesis of postsurgical pain.
Keywords: Postsurgical pain, CXCL12, NF-κB, extracellular signal-regulated kinase, spinal cord
Background
Postsurgical pain is a common consequence of most surgeries. Despite increased basic and clinical research have improved understanding of its pathologic mechanisms, optimal postsurgical pain therapy still remains a challenge for physicians.1 Previous studies have demonstrated that the surgery-induced upregulation of inflammatory mediators, including cytokines and chemokines, is involved in the generation of neuronal hyperexcitability and thus contributes to postoperative pain.2,3 The recruitment of leukocytes is a key step for the inflammatory process, and this recruitment process is controlled by chemotactic cytokines, also termed chemokines.4 The chemokine C-X-C motif ligand 12 (CXCL12, also named as stromal cell-derived factor-1, SDF-1) belongs to chemotactic superfamily. There are two receptors bind to CXCL12: the G-protein-coupled transmembrane (C-X-C motif) receptors, CXCR4 and CXCR7.5 The CXCL12/CXCR4 has been shown to be critical for inflammation, infectious diseases, angiogenesis, and tumors.6 Recently, the CXCL12/CXCR4 signaling pathway has been attracted much attention in the field of pain study because of its involvement in several types of chronic pain.7 It has been reported that CXCL12 and CXCR4 are widely distributed and constitutively expressed in dorsal root ganglia (DRG) neurons and satellite glial cells and in the spinal cord.8 Sustained mechanical allodynia occurs after a single intraplantar or intrathecal injection of CXCL12 in naïve rats.8–10 The administration of antiretroviral drugs11 and unilateral chronic constriction injury (CCI) of the sciatic nerve12 lead to the development of neuropathic pain, which is correlated with the upregulation of the expressions of both CXCL12 and CXCR4 in the DRGs. Intrathecal injection (i.t.) of AMD3100, a specific antagonist of CXCR4, or CXCL12 neutralizing antibody prevent the development of neuropathic pain and ameliorate established bone cancer pain.13 Using a spared nerve injury model combined with intrathecal and intraperitoneal injection of AMD3100 or CXCL12 neutralizing antibody, our recent work demonstrates that upregulation of chemokine CXCL12 in DRG and spinal cord contributes to the development and maintenance of neuropathic pain in rats.9 These findings imply that CXCL12/CXCR4 signaling might function as an important neuromodulator of both physiological and pathological pain. However, whether the CXCL12/CXCR4 signal pathway is involved in postsurgical pain has not been determined to date.
Compared with inflammatory and neuropathic pain, surgical pain is a unique acute pain state in which there are various central sensitization mechanisms, in particular, in the spinal cord.14 Previous studies have revealed the discrepancies between surgical incision and other pathologic pain models.15,16 The N-methyl-D-aspartate (NMDA)-dependent mechanism regulates the development of neuropathic and inflammatory pain.17,18 However, it has been reported that the NMDA-independent mechanism mediates the mechanical pain hypersensitivity induced by incision.19,20 Thus, although a number of studies have demonstrated that the CXCL12/CXCR4 signaling contributes to various types of chronic pain, whether it still plays a role in postsurgical pain process needs to be studied.
An animal model of postsurgical pain consisting of incision at the plantar hind paw has been developed in rats.21 This experimental model is characterized by spontaneous pain, allodynia and hyperalgesia, lasting for several days and corresponding with the time course of postsurgical pain in patients.1 Thus, in the current study, the plantar incision (PI) model in rat hind paw was used to investigate the role of CXCL12/CXCR4 signaling activation in spinal cord in the development of postsurgical pain process. We first observed the expressions of CXCL12 and its cognate receptor CXCR4 in spinal cord following PI. Then, the role of CXCL12/CXCR4 signaling in the induction of acute incisional pain was examined by intrathecal injection of AMD3100 or CXCL12 neutralizing antibody combined with pain-related behavioral test. Finally, the signal pathway of PI-induced upregulation of CXCL12 and the downstream molecules of CXCR4-mediated postsurgical pain were further determined.
Methods
Animal preparation
Male Sprague-Dawley rats weighing 200–300 g (purchased from the Laboratory Animal Center of Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China) were used. The rats were housed in separate cages with free access to food and water. The room temperature was maintained at 23 ± 2℃ under a natural light–dark cycle. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Zhengzhou University and were carried out in accordance with the guidelines of the National Institutes of Health on animal care.
Planter incision
An animal model of postsurgical pain was generated by PI as previously described.21 Male Sprague-Dawley rats were anesthetized with sevoflurane (2%–3%) vaporized through a nose cone. The plantar aspect of the left hind paw was scrubbed with 10% povidone-iodine three times. A 1-cm long incision, starting 0.5 cm from the heel and extending toward the toes, was made with a number-11 blade, through the skin and fascia of the plantar aspect of the left hind paw including the underlying muscle. The plantaris muscle was isolated, elevated slightly, and incised longitudinally, and then put it back to its original position. The exposed incision site of the plantaris muscle was desiccated and abraded with sterile gauze until hemorrhage was stopped. The wound was closed with two mattress sutures of 2-0 nylon. Rats were allowed to recover from the anesthesia before returning to their home cage. Sham animals were anesthetized and the left hind paw scrubbed with 10% povidone-iodine three times, but no incision was made. The incision was checked daily, and the rats which displayed wound infection or dehiscence were excluded from the study.
Intrathecal catheterization and drugs delivery
Drugs were delivered intrathecally. The intrathecal catheterization was performed according to our previous method.22 In brief, a polyethylene-10 (OD, 0.61 mm; ID, 0.28 mm) catheter was inserted into the rat’s subarachnoid space through L5–L6 intervertebral space, and the tip of the catheter was located at the L5 spinal segmental level. The CXCR4-specific antagonist AMD3100 and the NF-κB activation inhibitor PDTC were purchased from Sigma (St. Louis, USA) and freshly dissolved daily in normal sterile saline prior to use. Anti-CXCL12 neutralizing antibody (Abcam, ab25117) and anti-IgG antibody (for control) were purchased from Abcam (Massachusetts, USA) and diluted with sterile artificial cerebrospinal fluid containing 126.6 mM NaCl, 2.5 mM KCl, 2.0 mM MgCl2, and 1.3 mM CaCl2. The specific MEK (extracellular signal-regulated kinase (ERK)) inhibitor PD98059 was purchased from Sigma and was dissolved in sterile saline containing 10% DMSO. The intrathecal injection of drug was performed on 8:00 a.m. daily, the time which was 30 min before behavioral test. The doses of AMD3100 (5, 10, 20 µg/10 μl),9,13 anti-CXCL12 neutralizing antibody (4 µg/10 μl),8 PDTC (0.5 µg/10 μl),23,24 and PD98059 (10 µg/10 μl)25 used in this experiment were based on those used in previous studies.
Behavioral tests
The pain-related behavioral tests were performed according to our previous described methods.26,27 All rats were acclimated to the testing environment for at least three days prior to baseline measurement. To assess the mechanical sensitivity, the paw withdrawal threshold (PWT) was determined by applying mechanical stimuli to the plantar surface of the hind paw using von Frey hairs, and 50% PWT was determined using the up–down method.28 Heat hypersensitivity was determined by testing paw withdrawal latency (PWL) using the plantar test (7370, UgoBasile, Comeria, Italy) according to the method described by Hargreaves et al.29 Briefly, a radiant heat source beneath a glass floor was aimed at the plantar surface of the hind paw. Three measurements of latency were taken for each hind paw in each test session. The hind paw was tested alternately with greater than 5-min intervals between consecutive tests. The three measurements of latency per side were averaged as the result of per test.
Immunohistochemistry
Immunohistochemistry was done following the method described previously.27,30 Briefly, after defined survival times, control and plantar incisional rats were deeply anesthetized with ethyl ether and perfused from the ascending aorta with normal saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer. After perfusion, the lumbar 4-5 (L4–L5) spinal cord segments were removed and post fixed in same fixative for 3 h, and then replaced by 30% of sucrose phosphate-buffered saline over two nights. The transverse spinal sections (25 µm) were cut in a cryostat and prepared for immunofluorescence staining. The sections were randomly selected and put into different well of a 24-well plate. After washing with phosphate-buffered saline, the sections were blocked with 5% goat serum in 0.3% Triton X-100 for 1 h at 37℃ and incubated with primary antibody over night at 4℃. For double immunofluorescence staining, all of the above sections were treated by a mixture of goat anti-mouse FITC- (1: 200, Jackson ImmunoResearch) and goat anti-rabbit Cy3-conjugated secondary antibody (1:400, Jackson ImmunoResearch) for 1 h at 37℃. The stained sections were mounted onto slides and examined with an Olympus IX73 (Olympus Optical, Tokyo, Japan) fluorescence microscope and images were captured with a CCD spot camera. The primary antibodies used are as follows: rabbit anti-SDF-1α (1:100; Abcam ab25117, MA, USA) and rabbit anti-CXCR4 (1:200, Abcam ab7199). The following spinal cell specific marker were used: Glial fibrillary acidic protein (GFAP, a marker for satellite glial cell astrocyte, 1:200; Chemicon), monoclonal neuronal-specific nuclear protein (NeuN, a neuronal marker, 1:500; Chemicon), and OX42 (CD11b, microglia marker, 1:200; Chemicon). The quantification of CXCL12 and CXCR4 positive staining area in spinal cord were performed using a computerized image analysis system (NIH Image J) according to the method described previously.26 The specificity of anti-CXCL12 antibody was examined by pre-incubation with its antigen, which described by previous studies.26 To check the specificity of anti-CXCR4, anti-ERK, anti-p-p65, and anti-Akt antibodies, the primary antibodies were omitted during staining when performing control experiments, and further examined by Western blotting.
Western blotting
Western blotting was performed according to our previously published procedures.22 Briefly, the animals were sacrificed by decapitation at a designed time point. The L4–L5 spinal dorsal horns were harvested and placed temporarily in liquid nitrogen. Next, the samples were homogenized with ice-cold lysis buffer (10 mM Tris, 5 mM EGTA, 0.5% Triton X-100, 2 mM benzamidine, 0.1 mM PMSF, 40 µM leupeptin, 150 mM NaCl, 1% phosphatase inhibitor cocktail 2 and 3). The crude homogenate was centrifuged at 4℃ for 15 min at 3000 r/min, and the supernatants were collected. After the protein concentrations were measured, the samples were heated for 5 min at 99℃, and 30–60 µg protein was loaded onto 10%–12.5% SDS-polyacrylamide gels. The proteins were electrophoretically transferred onto PVDF membranes. The blotting membranes were blocked with 3% non-fat dry milk for 1 h and incubated overnight at 4℃ with the primary antibody. The following primary antibodies were used: rabbit anti-SDF-1α (1:500; Abcam ab25117, Massachusetts, USA), rabbit anti-CXCR4 (Abcam ab7199, 1: 1000), rabbit anti-p-ERK1/2 (Cell Signaling, 1:1000), rabbit anti-p-Akt, rabbit anti-Akt (Cell Signaling, 1:1000), rabbit anti-NF-kB p65, rabbit anti-NF-kB p-p65 (Cell Signaling, 1:1000) and mouse anti-β-actin (1:10,000; Sigma). The proteins were detected with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (BIORad, 1:3000), visualized using the chemiluminescence reagents provided with the ECL kit (Amersham Pharmacia Biotech, Piscataway, NJ) and exposed to film. The intensities of the blots were quantified via densitometry. The blot density of the control rats was set as 100%. The relative density values of the other groups were determined by dividing the optical density values of these groups by that of the control rats.
Statistical analysis
Statistical tests were performed with SPSS 10.0 (SPSS Inc., USA) and SigmaStat (Systat, San Jose, CA). All data were presented as mean ± SE. For behavioral analysis, two-way analysis of variance (ANOVA) with repeated measures followed by Tukey’s post hoc test for all groups and between groups and Student's t-test between two groups at the same time points were carried out. For Western blot and Immunohistochemistry data, the differences were tested using one-way ANOVA followed by individual post hoc comparisons (Tukey’s post hoc tests) or using Student's t-test if only two groups were applied. The P < 0.05 was considered significant.
Results
PI-induced pain-related hypersensitivity and upregulation of CXCL12 and CXCR4 in spinal cord
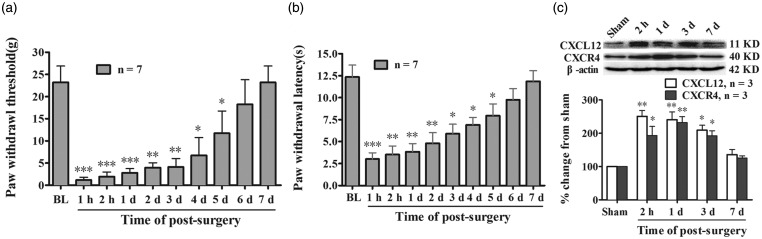
Consistent with previous study, PI produced a rapid mechanical allodynia and thermal hyperalgesia. Pain-related behavioral test revealed a clear reduction of PWT (compared with baseline value, 1 h, P < 0.001; 2 h, P < 0.001; 1 day, P < 0.001; 2 days, P < 0.01; 3 days, P < 0.01; 4 days, P < 0.05; 5 days, P < 0.05, two-way ANOVA, Figure 1(a)) and PWL (compared with baseline value, 1 h, P < 0.001; 2 h, P < 0.01; 1 day, P < 0.01; 2 days, P < 0.01; 3 days, P < 0.05; 4 days, P < 0.05; 5 days, P < 0.05, two-way ANOVA, Figure 1(b)), which started at 1 h after PI and persistent to the fifth day after surgery. In view of the potential role of CXCL12/CXCR4 signaling in acute pain, the protein expressions of CXCL12 and CXCR4 in L4–L5 spinal dorsal horn were examined at different time points after PI. The Western blotting data showed that PI induced robust increased expressions of CXCL12 and CXCR4 in spinal cord. Compared with sham-operated rats, the significant increased expression of CXCL12 started at 2 h and lasted to the third day (2 h, P < 0.01; 1 day, P < 0.01; 3 days, P < 0.05, one-way ANOVA, Figure 1(c)) after PI. In correlation with the change of CXCL12, PI also led to a clear enhanced expression of CXCR4 in spinal dorsal horn. Compared with sham group, the statistical difference of CXCR4 in PI rats occurred at 2 h and persistent to the third day after surgery (2 h, P < 0.05; 1 day, P < 0.01; 3 days, P < 0.05, one-way ANOVA, Figure 1(c)).
Figure 1.
Plantar incision (PI)-induced mechanical allodynia, thermal hyperalgesia, and upregulation of CXCL12 and CXCR4 in spinal cord. (a, b) Behavioral data showing reduction of paw withdrawal threshold (PWT) (a) and paw withdrawal latency (PWL) (b) following PI. *P < 0.05; **P < 0.01; ***P < 0.001 versus baseline value, two-way ANOVA. (c) Western blotting data showing increased expressions of CXCL12 and CXCR4 in spinal dorsal horn following PI. *P < 0.05; **P < 0.01 versus sham group one-way ANOVA.
BL: Baseline.
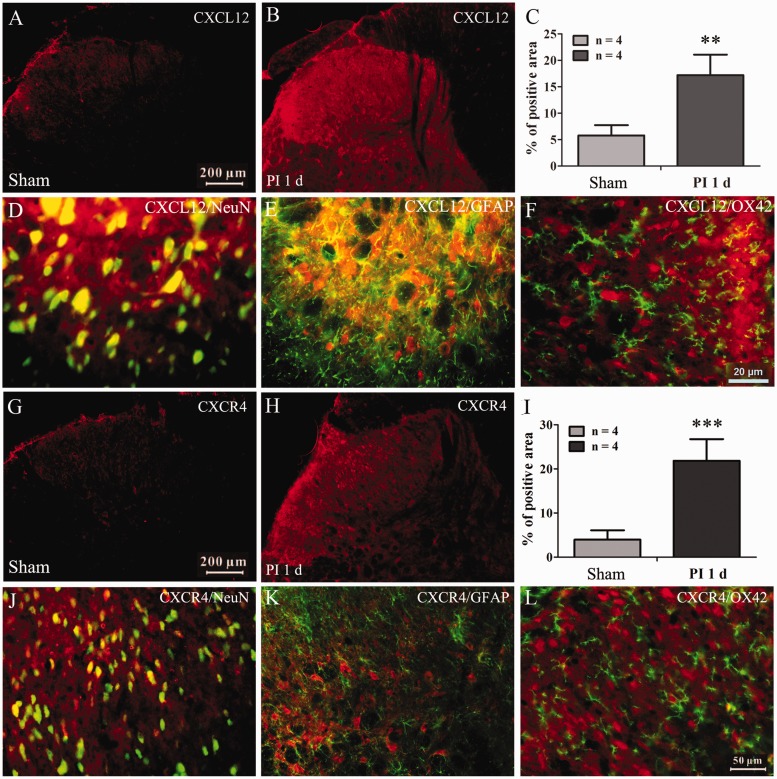
To further confirm the above results, the immuohistochemistry was performed to examine the expression and distribution of CXCL12 and CXCR4 in spinal cord following PI. The results showed that PI induced a significant increased expression of CXCL12 (compared with sham group, P < 0.01, Student's t-test, Figure 2(a) to (c)) and CXCR4 (P < 0.001, Student's t-test, Figure 2(g), 2(h), and 2(i)) in spinal dorsal horn. Double immunofluorescence staining revealed that the upregulated CXCL12 primarily co-localized with the neuronal marker NeuN (Figure 2(d)) and the astrocytic marker GFAP (Figure 2(e)), but not with the microglia marker OX42 (Figure 2(f)). The percentage of CXCL12 co-localized with neuron in spinal dorsal horn is about 71%, and the percentage of CXCL12 co-localized with astrocytes is about 48%. However, CXCR4 was exclusively co-localized with neuronal marker NeuN (the percentage is about 67% of total spinal dorsal horn neurons) (Figure 2(i), (k), and (l)).
Figure 2.
Distributions and cell-types of CXCL12 and CXCR4 expressed in spinal dorsal horn. (a) to (c) The immunofluorescence staining pictures showing increased expression of CXCL12 in spinal dorsal horn. **P < 0.01 versus sham group, Student's t-test. (d) to (f) Representative pictures showing the CXCL12 colocalized with neuronal marker NeuN (d) and astrocytic marker GFAP (e), but not microglia marker OX42 (f). (g) to (i) The immunofluorescence staining pictures showing an increased expression of CXCR4 in spinal dorsal horn. ***P < 0.01 versus sham group, Student's t-test. (j) to (l) Representative pictures showing the CXCR4 exclusively colocalized with neuronal marker NeuN (j), but not astrocytic (k) and microglia marker (l).
PI: Plantar incision.
The role of spinal CXCL12/CXCR4 signaling in the induction of postsurgical pain
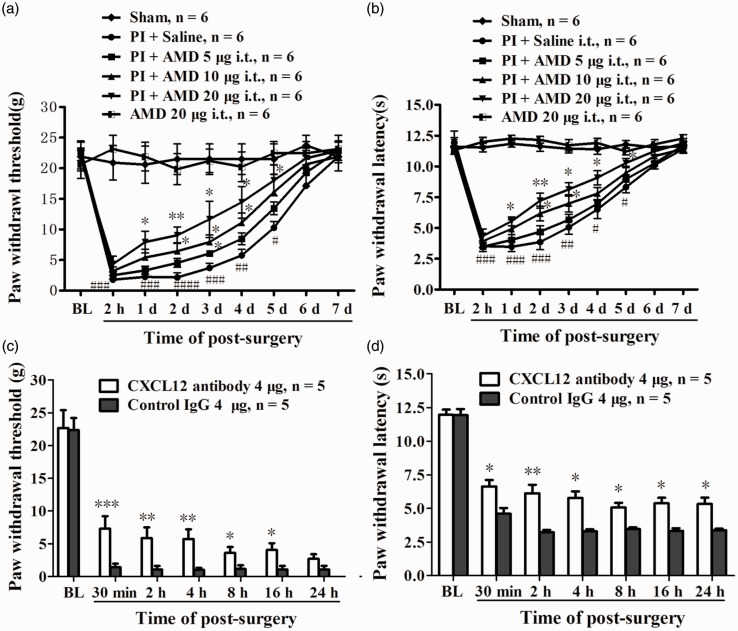
To evaluate the role of PI-induced upregulated CXCL12 and CXCR4 in spinal cord in the genesis of postsurgical pain, rats were treated intrathecally with different doses of AMD3100 (5, 10, 20 µg/10 μl), a specific antagonist of CXCR4, 30 min before PI and daily for five days. Compared with vehicle (i.t. 10 μl saline) group, AMD3100 treatment dose-dependently increased PWT and PWL in ipsilateral, but not contralateral, hind paw. Analysis of Student's t-test revealed the significant difference of PWT in high-dose group occurred at day 1, and lasted to 5th day after PI (compared with PI + saline group, 1 day, P < 0.05; 2 days, P < 0.01; 3 days, P < 0.05; 4 days, P < 0.05; 5 days, P < 0.05, Figure 3(a)). AMD3100 treatment also clearly increased PWL, the statistical difference occurred at day 1 and lasted to the fifth day after surgery in high-dose group (compared with PI + saline group, 1 day, P < 0.05; 2 days, P < 0.01; 3 days, P < 0.01; 4 days, P < 0.05; 5 days, P < 0.05, Figure 3(b)). The basal PWT and PWL in naïve rats were not changed by i.t. AMD3100 (20 µg) alone daily for five days (Figure 3(a) and 3(b)). To further define the above results, a procedure of intrathecal injection of CXCL12 neutralizing antibody (i.t. 4 µg/10 μl CXCL12 antibody 30 min before surgery) was performed in another group of rats. In control group, the rats received same dose of anti-IgG antibody. The results showed that a single dose of CXCL12 neutralizing antibody i.t. resulted in a significant reductions of PWT and PWL following PI. Compared to control group, the statistical difference of PWT (30 min, P < 0.001; 2 h, P < 0.01; 4 h, P < 0.01; 8 h, P < 0.05; 16 h, P < 0.05, Student's t-test, Figure 3(c)) and PWL (30 min, P < 0.05; 2 h, P < 0.01; 4 h, P < 0.05; 8 h, P < 0.05; 16 h, P < 0.05, 24 h, P < 0.05, Student's t-test, Figure 3(d)) started 30 min after surgery and maintained to the 16 h of post-surgery.
Figure 3.
The role of CXCL12/CXCR4 signaling in the pathogenesis of postsurgical pain. Prior intrathecal (i.t.) injection of AMD3100 partially prevented the reduction of PWT (a) and PWL (b) following PI. *P < 0.05; **P < 0.01; ***P < 0.001 versus PI + saline group at different time-points, Student's t-test. #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline value, two-way ANOVA. Prior i.t. administration of CXCL12 neutralizing antibody attenuated PI-induced mechanical allodynia (c) and thermal hyperalgesia (d). *P < 0.05; **P < 0.01; ***P < 0.001 versus control IgG group, Student's t-test.
Nuclear factor kappa B (NF-κB) activation mediates the PI-induced CXCL12 upregulation in spinal cord
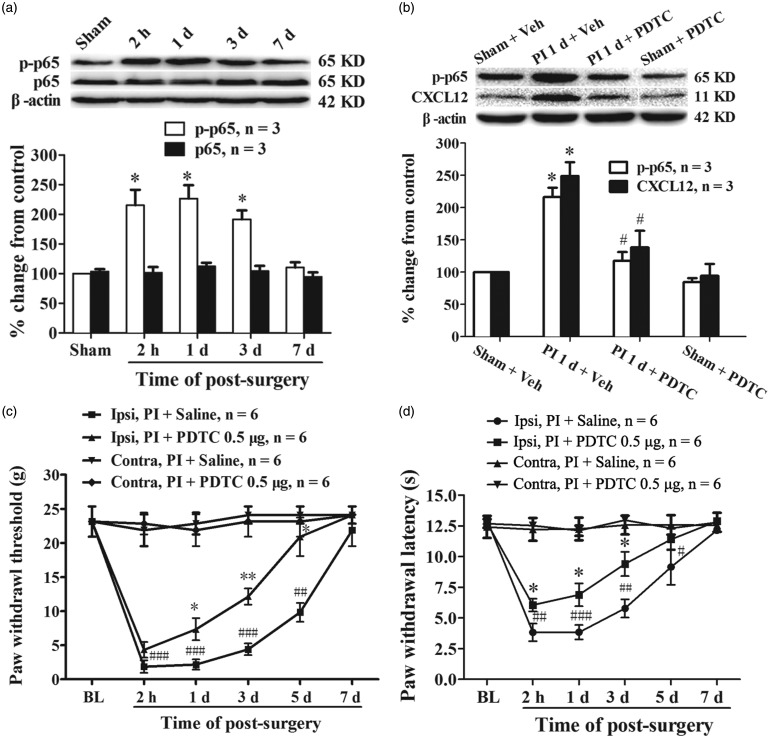
Previous studies have demonstrated that NF-κB signaling activation regulates the expression of many cytokines and chemokines.31 To determine whether NF-κB signaling involved in the PI-induced spinal CXCL12 upregulation, the phosphorylated NF-κB p65 level at different time points after PI was examined first. The results of Western blot showed that the expression of phosphorylated p65 at Ser311 was significantly increased, started at 2 h and persistent to the third day after surgery (compared with sham group, 2 h, P < 0.05; 1 day, P < 0.05; 3 days, P < 0.05, one-way ANOVA, Figure 4(a)). The total NF-κB p65 protein in spinal cord was not changed following PI (Figure 4(a)). Next, we examined whether the NF-κB activation involved in CXCL12 expression after PI. The result showed that intrathecal injection of PDTC (30 min before surgery and daily for two days), a specific inhibitor of NF-κB activation, suppressed PI-induced phosphorylation of NF-κB p65 in spinal cord (PI + PDTC vs. PI + vehicle, P < 0.05, one-way ANOVA, Figure 4(b)). The PI-induced upregulation of CXCL12 in spinal dorsal horn was inhibited following i.t. administration of PDTC. One-way ANOVA analysis revealed a significant reduction of CXCL12 expression in PI plus PDTC group (PI + PDTC vs. PI + vehicle, P < 0.05, Figure 4(b)). Behavior data analysis showed that i.t. administration of PDTC (500 ng/10 μl) attenuated PI-induced mechanical allodynia and thermal hyperalgesia. Compared with saline group, PDTC treatment significantly increased PWT (PI + PDTC vs. PI + saline, 1 day, P < 0.05; 3 days, P < 0.01; 5 days, P < 0.05, Student's t-test, Figure 4(c)) and PWL (PI + PDTC vs. PI + saline, 2 h, P < 0.05; 1 day, P < 0.05; 3 days, P < 0.05; Student's t-test, Figure 4(d)). These results suggest that upregulation of CXCL12 after PI might depend on NF-κB signaling activation.
Figure 4.
NF-κB activation mediated PI-induced upregulation of CXCL12 in spinal cord. (a) Western blotting data showing an increased level of phosphorylated NF-κB p65 (p-p65), but not total p65, in spinal cord following PI. *P < 0.05 versus sham group, one-way ANOVA. (b) Prior i.t. administration of PDTC inhibited activation of NF-κB and reduced CXCL12 expression in spinal cord following PI. *P < 0.05 versus sham + vehicle (Veh) group; #P < 0.05 versus PI + Veh group, one-way ANOVA. (c, d) Repetitive given rats of PDTC intrathecally prevented the reduction of PWT (c) and PWL (d) after PI. *P < 0.05; **P < 0.01 versus PI + saline group, Student's t-test. #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline value, two-way ANOVA.
Downstream pathway of CXCL12/CXCR4 signaling mediated postsurgical pain
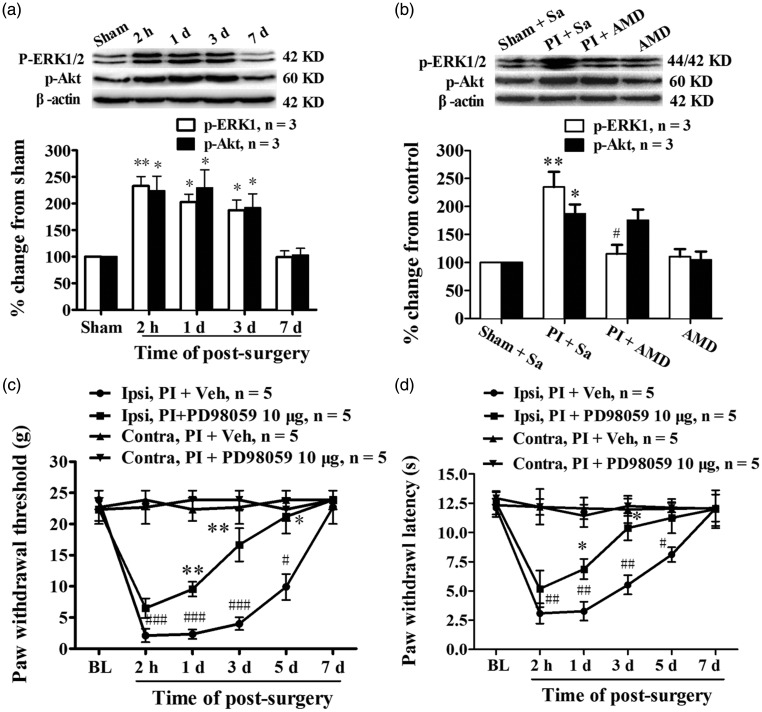
Previous studies have shown that the binding of CXCL12 to CXCR4 activates multiple intracellular signal pathways, including ERK and PI3K-Akt.32,33 Therefore, we assessed ERK and Akt activation in the spinal cord following PI. Analysis of Western blotting data showed an increased expression of phosphorylated ERK1/2 (p-ERK) and Akt (p-Akt) in spinal dorsal horn. Compared with sham group, the statistical difference of p-ERK was detected at 2 h and persistent to the third day after surgery (2 h, P < 0.01; 1 day, P < 0.05; 3 days, P < 0.05, one-way ANOVA, Figure 5(a)). The PI-induced significant increase of p-Akt also was detected at 2 h and lasted to the third day after surgery (2 h, P < 0.05; 1 day, P < 0.05; 3 days, P < 0.05, one-way ANOVA, Figure 5(a)). The expressions of total ERK and Akt were not changed significantly following PI (data not shown). Then, we examined whether CXCR4 blocker can suppress the PI-induced activation of ERK1/2 and Akt in spinal cord. Results from Western blot showed that prior i.t. administration of AMD3100 significantly reduced the phosphorylation level of PI-induced ERK1/2 (PI + AMD3100 vs. PI + saline, P < 0.05, one-way ANOVA, Figure 5(b)), but not Akt (PI + AMD3100 vs. PI + saline, P > 0.05, one-way ANOVA, Figure 5(b)), in spinal dorsal horn. Behavioral data showed that prior i.t. injection of PD098059 (10 µg/10 μl, 30 min before surgery and daily for five days) significantly prevented the PI-induced reductions of PWT (PI + PD98058 vs. PI + vehicle, 1 day, P < 0.01; 3 days, P < 0.01; 5 days, P < 0.05, Student's t-test, Figure 5(c)) and PWL (PI + PD98058 vs. PI + vehicle, 1 day, P < 0.05; 3 days, P < 0.05, Student's t-test, Figure 5(d)). It implied that ERK might be one of important downstream target in which CXCL12/CXCR4 mediated postoperative pain.
Figure 5.
ERK and PI3K-Akt signaling were activated in spinal cord after PI, and CXCL12/CXCR4-mediated postsurgical pain depended on ERK activation. (a) Western blotting data showing the time course of ERK and Akt activation in spinal cord after PI. *P < 0.05; **P < 0.01 versus sham group, one-way ANOVA. (b) Prior i.t. administration of AMD3100 prevented ERK, but not Akt, activation in spinal cord after PI. *P < 0.05; **P < 0.01 versus sham group. #P < 0.05 versus PI + Vehicle (Veh) group, one-way ANOVA. (c, d) Repetitive given rats of PD98059, a specific ERK kinase inhibitor, intrathecally prevented the reduction of PWT (c) and PWL (d) after PI. *P < 0.05; **P < 0.01 versus PI + Veh group, Student's t-test. #P < 0.05; ##P < 0.01; ###P < 0.001 versus baseline value, two-way ANOVA.
Discussion
Recent studies have revealed that the CXCL12/CXCR4 signaling activation plays a critical role in the development of several types of chronic pain. In the current study, we found, for the first time to our knowledge, that the CXCL12/CXCR4 signaling is required for the genesis of PI-induced postsurgical pain. Our results showed that the CXCL12 and its receptor CXCR4 were upregulated in spinal cord dorsal horn following rats receiving PI. Prior administration of AMD3100, a specific antagonist of CXCR4, or CXCL12 neutralizing antibody intrathecally attenuated PI-induced mechanical allodynia and thermal hyperalgesia. Blockage of NF-κB signaling activation by prior i.t. PDTC prevented the increased expression of CXCL12 and alleviated the postsurgical pain. PI also resulted in an enhanced phosphorylation of ERK1/2 in spinal cord, but inhibited by i.t. AMD3100 in advance. Taken together, these findings indicate that the upregulation of CXCL12/CXCR4, which might be mediated by NF-κB signaling, contributes to the postsurgical pain via ERK signaling activation.
The CXCL12/CXCR4 pair has attracted much attention regarding its involvement in nociceptive signal processing. Previous studies have found that CXCL12 and its cognate receptor CXCR4 are constitutively expressed in the DRG and spinal cord of naïve rats.8 The CXCL12 and CXCR4 expressions are upregulated in DRG and spinal cord in several types of chronic pain model.7 However, whether the PI, a model of postsurgical pain, can upregulate CXCL12 and CXCR4 in spinal cord still remains unclear. In the present study, significant increased expressions of CXCL12 and CXCR4 were detected at 2 h after PI and lasted to the third day of surgery. Results of double immunofluorescence showed that the CXCL12 expressed mainly in spinal neurons and astrocytes, whereas the CXCR4 expressed exclusively in spinal neurons. The cell types of CXCL12 and CXCR4 expressions in the present study were different from our recent work, in which the sciatic nerve injury induced a significant increased expression of CXCL12 in spinal neuron and CXCR4 in spinal neuron and astrocytes.9 This difference may be one of the discrepancies between acute pain and chronic neuropathic pain, which may be determined by different time course of pain lasting.
A great number of studies have demonstrated that the upregulated CXCL12 and CXCR4 in spinal cord contribute to the pathogenesis of several types of chronic pain.7 Postoperative incisional pain is characterized by persistent acute pain in the area of the cut and is associated with the release of proinflammatory cytokines and chemokines, which play important hyperalgesic and allodynic roles in various inflammatory conditions.1 To evaluate the role of PI-induced upregulation of CXCL12 and CXCR4 in spinal cord in the induction of postsurgical pain, rats were treated with AMD3100 intrathecally 30 min before PI and daily for five days. Our results showed that AMD3100 administration dose-dependently alleviated the PI-induced mechanical allodynia and thermal hyperalgesia. To further confirm the effects of AMD3100, a CXCL12 neutralizing antibody was used. The results showed that rats received i.t. bolus of anti-CXCL12 antibody 30 min prior to PI resulted in an increase of PWT and PWL. The significant difference started 1 h (30 min after PI) after injection and persistent to more than 16 h after surgery. It indicates that spinal CXCL12/CXCR4 signaling plays an important role in the genesis of postsurgical pain. Previous studies have shown that peripheral nerve injury or inflammation also lead to significant increased activations of CXCL12/CXCR4, ERK, and NF-kB signaling in DRG.9,34,35 Because the dural membrane in rats extends onto the capsule of the DRG, the proximal face of the DRG is in direct continuity with the subarachnoid space.30 Therefore, the effects of intrathecal injection of AMD3100 or CXCL12 neutralizing antibody may partially work in DRG in our present study. Recently, Yang and coworkers found that single intraplantar active SDF1 protein injection is sufficient to induce acute mechanical hyperalgesia and hyperalgesic priming through CXCR4. Peripheral treating with AMD3100 or knocking down CXCR4 by intraganglionar CXCR4 small interfering RNA (siRNA) injection prevent intraplantar bee venom (BV) injection-induced primary mechanical hyperalgesia and hyperalgesic priming.10,34 It suggests that CXCL12/CXCR4 signaling activation contributes to the establishment of plantar BV injection-induced acute pain. Using the lumbar 5 spinal nerve ligation (L5 SNL) model, Jiang and coworkers found that CXCL13, a member of same superfamily with CXCL12, and its receptor CXCR5 were persistently upregulated in spinal cord after L5 SNL. Inhibition of CXCL13 expression or genetically knock out CXCR5 in spinal cord effectively alleviates the neuropathic pain induced by L5 SNL.36 These results are in agreement with our recent study in which the CXCL12/CXCR4 signaling was found to play a critical role in the development and maintenance of neuropathic pain after sciatic nerve injury.9 It indicates that the C-X-C motif chemokines CXCL12 and CXCL13 may play similar role in the generation of chronic and acute pain.
It is well known that NF-κB activation plays a key role in regulating the expression of pro-inflammatory cytokines and chemokines after specific extracellular stimulation.31 It is also well established that the NF-κB family plays critical roles in inflammation, immunity, cell proliferation, and apoptosis, and has been implicated in the regulation of memory and neuroplasticity.31,37,38 Numerous studies have shown that NF-κB activation in the DRG and spinal dorsal horn contributes to the development of inflammatory and neuropathic pain.39 But few studies have observed the role of NF-κB signaling in postsurgical pain. Here, we found the level of phosphorylated NF-κB p65 significantly increased in spinal cord dorsal horn following PI. Pre administration of NF-κB activation inhibitor PDTC alleviated PI-induced mechanical allodynia and thermal hyperalgesia, and dramatically inhibited the expression of CXCL12 in spinal cord. It suggests that PI-induced upregulation of CXCL12 in spinal cord may depend on the NF-κB activation. Recently, Li et al.40 reported that NF-κB activation in spinal cord mediates paclitaxel-induced upregulation of CX3CL1. Xu et al.41 also found that NF-κB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. These studies suggest that NF-κB signaling is an important signal pathway to mediate the expression of chemokines after tissue and nerve injury.
Previous studies have shown that the binding of CXCL12 to CXCR4 activates several signaling pathways, including the ERK and phosphatidylinositol 3-kinase (PI3K)-Akt.42 It is well known that ERK43,44 and PI3K-Akt27,45 activation are required for the initiation and maintenance of neuropathic pain. Using a PI model, Shi et al.46 demonstrated that ERK activation in spinal cord contributes to the development of postsurgical pain. In the present study, we found that PI resulted in a significant augmentation of ERK1/2 and Akt phosphorylation, which parallelized to the increased expressions of CXCL12 and CXCR4 in spinal dorsal horn. However, the ERK1/2, but not Akt, activation following PI was prevented by i.t. administration of AMD3100 in advance. Prior i.t. injection of PD98059, a specific ERK kinase inhibitor attenuated the PI-induced postsurgical pain. It indicates that the CXCL12/CXCR4 mediated postsurgical pain might partially through the ERK signaling activation. Yang et al.34 reported recently that SDF-1/CXCR4 mediates intraplantar BV injection-induced hyperalgesic priming state through spinal ERK activation. Jiang et al.36 reported that CXCL13/CXCR5 signaling contributes to L5 SNL-induced neuropathic pain depending on ERK signaling activation. Our recent study also observed that CXCL12/CXCR4 signaling via ERK activation contributes to the development and maintenance of neuropathic pain following partial sciatic nerve injury in rats.9 It suggests that the ERK might be an important downstream kinase of CXCL12/CXCR4-mediated acute and chronic pain.
It has been reported that NF-κB47 and ERK48 signaling pathways are key upstream mechanisms for increased spinal NMDA receptor activation during the development of inflammatory or neuropathic pain, and the postsurgical pain develops in NMDA receptor-independent mechanism.16 The ERK25 and NF-κB49 in neuropathic and inflammatory pain displays a long-lasting activation (over two weeks) and lead to increased NMDA receptor activity in spinal cord which contributes to the development of chronic pain. In our present study, we also observed increased activation of NF-κB and ERK in spinal cord after PI, but the duration just persisted to the third day after surgery. Although other studies also observed the activation of ERK at the early stage of PI,46 the postsurgical pain generates in a mechanism of independent NMDA receptor activation.16 Therefore, the different duration of ERK and NF-κB activation in spinal cord may determine different mechanisms between acute and chronic pain. Ji and coworkers have observed a robust activation of ERK in spinal dorsal horn just 1 min after an intense noxious peripheral or C-fiber electrical stimulus, and inhibition of ERK phosphorylation by a MEK inhibitor reduced the second phase of formalin-induced pain behavior. Therefore, they think the ERK activation in the model probably involves regulation of neuronal excitability without changes in transcription because of its rapid activation.50 Therefore, in our present study, the CXCL12/CXCR4 signaling-mediated transient activation of ERK and NF-κB in spinal cord after PI may just change the neuronal excitability without affecting the NMDA receptor expression.
Conclusions
Our results reveal that PI-induced NF-κB activation mediates the upregulation of CXCL12 and CXCR4 in spinal dorsal horn. The increased release of CXCL12 from spinal neurons and astrocytes via act on CXCR4 in spinal neurons evoked ERK signal activation contributing to the generation of postsurgical pain. Therefore, the CXCL12/CXCR4 signaling may be a new target to the treatment of postsurgical pain.
Authors’ Contributions
WZ, LB, and JTX conceived of the project and designed the experiments. FX, CK, and JY carried out all experiments. FX, CK, and ZL analyzed the data. WZ and JTX supervised the overall experiment. CK and JTX revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (81571079, 81571082 and 81501070).
References
- 1.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America 2005; 23: 1–20. [DOI] [PubMed] [Google Scholar]
- 2.Wolf G, Livshits D, Beilin B, et al. Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun 2008; 22: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 3.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol 2005; 192: 444–462. [DOI] [PubMed] [Google Scholar]
- 4.Johnson Z, Schwarz M, Power CA, et al. Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol 2005; 26: 268–274. [DOI] [PubMed] [Google Scholar]
- 5.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 2005; 280: 35760–35766. [DOI] [PubMed] [Google Scholar]
- 6.Bonavia R, Bajetto A, Barbero S, et al. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol Lett 2003; 139: 181–189. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Wang X, Xia Z, et al. CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neurosci 2016; 27: 83–92. [DOI] [PubMed] [Google Scholar]
- 8.Reaux-Le GA, Rivat C, Kitabgi P, et al. Cellular and subcellular localization of CXCL12 and CXCR4 in rat nociceptive structures: physiological relevance. Eur J Neurosci 2012; 36: 2619–2631. [DOI] [PubMed] [Google Scholar]
- 9.Bai L, Wang X, Li Z, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016; 32: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Sun W, Luo WJ, et al. SDF1-CXCR4 signaling contributes to the transition from acute to chronic pain state. Mol Neurobiol 2017; 54: 2763–2775. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Zheng W, Ouyang H, et al. Mechanical allodynia induced by nucleoside reverse transcriptase inhibitor is suppressed by p55TNFSR mediated by herpes simplex virus vector through the SDF1 alpha/CXCR4 system in rats. Anesth Analg 2014; 118: 671–680. [DOI] [PubMed] [Google Scholar]
- 12.Dubovy P, Klusakova I, Svizenska I, et al. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 2010; 133: 323–337. [DOI] [PubMed] [Google Scholar]
- 13.Shen W, Hu XM, Liu YN, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014; 11: 75, DOI: 10.1186/1742-2094-11-75.:75-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011; 377: 2215–2225. [DOI] [PubMed] [Google Scholar]
- 15.Zahn PK, Umali E, Brennan TJ. Intrathecal non-NMDA excitatory amino acid receptor antagonists inhibit pain behaviors in a rat model of postoperative pain. Pain 1998; 74: 213–223. [DOI] [PubMed] [Google Scholar]
- 16.Zahn PK, Brennan TJ. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology 1998; 88: 143–156. [DOI] [PubMed] [Google Scholar]
- 17.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol 2006; 17: 592–604. [DOI] [PubMed] [Google Scholar]
- 18.Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain 2011; 4: 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu CZ, Hsieh G, Ei-Kouhen O, et al. Role of central and peripheral mGluR5 receptors in post-operative pain in rats. Pain 2005; 114: 195–202. [DOI] [PubMed] [Google Scholar]
- 20.Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain 2005; 114: 499–510. [DOI] [PubMed] [Google Scholar]
- 21.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 22.Xu JT, Zhao JY, Zhao X, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014; 124: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledeboer A, Gamanos M, Lai W, et al. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci 2005; 22: 1977–1986. [DOI] [PubMed] [Google Scholar]
- 24.Bai L, Zhai C, Han K, et al. Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 2014; 30: 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang ZY, Gerner P, Woolf CJ, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114: 149–159. [DOI] [PubMed] [Google Scholar]
- 26.Xu JT, Xin WJ, Zang Y, et al. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 2006; 123: 306–321. [DOI] [PubMed] [Google Scholar]
- 27.Xu JT, Tu HY, Xin WJ, et al. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 2007; 206: 269–279. [DOI] [PubMed] [Google Scholar]
- 28.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 30.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36: 57–68. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–734. [DOI] [PubMed] [Google Scholar]
- 32.Barbero S, Bonavia R, Bajetto A, et al. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res 2003; 63: 1969–1974. [PubMed] [Google Scholar]
- 33.Luo Y, Lathia J, Mughal M, et al. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem 2008; 283: 24789–24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang F, Sun W, Yang Y, et al. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation 2015; 12: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu ZH, Miao GS, Wang JN, et al. Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor-κB, phospho-extracellular signal-regulated kinase, and pro- and antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology 2016; 124: 934–944. [DOI] [PubMed] [Google Scholar]
- 36.Jiang BC, Cao DL, Zhang X, et al. CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest 2016; 126: 745–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattson MP. NF-kappaB in the survival and plasticity of neurons. Neurochem Res 2005; 30: 883–893. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109: S81–S96. [DOI] [PubMed] [Google Scholar]
- 39.Niederberger E, Geisslinger G. The IKK-NF-kappaB pathway: a source for novel molecular drug targets in pain therapy? FASEB J 2008; 22: 3432–3442. [DOI] [PubMed] [Google Scholar]
- 40.Li D, Huang ZZ, Ling YZ, et al. Up-regulation of CX3CL1 via nuclear factor-kappaB-dependent histone acetylation is involved in paclitaxel-induced peripheral neuropathy. Anesthesiology 2015; 122: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Zhu MD, Zhang X, et al. NFkappaB-mediated CXCL1 production in spinal cord astrocytes contributes to the maintenance of bone cancer pain in mice. J Neuroinflammation 2014; 11: 38–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floridi F, Trettel F, Di BS, et al. Signalling pathways involved in the chemotactic activity of CXCL12 in cultured rat cerebellar neurons and CHP100 neuroepithelioma cells. J Neuroimmunol 2003; 135: 38–46. [DOI] [PubMed] [Google Scholar]
- 43.Ji RR, Gereau RW, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramesh G. Novel therapeutic targets in neuroinflammation and neuropathic pain. Inflamm Cell Signal 2014; 1: e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popiolek-Barczyk K, Mika J. Targeting the microglial signaling pathways: New insights in the modulation of neuropathic pain. Curr Med Chem 2016; 23: 2908–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi XD, Fu D, Xu JM, et al. Activation of spinal ERK1/2 contributes to mechanical allodynia in a rat model of postoperative pain. Mol Med Rep 2013; 7: 1661–1665. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Lim G, Mao J, et al. Regulation of the trigeminal NR1 subunit expression induced by inflammation of the temporomandibular joint region in rats. Pain 2009; 141: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 2010; 16: 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chou CW, Wong GT, Lim G, et al. Spatiotemporal pattern of concurrent spinal and supraspinal NF-κB expression after peripheral nerve injury. J Pain 2011; 12: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]