Abstract
The disease course of BRAF (v-raf murine sarcoma viral oncogene homolog B1)-mutant melanoma has been drastically improved by the arrival of targeted therapies. NRAS (neuroblastoma RAS viral oncogene homolog)-mutated melanoma represents 15–25% of all metastatic melanoma patients. It currently does not have an approved targeted therapy. Metastatic patients receive immune-based therapies as first-line treatments, then cytotoxic chemotherapy like carboplatin/paclitaxel (C/P), dacarbazine (DTIC) or temozolomide (TMZ) as a second-line treatment. We will review current preclinical and clinical developments in NRAS-mutated melanoma, and analyze ongoing clinical trials that are evaluating the benefit of different targeted and immune-based therapies, either tested as single agents or in combination, in NRAS-mutant melanoma.
Keywords: NRAS-mutant melanoma, targeted therapies, metastatic melanoma, clinical trials
Introduction
Malignant melanoma represents less than 5% of all cutaneous malignancies but accounts for the majority of deaths from skin cancer.1 Due to its increasing incidence in White populations, in the USA, it is now the fifth leading cancer in men and the seventh in women.1
Patients diagnosed with localized melanoma at an early stage have a good chance of survival and are treated solely with surgery.2 Treatment of advanced or metastatic disease is dependent on the genotype of the melanoma with four distinct genetic categories including BRAF (v-RAF murine sarcoma viral oncogene homolog B1) mutant, NRAS (neuroblastoma RAS viral oncogene homolog) mutant, NF1 mutant and triple negative mutant melanoma (or wild type; WT) which includes melanomas with GNAQ or KIT mutations.3
The mitogen activated protein kinase (MAPK) cell signaling pathway, (also known as the RAS-RAF-MEK-ERK pathway) regulates cell growth, proliferation and differentiation in response to growth factors, cytokines and hormones and it is frequently altered in melanoma with 50% of metastatic cutaneous melanoma patients harboring a BRAF-activating mutation4 and 20–30% of them harboring an NRAS-activating mutation.5
The disease course of BRAF-mutant melanoma has been improved recently by the advent of targeted therapies, like BRAF inhibitors (BRAFi) (vemurafenib, dabrafenib, and encorafenib) that are used alone or in combination with MEK inhibitors (MEKis) (cobimetinib, trametinib, and binimetinib) and by the arrival of new immune-based therapies, that activate the immune system by targeting immune checkpoints (ipilimumab, nivolumab, pembrolizumab).6
NRAS-mutated melanoma currently does not have an approved targeted therapy and metastatic patients receive immune-based therapies as first-line treatment, then cytotoxic chemotherapy like carboplatin/paclitaxel (C/P), dacarbazine (DTIC) or temozolomide (TMZ) as a second-line treatment.6 We will review current preclinical and clinical developments in NRAS-mutated melanoma, and analyze ongoing clinical trials that are evaluating the benefit of different targeted and immune-based therapies, tested as single agents or in combination, in NRAS-mutant melanoma.
Characteristics of NRAS melanoma
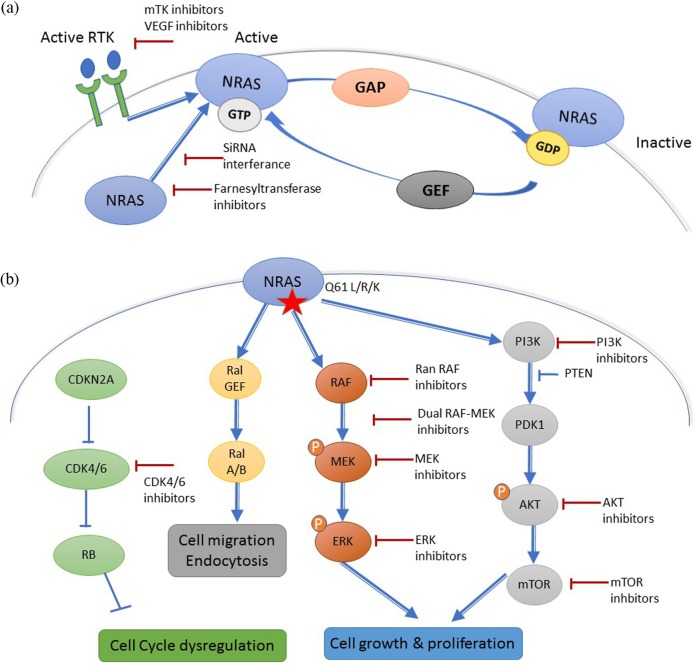
Three RAS family genes are known to be mutated in 20% of human cancer: NRAS, HRAS (Harvey Rat sarcoma virus) and KRAS (Kirsten Rat sarcoma virus).7 RAS proteins are small plasma membrane-associated guanosine 5’-triphosphate (GTP)-binding proteins that regulate cell growth by transmitting the signal from receptor tyrosine kinases (RTKs) at the cell surface to transcription factors and cell cycle proteins in the nucleus7 (Figure 1a). Oncogenic RAS proteins also have a role in tumor cell metabolism, microenvironment remodeling, and tumoral immune response evasion.8
Figure 1.
(a) Mechanism of NRAS activation. Receptor tyrosine kinase (RTK)-mediated activation requires dissociation of protein-bound GDP, a process that is accelerated by guanine nucleotide exchange factors (GEFs). The hydrolysis of GTP to GDP, that inactivates NRAS is accelerated by GTPase activating proteins (GAPs). (b) Downstream effectors of NRAS and different targeted therapy strategies.
GDP, ; GTP, guanosine 5’-triphosphate; VEGF, vascular endothelial growth factor.
Activated RTKs stimulate the passage from the inactive RAS-GDP to the active RAS-GTP with the help of guanine nucleotide exchange factors (GEFs), such as Son of Sevenless Ras/Rho Guanine Nucleotide Exchange Factor (SOS) that catalyze the exchange of Guanosine diphosphate (GDP) for GTP.7 GTPase activating proteins (RAS-GAPs), such as neurofibromin (NF1), inactivate RAS-GDP, and are considered as tumor suppressors.7 Activated RAS proteins stimulate different cell signaling pathways like the MAPK signaling pathway, the phosphoinositide 3-kinase (PI3K)/AKT pathway, and other factors like the RAL guanine nucleotide exchange factors (RAL-GEFs)8 (Figure 1a).
NRAS is very rarely mutated in uveal melanoma.9 In cutaneous melanoma, NRAS is most frequently mutated at hotspots in exon 1 (codon 12) and exon 2 (codon 61) which results in the prolongation of its active GTP-bound state.10 A glutamine to arginine/lysine/leucine substitution at position 61 (Q61R/K/L) accounts for 80% of all NRAS mutations in melanoma.9 No distinct clinical behavior was identified between NRAS exon 1 and exon 2 mutations.11 A BRAF V600E and an activating NRAS mutation were generally believed to be mutually exclusive, but can rarely occur in less than 1% of treatment-naïve melanoma patients.9
Contrarily to BRAF that is frequently mutated in benign nevi, NRAS is rarely mutated in benign melanocytic lesions, except in congenital nevi.12 At the time of initial diagnosis, NRAS-mutant cutaneous melanomas are generally located on the extremities, in older patients with more markers of chronic sun damage than BRAF-mutant melanoma13 even though the prevalence in older patients is disputed.14 Histologically NRAS-mutated melanomas are more frequently associated with a nodular subtype than BRAF melanomas, which are more frequently associated with an Superficial Spreading Melanoma (SSM) subtype.13 In patients with a metastatic disease, NRAS and BRAF mutations are associated with a higher risk of central nervous system involvement compared with WT BRAF and NRAS melanoma.9 Generally NRAS mutations are associated independently with decreased overall survival compared with WT melanoma9 even though these results have not been confirmed in all studies.8,11
Directly targeting NRAS
Due to the extremely high affinity of RAS to GTP and GDP, and the high intracellular concentrations of GTP, developing drugs that effectively compete with the nucleotide generally is considered to be an unrealistic approach.15 Most attempts to directly target RAS have focused on inhibiting the hydrolysis of GTP to GDP by trying to identify antagonists of GEFs or drug-like mimics of RAS-GAPs16 (Figure 1a). Until now these efforts have been largely unsuccessful, but research of a direct RAS-targeted therapy is still very active and recently small compounds that bind directly to the G-domain with inhibitory effects on mutated RAS function have been discovered and might permit the development of such drugs in the future.17
To be active, NRAS has to undergo post-translational modifications, like the farnesylation of a cysteine residue that permits its insertion to the plasma cell membrane where it is activated.18 Initial in vivo data suggested that farnesyl transferase inhibitors (FTIs) could reduce tumor growth in RAS-driven breast cancer and lymphoid tumors19 and that the FTI lonafarnib, could sensitize melanoma cells to RTK inhibitors like sorafenib.20 Unfortunately, these results were not confirmed in the clinical setting where two FTIs, lonafarnib and tipifarnib, progressed to advanced clinical trials but failed to show efficacy against NRAS and KRAS-driven cancers.16,21,22 Farnesyl transferase inhibition is considered to have failed in the clinics because, in the presence of FTI, NRAS and KRAS become substrates for geranylgeranyltransferase I (GGTase I) through a process known as alternative prenylation, and FTIs therefore do not effectively block RAS attachment to the plasma membrane.23 Dual FTI and GGTase I inhibitors have been tested in the clinical setting, but their development is limited by their toxicity.24 Other approaches to inhibit the localization of RAS to the plasma membrane have been attempted or are currently being evaluated in the preclinical or clinical setting but most of them are limited by toxicity16 or technological issues such as how to deliver siRNA using nanoparticle-based delivery systems.25
Targeting upstream effectors of NRAS
Under physiologic conditions, the interaction between an RTK and its ligand induces RTK dimerization, trans-phosphorylation, and activation which in turn stimulates RAS by recruiting GEFs (Figure 1a).
Tyrosine kinase inhibitors (TKIs) and monoclonal antibodies targeting upstream regulators of RAS have been tested in melanoma with limited clinical benefits when used as single agents (Table 1). Targeting downstream NRAS effectors has been associated with an upregulation of RTKs like EGFR, HER3, and ERRB3 in NRAS-mutated melanoma.26 Targeting RTKs with a TKI might therefore be efficacious in combination with MAPKi or PI3K-AKT-mTOR inhibitors to avoid targeted therapy-acquired resistance and avoid compensatory reactivation via RTK signaling.27
Table 1.
Ongoing and completed clinical trials testing mTKI in melanoma and advanced solid tumors.
| Drug | Combination agent | Control | Trial ID | Phase | Population | Status |
|---|---|---|---|---|---|---|
| Single agent mTKI | ||||||
| Amuvatinib | None | none | NCT00894894 | 1 | Solid tumors | Completed |
| Lenvatinib | None | none | NCT01136967 | 2 | Melanoma | Ongoing |
| Pazopanib | None | none | NCT00861913 | 2 | Melanoma | Ongoing |
| Sorafenib | None | none | NCT00119249 | 2 | Melanoma | Completed* |
| Combination mTKI with anti-mitotic chemotherapy | ||||||
| Lenvatinib | TMZ | TMZ | NCT00121680 | 1 | Melanoma | Completed 37 |
| Lenvatinib | DTIC | DTIC | NCT01133977 | 2 | Melanoma | Completed |
| Nintedanib | Paclitaxel | Paclitaxel | NCT02308553 | 2 | Melanoma | Ongoing |
| Pazopanib | Paclitaxel | none | NCT01107665 | 2 | Melanoma | Completed 36 |
| Sorafenib | DTIC | DTIC | NCT00110994 | 2 | Melanoma | Completed 30 |
| Sorafenib | Lacytarabin | none | NCT00498836 | 2 | Melanoma | Completed* |
| Sorafenib | TMZ | Sorafenib | NCT00602576 | 2 | Melanoma | Completed 31 |
| Sorafenib | Carboplatin + Abraxane | none | NCT00483301 | 2 | Melanoma | Completed* |
| Sorafenib | C/P | C/P | NCT00111007 | 3 | Melanoma | Completed 29 |
| Combination of two TKI | ||||||
| Lenvatinib | Golvatinib | Golvatinib | NCT01433991 | 1 | Solid tumors | Ongoing |
| Sorafenib | Bevacizumab | none | NCT00387751 | 2 | Melanoma | Completed 102 |
| Combination of a TKI with NRAS downstream effectors | ||||||
| Pazopanib | Trametinib | none | NCT01438554 | 1 | Solid tumors | Ongoing |
| Sorafenib | Tipifarnib or temsirolimus | none | NCT00281957 | 2 | Melanoma | Completed 22 |
| Combination of a TKI with immune based strategies | ||||||
| Lenvatinib | Pembrolizumab | none | NCT02501096 | 1 | Solid tumors | Ongoing |
| Sorafenib | Pegylated interferon α-2b | none | NCT00623402 | 2 | Melanoma | Completed 103 |
| Other combinations | ||||||
| Sorafenib | Tipifarnib or temsirolimus | none | NCT00281957 | 2 | Melanoma | Completed 22 |
| Sorafenib | Riluzole | none | NCT01303341 | 1 | Melanoma | Ongoing |
| Sorafenib | Tivantinib | none | NCT01178411 | 2 | Solid tumors | Ongoing |
| Sorafenib | Bortezomib | none | NCT01078961 | 1 | Melanoma | Completed 104 |
Red trials considered negative by the authors.
Green trials considered positive by the authors.
No published article.
(…) corresponds to the reference of the article that published the results of the study.
For instance sorafenib, a multi-TKI (mTKI), showed no clinical activity in melanoma when tested as a single agent28 or in combination with chemotherapy (C/P, DTIC, TMZ29–31), but combinations of sorafenib with Mesenchymal epithelial transition factor receptor (MET) inhibitors or with alpha-mangostin might be more promising to treat NRAS-mutant melanoma and are currently being tested in early clinical trials32 and in preclinical experiments.33
Axitinib and pazopanib, two other mTKIs, showed more promising results in phase II clinical trials in BRAF WT melanoma both when used as single agents34 and when used in combination with C/P,35,36 but have shown no benefit in NRAS-mutated melanoma. Ongoing trials are evaluating the safety of combining pazopanib with the MEKi trametinib [ClinicalTrials.gov identifier: NCT01438554].
The mTKI lenvatinib is currently being tested as a single agent in melanoma [ClinicalTrials.gov identifier: NCT01136936]. When lenvatinib is combined with TMZ, it has no clinical benefit,37 however, combined with DTIC it shows promising results in a phase II trial on metastatic melanoma, but not specifically in NRAS-mutant melanoma [ClinicalTrials.gov identifier: NCT01133977]. It is also currently being tested in combination with cMET inhibitor E7050 [ClinicalTrials.gov identifier: NCT01433991] and in combination with anti-PD1 pembrolizumab [ClinicalTrials.gov identifier: NCT02501096] in two phase I–II trials. Amuvatinib shows promising preclinical data in NRAS-mutant melanoma.38
Bevacizumab, a monoclonal antibody against VEGF-A was showed to be safe in phase II studies where it was combined with DTIC39 C/P,40,41 TMZ,42 fotemustine,43 everolimus,44 temsirolimus,45 ipilimumab,46 erlotinib47 or imatinib48 with limited clinical activity in NRAS-mutant melanoma. Vatalanib, another VEGF antibody seems to have no clinical activity as a single agent49 and is currently being tested in combination with everolimus [ClinicalTrials.gov identifier: NCT00655655].
Anti-integrin alphavbeta antibodies (etaracizumab, intetumumab) showed limited clinical activity compared with DTIC in metastatic melanoma.50,51
Targeting downstream effectors of NRAS
Targeting the MAPK signaling pathway with single agents
RAS activates the MAPK signaling pathway by inducing a conformational change and activation of BRAF, CRAF or ARAF.52 Upon activation, homo or heterodimers of RAF phosphorylate MEK that then phosphorylates the transcription factor ERK that enters the nucleus and activates cell behaviors like proliferation and differentiation53 (Figure 1b). Targeting RAS MAPK downstream effectors therefore seems a promising approach.
BRAFi were the first targeted therapies to be approved for BRAF-mutant melanoma.6 Unfortunately, first and second generation BRAFi cannot be used as single agents to treat NRAS-mutant melanoma because while these inhibitors are effective at shutting down ERK signaling mediated by mutant-BRAF, they paradoxically upregulate ERK activity in the presence of oncogenic RAS, by stimulating BRAF–CRAF heterodimerization.53 Pan RAF inhibitors (PRis) (TAK-632, LY3009120, Compound A) show interesting preclinical data in this setting,54 as single agents55 but also in combination with MEKis.56 Ongoing phase I clinical trials are testing PRi alone (CCT3833 in ClinicalTrials.gov identifier: NCT02437227; LY3009120 in ClinicalTrials.gov identifier: NCT02014116) or in combination with an anti-PD1 antibody (LXH254+PDR001, ClinicalTrials.gov identifier: NCT02607813).
RAF can also be inhibited in NRAS-mutant melanoma with a dual RAF/MEKi (RO5126766) that stabilizes the RAF-MEK dimer and therefore blocks the phosphorylation and release of RAF.57 A phase I study showed that RO5126766 has manageable toxicity with encouraging preliminary antitumor activity.58
Targeting MEK1/2 in NRAS-mutated melanoma is currently the most developed targeted therapy approach. MEKis are orally bioavailable and either ATP-competitive or non-ATP-competitive, allosteric binding inhibitors of MEK.59
The first generation of MEKis (CI-1040, PD-901) showed limited clinical benefit in unselected melanoma patients as single agents60,61 and also in combination with docetaxel in WT melanoma.62 Second and third generation MEKis (trametinib, binimetinib, selumetinib, pimasertib, cobimetinib) seem to have a safer toxicity profile and a more promising clinical activity and are therefore being tested in phase II/III clinical trials.59
In a phase III clinical trial, the MEKi binimetinib recently showed its superiority compared with DTIC in NRAS-mutant melanoma (SMR, 2016;63 ASCO, 201664) even though its benefit was small [progression-free survival of 2.8 months with binimetinib compared with 1.5 months for DTIC; hazard ratio (HR) 0.62 (95% confidence interval (CI) 0.47–0.8)]. Pimaseritib showed promising results in a phase I clinical trial and is being in compared with DTIC in NRAS-mutated melanoma, in a completed but not published phase II trial [ClinicalTrials.gov identifier: NCT 0193068]. Trametinib is United States Food and Drug Administration (US FDA)-approved in combination with dabrafenib in BRAF-mutant melanoma. It has not been specifically tested in NRAS melanoma, but in a phase I trial for unselected melanoma patients, two out of seven patients with an NRAS-mutated melanoma achieved stable disease with trametinib treatment.65
According to preclinical data, the next generation MEKi GDC-0623 [ClinicalTrials.gov identifier: NCT01106599] and G-573 might be more effective in RAS-mutated cells compared with BRAF-mutated cells66 but TAK-733 showed limited tumor activity in a phase I trial, with no further development currently planned.67
Finally, preclinical data suggest that ERK inhibitors (ERKis) might be interesting in NRAS-mutant melanoma as ERK represents the final single node in the MAPK signaling pathway for potential inhibition.68 Several ERKis are currently being developed in the preclinical setting and in phase I clinical trials as single agents or in combination with chemotherapy or MEKis: BVD-523 [ClinicalTrials.gov identifiers: NCT02608229, NCT02296242], SCH772984,69 LTT462 [ClinicalTrials.gov identifiers: NCT02711345]; CC-90003 [ClinicalTrials.gov identifier: NCT0231012] and GDC 0994 [ClinicalTrials.gov identifiers: NCT01875705, NCT02457793].70
Combining MAPKi and the PI3K-AKT-mTOR inhibitors
NRAS not only activates the MAPK signaling pathway, but also activates the PI3K-AKT-mTOR cell signaling pathway, RAL pathways and cell cycle regulatory proteins.8 This may explain why MEKis as single agents are less effective in NRAS-mutated melanoma than BRAFis are in BRAF-mutant melanoma.8
Multiple classes of inhibitors of PI3K-AKT-mTOR are available including PI3K inhibitors (pan-isoform and isoform specific), dual PI3K-mTOR inhibitors, AKT inhibitors (AKTis) and mTOR inhibitors (mTORC1 and mTORC1/2) (mTORis).71 Combining MAPKis with these inhibitors demonstrated promising preclinical in vitro and in vivo results in NRAS-mutant melanoma,72 however, these results have yet to be translated into the clinical setting. Many clinical trials combining MAPKis and inhibitors of PI3K-AKT-mTOR have been or are currently being tested (Table 2). Unfortunately, this approach is limited by overlapping toxicities and compensatory signaling within and between cell signaling pathways that results in insufficient plasma drug levels of PI3-AKT-mTOR inhibitors for antitumor activity.73–75 This could be overcome by intermittent high dose administration of PI3K-AKT-mTOR inhibitors associated with continuous MEKi administration as suggested by preclinical data.76
Table 2.
PI3K-AKT-mTOR inhibitors that have been tested in combination with MEK inhibitors in the clinical setting.
| PI3Ki | MEKi | AKT inhibitor | MEKi | Dual PI3K/mTOR | MEKi | mTOR | MEKi |
|---|---|---|---|---|---|---|---|
| BKM120 | MEK162 | GSK2110183 | GSK1120212 | BEZ235 | MEK162 | RAD001 | GSK1120212 |
| BKM120 | GSK1120212 | GSK2141795 | GSK1120212 | SAR245409 | MSC1936369B | CCI-779 | MSC1936369B |
| BAY80-6946 | BAY86-9766 | MK-2206 | AZD6244 | PF-04691502 | PD-0325901 | CCI-779 | AZD6244 |
| BYL719 | MEK162 | GDC-0068 | GDC-0973 | AZD2014 | AZD6244 | ||
| GDC-0941 | GDC-0973 | MK 2206 | AZD6244 | ||||
| GSK2126458 | GSK1120212 |
AZD2014 (vistusertib); AZD6244 (selumetinib); BAY80-6946 (copanlisib); BAY86-9766 (refametinib); BEZ235 (dactolisib); BKM120 (buparlisib); BYL719 (alpelisib); CCI-779 (temsirolimus); GDC-0068 (ipatasertib); GDC-0941 (taselisib); GDC-0973 (cobimetinib); GSK1120212 (trametinib); GSK2110183 (afuresertib); GSK2141795 (uprosertib); GSK2126458 (omipalisib); MEK162 (binimetinib); MSC1936369B (pimasertib); RAD001 (everolimus); SAR245409 (voxtalisib).
Combining MAPKis with cell cycle regulator protein inhibitors
NRAS induces the expression of cyclin D1 that regulates cell cycle regulators like cyclin-dependent kinase 4/6 (CDK4/6)77 that are fundamental to RAS-induced transformation.8 CDK4/6 inhibitors are currently being tested in combination with MEKis with encouraging early clinical results. Ribociclib (LEE011) is being tested in combination with binimetinib in an encouraging phase II trial78 and palbociclib is being tested in combination with trametinib.79
Wee1 is a kinase that inactivates the Cyclin B Cell division control protein kinase (CDC)/cyclin B complex that regulates the G2 cell cycle checkpoint.80 Combining Wee1 inhibitor with an mTOR inhibitor like rapamycin has shown promising preclinical data in NRAS-mutated melanoma81 and the Wee1 inhibitor AZD-1775 is currently being tested in phase I trials [ClinicalTrials.gov identifiers: NCT02610075; NCT02617277].
Polo-kinase 1 (PK1) is overexpressed in NRAS-mutant melanoma and regulates the cell cycle.82 PK1 inhibitors have shown disappointing clinical activity as single agents, but preclinical data suggest they may be interesting in combination with MEKi in NRAS-mutant melanoma.83,84
Combining MAPKis and RalGEF inhibitors
RAS activates the RalGEF pathway. TANK-binding kinase 1 (TBK1)is activated downstream of RALB and has shown promising preclinical activity in NRAS-mutant melanoma when combined with MEKis.85,86
Other combination of targeted therapies
ROCK 1/2 are RHO GTPase-activated serine/threonine kinases that are involved in RAS tumor proliferation. Preclinical data suggest that ROCK inhibition could increase MEKi antitumoral activity in vivo.87
Preclinical data suggests combining ERβ inhibition with MAPKi or PI3K-AKT-mTOR inhibition could be interesting in NRAS melanoma.88
Targeting the immune system
The arrival of immune-based therapies for the treatment of melanoma has revolutionized the standard of care and are now the first-line treatment for NRAS and WT melanoma.89 Interleukin (IL)2 and anti-CTLA4 antibody (ipilimumab) were the first immunotherapies approved by the US FDA to treat metastatic melanoma, with durable responses seen in 5–15% of patients despite severe acute toxicities.90,91 More recently therapeutic approaches aimed at activating antitumor immunity through blockade of the immune checkpoint PD1 with nivolumab and pembrolizumab have showed objective responses in 25–50% of patients in early trials.92,93
Due to a distinct immune microenvironment compared with BRAF-mutant melanoma,94 NRAS-mutant melanoma may be associated with more frequent responses in patients treated by IL2, ipilimumab, and anti-PD1.95–97
In vitro and in vivo, MEKis enhance melanoma antigen expression and reactivity to antigen-specific T-lymphocytes leading to a synergy with immune checkpoint blockade in murine models.98,99 This gives a strong rationale to combine targeted and immune-based strategies100 in NRAS-mutated melanoma with numerous ongoing trials.101
Conclusion
NRAS has often been considered an undruggable target because even though its role in cancer has been demonstrated for more than 25 years, no targeted therapy has been approved despite extensive efforts in melanoma and other RAS-mutated malignancies. This has recently changed with the advent of new MEKis that are tested in combination with a variety of drugs that use different approaches: inhibition of upstream RAS effectors, inhibition of PI3K-AKT-mTOR, inhibition of cell cycle regulators and activation of anti-tumor immunity. As each combination of treatments pursues its clinical development though phase I, II and III clinical trials, the challenge will be to choose in what order to use them in NRAS-mutant melanoma patients.
Footnotes
Funding: Amélie Boespflug is currently being funded by a graduate grant of the Fondation pour la Recherche Médical (FRM), France (FDM20150633361).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Amélie Boespflug, Hospices Civils de Lyon, Dermatology Unit, Lyon, France; Department of Medicine, Claude Bernard Lyon–1 University, Lyon, France; Cancer Research Center of Lyon, Claude Bernard Lyon–1 University, INSERM1052, CNRS 5286, Lyon, France.
Julie Caramel, Cancer Research Center of Lyon, Claude Bernard Lyon–1 University, INSERM1052, CNRS 5286, Lyon, France.
Stephane Dalle, Hospices Civils de Lyon, Dermatology Unit, Lyon, France; Department of Medicine, Claude Bernard Lyon–1 University, Lyon, France; Cancer Research Center of Lyon, Claude Bernard Lyon–1 University, INSERM1052, CNRS 5286, Lyon, France.
Luc Thomas, Service de Dermatologie, CH Lyon Sud, 165 Chemin du Grand Revoyet, 69495 Pierre Bénite, Cedex, France.
References
- 1. Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol 2014; 170: 11–19. [DOI] [PubMed] [Google Scholar]
- 2. Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amann VC, Ramelyte E, Thurneysen S, et al. Developments in targeted therapy in melanoma. European Journal of Surgical Oncology (EJSO) 2017; 43: 581–593. [DOI] [PubMed] [Google Scholar]
- 4. Baiter M, Schuler G, Hartmann A, et al. Pathogenetic implications of BRAF mutation distribution in stage IV melanoma patients. Dermatology (Basel, Switzerland) 2015; 231: 127–133. [DOI] [PubMed] [Google Scholar]
- 5. Zhang T, Dutton-Regester K, Brown KM, et al. The genomic landscape of cutaneous melanoma. Pigment Cell Melanoma Res 2016; 29: 266–283. [DOI] [PubMed] [Google Scholar]
- 6. Ranchon F, Boespflug A, Rioufol C, et al. New treatments for cutaneous metastatic melanoma: MAPK pathway-targeted and immune based therapies. Anti-Cancer Agents Med Chem 2015; 15: 461–467. [DOI] [PubMed] [Google Scholar]
- 7. Fedorenko I V, Gibney GT, Smalley KSM. NRAS mutant melanoma: biological behavior and future strategies for therapeutic management. Oncogene 2013; 32: 3009–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandalà M, Merelli B, Massi D. NRAS in melanoma: targeting the undruggable target. Crit Rev Oncol Hematol 2014; 92: 107–122. [DOI] [PubMed] [Google Scholar]
- 9. Jakob JA, Bassett RL, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012; 118: 4014–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mor A, Philips MR. Compartmentalized RAS/MAPK signaling. Annu Rev Immunol 2006; 24: 771–800. [DOI] [PubMed] [Google Scholar]
- 11. Bucheit AD, Syklawer E, Jakob JA, et al. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer 2013; 119: 3821–3829. [DOI] [PubMed] [Google Scholar]
- 12. Ross AL, Sanchez MI, Grichnik JM. Molecular nevogenesis. Dermatology Research and Practice 2011; 2011: 463184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellerhorst JA, Greene VR, Ekmekcioglu S, et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res 2011; 17: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J-H, Choi J-W, Kim Y-S. Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: a meta-analysis. Br J Dermatol 2011; 164: 776–784. [DOI] [PubMed] [Google Scholar]
- 15. Chang EH, Gonda MA, Ellis RW, et al. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci USA 1982; 79: 4848–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox AD, Der CJ, Philips MR. Targeting RAS Membrane Association: Back to the Future for Anti-RAS Drug Discovery? Clin Cancer Res 2015; 21: 1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcus K, Mattos C. Direct attack on RAS: intramolecular communication and mutation-specific effects. Clin Cancer Res 2015; 21: 1810–1818. [DOI] [PubMed] [Google Scholar]
- 18. Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov 2007; 6: 541–555. [DOI] [PubMed] [Google Scholar]
- 19. Mangues R, Corral T, Kohl NE, et al. Antitumor effect of a farnesyl protein transferase inhibitor in mammary and lymphoid tumors overexpressing N-RAS in transgenic mice. Cancer Res 1998; 58: 1253–1259. [PubMed] [Google Scholar]
- 20. Meier FE, Niessner H, Flaherty K, et al. Effect of the farnesyl transferase inhibitor lonafarnib on sensitivity of melanoma cells to the multikinase inhibitor sorafenib and on Rheb farnesylation and mTOR signaling. J Clin Oncol 2009; 27(Suppl. 15): abstract 9077. [Google Scholar]
- 21. Chow LQM, Eckhardt SG, O’Bryant CL, et al. A phase I safety, pharmacological, and biological study of the farnesyl protein transferase inhibitor, lonafarnib (SCH 663366), in combination with cisplatin and gemcitabine in patients with advanced solid tumors. Cancer Chemother Pharmacol 2008; 62: 631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Margolin KA, Moon J, Flaherty LE, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438). Clin Cancer Res 2012; 18: 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whyte DB, Kirschmeier P, Hockenberry TN, et al. K- and N-RAS are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 1997; 272: 14459–14464. [DOI] [PubMed] [Google Scholar]
- 24. Brock EJ, Ji K, Reiners JJ, et al. How to target activated RAS proteins: direct inhibition vs. induced mislocalization. Mini Rev Med Chem 2016; 16: 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis ME, Zuckerman JE, Choi CHJ, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010; 464: 1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fedorenko I V, Fang B, Munko AC, et al. Phosphoproteomic analysis of basal and therapy-induced adaptive signaling networks in BRAF and NRAS mutant melanoma. Proteomics 2015; 15: 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Capparelli C, Rosenbaum S, Berman-Booty LD, et al. ErbB3-ErbB2 complexes as a therapeutic target in a subset of wild-type BRAF/NRAS cutaneous melanomas. Cancer Res 2015; 75: 3554–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a phase II randomised discontinuation trial analysis. Br J Cancer 2006; 95: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009; 27: 2823–2830. [DOI] [PubMed] [Google Scholar]
- 30. Eisen T, Marais R, Affolter A, et al. Sorafenib and dacarbazine as first-line therapy for advanced melanoma: phase I and open-label phase II studies. Br J Cancer 2011; 105: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amaravadi RK, Schuchter LM, McDermott DF, et al. Phase II trial of temozolomide and sorafenib in advanced melanoma patients with or without brain metastases. Clin Cancer Res 2009; 15: 7711–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puzanov I, Sosman J, Santoro A, et al. Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Invest New Drugs 2015; 33: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia Y, Li Y, Westover KD, et al. Inhibition of cell proliferation in an NRAS mutant melanoma cell line by combining sorafenib and α-mangostin. PloS one 2016; 11: e0155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fruehauf J, Lutzky J, McDermott D, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res 2011; 17: 7462–7469. [DOI] [PubMed] [Google Scholar]
- 35. Algazi AP, Cha E, Ortiz-Urda SM, et al. The combination of axitinib followed by paclitaxel/carboplatin yields extended survival in advanced BRAF wild-type melanoma: results of a clinical/correlative prospective phase II clinical trial. Br J Cancer 2015; 112: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fruehauf JP, Alger B, Jakowatz JG, et al. A phase II single-arm study of pazopanib and paclitaxel as first-line treatment for patients with advanced melanoma. J Clin Oncol 2014; 32(Suppl. 5): abstract 9036. [Google Scholar]
- 37. Hong DS, Kurzrock R, Falchook GS, et al. Phase 1b study of lenvatinib (E7080) in combination with temozolomide for treatment of advanced melanoma. Oncotarget 2015; 6: 43127–43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fedorenko I V, Fang B, Koomen JM, et al. Amuvatinib has cytotoxic effects against NRAS-mutant melanoma but not BRAF-mutant melanoma. Melanoma Res 2014; 24: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrucci PF, Minchella I, Mosconi M, et al. Dacarbazine in combination with bevacizumab for the treatment of unresectable/metastatic melanoma. Melanoma Res 2015; 25: 239–245. [DOI] [PubMed] [Google Scholar]
- 40. Kim KB, Sosman JA, Fruehauf JP, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2012; 30: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spitler LE, Boasberg P, Day SO, et al. Phase II study of nab-paclitaxel and bevacizumab as first-line therapy for patients with unresectable stage III and IV melanoma. Am J Clin Oncol 2015; 38: 61–67. [DOI] [PubMed] [Google Scholar]
- 42. von Moos R, Seifert B, Simcock M, et al. First-line temozolomide combined with bevacizumab in metastatic melanoma: a multicentre phase II trial (SAKK 50/07). Ann Oncol 2012; 23: 531–536. [DOI] [PubMed] [Google Scholar]
- 43. Del Vecchio M, Mortarini R, Canova S, et al. Bevacizumab plus fotemustine as first-line treatment in metastatic melanoma patients: clinical activity and modulation of angiogenesis and lymphangiogenesis factors. Clin Cancer Res 2010; 16: 5862–5872. [DOI] [PubMed] [Google Scholar]
- 44. Hainsworth JD, Infante JR, Spigel DR, et al. Bevacizumab and everolimus in the treatment of patients with metastatic melanoma: a phase 2 trial of the Sarah Cannon Oncology Research Consortium. Cancer 2010; 116: 4122–4129. [DOI] [PubMed] [Google Scholar]
- 45. Slingluff CL, Petroni GR, Molhoek KR, et al. Clinical activity and safety of combination therapy with temsirolimus and bevacizumab for advanced melanoma: a phase II trial (CTEP 7190/Mel47). Clin Cancer Res 2013; 19: 3611–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2014; 2: 632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mudigonda TV, Wyman K, Spigel DR, et al. A phase II trial of erlotinib and bevacizumab for patients with metastatic melanoma. Pigment Cell Melanoma Res 2016; 29: 101–103. [DOI] [PubMed] [Google Scholar]
- 48. Flaherty KT, Hamilton BK, Rosen MA, et al. Phase I/II trial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers. Oncologist 2015; 20: 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cook N, Basu B, Biswas S, et al. A phase 2 study of vatalanib in metastatic melanoma patients. Eur J Cancer 2010; 46: 2671–2673. [DOI] [PubMed] [Google Scholar]
- 50. Hersey P, Sosman J, O’Day S, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin α v β 3, ± dacarbazine in patients with stage IV metastatic melanoma. Cancer 2010; 116: 1526–1534. [DOI] [PubMed] [Google Scholar]
- 51. O’Day S, Pavlick A, Loquai C, et al. A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer 2011; 105: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vojtek AB, Hollenberg SM, Cooper JA. Mammalian RAS interacts directly with the serine/threonine kinase RAF. Cell 1993; 74: 205–214. [DOI] [PubMed] [Google Scholar]
- 53. Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014; 14: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kortum RL, Morrison DK. Path Forward for RAF Therapies: Inhibition of Monomers and Dimers. Cancer Cell 2015; 28: 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peng S-B, Henry JR, Kaufman MD, et al. Inhibition of RAF isoforms and active dimers by LY3009120 leads to anti-tumor activities in RAS or BRAF mutant cancers. Cancer Cell 2015; 28: 384–398. [DOI] [PubMed] [Google Scholar]
- 56. Atefi M, Titz B, Avramis E, et al. Combination of pan-RAF and MEK inhibitors in NRAS mutant melanoma. Mol Cancer 2015; 14: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wada M, Horinaka M, Yamazaki T, et al. The dual RAF/MEK inhibitor CH5126766/RO5126766 may be a potential therapy for RAS-mutated tumor cells. PloS One 2014; 9: e113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martinez-Garcia M, Banerji U, Albanell J, et al. First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin Cancer Res 2012; 18: 4806–4819. [DOI] [PubMed] [Google Scholar]
- 59. Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol 2013; 6: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012; 18: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004; 22: 4456–4462. [DOI] [PubMed] [Google Scholar]
- 62. Gupta A, Love S, Schuh A, et al. DOC-MEK: a double-blind randomized phase II trial of docetaxel with or without selumetinib in wild-type BRAF advanced melanoma. Ann Oncol 2014; 25: 968–974. [DOI] [PubMed] [Google Scholar]
- 63. Arance A, Dutriaux C, Gutzmer R, et al. Efficacy of binimetinib in patients with NRAS-mutant melanoma: subgroup analysis of the phase 3 NEMO study. In: Poster presentation at the 13th international congress of the SMR annual congress, Boston, MA, 6–9 November 2016. [Google Scholar]
- 64. Dummer R, Schadendorf D, Ascierto PA, et al. Results of NEMO: a phase III trial of binimetinib (BINI) vs dacarbazine (DTIC) in NRAS-mutant cutaneous melanoma. J Clin Oncol 2016; 34(Suppl.): abstract 9500. [Google Scholar]
- 65. Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hatzivassiliou G, Haling JR, Chen H, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature 2013; 501: 232–236. [DOI] [PubMed] [Google Scholar]
- 67. Adjei AA, LoRusso P, Ribas A, et al. A phase I dose-escalation study of TAK-733, an investigational oral MEK inhibitor, in patients with advanced solid tumors. Invest New Drugs 2017; 35: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jha S, Morris EJ, Hruza A, et al. Dissecting therapeutic resistance to ERK inhibition. Mol Cancer Ther 2016; 15: 548–559. [DOI] [PubMed] [Google Scholar]
- 69. Lim J, Kelley EH, Methot JL, et al. Discovery of 1-(1 H -Pyrazolo[4,3- c ]pyridin-6-yl)urea Inhibitors of Extracellular Signal-Regulated Kinase (ERK) for the treatment of cancers. J Med Chem 2016; 59: 6501–6511. [DOI] [PubMed] [Google Scholar]
- 70. Blake JF, Burkard M, Chan J, et al. Discovery of (S)-1-(1-(4-Chloro-3-fluorophenyl)-2-hydroxyethyl)-4-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)pyridin-2(1H)-one (GDC-0994), an Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) inhibitor in early clinical development. J Med Chem 2016; 59: 5650–5660. [DOI] [PubMed] [Google Scholar]
- 71. Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res 2013; 19: 5310–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Posch C, Moslehi H, Feeney L, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci USA 2013; 110: 4015–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tolcher AW, Bendell JC, Papadopoulos KP, et al. A phase IB trial of the oral MEK inhibitor trametinib (GSK1120212) in combination with everolimus in patients with advanced solid tumors. Ann Oncol 2015; 26: 58–64. [DOI] [PubMed] [Google Scholar]
- 74. Bedard PL, Tabernero J, Janku F, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res 2015; 21: 730–738. [DOI] [PubMed] [Google Scholar]
- 75. Grilley-Olson JE, Bedard PL, Fasolo A, et al. A phase Ib dose-escalation study of the MEK inhibitor trametinib in combination with the PI3K/mTOR inhibitor GSK2126458 in patients with advanced solid tumors. Invest New Drugs 2016; 34: 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K Inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res 2012; 72: 210–219. [DOI] [PubMed] [Google Scholar]
- 77. Winston JT, Coats SR, Wang YZ, et al. Regulation of the cell cycle machinery by oncogenic ras. Oncogene 1996; 12: 127–134. [PubMed] [Google Scholar]
- 78. Sosman JA, Kittaneh M, Lolkema MPJK, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity. J Clin Oncol 2014; 32(Suppl. 5): abstract 9009. [Google Scholar]
- 79. Siravegna G, Russo M, Blaszkowsky LS, et al. PR01 Acquisition of resistance to anti-EGFR therapy drives genomic heterogeneity and lesion-specific responses in colorectal cancer. Mol Cancer Ther 2016; 14(Suppl. 2): PR01. [Google Scholar]
- 80. Schellens JH, Leijen S, Shapiro GI, et al. A phase I and pharmacological study of MK-1775, a Wee1 tyrosine kinase inhibitor, in both monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol 2009; 27(Suppl. 15): abstract 3510. [Google Scholar]
- 81. Weisberg E, Nonami A, Chen Z, et al. Identification of Wee1 as a novel therapeutic target for mutant RAS-driven acute leukemia and other malignancies. Leukemia 2015; 29: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jalili A, Moser A, Pashenkov M, et al. Polo-like kinase 1 is a potential therapeutic target in human melanoma. J Invest Dermatol 2011; 131: 1886–1895. [DOI] [PubMed] [Google Scholar]
- 83. Posch C, Cholewa BD, Vujic I, et al. Combined inhibition of MEK and Plk1 has synergistic antitumor activity in NRAS mutant melanoma. J Invest Dermatol 2015; 135: 2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin C-C, Su W-C, Yen C-J, et al. A phase I study of two dosing schedules of volasertib (BI 6727), an intravenous polo-like kinase inhibitor, in patients with advanced solid malignancies. Br J Cancer 2014; 110: 2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vu HL, Aplin AE. Targeting TBK1 inhibits migration and resistance to MEK inhibitors in mutant NRAS melanoma. Mol Cancer Res 2014; 12: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chien Y, Kim S, Bumeister R, et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 2006; 127: 157–170. [DOI] [PubMed] [Google Scholar]
- 87. Vogel CJ, Smit MA, Maddalo G, et al. Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and ROCK. Pigment Cell Melanoma Res 2015; 28: 307–317. [DOI] [PubMed] [Google Scholar]
- 88. Marzagalli M, Montagnani Marelli M, Casati L, et al. Estrogen receptor β in melanoma: from molecular insights to potential clinical utility. Front Endocrinol 2016; 7: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ranchon F, Boespflug A, Rioufol C, et al. New treatments for cutaneous metastatic melanoma: MAPK pathway-targeted and immune based therapies. Anti-Cancer Agents Med Chem 2015; 15: 461–467. [DOI] [PubMed] [Google Scholar]
- 90. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 91. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- 92. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016; 315: 1600–1609. [DOI] [PubMed] [Google Scholar]
- 93. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17: 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Clancy T, Hovig E. Profiling networks of distinct immune-cells in tumors. BMC Bioinformatics 2016; 17: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res 2015; 3: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother 2012; 35: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mangana J, Cheng PF, Schindler K, et al. Analysis of BRAF and NRAS mutation status in advanced melanoma patients treated with anti-CTLA-4 antibodies: association with overall survival? PloS One 2015; 10: e0139438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70: 5213–5219. [DOI] [PubMed] [Google Scholar]
- 99. Liu L, Mayes PA, Eastman S, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 2015; 21: 1639–1651. [DOI] [PubMed] [Google Scholar]
- 100. Cooper ZA, Reuben A, Austin-Breneman J, et al. Does it MEK a difference? Understanding immune effects of targeted therapy. Clin Cancer Res 2015; 21: 3102–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tchekmedyian N, Gray JE, Creelan BC, et al. Propelling immunotherapy combinations into the clinic. Oncology (Williston Park, NY) 2015; 29: pii: 214814. [PubMed] [Google Scholar]
- 102. Mahalingam D, Malik L, Beeram M, et al. Phase II study evaluating the efficacy, safety, and pharmacodynamic correlative study of dual antiangiogenic inhibition using bevacizumab in combination with sorafenib in patients with advanced malignant melanoma. Cancer Chemother Pharmacol 2014; 74: 77–84. [DOI] [PubMed] [Google Scholar]
- 103. Egberts F, Gutzmer R, Ugurel S, et al. Sorafenib and pegylated interferon-α2b in advanced metastatic melanoma: a multicenter phase II DeCOG trial. Ann Oncol 2011; 22: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 104. Sullivan RJ, Ibrahim N, Lawrence DP, et al. A phase I trial of bortezomib and sorafenib in advanced malignant melanoma. Oncologist 2015; 20: 617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]