Abstract
Breast cancer is a classical hormone-dependent tumour; therefore, endocrine therapy is the mainstay of treatment for hormone receptor-positive, human epidermal growth factor 2-negative advanced breast cancer. Until recently, classical endocrine agents such as tamoxifen, steroidal and nonsteroidal aromatase inhibitors and fulvestrant have been widely used in postmenopausal patients to treat locally advanced or metastatic disease. However, for patients with this subtype of breast cancer, the landscape of endocrine therapy is rapidly changing. Therapies targeting oestrogen modulation have evolved in recent years following the introduction of targeted agents, mTOR and CDK 4/6 inhibitors that are administered in combination with hormone therapy. As a result, options for endocrine therapy have expanded in recent years, and a variety of single-agent or combinations of targeted drugs and endocrine therapies are accepted. Fulvestrant is a selective oestrogen receptor downregulator (SERD) which was introduced to clinical practice in 2002, initially with the indication to treat postmenopausal women with hormone-receptor-positive advanced breast cancer as second-line therapy postdisease progression after aromatase inhibitors or tamoxifen. Additionally, fulvestrant has also been shown to be active in patients previously untreated with endocrine therapy, either both in the neoadjuvant and the metastatic setting, alone or in combination with other targeted therapies. Currently, the standard dose is 500 mg, which is administered with a loading dose. Fulvestrant received a new FDA indication in December 2016, in combination with palbociclib, both in pre/peri/postmenopausal women with breast cancer progressing after endocrine therapy.
This manuscript aims to give an overview of new efficacy data and the current role of fulvestrant in the systemic therapy of hormone-receptor-positive advanced breast cancer, in the context of other available therapeutic modalities.
Keywords: advanced breast cancer, endocrine therapy, fulvestrant, selective oestrogen receptor downregulator
Introduction
The highest incidence rates of breast cancer are found in postmenopausal women, and approximately 67–70% of all metastatic breast cancers are oestrogen-receptor (ER) or progesterone-receptor (PR) positive, which are potentially sensitive to endocrine therapy.1 The treatment of hormone-receptor-positive (HR+ve), human epidermal growth factor 2-negative (HER2–ve) advanced breast cancer (locally advanced or metastatic) is primarily palliative, that is, optimal therapy should aim to prolong survival, improve or at least maintain the quality of life, and delay the initiation of chemotherapy. Treatment selection is mainly based on four factors: the extent of disease, prior response to adjuvant endocrine therapy, the patient’s clinical status and preferences. According to major international guidelines, endocrine therapy is regarded as the cornerstone treatment for HR+ve/HER2–ve breast cancers, and should be considered in the majority of patients with locally advanced or metastatic tumours, excluding those with life-threatening disease, patients experiencing visceral crisis or those with proof of prior endocrine resistance.2,3
There are several classical endocrine therapies for the treatment of HR+ve/HER2–ve advanced breast cancer in postmenopausal women: selective ER modulators (SERMs) that act by blocking the ER (e.g. tamoxifen), nonsteroidal and steroidal aromatase inhibitors (AIs), which reduce oestrogen levels by inhibiting the peripheral synthesis of oestrogen (e.g. anastrozole, letrozole and exemestane), and the selective ER downregulator (SERD) (fulvestrant).3,4 Options for endocrine therapy have expanded in recent years, along with the availability of new and more effective agents.
A deeper understanding of biological pathways contributing to hormone resistance has led to the approval of targeted agents, such as mammalian target of rapamycin (mTOR) and cyclin-dependent kinase 4, 6 (CDK4/6) inhibitors. One of the most important advances in the management of HR+ve/HER2–ve advanced breast cancer over the last 5 years has been the introduction of everolimus and palbociclib, administered in combination with an endocrine agent.5 As a consequence, a variety of single-agent or combinations of targeted drugs and endocrine therapies are accepted. However, the optimal choice and sequence of endocrine therapies have not been clearly defined.
Given the evolving role of fulvestrant in the management of breast cancer, the present manuscript aims to review its clinical efficacy data and its possible current role in the systemic therapy of locally advanced or metastatic HR+ve/HER2–ve breast cancer, in association with other therapeutic modalities.
Fulvestrant
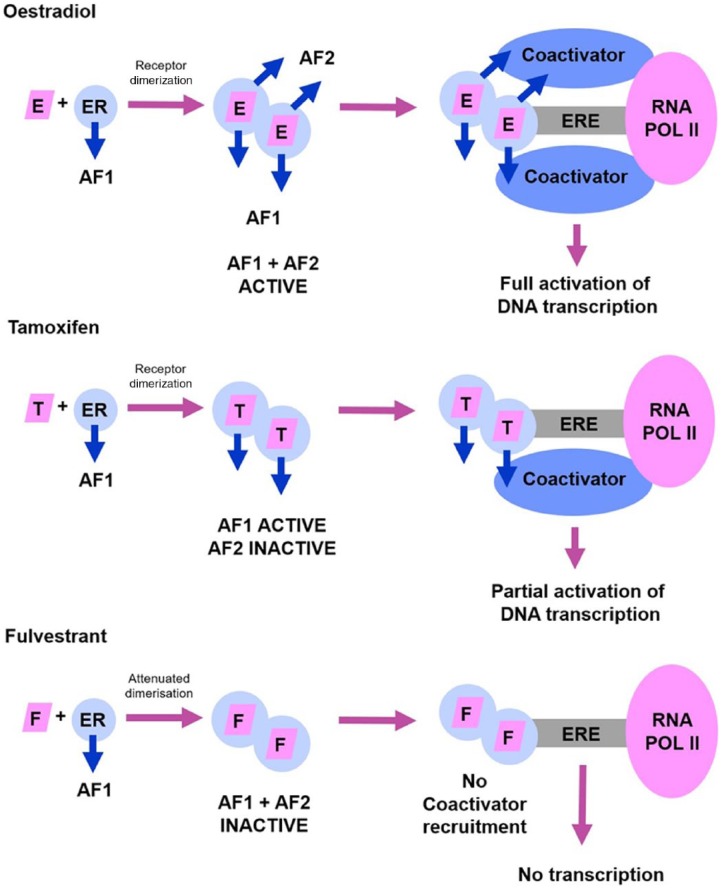
Fulvestrant is a pure oestrogen receptor (ER) antagonist, exerting selective ER downregulation (SERD), and competitively binding to the ER. This binding attenuates the ability of the ER to activate or inhibit gene transcription through impaired dimerization, increased receptor turnover, and disrupted nuclear localization. Fulvestrant has a binding affinity 100 times greater than the affinity of tamoxifen. However, in contrast to tamoxifen, binding of fulvestrant to the ER induces a rapid degradation making the receptor unavailable or unresponsive to oestrogen; the drug attenuates the ability of the ER to activate gene transcription. An important characteristic of fulvestrant, distinguishing its mode of action from that of tamoxifen, is that fulvestrant consistently reduces oestrogen and progesterone receptor levels in the tumour as well, without having agonist effects.6,7 Figure 1 shows a schematic representation of the action of oestradiol in comparison with that of tamoxifen and fulvestrant.
Figure 1.
A schematic representation comparing the action of oestradiol (E) with that of tamoxifen (T) and fulvestrant (F).
AF1, activation function 1; AF2, activation function 2; E, oestradiol; ER, oestrogen receptor; ERE, oestrogen receptor response element; F, fulvestrant; RNA POL II, ribonucleic acid polymerase II; T, tamoxifen.
In early trials, fulvestrant has been shown to be active in patients with metastatic breast cancer previously treated with endocrine therapy. The phase III, multicentre, randomized European and American trials (Trial 0020 and Trial 0021) compared a once-monthly intramuscular injection of low-dose fulvestrant (250 mg) with a once-daily oral 1 mg dose of anastrozole in postmenopausal women with advanced breast carcinoma who previously had disease progression after receiving endocrine therapy. In both trials the majority of patients progressed on tamoxifen. The median time to progression (TTP) was 5.5 months in the fulvestrant group and 4.1 months in the anastrozole arm [hazard ratio: 0.95; confidence interval (CI): 0.82–1.10; p = 0.48], and overall response rate (ORR) was 19.2% and 16.5% for fulvestrant and anastrozole, respectively (hazard ratio: 0.95; CI: 2.27–9.05; p = 0.31). At an extended follow up, the median overall survival (OS) was similar between both treatment groups (27.4 and 27.7 months, respectively). The results demonstrated non-inferiority of fulvestrant in comparison with anastrozole, and fulvestrant 250 mg was registered as an option for postmenopausal patients with endocrine-sensitive advanced breast cancer who had progressed on prior endocrine therapy.8–11
The efficacy and tolerability of fulvestrant have been also demonstrated in the neoadjuvant setting. The NEWEST (Neoadjuvant Endocrine Therapy for Women with Estrogen-Sensitive Tumors) phase II trial was designed to compare fulvestrant at 500 mg with 250 mg as neoadjuvant endocrine therapy in terms of biological activity, such as expression of ER, PR, Ki-67, and ORR in postmenopausal patients with locally advanced breast cancer. In this study, a greater suppression of ER (–50.3 versus –13.7%; p < 0.0001), and PR (–80.5 versus –46.3%; p = 0.0018) was detected at week 4 for fulvestrant 500 versus 250 mg. The NEWEST trial provided the first evidence of greater biological activity for fulvestrant 500 versus 250 mg in depleting ER expression and tumour growth.12
Summing up, fulvestrant was initially approved as a 250 mg monthly dose; later, however, a high dose (HD, i.e. 500 mg) of fulvestrant has proved to be more effective than 250 mg, without showing significant differences in toxicity profile. In accordance with the European Medicine Agency (EMA) product information, fulvestrant (Faslodex) is indicated to treat postmenopausal women with ER+ve, locally advanced or metastatic breast cancer either for disease relapse on or after adjuvant antioestrogen therapy, or for disease progression on antioestrogen therapy.13 In December 2016, the US Food and Drug Administration (FDA) extended the label of fulvestrant for treating HR+ve/HER2–ve advanced or metastatic breast cancer, in combination with palbociclib in women with disease progression following endocrine therapy.14 Table 1 shows all relevant studies regarding the current and possible future role of fulvestrant in the treatment of HR+ve advanced breast cancer.
Table 1.
Phase II/III. Clinical studies with fulvestrant.
| Trial | Phase | Menopausal status | n | Regimen | Treatment line | Primary endpoint | CBR or ORR (%) | Survival (months) | |
|---|---|---|---|---|---|---|---|---|---|
| OS | PFS or TTP | ||||||||
| Fulvestrant single agent | |||||||||
| CONFIRM Di Leo et al.15,16
[2010, 2014] |
III | Postmenopausal | 736 | Fulvestrant 500 mg, fulvestrant 250 mg |
Second | TTP | 45.6 versus 39.6 (CBR) | 26.4 versus 22.3 p = 0.02 | 6.5 versus 5.5 p = 0.006 |
| FIRST Robertson et al.18–20 [2009, 2012, 2015] Ellis et al.21 [2015] |
II | Postmenopausal | 205 | Fulvestrant 500 mg, anastrozole 1 mg |
First | CBR | 72.5 versus 67.0 (CBR) p = 0.386 |
54.1 (n = 86) versus 48.4 (n = 84) p = 0.04 | 23.4 versus 13.1 p = 0.01 |
| FALCON Robertson et al.22 [2017] | III | Postmenopausal | 462 | Fulvestrant 500 mg, anastrozole 1 mg |
First | PFS | 78 versus 74 (CBR) p = 0.3045 |
NR | 16.6 versus 13.8 p = 0.0486 |
| Fulvestrant in combination with other endocrine therapy | |||||||||
| FACT Bergh et al.23 [2012] | III | Postmenopausal | 514 | Fulvestrant 250 mg + anastrozole, anastrozole |
First | TTP | NR | 38.2 versus 37.8 p = 1.00 | 10.8 versus 10.2 p = 0.91 |
| SWOG 0226 Mehta et al.24 [2012] |
III | Postmenopausal | 694 | Fulvestrant 250 mg + anastrozole, anastrozole, fulvestrant |
First | PFS | 73 versus 70 (CBR) | 47.7 versus 41.3 p = 0.05 | 15 versus 13.5 p = 0.007 |
|
Fulvestrant in combination with targeted agents
fulvestrant + CDK inhibitor | |||||||||
| PALOMA 3 Turner et al.34 [2015] Cristofanilli et al.35 [2016] |
III | Pre/peri/ postmenopausal |
521 | Fulvestrant 500 mg + palbociclib, Fulvestrant 500 mg + placebo |
Second | PFS | 24.6 versus 10.9 (CBR) p = 0·0012 |
NR | 9.5 versus 4.6 p < 0.001 |
| Fulvestrant + mTOR inhibitor | |||||||||
| PrECOG 0102 Kornblum et al.36 [2016] |
II | Postmenopausal | 131 | Fulvestrant 500 mg + everolimus, fulvestrant 500 mg + placebo |
Second | PFS | NR | 24.8 not reached in the placebo arm |
10.4 versus 5.1 p = 0.02 |
| Fulvestrant + pan-PI3K inhibitor | |||||||||
| BELLE 2 Baselga et al.37 [2015] |
III | Postmenopausal | 1147 | Fulvestrant 500 mg + buparlisib, fulvestrant 500 mg + placebo |
Second | PFS | 11.8 versus 7.7 (ORR) | NR | 6.9 versus 5.0 p < 0.001 |
| FERGI Krop et al.39 [2016] |
II | Postmenopausal | 168 | Fulvestrant 500 mg + pictilisib, fulvestrant 500 mg + placebo |
Second | PFS | 7.9 versus 6.3 (ORR) | NR | 6.6 versus 5.1 p = 0.096 |
| BELLE 3 Di Leo et al.38 [2016] | III | Postmenopausal | 432 | Fulvestrant 500 mg + buparlisib, fulvestrant 500 mg, fulvestrant 500 mg + placebo |
Second | PFS | 7.6 versus 2.1 (ORR) |
7.6 versus 2.1 | 3.9 versus 1.8 p < 0.001 |
| LEA Martín et al.40 [2015] |
III | Postmenopausal | 380 | Fulvestrant 250 mg or letrozole + bevacizumab, fulvestrant 250 mg or letrozole + placebo |
First | PFS | 76.8 versus 67.4 (CBR) p = 0.041 | 52.1 versus 51.8 p = not stated | 19.3 versus 14.4 p = 0.126 |
| Fulvestrant + EGFR, HER2 inhibitor | |||||||||
| CALGB 40302 Burstein et al.41 [2014] |
III | Postmenopausal | 291 | Fulvestrant 500 mg + lapatinib, fulvestrant 500 mg + placebo | First | PFS | NR | 30 versus 26.4 p = 0.25 | 4.7 versus 3.8 p = 0.37 |
| Fulvestrant + IGFR inhibitor | |||||||||
| Robertson et al.42 [2013] | II | Postmenopausal | 156 | Fulvestrant 250 mg or exemestane + ganitumab, fulvestrant 250 mg or exemestane + placebo |
Second | PFS | NR | 22.2 not reached p = 0.025 favours placebo |
5.7 versus 3.9 p = 0.44 |
| Fulvestrant + RET, VEGFR and EGFR TKI inhibitor | |||||||||
| OCOG -ZAMBONEY Clemons [2014] | II | Postmenopausal | 129 | Fulvestrant 500 mg + vandetanib, fulvestrant 500 mg + placebo |
First | PFS | NR | 73.7 versus 69.1 | 6 versus 4.8 p = 0.47 |
CDK, cyclin-dependent kinase; CBR, clinical benefit rate; ORR, overall response rate; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor type 2; IGFR, insulin-like growth factor receptor; mTOR, mammalian target of rapamycin; N, number of patients; NR, not reported; NS, not significant; OS, overall survival; PFS, progression-free survival; PI3K, phosphoinositide 3-kinase; RET, rearranged during transfection; TKI, tyrosine kinase inhibitor; TTP, time to progression; VEGFR, vascular endothelial growth factor receptor.
Fulvestrant high dose 500 mg
Fulvestrant was initially approved for use at a dose of 250 mg every 28 days. Preoperative studies showed a dose-dependent downregulation of ER, PR and proliferation-related antigen KI67 by fulvestrant. In addition, other trials suggested a dose-response effect for fulvestrant, assuming that doses higher than 250 mg may have a greater effect on ER positive breast cancers.
Preclinical and clinical data led to the activation of a very important trial in this context, that is, the phase III Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM), which compared the then-approved fulvestrant 250 mg with fulvestrant HD 500 mg in postmenopausal patients with ER+ve advanced breast cancer, having recurred after or progressed while on prior endocrine therapy. In this second-line trial, 736 patients were randomly assigned to fulvestrant 250 mg or fulvestrant HD 500 mg. Di Leo et al. reported benefits in progression-free survival (PFS) (6.5 versus 5.5 months; Hazard ratio: 0.80; p = 0.006), in terms of ORR (9.1% versus 10.2%) and CBR (45.6% versus 39.6%), in favour of the higher-dose regimen in patients who experienced progression after previous endocrine therapy with tamoxifen or AI. Fulvestrant HD 500 mg has been well tolerated, and no substantial difference in incidence and severity of adverse events has been observed between the two treatment groups. Serious adverse events reported in more than two patients were bronchitis (0.6%), dyspnoea (0.6%), and vomiting (0.8%) in the 500 mg group, whereas no cases were reported in the 250 mg arm. A total of 145 patients participated in the QOL substudy, and in terms of physical well-being, functional well-being, and breast cancer subscale, no significant difference has been detected between the two study arms.15 A longer unplanned OS analysis showed that fulvestrant HD resulted in a 4.1-month significant increase in median OS, with a 19% relative reduction in the risk of death (26.4 versus 22.3 months; hazard ratio: 0.81%; p = 0.016). Following the publication of these results, fulvestrant HD 500 mg has been regarded as the approved and preferred schedule for this drug.16 Primary tumours from a subset of patients enrolled in the CONFIRM trial were evaluated in the transCONFIRM study in order to identify a gene signature of response to fulvestrant in advanced breast cancer. For this purpose, the established gene signature of the PAM50 intrinsic subtype predictor and the Oncotype DX recurrence scoring risk classifier were also examined as predictors of response to fulvestrant. Moreover, Jeselsohn et al. reported that increased EGF pathway and FOXA1 transcriptional signalling are associated with a decreased response to fulvestrant. Furthermore, they identified a novel set of 37 genes with an expression independently associated with PFS, demonstrating that high expression of the TFAP2C gene (a well-known regulator of ER activity) is related to a decreased response to fulvestrant. The negative predictive value of TFAP2C expression, therefore, suggests further validation of fulvestrant treatment as a predictive biomarker in metastatic breast cancer.17
The efficacy of the first-line fulvestrant HD 500 mg regimen has been evaluated in two clinical trials. The phase II, open-label study (FIRST) was designed to evaluate fulvestrant HD 500 mg in comparison with anastrozole 1 mg as first-line endocrine therapy for postmenopausal women with HR+ve/HER2–ve advanced breast cancer, and included a total of 205 breast cancer patients not having been treated for metastatic disease. The primary endpoint of this trial was the clinical benefit rate (CBR), so OS was not defined as an endpoint in the original protocol. Robertson et al. reported that fulvestrant HD therapy was at least as effective as anastrozole, as there were no significant differences regarding either the clinical benefit rate (CBR) (72.5% versus 67.0%) or the ORR (36.0% and 35.5%); therefore, findings have confirmed the clinical efficacy of both drugs.18,19 In contrast, a follow-up analysis detected a significantly longer TTP for fulvestrant (23.4 versus 13.1 months, hazard ratio: 0.66; p = 0.01), with results suggesting advantages for patients treated with fulvestrant. OS was assessed by protocol amendment and the results indicated that fulvestrant HD extends OS versus anastrozole (54.1 versus 48.4 months, hazard ratio 0.70, 0.50 to 0.98; 95% CI, p = 0.04). In subgroup analyses, OS was increased with fulvestrant 500 mg versus anastrozole for endocrine therapy-naïve patients, but was found to be equivalent between treatments for patients having received prior endocrine therapy. Fulvestrant HD was generally well tolerated, with a tolerability profile similar to that of anastrozole. In this study, two serious adverse events have been considered as treatment related, namely, one case of hypertension and one case of pulmonary embolism, both occurring in the fulvestrant 500 mg treatment group.20,21
Based on these results, investigation of fulvestrant in first-line therapy in comparison with anastrozole continued in the phase III FALCON, involving hormonal-therapy-naïve breast cancer patients: 462 postmenopausal women with locally advanced or metastatic HR+ve/HER2–ve breast cancer were randomized to receive fulvestrant 500 mg or anastrozole 1 mg. The primary endpoint was PFS and the results showed a significantly longer PFS in the fulvestrant group than in the anastrozole group (16 versus 13.8 months, hazard ratio 0.797, 95% CI: 0.637–0.999, p = 0.0486). Subgroup analysis, however, showed an even greater impact on PFS in patients without visceral metastases, who benefited substantially more from fulvestrant than with anastrozole (22.3 versus 13.8 months, p = 0. 0486). Overall, the FALCON study showed that fulvestrant had superior efficacy compared with anastrozole in completely endocrine-therapy-naïve patients with HR+ve advanced breast cancer. Enhanced treatment effects with fulvestrant were seen in patients with nonvisceral disease compared with those with visceral disease. As regards tolerability, the most common adverse events were arthralgia (17% in the fulvestrant group versus 10% in the anastrozole group) and hot flushes (11% in the fulvestrant group versus 10% in the anastrozole group).22
Fulvestrant in combination with other endocrine therapies
As the available endocrine agents in the treatment of breast cancer have different mechanisms of action, a combination of drugs is a logical approach to improve the effectiveness of endocrine therapy. Furthermore, preclinical data support that fulvestrant may have a higher efficacy in a low-oestrogen environment, and it has also been shown that the combination of fulvestrant and an aromatase inhibitor might be an optimal therapy in comparison with either agent alone, producing complete oestrogen blockade by downregulating the oestrogen receptor and inhibiting oestrogen synthesis. Therefore, the combination of endocrine agents with a different mechanism of action may delay the development of resistance by preventing the activation of growth factor pathways and cross-talk with ER. For this reason, two randomized trials were conducted to determine whether the combination of fulvestrant and anastrozole might be superior to anastrozole therapy in the treatment of metastatic HR+ve breast cancer.
A phase III trial, Fulvestrant and Anastrozole in Combination Trial (FACT) randomized 514 patients to the combination of fulvestrant 250 mg (low-dose regimen) plus anastrozole versus anastrozole alone at the first relapse of endocrine-responsive breast cancer. It is important to note that approximately two thirds of the randomized patients in this study had received adjuvant antioestrogens. Bergh et al. reported that all evaluated efficacy outcomes (e.g. TTP, OS, ORR, CBR) were similar between both treatment arms. No difference was observed in TTP (10.8 versus 10.2 months, hazard ratio = 0.99; 95% CI: 0.81 to 1.20; p = 0.91) and OS (37.8 versus 38.2 months, hazard ratio = 1.0; 95% CI: 0.76 to 1.32; p = 1.00). The investigators concluded that fulvestrant 250 mg in combination with anastrozole offered no clinical efficacy advantage over anastrozole in metastatic breast cancer patients. In this trial, a relatively high proportion of patients had received previous adjuvant antioestrogen therapy. Adverse events of interest, respectively, were gastrointestinal disorders (25.2% versus 28.9%), joint disorders (27.6% versus 26.6%), hot flushes (13.8% versus 24.6%) and thromboembolic events (1.6% versus 2.3%).23
In a similarly designed, randomized, phase III first-line trial, SO226, conducted by the Southwest Oncology Group (SWOG) Cooperative Group, 694 postmenopausal patients were randomly assigned to anastrozole alone or the combination of fulvestrant 250 mg (low-dose regimen) plus anastrozole. Improved PFS (15 versus 13.5 months; hazard ratio 0.80; 95% CI: 0.68–0.94; p = 0.007) and OS (47.7 versus 41.3 months; hazard ratio 0.81; 95% CI: 0.65–1.00; p = 0.05) have been reported in favour of the combination treatment, despite having administered fulvestrant in a dose below the current standard and also, in spite of nearly half of the patients in the anastrozole arm crossing over to fulvestrant after progression. Differences in PFS were even higher in patients without prior adjuvant tamoxifen therapy, representing 59.7% of patients. Overall, data suggest that the benefit from combination therapy might have occurred in patients with untreated, de novo metastatic breast cancer. In general, toxic effects did not differ significantly in grade between the two groups, and the most common grade 3 side effects were musculoskeletal pain (2.8%), influenza-like symptoms (2.4%), gastrointestinal disturbances (1.5%), and haematologic effects (1.5%).24
Fulvestrant in combination with targeted agents
Although the majority of patients with ER+ve breast cancer can be successfully treated with endocrine therapies, a substantial subset of this population will relapse and become refractory to such therapeutic approaches. While major advances have been made to understand molecular interactions of hormone signalling with other important growth factors, metabolic and cell-division pathways of HR+ve breast cancer, endocrine resistance, both primary and secondary, is still a burning clinical problem.25
Whereas most patients with HR+ve breast cancer benefit from first-line endocrine therapy, many of them develop resistance to these drugs because their tumours take advantage of alternative growth pathways. The resistance may be due to several factors, such as activating mutations in the ESR1 gene that encodes ER, the increased activity of CDK4/6, and the upregulation of signalling pathways such as phosphoinositide-3-kinase (PI3K)/Akt/mTOR and human epidermal growth factor receptor type 2 (HER2)/mitogen-activated protein kinase (MAPK).3,26,27 Identification of molecular and genomic alterations leading to endocrine resistance has enhanced the development of targeted therapies, changing the landscape of HR+ve/ HER2–ve advanced breast cancer treatment. The combination of endocrine therapy with targeted agents is a developing field, targeting the PI3K/AKT/mTOR pathway, cell cycle regulation and the cross-talk between HR and growth-factor-receptor-signalling mechanisms, which might increase or restore endocrine sensitivity. Recently, combination therapies of AIs with targeted agents for mTOR (everolimus) and CDK 4/6 have been approved for metastatic HR+ve breast cancer.28–30 Studies suggest that activating mutations in ESR1 represents a key mechanism in acquired resistance and could provide one more reason why tumours often become resistant to endocrine therapy. However, mutations in ESR1 are common in patients with metastatic breast cancer who were previously treated with an AI. Preclinical and clinical investigations have demonstrated that ESR1 mutations can promote oestrogen-independent ER activity and finally, tumour growth in patients who have acquired resistance. Moreover, there are data supporting that the mutant ER protein is susceptible to degradation with fulvestrant. Thus, the combination of CDK 4/6 or mTOR inhibitor with fulvestrant may provide a superior efficacy by lowering ER expression in patients who have previously received an aromatase inhibitor.31,32
Fulvestrant in combination with cyclin-dependent kinase 4/6 inhibitor
Deregulation of the cyclin D-CDK4/6-retinoblastoma (Rb) pathway and the increased activity of CDK 4/6 are commonly seen in HR+ve breast cancer. The growth of HR+ve breast cancer depends on CDK4 and CDK6, which promote progression from the G1 phase to the S phase of the cell cycle. CDK 4/6 inhibitors could stop the growth of HR+ve breast cancer cells, even those resistant to endocrine therapy. The inhibition of CDK4/6 has been established as a new therapeutic strategy to enhance the efficacy of endocrine therapy and to reverse both primary and secondary resistance.33 Palbociclib is the first drug in its class, that is, selective inhibitors of CDK 4/6, introduced into clinical practice.30,34
The phase III Paloma-3 trial was designed to compare the combination of palbociclib (125 mg per day orally for 3 weeks, followed by 1 week off) and fulvestrant with fulvestrant monotherapy. Fulvestrant was administered at a rate of 500 mg per standard of care every 14 days for the first three injections and then every 28 days. The study enrolled 521 patients, both premenopausal and postmenopausal HR+ve metastatic women having relapsed or progressed during prior endocrine therapy. In premenopausal or perimenopausal patients, ovarian suppression was induced by the administration of goserelin for the duration of study treatment. Turner et al. reported significant improvements in PFS (9.5 versus 4.6 months; hazard ratio 0.46, 95% CI: 0.36–0.59, p < 0.001) and CBR (34% versus 19%; p = 0.001) in favour of the palbociclib and fulvestrant combination arm. The most common grade 3 or 4 adverse events were neutropenia (65% in the fulvestrant plus palbociclib group and 1% in the fulvestrant plus placebo group), anaemia (3% versus 2%), and leucopenia (28% versus 1%). Neutropenia observed with palbociclib was generally asymptomatic, resulting in a very low incidence of febrile neutropenia, and could be managed effectively by dose reduction, interruption, or cycle delay without compromising efficacy. An extended follow-up subgroup and biomarker analysis has also shown that fulvestrant plus palbociclib was associated with a significant improvement in PFS when compared with fulvestrant alone, irrespective of the degree of HR expression level, and the PIK3CA mutational status of the tumour.34,35
Fulvestrant in combination with mammalian target of rapamycin inhibitor
Inhibition of mTOR, one of the key pathways in endocrine resistance, along with a complete blockade of the oestrogen receptor and a selective oestrogen receptor downregulator are emerging strategies for the treatment of endocrine resistance. The combination of everolimus, the first mTOR inhibitor introduced into clinical practice, and endocrine therapy represents an important strategy to overcome endocrine resistance.29 At present, everolimus is approved for the treatment of postmenopausal advanced HR+ve/HER2–ve breast cancer in combination with exemestane.
PrECOG 0102 was a multicentre phase II study designed to evaluate the combination of everolimus with fulvestrant versus fulvestrant single agent, as a second-line therapy in 131 postmenopausal women with HR+ve/HER2–ve advanced breast cancer previously treated with an aromatase inhibitor for metastatic disease or relapsing on adjuvant aromatase inhibitor. Kornblum et al. reported a statistically significant improvement in median PFS for the combination of the selective oestrogen receptor downregulator, fulvestrant and an mTOR inhibitor, everolimus, compared with fulvestrant alone (5.1 versus 10.4 months; hazard ratio: 0.61, 95% CI: 0.40–0.92; p = 0.02) in women with breast cancer resistant to aromatase inhibitor therapy. The combination was associated with greater toxicity; the most frequent grade 3 adverse events were stomatitis (9%), pneumonitis (6%), fatigue (5%), and hyperglycaemia (6%). The tolerability profile of both drugs was consistent with that seen in other studies.36 Nonetheless, the PrECOG 0102 trial results need to be confirmed by larger studies.
Fulvestrant in combination with pan-PI3K inhibitors
Activation of the PI3K pathway is a hallmark of HR+ve breast cancer cells that are resistant to endocrine therapy; approximately one third of these tumours harbours activating mutations of the phosphoinositol-3 (PI3) kinase (PIK3CA) catalytic subunit of PI3K. Thus, the dual blockade of the ER pathway and PIK3CA is a promising strategy to overcome resistance.
In a phase III (BELLE-2) second-line trial, 1147 patients with HR+ve/HER2–ve advanced breast cancer having progressed while on or following treatment with an aromatase inhibitor were randomized to fulvestrant with either placebo or an oral pan-PI3K inhibitor, buparlisib. PIK3CA mutation status was assessed in archival tumour tissue, and in circulating tumour DNA at trial entry, in a subset 587 patients. Baselga et al. detected a modest PFS improvement following the combination of buparlisib plus fulvestrant (6.9 versus 5.0 months; hazard ratio = 0.78; p < 0.001) in the entire study population. In patients with PI3K-activated tumours detected in archival samples, the median PFS was 6.8 months with the combination and 4.0 months with fulvestrant alone (hazard ratio = 0.76; p = 0.014). Among the patients for whom PIK3CA status was examined by liquid biopsy in circulating tumour DNA, mutations have been found in 200 cases. Interestingly, in this subset, there was an even more remarkable difference in the PFS, favouring the combination treatment group (7.0 versus 3.2 months; p < 0.001). Doubling in the median PFS with the addition of pan-PI3K inhibitors to fulvestrant could have important clinical implications. In contrast, among patients with wild-type PIK3CA, no benefits have been seen following the addition of the PI3K inhibitor (PFS 6.8 months in both arms).37 This study also suggests that liquid-biopsy-based PIK3CA sampling is more relevant and predictive than mutations detected in tumours. The most common toxicities observed with the combination were transaminitis, hyperglycaemia, rash, and mood disorders which frequently led to treatment discontinuations. Treatment duration was reduced by discontinuation as well. Currently, buparlisib is being evaluated in a phase III Study (BELLE-3), which is recruiting patients progressing on AI, and on mTOR inhibitor.38
The phase II FERGI investigated the activity of pictilisib, another pan-PI3K inhibitor, in combination with fulvestrant in 168 postmenopausal women with HR+ve/HER2–ve breast cancer resistant to treatment with an aromatase inhibitor in the adjuvant or metastatic setting. The addition of pictilisib to fulvestrant did not significantly improve PFS (6.6 versus 5.1 months, hazard ratio 0.74; p = 0.096), regardless of PI3K mutation. Dosing of pictilisib was limited by toxicity, potentially limiting its efficacy.39
Fulvestrant in combination with vascular endothelial growth factor antibody
The phase III LEA trial tested whether combining an anti-vascular endothelial growth factor (VEGF) antibody with endocrine therapy could potentially delay the development of resistance. The study evaluated the addition of bevacizumab to letrozole or fulvestrant as a first-line therapy in 380 postmenopausal patients with HR+ve/HER2–ve advanced breast cancer. The combination of VEGF antibody plus endocrine therapy has not resulted in a statistically significant increase in PFS (14.4 months in the endocrine treatment arm and 19.3 months in the endocrine treatment plus bevacizumab, treatment group, hazard ratio 0.83; p = 0.126) or OS.40
Fulvestrant in combination with endothelial growth factor receptor–human epidermal growth factor receptor type 2-targeted therapy
Resistance to endocrine agents could be associated with acquired overexpression of EGFR or HER2; evidence has indicated that the dual EGFR- and HER2-targeting agents can resensitize breast cancers to endocrine treatments. The phase III trial Cancer and Leukaemia Group B 40302B (CALGB 40302/Alliance) CALGB investigated the addition of dual EGFR-HER2 inhibitor, lapatinib to fulvestrant, in comparison with the mono-agent lapatinib in women with advanced HR+ve/HER2–ve or HER2+ve breast cancer that proved to be resistant to endocrine therapy. Burstein et al. reported no benefit from the addition of lapatinib to fulvestrant in either PFS (4.7 versus 3.8 months, hazard ratio = 1.04, p = 0.37), or OS (30 versus 26.4 months, hazard ratio = 0.91, p = 0.25). Similarly, no significant improvement in PFS (5.9 versus 3.3 months; p = 5.53) was observed in the HER2+ve subgroup of patients.41
Fulvestrant in combination with an insulin-like growth factor receptor antibody
In a phase II second-line trial, a monoclonal IgG1 antibody that blocks IGF-1R, ganitumab, has been tested in combination with either fulvestrant or exemestane in postmenopausal women with advanced HR+ve breast cancer. Robertson et al. reported no clinical benefit from the addition of targeted therapy to endocrine agent; on the contrary, a decrease in OS has been observed with ganitumab-based therapy.42
Fulvestrant in combination with a rearranged-during-transfection, vascular endothelial growth factor receptor, and epidermal growth factor receptor tyrosine kinase inhibitor
A phase II, placebo-controlled trial added vandetanib to fulvestrant in women with bone-only or bone-predominant HR+ve metastatic breast cancer. A biomarker of bone turnover, urine N-telopeptide (uNTx), has been used to assess whether vandetanib improved uNTx response when added to fulvestrant in patients with bone metastases. No difference was detected between groups for PFS (hazard ratio = 0.95, 95% CI: 0.65–1.38) or OS (hazard ratio = 0.69, 95% CI: 0.37–1.31). In addition, investigators also concluded that adding vandetanib to fulvestrant did not result in an improvement of biomarker response, PFS or OS in patients with bone metastases.43
Expert commentary
All current clinical consensus guidelines (e.g. ASCO, NCCN, ESMO-ABC3) recommend that the preferred treatment for HR+ve/HER–ve metastatic breast cancer should be endocrine therapy in the majority of cases, even in the presence of asymptomatic visceral metastases. Endocrine therapy is the recommended first option for patients who do not have aggressive disease, and should be continued for up to three lines of therapy unless there is visceral crisis or proof of endocrine resistance. Although patients with rapidly progressing disease or visceral crisis are candidates for chemotherapy, endocrine therapy may be administered as maintenance therapy, after gaining control of the disease.2,3 The landscape of endocrine therapy for advanced HR+ve/HER2–ve breast cancer is rapidly changing. Therapies targeting oestrogen modulation have evolved in recent years with the introduction of targeted agents (e.g. mTOR and CDK 4/6 inhibitors) administered in combination with hormone therapy. The increased use of nonsteroidal AI therapy in the adjuvant setting and the growing number of therapeutic options makes it difficult to determine the best choice and the optimal sequence of endocrine therapy.27
Fulvestrant, as a SERD, has a unique mode of action and is an active compound in the treatment of HR+ve/HER–ve locally advanced and metastatic breast cancer, with an excellent tolerability profile.44 It has been introduced into clinical practice since 2002, and initially, its approved dose was 250 mg. The currently approved standard dose is HD 500 mg, and it should be administered every 14 days for the first three injections and then every 28 days. Until recently, fulvestrant was indicated for the treatment of postmenopausal women with ER+ve advanced breast cancer as a second-line therapy postdisease progression after aromatase inhibitors or tamoxifen. However, since December 2016, FDA approval has been extended to the combination of fulvestrant with palbociclib in women with disease progression after endocrine therapy.
Fulvestrant first line
Recent data on efficacy and tolerability support the use of fulvestrant in the first-line setting, as a monotherapy or in combination with anastrozole, both with preferential consideration in endocrine-naïve patients. Evidence from the recently published FALCON phase III study results has shown that fulvestrant significantly improved PFS in comparison with anastrozole (16.6 versus 13.8 months; p = 0.0486), and the PFS difference was markedly better in patients with nonvisceral disease (22.3 versus 13.8 months; p = 0.0092).22 These data proved to be consistent with the results of FIRST phase II trial and confirm the superiority of fulvestrant versus anastrozole.18,19
Another first-line therapeutic option with fulvestrant is represented by the combination of fulvestrant 250 mg (low-dose regimen) and anastrozole. The SWOG 0226 phase III trial showed a significant improvement in PFS (15 versus 13.5 months; p = 0.007) and marginally significant OS (47.7 months versus 41.3 months; p = 0.049) in favour of fulvestrant plus anastrozole, despite the use of a dose of fulvestrant below the current standard. Differences in PFS were higher in the combination subset of patients who did not receive prior endocrine therapy.24 However, similar improvements were not seen in the FACT trial.23 Given the improved survival observed in the SWOG 0226 study, fulvestrant (500 mg on day 1, 250 mg on days 14 and 28 and monthly thereafter) plus anastrozole could be recommended for use as a first-line therapy for the minority of postmenopausal, endocrine therapy-naïve patients. In view of these results, fulvestrant single agent and in combination with anastrozole are effective and safe options for the initial treatment of postmenopausal women with advanced HR+ve/HER–ve breast cancer.24 It should be mentioned that fulvestrant has no approval for the first-line setting, which could be of importance for its implementation in clinical practice.
Presently, with the rapidly changing landscape of endocrine therapy, selecting first-line therapy for postmenopausal HR+ve/HER2–ve advanced breast cancer has become complex and presents clinical challenges. Additionally, the increased use of AI therapy in the adjuvant setting has also complicated choices. However, at the initiation of first-line endocrine therapy, the hardest question is probably whether to use monotherapy or combination therapy. Both single-agent therapy (AI, tamoxifen, fulvestrant) and the combination of different agents (endocrine therapy plus other endocrine agent, or endocrine therapy in combination with a targeted agent) are reasonable alternatives.
Firstly, AIs may provide better disease control compared with tamoxifen (PFS 10.7 versus 6.4 months; p = 0.022) in first-line therapy. In recent years, there has been a shift towards the use of aromatase inhibitors and there is a broad consensus that AIs should be part of first-line therapy. Secondly, the FALCON trial demonstrated the superiority of fulvestrant versus anastrozole, and improved PFS and OS survival data support the use of fulvestrant plus anastrozole as an initial therapy. Finally, a very reasonable first-line treatment option is represented by the combination of a CDK4/6 inhibitor, palbociclib and letrozole. Data from one phase II and two phase III clinical trials show that adding a CDK4/6 inhibitor (palbociclib or ribociclib) to letrozole results in significant improvements in PFS versus an AI. Very recently, the first results of the phase III PALOMA-2 trial have shown a greater than 10-month PFS gain (PFS 24.8 versus 14.5 months; p < 0.001) for the addition of palbociclib to letrozole.45 Quite similarly, in the MONALEESA-2 trial, patients receiving initial systemic treatment with ribociclib in combination with letrozole had a significantly longer PFS in comparison with those receiving letrozole.46 It is important to mention that although each endocrine therapy improves PFS, none with the exception of anastrozole plus fulvestrant have demonstrated improved OS to date.
Concerning available endocrine therapies, fulvestrant plus anastrozole as first-line therapy could be recommended for the minority of postmenopausal, endocrine-therapy-naïve patients. The other option in this setting, represented by single-agent-fulvestrant monotherapy, could be a good option similarly in patients who are completely endocrine naïve, or who relapse on or shortly after the completion of adjuvant AI or tamoxifen therapy. Fulvestrant single agent could also be favoured in low-risk patients, with very limited, bone-only, or with nonvisceral disease. Furthermore, fulvestrant monotherapy might be a choice for patients with comorbidities, and for those unable to tolerate combination targeted therapy with an eventually higher rate of myelosuppression, or in situations where targeted therapies are not available.
Fulvestrant second line and beyond
The choice of second-line endocrine therapy should take into consideration prior agent exposure and response to previous hormone therapy. In second-line therapy, several options exist, so both single-agent therapy (exemestane or fulvestrant) and the combination of endocrine therapy plus a targeted agent (mTOR or CDK 4/6 inhibitor) could be considered. For the second-line setting, nonsteroidal AI exemestane and fulvestrant proved to be equally effective. The use of fulvestrant HD (500 mg) as monotherapy in second-line treatment is supported by the evidence provided by the CONFIRM study. The trial showed benefits both in PFS (6.5 versus 5.5 months; p = 0.05) and OS (26.4 months with fulvestrant HD 500 mg and 22.3 months with 250 mg dose regimen p = 0.05) in patients having experienced progression after previous endocrine therapy with tamoxifen or AIs.15,16 Fulvestrant received a new indication in 2016, in combination with CDK 4/6 inhibitor, palbociclib on the basis of the PALOMA-3 trial. The trial demonstrated a doubling in PFS (9.5 versus 4.6 months; p < 0.0001) with the combination of fulvestrant and palbociclib in patients with prior exposure to AIs, and the therapy proved to be well tolerated, even though toxicities included an increase in grade 3 and 4 neutropenia. Importantly, this treatment is an option for both postmenopausal and pre/perimenopausal patients, although the treatment should be administered for the latter in combination with ovarian-function suppression, with a luteinising-hormone-releasing-hormone (LHRH) agonist.34,35
In second line, another possible choice is the combination of everolimus and exemestane. This combination has shown a significant PFS gain (7.8 versus 3.2 months; p < 0.001) in the Bolero 2 trial, although the efficacy benefit with everolimus came with an increased incidence of mTOR inhibitor-associated toxicities.29 Thus, when recommending everolimus plus exemestane, an important consideration might be the selection of the most appropriate, medically fit patients with prior exposure to nonsteroidal aromatase inhibitors. Very recently, the results of the combination of fulvestrant plus everolimus became available; however, the combination is not approved, and has not been introduced into clinical practice. Data on efficacy and tolerability support the use of the second-line therapy fulvestrant as a monotherapy, or in combination with the CDK4/6 inhibitor, palbociclib. In the context of the availability of other treatment choices (e.g. monotherapy or combination, mTOR or CDK4/6 inhibitor), when choosing a specific agent over another treatment, decisions may be guided by taking distinct adverse-event profiles of the drugs, patient performance status, comorbidities, and preferences into account.
Conclusions
Fulvestrant, as a SERD, with a unique mode of action is an active compound that showed increased efficacy for the treatment of patients with HR+ve/HER2–ve advanced breast cancer, alone or in combination with other endocrine agents or targeted therapies. Fulvestrant should be administered using the 500 mg dose with a loading schedule, and may be considered with LHRH agonists for premenopausal women. Taking the efficacy and tolerability of the drug with new standard regimens, it is a good alternative therapeutic choice for patients who need a well-tolerated therapy, in the context of providing a balance of efficacy, safety, patient preferences and effect on the quality of life. A potential advantage of fulvestrant is that it may improve treatment compliance due to its monthly parenteral administration compared with daily oral intakes of other endocrine therapies. Fulvestrant alone or in combination with an AI appears to work best early, especially in endocrine-naïve cases. Fulvestrant in combination with a CDK inhibitor should be preferred in patients with prior exposure to an AI. At present, there are well-defined indications for fulvestrant in the therapeutic algorithm of advanced ER+ve breast cancer, but the optimal position has not yet been defined. Recent studies offer hope that identifying biomarkers will lead to a more accurate selection of patients likely to benefit the most from fulvestrant monotherapy or from existing combinations. Additionally, research is continuing to evaluate the full potential of fulvestrant in advanced breast cancer.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Boér reports having an advisory role for Amgen, GlaxoSmithKline, Novartis, Pfizer and Roche.
References
- 1. Setiawan VW, Monroe KR, Wilkens LR, et al. Breast cancer risk factors defined by oestrogen and progesterone receptor status: the multi-ethnic cohort study. Am J Epidemiol 2009; 15: 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 2017; 31: 244–259. [DOI] [PubMed] [Google Scholar]
- 3. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016; 34: 3069–3103. [DOI] [PubMed] [Google Scholar]
- 4. Ciruelos E, Pascual T, Arroyo Vozmediano ML, et al. The therapeutic role of fulvestrant in the management of patients with hormone receptor-positive breast cancer. Breast 2014; 23: 201–208. [DOI] [PubMed] [Google Scholar]
- 5. Kümler I, Knoop AS, Jessing CA, et al. Review of hormone-based treatments in postmenopausal patients with advanced breast cancer focusing on aromatase inhibitors and fulvestrant. ESMO Open 2016; 1: e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer 2004; 90(Suppl. 1): S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wakeling AE. Similarities and distinctions in the mode of action of different classes of anti-oestrogens. Endocr Relat Cancer 2000; 7: 17–28. [DOI] [PubMed] [Google Scholar]
- 8. Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol 2002; 20: 3396–3403. [DOI] [PubMed] [Google Scholar]
- 9. Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 2002; 20: 3386–3395. [DOI] [PubMed] [Google Scholar]
- 10. Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women – a prospective combined analysis of two multicenter trials. Cancer 2003; 98: 229–238. [DOI] [PubMed] [Google Scholar]
- 11. Vergote I, Robertson JFR. Fulvestrant is an effective and well-tolerated endocrine therapy for postmenopausal women with advanced breast cancer: results from clinical trials. Br J Cancer 2004; 90(Suppl. 1): S11–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuter I, Gee JM, Hegg R, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized phase II study. Breast Cancer Res Treat 2012; 133: 237–246. [DOI] [PubMed] [Google Scholar]
- 13. EMA. Faslodex. Product information, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000540/WC500021174.pdf (accessed 28 February 2017).
- 14. FDA. Faslodex. Injection label, http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021344s029lbl.pdf (accessed 28 February 2017).
- 15. Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with oestrogen receptor-positive advanced breast cancer. J Clin Oncol 2010; 28: 4594–4600. [DOI] [PubMed] [Google Scholar]
- 16. Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs. 250 mg in the randomised CONFIRM trial. J Natl Cancer Inst 2014; 106: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeselsohn R, Barry WT, Migliaccio I, et al. TransCONFIRM: identification of a genetic signature of response to fulvestrant in advanced hormone receptor-positive breast cancer. Clin Cancer Res 2016; 22: 5755–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol 2009; 27: 4530–4535. [DOI] [PubMed] [Google Scholar]
- 19. Robertson JF, Lindemann JP, Llombart-Cussac A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomised ‘FIRST’ study. Breast Cancer Res Treat 2012; 136: 503–511. [DOI] [PubMed] [Google Scholar]
- 20. Robertson JF, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole as first-line treatment for advanced breast cancer: overall survival from the phase II ‘FIRST’ study [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 9–13 December 2014; San Antonio, TX Philadelphia, PA: AACR; Cancer Res 2015; 75(9 Suppl): Abstract nr S6–04. [Google Scholar]
- 21. Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol 2015; 33: 3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robertson JF, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase III trial. Lancet 2017; 388: 2997–3005. [DOI] [PubMed] [Google Scholar]
- 23. Bergh J, Jönsson PE, Lidbrink EK, et al. FACT: an open-label randomised phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012; 30: 1919–1925. [DOI] [PubMed] [Google Scholar]
- 24. Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012; 367: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santa-Maria CA, Gradishar WJ. Changing treatment paradigms in metastatic breast cancer: lessons learned. JAMA Oncol 2015; 1: 528–534. [DOI] [PubMed] [Google Scholar]
- 26. Poggio F, Lambertini ME, Vaglica M, et al. Role of fulvestrant in the treatment of postmenopausal metastatic breast cancer patients. Expert Rev Clin Pharmacol 2016; 9: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 27. Pritchard KI, Chia SK, Simmons C, et al. Enhancing endocrine therapy combination strategies for the treatment of postmenopausal HR+/HER2– advanced breast cancer. Oncologist 2017; 22: 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beaver JA, Park BH. The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol 2012; 8: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone- receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 2014; 25: 2357–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Finn RS, Crown JP, Lang J, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase II study. Lancet Oncol 2015; 16: 25–35. [DOI] [PubMed] [Google Scholar]
- 31. Fribbens C, O’ Leary B, Kilburn L, et al. Plasma ESR1 mutations and the treatment of oestrogen receptor-positive advanced breast cancer. J Clin Oncol 2016; 34: 2961–2968. [DOI] [PubMed] [Google Scholar]
- 32. Ladd B, Mazzola AM, Bihani T, et al. Effective therapies in preclinical endocrine-resistant breast cancer models harbouring ER mutations. Oncotarget 2016; 7: 54120–54136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicentre, randomised, placebo-controlled, phase III study (PALOMA-3). Oncologist 2016; 21: 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 35. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase III randomised controlled trial. Lancet Oncol 2016; 17: 425–439. [DOI] [PubMed] [Google Scholar]
- 36. Kornblum N, Manola J, Klein P, et al. PrECOG 0102: A randomized, double-blind, phase II trial of fulvestrant plus everolimus or placebo in post-menopausal women with hormone receptor (HR)-positive, HER2-negative metastatic breast cancer (MBC) resistant to aromatase inhibitor (AI) therapy. Paper presented at 2016 San Antonio Breast Cancer Symposium, 7 December 2016; San Antonio, TX. Abstract S1–02. [Google Scholar]
- 37. Baselga J, Im SA, Iwata H, et al. PIK3C: a status in circulating tumour DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR1/HER2– advanced breast cancer (BC): first results from the randomized, phase III BELLE-2 trial. Paper presented at 2015 San Antonio Breast Cancer Symposium, 8–12 December 2015; San Antonio, TX. Abstract S6–01. [Google Scholar]
- 38. Di Leo A, Keun SL, Ciruelos E, et al. BELLE-3: A phase III study of buparlisib + fulvestrant in postmenopausal women with HR+, HER2–, aromatase inhibitor-treated, locally advanced or metastatic breast cancer, who progressed on or after mTOR inhibitor-based treatment. Paper presented at 2016 San Antonio Breast Cancer Symposium, 6–10 December 2016; San Antonio, TX. S4–07. P4–22–01. [Google Scholar]
- 39. Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase II trial. Lancet Oncol 2016; 17: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martín M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol 2015; 33: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 41. Burstein JH, Cirrincione CT, Barry WT, et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: A randomised, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer—CALGB 40302 (Alliance). J Clin Oncol 2014; 32: 3959–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone- receptor-positive breast cancer: a randomised, controlled, double-blind, phase II trial. Lancet Oncol 2013; 14: 228–235. [DOI] [PubMed] [Google Scholar]
- 43. Clemons MJ, Cochrane B, Pond GR, et al. Randomised, phase II, placebo-controlled, trial of fulvestrant plus vandetanib in postmenopausal women with bone only or bone predominant, hormone-receptor-positive metastatic breast cancer (MBC): the OCOG ZAMBONEY study. Breast Cancer Res Treat 2014; 146: 153–162. [DOI] [PubMed] [Google Scholar]
- 44. Telford C, Jones N, Livings C, et al. Network meta-analysis comparing overall survival for fulvestrant 500 mg versus alternative therapies for treatment of postmenopausal, oestrogen receptor-positive advanced breast cancer following failure on prior endocrine therapy. Clin Breast Cancer 2016; 16: 188–195. [DOI] [PubMed] [Google Scholar]
- 45. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 46. Hortobagyi GN, Salomon M, Stemmer SM, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]