Abstract
Background
Sarcoidosis is a granulomatous disease of unknown etiology and coccidioidomycosis is a granulomatous fungal infection endemic to the southwestern United States. Clinical observations on patients with sarcoidosis and coccidioidomycosis simultaneously led to the hypothesis that sarcoidosis can be caused by the fungus Coccidioides.
Methods
Two patients with sarcoidosis and coccidioidomycosis were studied, one prospectively (ie, a patient with sarcoidosis was predicted to develop coccidioidomycosis) and the other retrospectively (ie, a patient with coccidioidomycosis develops sarcoidosis). In addition, a literature review found 5 patients with these 2 diseases, and they were analyzed to establish an archived relationship between the 2 entities. In addition, polymerase chain reaction (PCR) testing for Coccidioides deoxyribonucleic acid (DNA) was performed on tissue from 15 Arizona patients diagnosed with sarcoidosis.
Results
Patient 1 was diagnosed with sarcoid in Arizona and followed prospectively. There was no evidence for coccidioidomycosis at diagnosis. This patient was observed for 8 years before he was documented to have disseminated coccidioidomycosis. Patient 2 was retrospectively studied. He was diagnosed with disseminated coccidioidomycosis, but after 3 years, while on antifungal therapy, he developed respiratory failure. A lung biopsy was consistent with sarcoidosis with no evidence of Coccidioides; cultures, histopathology, and serology were all negative. Paradoxically, PCR testing for Coccidioides DNA on tissue taken at the time of the sarcoidosis diagnosis were all negative, including Patient 2 who had proven coccidioidomycosis.
Conclusions
The 2 study patients and 5 case reports from the literature support the hypothesis that Coccidioides can cause sarcoidosis in Arizona. There are similar reports in the literature that Histoplasma can be associated with sarcoidosis. Based on these observations, we propose that sarcoidosis can be caused by endemic fungi in different areas of the United States, coccidioidomycosis in the Southwest and histoplasmosis in the Midwest.
Keywords: cell-mediated immunity, coccidioidomycosis, histoplasmosis, sarcoidosis
Sarcoidosis was first described in 1899, 117 years ago [1]. It is a multisystem disease characterized histologically by the presence of noncaseating epithelioid granulomas. Despite multiple investigations since its description, the cause of sarcoid has not been identified [2]. Based on clinical observations beginning over 14 years ago (2002), a hypothesis was formulated about a potential relationship between coccidioidomycosis (coccy) and sarcoidosis (sarcoid) [3]. Those initial observations were based on a series of 4 patients in Arizona who were noted to have 2 granulomatous diseases simultaneously, both sarcoid and a Coccidioides infection. The occurrence of these 2 granulomatous processes simultaneously raised the possibility of a potential etiologic relationship between the 2 entities. The usual assumption was that steroid-treated sarcoid patients in Arizona became immunosuppressed, were exposed to Coccidioides and developed coccy. However, an alternative possibility was postulated that certain patients manifest their Coccidioides infection as clinical sarcoid. These sarcoid patients would then receive steroids for sarcoid and eventually the immunosuppression would result in the expression of an underlying coccy infection. The latter scenario between coccy and sarcoid was hypothesized by the authors at the start of this research in 2013 [4].
CASE REPORTS
Case Report 1 is an Arizona patient of the first author who was diagnosed with sarcoid in 2000 and his clinical course was prospectively documented over 8 years during which time he developed disseminated coccy.
Case Report 1
A 50-year-old white male policeman presented in November 2000 with hypercalcemia, thrombocytopenia, and splenomegaly. He was eventually hospitalized in December 2000 and underwent a splenectomy mainly because of a persistent thrombocytopenia. The resected spleen histopathology was consistent with noncaseating granulomatous inflammation typical of sarcoid. Three pathologists (2 second opinion pathologists) were in agreement with the diagnostic histopathology. Workup for a granulomatous process, including a Coccidioides infection, was done in the hospital and the results were negative. No spherules were observed on spleen histopathology and Coccidioides serology was negative. Postoperatively, he was started on prednisone for the sarcoid and prospectively observed as an outpatient for a Coccidioides infection. His subsequent clinical course is outlined in Table 1. At an outpatient visit in June 2001, the patients Coccidioides complement fixation (CF) titer seroconverted from negative to positive at 1:8 with a positive immunoglobulin IgM (all Coccidioides serologies on this patient were performed by the University of California at Davis, Coccidioidomycosis Serology Laboratory in Davis). At the time of the seroconversion the patient was asymptomatic, feeling better, back to work, and being tapered off steroids. Because the patient had seroconverted from negative to positive, he was transiently placed on fluconazole even though he was asymptomatic. The patient returned to work and eventually was taken off steroids in October 2001. After being taken off the steroids, he declined to continue taking the fluconazole. Over the next 2 years, while off steroids, the patient’s coccy CF titers decreased to 1:2 in March 2002. He was working without missing any work and had no significant fevers or evidence of symptomatic coccy. Between October 2001 and June 2002, the patient was off steroids, but he was placed back on them again by a rheumatologist because of an acute polyarticular arthritis. The patient had no significant fevers with the onset of the arthritis; however, while on steroids, his coccy CF titers had increased to a high of 1:32. Titers ≥1:32 are generally only seen in patients with disseminated coccy [5]. Aside from his arthritis, he was otherwise asymptomatic for coccy, specifically denying any fever, chills or sweats. His arthritis gradually improved, the steroids were tapered down and discontinued 18 months later in December 2003. His coccy CF titers gradually decreased to a low of 1:4 on March 2006. It is worth noting that his angiotensin- converting enzyme (ACE) levels were highest when his coccy CF titers were the highest. He was asymptomatic relative to coccy between June 2006 and June 2008. However, in June 2008, the patient developed swelling of his right sternoclavicular joint but did not complain of fever, chills or sweats. Aspiration of that joint grew Coccidioides; his coccy CF titer then was 1:8. He was placed on oral fluconazole 400 mg daily, which appeared to control the coccy. Between June 2008 and September 2009, his coccy CF titer decreased from 1:8 to 1:2, the right sternoclavicular joint improved and he returned to work. The patient was infrequently observed because his coccy was controlled. He missed an appointment and upon inquiring, he had died in 2009 because of “cancer of the brain”. No post mortem was done. This patient was observed prospectively from December 2000 through June 2008 before he developed culture-proven disseminated coccy associated with his sarcoid as hypothesized.
Table 1.
Clinical Course For Case 1 (2000–2009)
| Date | Clinical Observations | Coccy CF titer-IgGa | ACEb |
|---|---|---|---|
| November 2000 | Splenomegaly and hypercalcemia | Neg | 37 |
| December 2000 | Splenectomy; diagnosis of sarcoid | ||
| January 2001 | Started steroids | ||
| March 2001 | Transient eosinophilia (10%) | Neg | — |
| June 2001 | Asymptomatic (started fluconazole) | 1:8 (IgM+) | 83 |
| August 2001 | Asymptomatic | 1:8 | — |
| October 2001 | Asymptomatic (stopped steroids) | 1:16 | — |
| December 2001 | Asymptomatic (stopped fluconazole) | 1:4 | 108 |
| March 2002 | Asymptomatic | 1:2 | — |
| June 2002 | Arthritis (had been started on steroids) | 1:32 | 44 |
| August 2002 | Arthritis | 1:32 | 63 |
| October 2002 | Arthritis | 1:32 | 75 |
| May 2003 | Arthritis | 1:32 | 109 (highest) |
| December 2003 | Arthritis resolved (stopped steroids) | 1:32 | 90 |
| June 2004 | Asymptomatic | 1:32 | 84 |
| January 2005 | Asymptomatic | 1:16 | 89 |
| August 2005 | Asymptomatic | 1:8 | 61 |
| March 2006 | Asymptomatic | 1:4 | — |
| October 2006 | Asymptomatic | 1:16 | 54 |
| December 2006 | Melanoma diagnosed | 1:8 | 70 |
| April 2007 | Asymptomatic | 1:8 | 73 |
| December 2007 | Asymptomatic | 1:8 | — |
| June 2008 | Coccidioides recovered on culture from the sternoclavicular joint (started fluconazole) | 1:8 | — |
| December 2008 | Coccy controlled | 1:8 | 52 |
| May 2009 | Coccy controlled | 1:8 | — |
| September 2009 | Coccy controlled | 1:2 (IgM+) | — |
| December 2009 | Expired from “brain cancer” |
Abbreviations: ACE, angiotensin-converting enzyme; CF, complement fixation; coccy, coccidioidomycosis; Ig, immunoglobulin; Neg, negative.
aComplement fixation titer to Coccidioides (normal = negative).
bAngiotensin-converting enzyme level (normal 9–67 U/mL).
Case Report 2
A 34-year-old African American male was diagnosed with disseminated coccy in 2009. He was born in California but moved to Arizona in 1996. In 2009, he was incarcerated in Arizona but transferred to a prison in Oklahoma City. Shortly after arriving there, he presented to the jail infirmary with myalgias, weight loss, night sweats, pain and swelling of his left elbow. He was hospitalized and his left elbow was aspirated. Cultures of the elbow aspiration grew Coccidioides. His coccy CF serology was positive at 1:8. While in jail he was started on oral fluconazole. He was released from jail and returned to Arizona in late 2009. Initially, his clinical course was complicated by presumed noncompliance with oral antifungal agents. However, despite becoming more compliant, his clinical course between 2009 and 2012 was suggestive of refractory disease. He had been treated initially with oral fluconazole, then oral voriconazole, followed by intravenous amphotericin B as an outpatient over those 3 years. Although he was compliant with his antifungal medications, they did not seem to control or resolve his symptoms.
In 2012, despite amphotericin B as an outpatient, he became seriously ill with fevers and respiratory failure, which required hospitalization and mechanical ventilation. During that hospitalization he underwent a lung biopsy. The histopathology from the biopsy revealed noncaseating granulomas consistent with sarcoid. Acid-fast bacillus and Gomori methenamine silver stains were negative. There was no histopathologic or culture evidence of Coccidioides [6].
His coccy serology had become negative and multiple cultures for Coccidioides were also negative. The histopathologic changes and clinical findings were consistent with sarcoid without evidence of an active Coccidioides infection. The patient was started on corticosteroids and maintained on amphotericin B. He clinically improved, was extubated and eventually placed on supplemental oxygen and oral voriconazole. Between 2012 and 2015, he clinically improved and his “sarcoid/coccy” was controlled. He was off oxygen supplementation and on maintenance voriconazole with slowly lowered doses of corticosteroids.
LITERATURE REVIEW
Based on observations made in the previous 2 case reports, we believed that if there was a relationship between coccy and sarcoid, there should be other reports in the older literature about similar patients. Five case reports from 1955 to 1997 [7–11] were found and reviewed providing retrospective insights into the pathogenesis of these 2 entities, generally supporting the hypothesis that there is a relationship between coccy and sarcoid.
Case Report 3
A 29-year-old black male with a history of performing farm work in Bakersfield, California was diagnosed with coccy in 1950 [7]. Coccidioides was cultured from a posterior neck lymph node and his right tibia. His cultures were positive and the coccy CF titer was positive at 1:128. His infection eventually improved with no antifungal treatment. Three years later, an enlarged cervical lymph node was biopsied and found to be consistent with sarcoid. The authors thought the observation of the Coccidioides infection and sarcoid were unrelated and the coccy was incidental to his sarcoid.
This patient is similar to Case Report 2, who had well documented disseminated coccy, and 3 years later he had a lung biopsy that showed sarcoid with no evidence of coccy.
Case Report 4
A 23-year-old black male with a history of driving through the San Joaquin Valley in California in December 1960 developed significant epididymitis 6 months later in June 1961 [8]. He subsequently had resection of the epididymis. The histopathology showed noncaseating granulomas, no acid-fast organisms or spherules. He was diagnosed with coccy based on a coccy CF titer at 1:8. However, no organisms were cultured or seen. A year later in 1962, he developed right paratracheal and hilar lymphadenopathy. A lymph node biopsy showed epithelioid granulomas consistent with sarcoid. There were no spherules. His coccy skin test was negative, and his coccy CF titer had increased from 1:8 to 1:128. The authors thought their patient had a “sarcoid syndrome” associated with his Coccidioides infection. He was treated with both steroids and amphotericin B with improvement.
This patient had disseminated coccy involving the epididymis in 1961. In 1962 he developed a “sarcoid syndrome.” The only evidence for coccy was a coccy CF titer rise from 1:8 to 1:128. It is notable that his coccy skin test was reported negative. If no CF serology for coccy had been done, this patient would have fit the diagnosis of sarcoid.
Case Report 5
A 30-year-old white male from Phoenix was admitted in 1951 for fever and lymphadenopathy [9]. A lymph node biopsy was consistent with sarcoid and he was treated with steroids. In 1961, 10 years later, he presented with bilateral infiltrates on chest x-ray and a right femur lesion. Sputum cultures for Coccidioides were negative, but his coccy CF titer was positive at 1:16. He was treated with amphotericin B and improved. The authors thought their patient developed coccy related to the immunosuppression of the sarcoid treatment.
This case is similar to Case Report 1, only by prospectively observing this patient for 10 years would a relationship between coccy and sarcoid have been considered.
Case Report 6
A 27-year-old male medical student from Maine was evaluated in 1956 for cough, fatigue and with bilateral hilar lymphadenopathy [10]. A lymph node biopsy was consistent with sarcoid. At that time, a histoplasmosis CF titer was positive at 1:64. Serologies for blastomycosis and coccy were negative. After graduating from medical school, the physician went into practice in Arizona in 1961 and promptly developed coccy, which resolved spontaneously. In 1967, he was found to have an abnormal chest x-ray, prompting a lymph node biopsy, which was diagnostic of sarcoid and he was briefly treated with steroids. Four years later, he returned to Maine in 1971. In 1972, he was admitted to the hospital for fever, headaches and malaise. Serum coccy CF titers were negative. There was evidence of pulmonary fibrosis and he was started on corticosteroid therapy. The patient subsequently experienced a “septic syndrome” with altered mental status. A workup for an infectious process, including a lumbar puncture, was reported negative. The process progressed and the patient died. At the post mortem examination, there were Coccidioides organisms in his lungs and central nervous system. Post mortem cerebrospinal fluid revealed a coccy CF titer at 1:128, consistent with disseminated coccy with involvement of the central nervous system.
The findings in this patient are particularly interesting. A diagnosis of sarcoid was made twice in this patient. In 1956, in Maine where there is no endemic Coccidioides, his coccy CF titer was negative and his histoplasmosis CF test was positive. An interpretation of this patient’s entire clinical course is that he was predisposed to develop sarcoid and it was triggered by 2 different fungi. The first episode of sarcoid was associated with histoplasmosis. Then, after arriving in Arizona in 1961, he developed an acute Coccidioides infection that resolved without obvious problems. In 1967, while in Arizona, he was diagnosed with sarcoid a second time. He returned to Maine in 1971 (after living in Arizona 10 years) where he died in 1972 without a clinically obvious diagnosis. However, at post mortem examination, he was found to have disseminated coccy. This patient’s course with 2 episodes of sarcoid associated with 2 different systemic fungi supports the hypothesis that there may be persons predisposed to having fungal agents trigger a sarcoid response.
Case Report 7
A 52-year-old male was evaluated in 1985 for fevers [11]. A chest x-ray showed hilar adenopathy and a right mid-lung infiltrate. Bronchoscopy cultures grew Coccidioides; a coccy CF titer was positive at 1:256. He was treated with amphotericin B with improvement. Five years later in 1990, he developed skin lesions on his back, which were biopsied and believed to be consistent with sarcoid. He was treated with prednisone and ketoconazole and the skin lesions improved. In 1992, the lesions recurred on his back with similar lesions now on his legs. A chest x-ray showed interstitial infiltrates and hilar adenopathy. Cultures of the skin lesions were negative for Coccidioides, and no organisms were seen on biopsy of the skin lesions. He was started again on prednisone with improvement. In 1995, the patient developed fevers and fatigue. His coccy CF titer was positive at 1:128, and he was treated with fluconazole with improvement. In 1996, the skin lesions returned and he was retreated with steroids and fluconazole with improvement. The authors did not believe that the sarcoid skin lesions were associated with Coccidioides.
This patient had well documented coccy in 1985. Five years later in 1990, he developed skin lesions on his back that were biopsied, and the histopathology was consistent with sarcoid. The skin lesions were treated with oral steroids and empirical ketoconazole with improvement. The skin lesions recurred in 1992 associated with an abnormal chest x-ray with interstitial infiltrates and hilar adenopathy. No lymph node biopsy was done. Cultures for coccy were negative and serology was intermittently positive. In 1995, he had a coccy recurrence, which was treated with fluconazole and no steroids. The coccy improved; however, the skin lesions recurred in 1996. He was then retreated with both steroids and fluconazole with improvement. The authors did not believe there was a relationship between the sarcoid and coccy, but the 2 were clinically intertwined over 10 years.
METHODS
Polymerase Chain Reaction Testing For Coccidioides Deoxyribonucleic Acid
Purpose
Polymerase chain reaction (PCR) testing for Coccidioides deoxyribonucleic acid (DNA) has recently become available [12]. Detecting Coccidioides DNA in archived tissue obtained at the time of a sarcoid diagnosis would provide additional evidence that Coccidioides could be etiologic in sarcoid.
Methods
The methodology for the molecular identification of Coccidioides DNA was performed as previously described [12]. Sarcoid tissue specimens were identified by chart review. Formalin-fixed, paraffin-embedded (FFPE) tissue sections from 14 patients diagnosed with sarcoid were tested for Coccidioides DNA using PCR. In addition, a FFPE specimen obtained from the lung biopsy tissue from case 2 was also tested.
Results
There were 15 FFPE tissue specimens available from Arizona patients diagnosed with sarcoid. All of the PCR tests for Coccidioides DNA on these specimens were negative. A positive control FFPE tissue with known Coccidioides was positive.
Discussion
Identifying Coccidioides DNA in the sarcoid diagnostic tissues would help to support a relationship between coccy and sarcoid. Of interest, the lung biopsy from Patient 2 with known coccy was also negative, suggesting that Coccidioides DNA can be sequestered somehow with sarcoid. In addition, using FFPE tissues specifically for PCR testing can reduce the sensitivity by approximately 30% [12].
DISCUSSION
The hypothesis at the beginning of this research was that an infection by the Arizona-endemic fungus, Coccidioides, could cause sarcoid. We have presented 2 patients, one studied prospectively and the other retrospectively, who were both diagnosed with sarcoid and coccy in Arizona. In addition, we found 5 other case reports of coccy and sarcoid, which were analyzed from the old literature (1955–1997) and also supported the study hypothesis. A conclusion reached early in the research was that sarcoid was not the result of a single etiologic agent. If Coccidioides caused sarcoid, there had to be other etiologies for sarcoid because that fungus had a very restricted area of endemicity, being found mainly in the southwestern United States. However, throughout the world, there are many systemic fungal agents, similar to Coccidioides, capable of causing a granulomatous immune response resulting in the noncaseating granulomas diagnostic of sarcoid. Systemic fungi such as Blastomyces [13], Cryptococcus [14], and Histoplasma [15] have all been reported as being associated with sarcoid, but none have been shown to be causative. However, the sheer number of independent reports between these fungi and sarcoid tends to support a relationship.
The strength of an etiologic association between the fungus Coccidioides and sarcoid is best illustrated in Patient 1. He was observed prospectively—meaning that based on previous clinical observations between coccy and sarcoid patients, it was predicted that he would develop coccy at some point after his sarcoid diagnosis. Studies that are prospective provide the best epidemiological information on the etiology of a disease [16]. At the time of his sarcoid diagnosis, there was no detectable evidence for coccy. After being on steroids for sarcoid a few months, the patient clinically improved. However, at the same time, his Coccidioides blood tests seroconverted from negative to positive with an IgM antibody. The latter result represents evidence of an acute subclinical infection with Coccidioides. It is remarkable that he was asymptomatic and clinically improving at the time of the seroconversion. It is presumed that there was an individual protective immunological response generated by this patient that was capable of preventing dissemination of the fungus. In general, patients with acute coccy, while being treated simultaneously with steroids, would be more likely to become symptomatic with clinical deterioration. However, this patient was asymptomatic despite steroids, felt well and was able to return to work. We hypothesize that this early symbiotic relationship between Coccidioides and sarcoid was the result of a cell-mediated immune (CMI) response unique to that patient, which enabled him to suppress any significant clinical evidence of coccy. However, he was able to generate a coccy humoral CF antibody response. Several years after the diagnosis of his sarcoid, the patient was again placed on steroids, and this time his coccy CF titers climbed to a 1:32 titer, which is most often only observed in patients with Coccidioides dissemination [5]. While on the steroids, his coccy CF titers continued at 1:32 for several years, without other obvious evidence of disseminated infection. Documentation of disseminated coccy was eventually confirmed when Coccidioides was recovered from his sternoclavicular joint 8 years after his sarcoid diagnosis.
Angiotensin-converting enzyme levels were also observed in this patient. Angiotensin-converting enzyme levels are often used to support the diagnosis of sarcoid and follow the response to therapy [17]. In the course of evaluating ACE levels in other diseases, approximately 70% of patients with leprosy were found to have elevated ACE levels [18]. In the latter study, there were also 13 sera from patients with coccy that were tested. One of the 13 coccy sera had a markedly elevated ACE at 98.9 U/mL. The reason for the elevated level in that 1 serum was unknown; however, the possibility of concomitant sarcoid with a Coccidioides infection was suggested by the authors. Elevated ACE levels have also been observed in histoplasmosis [19].
The highest ACE level over the course of Case Report 1 was 109 U/mL (Table 1) and associated with the highest coccy CF titer of 1:32, which occurred while he was on steroids. It is worth noting that 4 years later, off steroids, Coccidioides was cultured from his sternoclavicular joint, his coccy CF titer had dropped from 1:32 to 1:8, and the ACE concomitantly fell to 52 U/mL. This observation suggests that his immune response to coccy was waning and allowed the underlying fungal infection to manifest. These ACE observations in sarcoid and coccy have not been documented before and the mechanism is unknown. There has been a suggestion that as the ACE-secreting cells mature there is a lowering of ACE levels and a weakening of the CMI response [18].
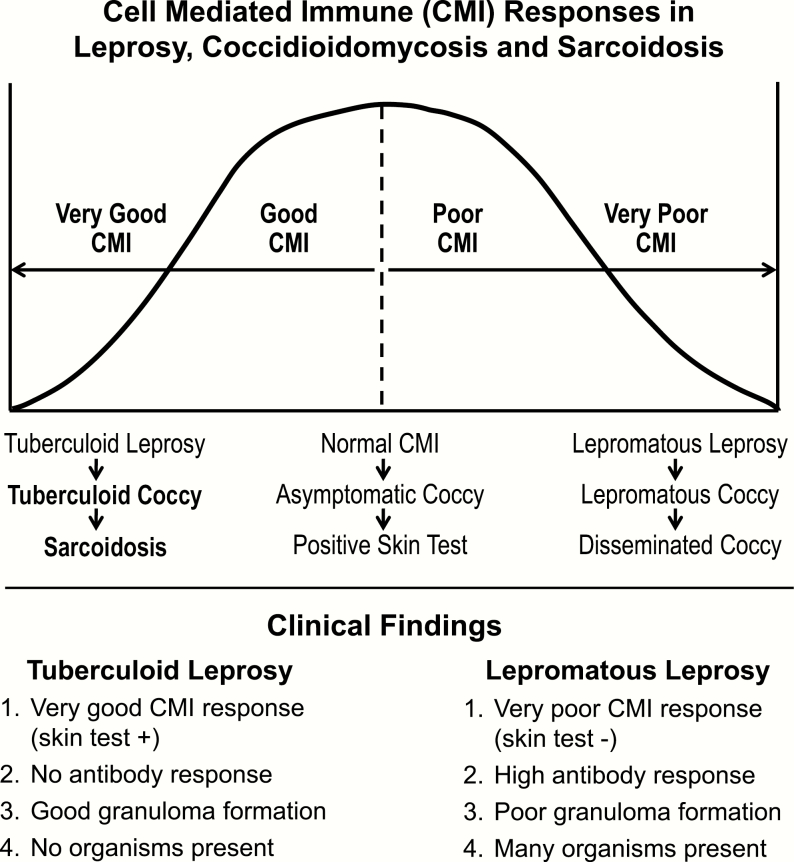
Based on our clinical observations, we hypothesize that “Coccidioides-induced” sarcoid is the result of a unique individual CMI response to the fungal infection. Figure 1 illustrates the bell-shaped curve that represents the range of the human CMI response in leprosy, the prototypical infectious disease used to illustrate the human CMI response to Mycobacterium leprae. In (Figure 1), Coccidioides has been substituted for leprosy to illustrate the presumed CMI responses to a Coccidioides infection and the genesis of sarcoid. The spectrum of human CMI responses to both M leprae and Coccidioides is likely genetically determined, and a genetic predisposition for developing sarcoid has been observed in families [20] and certain racial groups [21]. Basically, the spectrum of CMI responses goes from a very poor response, resulting in disseminated coccy, to those who have an exceptionally good response, representing the small unique subset of individuals who develop sarcoid. The immune response for the majority of Coccidioides-infected individuals falls into the middle of the CMI response curve. These are the majority of patients with coccy who tend to do well and resolve their infection without significant complications. The sarcoid/coccy patients are hypothesized to be those who are at the extreme “tuberculoid coccy” end of the immunologic bell-shaped curve. By comparison, those patients on the opposite “lepromatous coccy” end are those patients who have disseminated coccy, reflecting a poor CMI response.
Figure 1.
Hypothetical cell-meditated immune (CMI) response between leprosy, coccidioidomycosis and sarcoidosis.
To illustrate the differences in the CMI response between tuberculoid and lepromatous coccy, there are certain clinical factors that characterize the human CMI response to leprosy, which can be applied to the CMI response to coccy. There are 4 important clinical factors in the immune response to M leprae, or to a Coccidioides infection, and they are shown in (Figure 1). Patients on the lepromatous coccy end of the CMI spectrum tend to do poorly and develop disseminated coccy. In these patients, the infecting Coccidioides organisms are present in large numbers, the granulomatous response is poor, and their Coccidioides skin test reaction is poor. The latter results in a compensatory immune response with the production of humoral antibody, which in coccy is expressed by the development of CF antibodies. The presence of a CF antibody response in coccy is generally a gauge of the severity of the underlying infection and reflects a poor CMI response. In general, the higher the CF antibody titer, the worse the clinical disease.
Those unique individuals who develop an asymptomatic Coccidioides infection fall into the tuberculoid end of the CMI spectrum, presumptively these individuals are genetically predisposed to develop sarcoid. As a group, we hypothesize the tuberculoid coccy patients have a very good CMI response, which is expressed as sarcoid. Clinically, their infection is characterized by the following: (1) there are no organisms present; (2) the Coccidioides skin test is positive; (3) the granulomas are manifest as noncaseating; and (4) there is no compensatory humoral CF antibody response. By contrasting these 4 observations in patients at either the tuberculoid or lepromatous extremes of the human CMI response helps explain why a diagnosis of coccy could not be made at the time of the sarcoid diagnosis, even though the sarcoid response was the result of a Coccidioides infection. In previous studies, sarcoid complicated by coccy had been categorized as an opportunistic infection, but the expression of this infection, in this setting, is the result of CMI senescence, which eventually allows the infection to be expressed as illustrated by the clinical course of Patient 1.
In a different clinical situation, Patient 2 had documented disseminated coccy for over 3 years when he developed respiratory failure requiring the intensive care unit and a ventilator. During the latter, he had a lung biopsy that was consistent with sarcoid. The clinical setting of Patient 2 suggests that a tuberculoid coccy response can take place in a patient who has documented coccy, and their immune system is able to evolve with no obvious evidence of a Coccidioides infection. Of note, the PCR test for Coccidioides DNA was negative in Patients 2’s lung tissue biopsy despite a 3-year history of active disseminated coccy. The mechanisms for how this can occur is unclear, but a very similar clinical scenario was described in 1955 in Case Report 3 [7].
We were unable to demonstrate Coccidioides DNA in the biopsy tissues obtained from Arizona sarcoid patients at the time of their diagnosis. If Coccidioides DNA had been demonstrated in the tissues of these patients, it would have helped to substantiate that Coccidioides was etiologic. However, the absence of DNA does not negate the association between Coccidioides and sarcoid. In patients with tuberculoid leprosy (also known as paucibacillary leprosy), it can be difficult to detect the DNA of the causative agent M leprae [22]. Similar things may be occurring in tuberculoid coccy, contributing to the difficulty in recognizing the pathogenesis of sarcoid as being due to coccy (or any other agent).
Evidence has been presented that suggests Coccidioides can cause sarcoid. However, what about the patients with sarcoid outside the endemic area for Coccidioides? In 1952, there was a report of an association between Histoplasma capsulatum infections and sarcoid [15]. The authors assumed that the sarcoid was due to Histoplasma and went on to do another investigation of 22 sarcoid patients living outside the endemic area for histoplasmosis. The latter studies were negative for any obvious relationship to Histoplasma. Over the years, a potential relationship between Histoplasma and sarcoid is well known in the endemic area [23]. The similar observations made with both Histoplasma and Coccidioides suggest an etiologic connection may exist between sarcoid and at least those 2 systemic fungi. It is presumed that the mechanisms for how these systemic fungi are capable of triggering a sarcoid response would be similar.
CONCLUSIONS
Have we established a cause and effect between Coccidioides and sarcoid? It is clear from the clinical observations that sarcoid histopathology can occur with a Coccidioides infection. The data from the prospectively studied Patient 1 significantly impacts the sarcoid etiology question. Even though he had no evidence of coccy at the sarcoid diagnosis, he developed an asymptomatic seroconversion to coccy shortly after starting steroids. In theory, he could have been infected by Coccidioides in the few weeks between the sarcoid diagnosis and when the serologic evidence for the infection appeared. However, the beauty of a prospective study is that it was hypothesized he would have evidence of coccy at some point in his sarcoid course and it happened. In addition, the seroconversion to coccy was asymptomatic, which would be very unusual in a patient with acute coccy on steroids. In keeping with our hypothesis, perhaps the use of steroids triggered the asymptomatic seroconversion and the appearance of coccy antibody. Had Patient 1 not been observed and tested prospectively for evidence of an asymptomatic Coccidioides infection, it would not have been documented. Proof of actual disseminated coccy was not demonstrated until 8 years later when the organism was cultured from a sternoclavicular joint. Documentation of the clinical course prospectively in this Arizona sarcoid patient was quite revealing and illustrated what can happen in a patient with sarcoid due to coccy. It is worth noting that the course of Patient 1 was comparable to that of Patient 5, where there was a 10-year difference between the diagnosis of sarcoid and the expression of disseminated coccy [9]. The long time periods between the sarcoid diagnosis and expression of coccy tends to help explain why finding the cause of sarcoid has been so difficult to establish. There were 8 and10 years, respectively, between the sarcoid diagnosis and the expression of coccy in those 2 patients. In contrast, Patient 2 was studied retrospectively, illustrating that sarcoid histopathology, with no organisms, a negative Coccidioides DNA by PCR, and a negative coccy CF serology can also be seen in patients with a prior documented Coccidioides infection. Information from all 7 patients provides a strong basis for further studies on the relationship between coccy and sarcoid. The clinical observations made on Patients 1 and 2 were supported by the historical information obtained from the 5 case reports of coccy and sarcoid that were documented in the medical literature between 1955 and 1997 [7–11]. The sarcoid purists will say we have not defined the cause of sarcoid; however, we believe we have clinically demonstrated that coccy can cause sarcoid, but we have not determined the mechanisms that result in the sarcoid pathophysiology.
Acknowledgments
We thank Dr. Demosthenes Pappagianis (Department of Medical Microbiology and Immunology, University of California, Davis) for encouragement and review of our work.
Financial support. This work was funded by an unrestricted grant from the University of Arizona Foundation (to T. K. and I. Y., 2013) as research support for the Scholarly Project of I. Y. (then a first-year medical student) with T. K. as mentor.
Potential conflicts of interest. Both authors: no reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Boeck C. Multiple benign sarcoid of the skin. Arch Dermatol 1982; 118:711–20. [DOI] [PubMed] [Google Scholar]
- 2. Chen ES, Moller DR. Etiologic role of infectious agents. Semin Respir Crit Care Med 2014; 35:285–95. [DOI] [PubMed] [Google Scholar]
- 3. Kuberski T. Coccidioidomycosis as a cause of sarcoidosis in Arizona. Proceedings of the Forty-sixth Annual Coccidioidomycosis Study Group Meeting.Davis, CA: April 6, 2002. (Abstract: 19). [Google Scholar]
- 4. Yourison I, Kuberski T. Clinical evidence for coccidioidomycosis as an etiology for sarcoidosis. Proceedings of the Fifty-seventh Annual Coccidioidomycosis Study Group Meeting.Pasadena, CA: April 6, 2013. (Abstract: 6) . [Google Scholar]
- 5. Smith CE, Saito MT, Simons SA. Pattern of 39,500 serologic tests in coccidioidomycosis. J Am Med Assoc 1956; 160:546–52. [DOI] [PubMed] [Google Scholar]
- 6. Yourison I, Kuberski T. Sarcoid histopathology in coccidioidomycosis. Proceedings of the Fifty-ninth Annual Coccidioidomycosis Study Group Meeting. San Diego, CA: April 11, 2015. (Abstract: 3) . [Google Scholar]
- 7. Ellis FW. Coexistent arrested disseminated coccidioidomycosis and Boeck’s sarcoid. Calif Med 1955; 82:400–4. [PMC free article] [PubMed] [Google Scholar]
- 8. Bacharach T, Zalis EG. Sarcoid syndrome associated with coccidioidomycosis. Am Rev Respir Dis 1963; 88:248–51. [DOI] [PubMed] [Google Scholar]
- 9. Lipschultz BM, Liston HE. Steroid induced disseminated coccidioidomycosis. Report of two cases. Dis Chest 1964; 46:355–9. [DOI] [PubMed] [Google Scholar]
- 10. Lord GP. Pulmonary sarcoidosis complicated by cryptococcosis and coccidioidomycosis. J Maine Med Assoc 1974; 65:236–40. [PubMed] [Google Scholar]
- 11. Sharma OP, Arora A. Coccidioidomycosis and sarcoidosis. Multiple recurrences. West J Med 1997; 166:345–7. [PMC free article] [PubMed] [Google Scholar]
- 12. Binnicker MJ, Buckwalter SP, Eisberner JJ, et al. et al. Detection of Coccidioides species in clinical specimens by real-time PCR. J Clin Microbiol 2007; 45:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiatt JS, McCain MD, Lide TN. Blastomycosis complicating Boeck’s sarcoid. Report of a case. NC Med J 1949; 10:650–6. [PubMed] [Google Scholar]
- 14. Bernard LA, Owens JC. Isolated cryptococcosis associated with Boeck’s sarcoid. Report of a case treated with amphotericin B. Arch Intern Med 1960; 106:101–11. [DOI] [PubMed] [Google Scholar]
- 15. Israel HL, DeLamater E, Sones M, et al. et al. Chronic disseminated histoplasmosis. An investigation of its relation to sarcoidosis. Am J Med 1952; 12:252–60. [DOI] [PubMed] [Google Scholar]
- 16. Nagurney JT, Brown DF, Sane S, et al. et al. The accuracy and completeness of data collected by prospective and retrospective methods. Acad Emerg Med 2005; 12:884–95. [DOI] [PubMed] [Google Scholar]
- 17. Lieberman J. Elevation of serum angiotensin-converting enzyme (ACE) level in sarcoidosis. Am J Med 1975; 59:365–72. [DOI] [PubMed] [Google Scholar]
- 18. Lieberman J, Rea TH. Serum angiotensin-converting enzyme in leprosy and coccidioidomycosis. Ann Intern Med 1977; 87:423–5. [DOI] [PubMed] [Google Scholar]
- 19. Ryder KW, Jay SJ, Kiblawi SO, Hull MT. Serum angiotensin converting enzyme activity in patients with histoplasmosis. JAMA 1983; 249:1888–9. [PubMed] [Google Scholar]
- 20. Rybicki BA, Iannuzzi MC, Frederick MM, et al. et al. Familial aggregation of sarcoidosis. Am J Respir Crit Care Med 2001; 164:2085–91. [DOI] [PubMed] [Google Scholar]
- 21. Rybicki BA, Major M, Popovich J, et al. et al. Racial differences in sarcoidosis: a 5-year study in a health maintenance organization. Am J Epidemiol 1997; 145:234–41. [DOI] [PubMed] [Google Scholar]
- 22. Martinez AN, Britto CF, Nery JA, et al. et al. Evaluation of real-time and conventional PCR targeting complex 85 genes for detection of Mycobacterium leprae DNA in skin biopsy samples from patients diagnosed with leprosy. J Clin Microbiol 2006; 44:3154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wheat LJ, French ML, Wass JL. Sarcoidlike manifestations of histoplasmosis. Arch Intern Med 1989; 149:2421–6. [PubMed] [Google Scholar]