Abstract
Dental caries is prevalent, and secondary caries causes restoration failures. This article reviews recent studies on developing a new generation of bioactive resins with anticaries properties. Extensive effects were made to develop new antimicrobial composites, bonding agents, and other resins containing quaternary ammonium methacrylates to suppress plaque buildup and bacterial acid production. The effects of alkyl chain length and charge density and the antimicrobial mechanisms for chlorhexidine, nano-silver, quaternary ammonium methacrylates, and protein-repellent agents were discussed. Synergistic effects of contact-killing and protein-repellent properties were shown to yield the greatest biofilm-inhibition effects. The combination of antimicrobial, protein-repellent, and calcium phosphate nanoparticle remineralization was suggested to provide maximal anticaries effects. In addition, for use orally, cytotoxicity and biocompatibility were important considerations for the new bioactive materials. Furthermore, rather than kill all bacteria, it would be more desirable to modulate the oral biofilm compositions via bioactive resins to suppress cariogenic/pathogenic species and promote benign species. For widespread clinical use of the new antimicrobial and therapeutic materials, whether they would induce bacterial drug resistance needs to be determined, which requires further study. Nonetheless, the new generation of bioactive anticaries resins with therapeutic and biofilm acid-inhibiting properties has the potential to substantially benefit oral health.
Keywords: anti-caries, antibacterial, composites and adhesives, therapeutic, remineralization, modulating biofilm compositions
Introduction
Acidogenic bacteria with fermentable carbohydrates are responsible for caries (Zaura and ten Cate 2015). Secondary caries is a main reason for restoration failures (Sakaguchi 2005). The replacement of failed restorations accounts for half of all restorations placed (Jokstad et al. 2001). Therefore, it is beneficial to develop new materials to reduce/eliminate bacterial acids. Restorative materials interact with oral biofilms; therefore, it is promising for new materials to have functionalities/therapeutic capabilities. These functionalities include 1) inhibiting biofilm adhesion, 2) discouraging biofilm growth, 3) affecting biofilm metabolism, and 4) adjusting microbial ecosystem (Bayne et al. 2013). Meritorious reviews on composites, bonding agents, and antimicrobial materials are available (Imazato 2003; Spencer et al. 2010; Ferracane 2011; Pashley et al. 2011; Imazato et al. 2012; Ferracane and Giannobile 2014). The present review focuses on the development of a new generation of bioactive resins with antimicrobial, remineralizing, and biofilm-modulating functions.
Antimicrobial Resin Composites
Composites are increasingly used due to their aesthetics and direct filling capabilities (Ferracane 2011; Bayne et al. 2013). However, composites accumulate more biofilms and plaque, with acid production leading to recurrent caries at the margins (Beyth et al. 2007; Cocco et al. 2015). Indeed, secondary caries is a main reason for the failure of composite restorations (Jokstad et al. 2001). Antimicrobial agents in resins include releasing and nonreleasing agents. Chlorhexidine (CHX) and silver (Ag) particles in resins were releasing agents in various studies (Cheng et al. 2011; Fan et al. 2011; Cheng et al. 2012; Cheng et al. 2015). A method was developed to encapsulate and release CHX from a composite with mesoporous silica nanoparticles (Zhang et al. 2014). An antimicrobial mechanism of CHX was suggested—namely, that when bacterial cells contact CHX, the outer cell membrane of the bacteria is rapidly damaged, although the damage is insufficient to induce cytolysis or apoptosis. CHX then traverses the outer membrane of the bacterial cell, presumably by passive diffusion, and subsequently attacks the bacterial cytoplasmic or inner cell membrane. This damages the semipermeable membrane, followed by leakage of the cytoplasm, leading to apoptosis. Indeed, low concentrations of CHX affected the bacterial cell membrane integrity, while high concentrations of CHX caused the congealing of the cytoplasm (McDonnell and Russell 1999).
Ag has also been incorporated into resins. A study reported sol-gel bioglass containing Ag, which showed antimicrobial activity against Escherichia coli and Streptococcus mutans (Chatzistavrou et al. 2014). Nanoparticles of Ag (NAg) possessed potent antimicrobial properties due to their small sizes and high surface areas (Cheng et al. 2012). NAg were incorporated into resins, which greatly reduced biofilm growth without negatively affecting the bond strength and material color (Cheng et al. 2012). Regarding the antibacterial mechanism, it was suggested that Ag ions could inactivate the vital enzymes of bacteria to cause the bacterial DNA to lose its replication ability, leading to cell death (Morones et al. 2005).
A limitation of these releasing systems is the uncontrolled burst release lasting for only a short time. This shortcoming could be overcome via covalently bonded (“immobilized” and “nonreleasing”) agents. Nonreleasing antimicrobial agents are immobilized in resins (Imazato 2003; Imazato et al. 2012). This is achieved by copolymerizing quaternary ammonium methacrylates (QAMs) in the resin matrix. 12-Methacryloyloxydodecyl-pyridinium bromide (MDPB) was copolymerized in the resin to provide durable contact inhibition against bacteria (Imazato 2003). Other researchers reported quaternary ammonium polyethylenimine nanoparticles (Beyth et al. 2006; Beyth et al. 2010), methacryloxyethyl cetyl dimethyl ammonium chloride–containing adhesive (Li et al. 2009), antimicrobial glass ionomer cements (Xie et al. 2011), antimicrobial composites (Xu et al. 2012), and antimicrobial nanocomposite and adhesive (Cheng et al. 2012). The antimicrobial mechanism of quaternary ammonium salts is via their binding to cell membranes, which causes cytoplasmic leakage (Imazato 2003; Beyth et al. 2006). When the negatively charged bacterial cell contacts the positively charged (N+) sites of a QAM resin, the electric balance of the cell membrane could be disturbed, and the bacterium could explode under its own osmotic pressure (Imazato 2003; Beyth et al. 2006). Recently, a series of QAMs with alkyl chain lengths of 3, 6, 12, 16, and 18 were synthesized (Li, Weir, and Xu 2013). For short-chained quaternary ammonium, the antimicrobial activity appeared to rely on positively charged ammonium groups coupling with negatively charged bacterial membranes to disrupt membrane functions, alter the balance of essential ions (i.e., K+, Na+, Ca2+, and Mg2+), interrupt protein activity, and damage bacterial DNA (Simoncic and Tomsic 2010). Increasing chain length reduced the metabolic activity and acid production of saliva-derived microcosm biofilms. Dimethylaminohexadecyl methacrylate (DMAHDM) with chain length 16 showed the strongest antimicrobial potency, as shown in Figure 1 (Zhang, Cheng, et al. 2016). This mechanism was likely a result of the long-chained QAM having double-killing effects: 1) the positively charged ammonium groups and 2) the additional antimicrobial activity by the long alkyl chain inserting into bacterial membranes, resulting in disruption of bacterial cells (Simoncic and Tomsic 2010; Li, Weir, and Xu 2013). Regarding long-term durability, a recent study tested antibacterial composites after water aging for 1 d and 3, 6, 9, and 12 mo (Cheng et al. 2016). After each water-aging time, the antibacterial properties of the composites were tested via a dental plaque biofilm model with human saliva as inoculum. The antibiofilm activity was maintained after 12 mo of water aging, showing no significant decrease with increasing time (P > 0.1). However, tests lasting >1 y are still lacking. Hence, the assumption that covalently immobilized or nonreleasing agents are indeed not being released has not been strictly verified in the long term. It is possible that some compounds in covalently bonded resins may be released in small quantities after exposure to enzymes and oxidative agents from saliva and bacteria.
Figure 1.
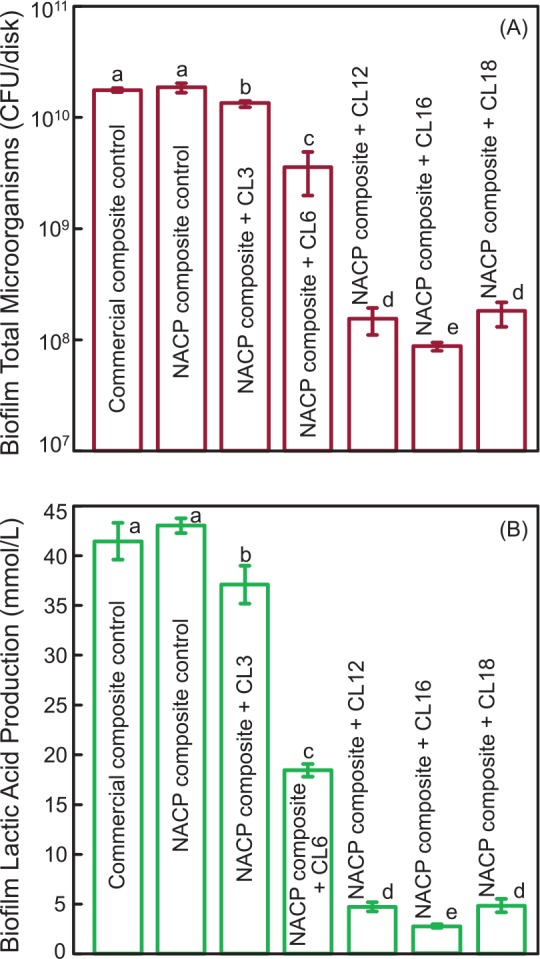
Effects of alkyl chain length (CL) of quaternary ammonium methacrylates in composite on biofilms: (A) colony-forming units (CFU) of 2-day biofilms on composite; (B) lactic acid production (mean ± SD; n = 6). In each plot, values with dissimilar letters are significantly different (P < 0.05). Note the log scale for the y-axis (A). NACP, nanoparticles of amorphous calcium phosphate. (Adapted from Zhang, Cheng, et al. 2016, with permission.)
One limitation of QAM-containing resins is that salivary protein coating on the resin surface would reduce the efficacy of “contact killing” of QAM by reducing the contact of bacteria with the resin (Imazato 2003). Therefore, it would be beneficial to develop protein-repellent resins. This could produce 2 benefits. First, by reducing salivary protein coating on resin, the QAM sites are exposed to bacteria to enhance contact killing. Second, during biofilm formation, protein adsorption on the surface is a prerequisite for bacteria to attach (Donlan and Costerton 2002). Therefore, protein-repellent resins would make it more difficult for bacteria to attach. A previous study showed that poly(ethylene glycol) and methacrylate monomers with pyridinium groups were immobilized to produce protein-repellent functions (Muller et al. 2009). Another approach used 2-methacryloyloxyethyl phosphorylcholine (MPC), which is a methacrylate with a phospholipid polar group (Lewis 2000). The protein-repellent mechanism is related to the fact that hydrophilic material surfaces usually have less protein adsorption than do hydrophobic surfaces (Cheng et al. 2007). MPC is a methacrylate with a phospholipid polar group in the side chain (Ishihara et al. 1998). Phospholipids are a major component of all cell membranes, and they can form lipid bilayers (Mashaghi et al. 2013). Phospholipid molecules consist of hydrophilic heads and hydrophobic tails. When placed in water, phospholipids will orient themselves into a bilayer in which the nonpolar tail faces the inner area and the polar head faces outward and interacts with water. MPC polymers are hydrophilic and, when in the hydrated state, have an abundance of free water but no bound water. The presence of bound water would cause protein adsorption (Ishihara et al. 1998). However, the large amount of free water around the phosphorylcholine groups is considered to detach proteins effectively, thereby repelling protein adsorption (Ishihara et al. 1998). Indeed, MPC has been shown to have strong protein repellency and is used in artificial blood vessels, artificial hip joints, and microfluidic devices. Recently, MPC was copolymerized in a dental composite that became protein repellent (Zhang, Ma, et al. 2015). It had protein adsorption that was only 1/10 that of a commercial composite. Indeed, a synergistic effect was achieved when MPC and DMAHDM were both used in the resin by repelling salivary proteins from resin, thereby increasing the contact inhibition efficacy of DMAHDM (Zhang, Ma, et al. 2015). This synergy via MPC and DMAHDM achieved much stronger biofilm inhibition effects than either MPC or DMAHDM alone when tested via a dental plaque microcosm biofilm model with human saliva as inoculum (Zhang, Ma, et al. 2015).
Antimicrobial Dental Bonding Agents
Composite restorations are bonded to tooth structures via adhesives. The interface is the weak link, often with microgaps trapping biofilms, producing acids, and leading to secondary caries (Spencer et al. 2010; Pashley et al. 2011). Efforts were made to improve the chemical compositions of bonding agents and increase the bond strength (Spencer et al. 2010; Van Meerbeek et al. 2011). In addition, antibacterial bonding agents were synthesized to eradicate 1) the residual bacteria in the tooth cavity and 2) the new invading bacteria at the margins, demonstrating antibacterial activity without compromising the bond strength (Imazato 2003). Adhesives containing dimethylaminododecyl methacrylate (DMADDM) inhibited the growth, acid, and exopolysaccharides of S. mutans biofilms, with no adverse effect on dentin bond strength (Wang et al. 2014).
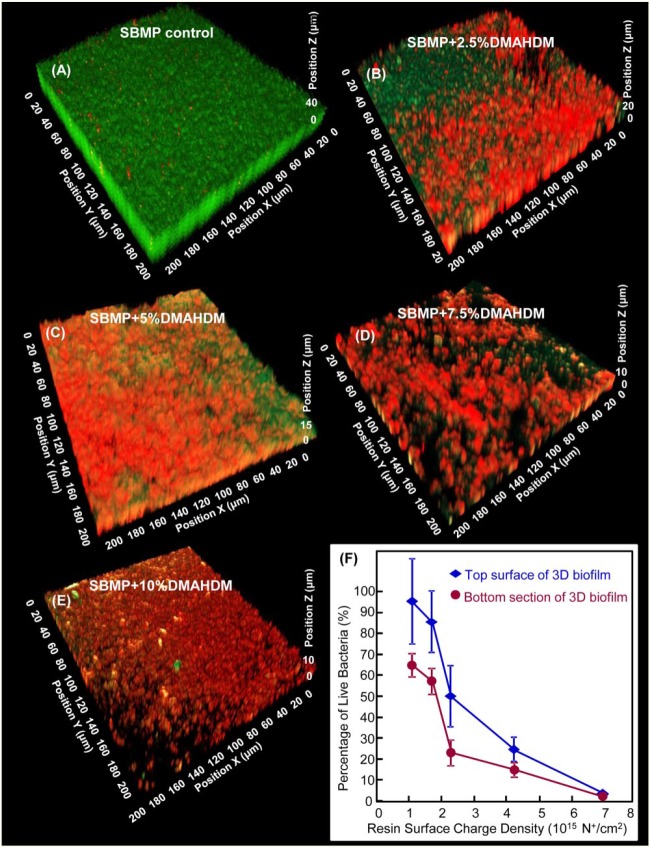
The effects of quaternary amine charge density were investigated for bonding agents with 3-dimensional biofilms of S. mutans with confocal laser scanning microscopy (Zhou et al. 2016). Biofilm thickness and volume decreased with increasing charge density. With 10% DMAHDM, which produced a resin charge density of approximately 7 × 1015 N+/cm2, bacteria in the entire 3-dimensional biofilm were killed, including bacteria away from resin surface. This yielded a live-bacteria percentage of nearly 0% throughout the biofilm, as shown in Figure 2 (Zhou et al. 2016). Therefore, the charge density is an important parameter for antibacterial resins because a higher DMAHDM concentration in resin produced more positive charges to interact with bacteria, thereby providing a stronger antimicrobial function. The resin with 10% DMAHDM killed the entire 3-dimensional biofilm, likely due to the following reason. Previous studies indicated a stress condition or challenge in bacteria that could trigger a built-in suicide program—that is, programmed cell death (PCD)—in the biofilm (Gerdes et al. 2005; Murata et al. 2007). A biofilm challenged by antimicrobial agents may trigger PCD in the neighboring bacteria. Indeed, oral biofilms on a QAM composite were killed not only on resin surface via contact inhibition but also in the outer, more remote parts of the biofilm away from resin surface (Beyth et al. 2010). These results were possibly due to an intracellularly mediated death program, in which the bacterial lysis by QAM on resin may function as a stressful condition triggering PCD to bacteria farther away in the biofilm. Furthermore, this mechanism of an intracellularly mediated death program to trigger PCD to the bacteria farther away in the biofilm may be dependent on resin charge density. Figure 2 suggests that only when a minimum charge density was exceeded would this PCD be triggered. The reason is that SBMP + 10% DMAHDM resulted in the death of the entire biofilm, while SBMP + 7.5% DMAHDM had a biofilm that was almost entirely dead, and SBMP + 2.5% DMAHDM and SBMP + 5% DMAHDM had biofilms that were mostly alive in areas away from resin surface (Zhou et al. 2016). Further studies are needed to investigate the bioactive resin-biofilm interactions and evaluate antimicrobial bonding agent in vivo.
Figure 2.
Relationship between 3-dimensional (3D) biofilms and resin charge density. Confocal laser scanning microscopy image of 3D biofilms cultured for 2 d on (A) Scotchbond Multi-Purpose (SBMP; 3M), (B) SBMP + 2.5% dimethylaminohexadecyl methacrylate (DMAHDM), (C) SBMP + 5% DMAHDM, (D) SBMP + 7.5% DMAHDM, (E) SBMP + 10% DMAHDM. The x- and y-axes are parallel to resin surface. The z-axis is perpendicular to resin surface. Live bacteria were stained green. Dead bacteria were stained red. (F) Percentage of live bacteria in 3D biofilm versus resin quaternary amine charge density, which was increased with increasing the percentage of DMAHDM in resin. The top and bottom biofilm surfaces were both plotted (mean ± SD; n = 6). (Adapted from Zhou et al. 2016, with permission.) This figure is available in color online.
Root Restorations That Can Inhibit Some Periodontal Pathogens
Another area of need is that, as the world population ages, there is an increasing incidence of root caries among the elderly who retain more of their teeth; however, older people are at a higher risk for root caries (Curzon and Preston 2004). Gingival recession leads to more exposure of tooth root surfaces, which have a higher solubility to biofilm acids than coronal enamel. Indeed, root caries occurrence in the United States increased rapidly with age, from 7% among young people to 56% in seniors >75 y of age (Curzon and Preston 2004). Class V restorations with subgingival margins are difficult to clean and may provide pockets for periodontal bacterial growth. Therefore, there is a need to develop bioactive composites and adhesives for class V restorations that can inhibit cariogenic and periodontal pathogens.
Two promising approaches were recently reported. The first involved the development of a bioactive class V composite. The resin matrix consisted of ethoxylated bisphenol A dimethacrylate and pyromellitic glycerol dimethacrylate, which could recharge and re-release calcium and phosphate ions. Triple agents (DMAHDM, MPC, and nanoparticles of amorphous calcium phosphate [NACP]) were incorporated into resin (Wang et al. 2016). Four periodontitis-related species were tested: Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum. The composite with 3% DMAHDM and 3% MPC achieved a potent antimicrobial efficacy, with much greater reduction in biofilms than DMAHDM or MPC alone. Biofilm CFU was reduced by 4 orders of magnitude via 3% DMAHDM + 3% MPC, as shown in Figure 3 (Wang et al. 2016). Therefore, this composite is promising for class V restorations, especially with subgingival margins to inhibit periodontal pathogens, combat periodontitis, and protect the periodontium (Wang et al. 2016).
Figure 3.
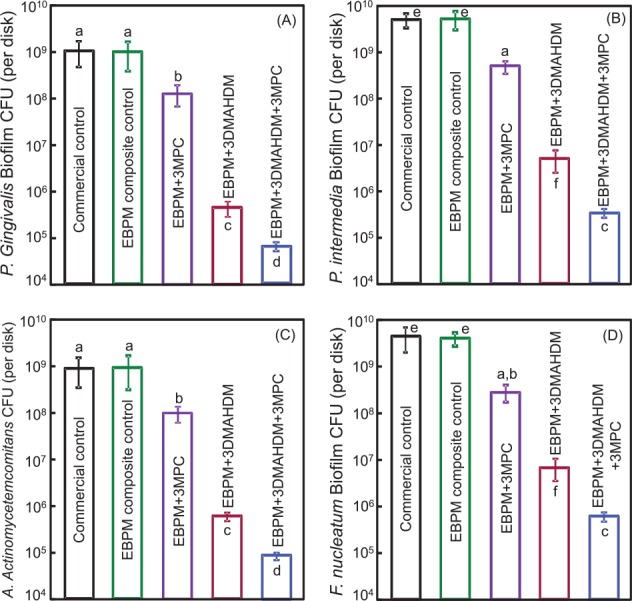
Colony-forming units (CFU) of 2-day biofilms on composites for 4 periodontitis-related species: (A) Porphyromonas gingivalis, (B) Prevotella intermedia, (C) Aggregatibacter actinomycetemcomitans, and (D) Fusobacterium nucleatum (mean ± SD; n = 6). Note the log scale for the y-axis. Bars with dissimilar letters are significantly different (P < 0.05). 3MPC, the composite contained 3% MPC (2-methacryloyloxyethyl phosphorylcholine); 3DMAHDM, the composite contained 3% DMAHDM (dimethylaminohexadecyl methacrylate); EBPM, the resin consisted of ethoxylated bisphenol A dimethacrylate and pyromellitic glycerol dimethacrylate. (Adapted from Wang et al. 2016, with permission.)
In the second approach, a bioactive adhesive was formulated containing MPC, DMAHDM, and NACP to seal the exposed root dentin where cementum was lost, by forming a resinous coating to protect root surfaces, remineralize, and neutralize acids (Zhang, Melo, et al. 2015). The resin containing triple agents had 95% reduction in protein adsorption, a strong bond strength to dentin, and a potent antimicrobial function, with biofilm colony-forming units (CFU) being 4 log lower than commercial control. Therefore, this bioactive adhesive is promising to coat the exposed roots to remineralize and inhibit plaque acids (Zhang, Melo, et al. 2015). These results indicate a promising trend of using bioactive and therapeutic resins for treating root caries. The new bioactive resins have the potential to not only inhibit cariogenic bacterial acid production and protect the roots but also suppress periodontal pathogens, combat periodontitis, and protect the periodontium.
Antimicrobial and Remineralization Combination
Two main strategies to combat caries are suppressing biofilm acids and promoting remineralization. Therefore, it would be ideal to develop resins by combining both these benefits. This can be achieved by incorporating calcium phosphate particles with antibacterial agents into resins. Amorphous calcium phosphate composite could release calcium and phosphate ions and remineralize lesions (Langhorst et al. 2009). However, composites containing traditional calcium phosphate particles were mechanically weak and could not be used as bulk restoratives. NACP were incorporated into resin with high levels of calcium and phosphate ion release and mechanical properties matching those of commercial composite controls (Xu et al. 2011). NACP were incorporated into an adhesive containing 5% DMADDM (Chen et al. 2014). Increasing NACP filler level from 10% to 40% in adhesive increased the calcium and phosphate ion release by an order of magnitude, without compromising the dentin bond strength. The adhesive not only had calcium and phosphate ions for remineralization but also strong antimicrobial activities to suppress biofilm acid production when tested via a dental plaque microcosm biofilm model with human saliva as inoculum (Chen et al. 2014).
The dual-agent combination recipe was expanded to include triple and quadruple agents to further increase the anticaries capability of resins. Melo et al. (2016) incorporated 3 agents into composites, including 2 antibacterial agents (NAg, DMAHDM) and a remineralization agent (NACP), for better anticaries effects than through combinations of 2 agents. In another study, quadruple agents (MPC, DMAHDM, NAg, NACP) were incorporated into an resin-modified glass ionomer (Zhang, Weir, et al. 2016). Increasing the filler level of NACP increased calcium and phosphate ion release. Adding MPC into a resin-modified glass ionomer reduced protein adsorption by an order of magnitude. By adding DMAHDM and NAg together, biofilm CFU was 3 log less than commercial control when tested via a dental plaque microcosm biofilm model with human saliva as inoculum (Zhang, Weir, et al. 2016). While further studies are needed, the method of incorporating multiple bioactive agents into resins may have wide applicability to dental cements, sealants, binding agents, and composites to inhibit caries.
Cytotoxicity and Biocompatibility
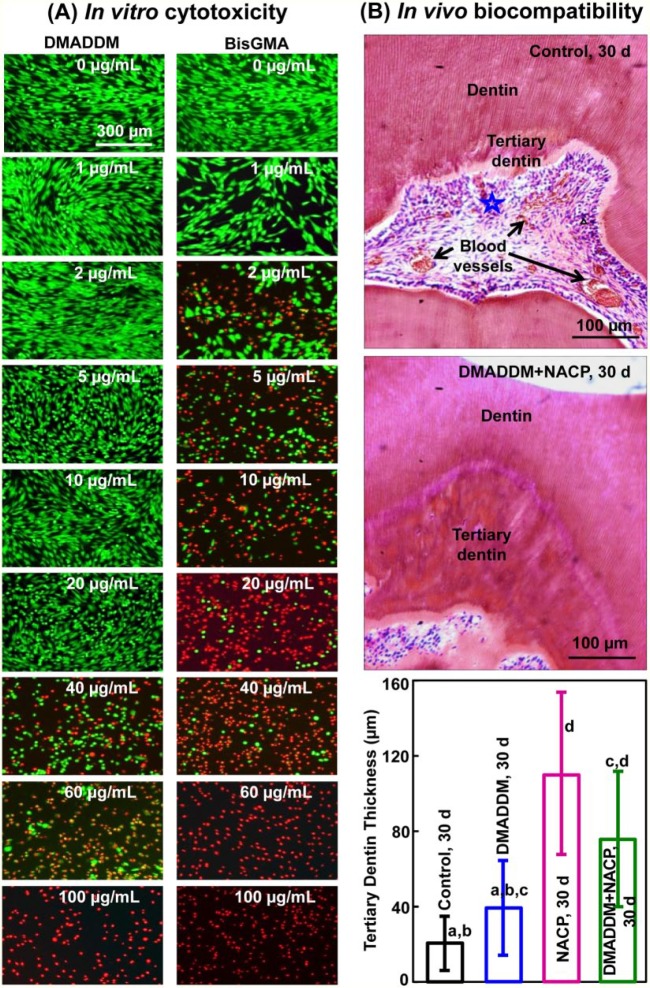
Investigation of the toxicity of the antimicrobial monomers and resins is a prerequisite before their use in oral restorations. MDPB exhibited a low level of toxicity to human pulpal cells, similar to triethyleneglycol dimethacrylate (Imazato et al. 1999). The incorporation of MDPB into a primer did not increase the toxicity on pulpal cells (Imazato et al. 2000). The inhibitory effects of MDPB on the proliferation and mineralization of odontoblast-like MDPC-23 mouse cells were lower than those of bisphenol A glycidyl methacrylate (BisGMA), indicating that MDPB had better biocompatibility than BisGMA (Nishida et al. 2010). For DMADDM, its median lethal concentration was between 20 and 40 μg/mL, 20-fold higher than that of BisGMA control, indicating a much lower cytotoxicity than BisGMA (Li, Weir, Fouad, et al. 2013). This is consistent with the live/dead results in vitro showing that DMADDM had less cell cytotoxicity than BisGMA (Fig. 4A; Li, Weir, Fouad, et al. 2013).
Figure 4.
Cytotoxicity and biocompatibility. (A) Representative live/dead images of human gingival fibroblasts in vitro cultured in medium containing monomers. The top titles indicate the monomers; the various monomer concentrations in the culture medium are labeled in the images. Live cells were stained green, and dead cells were stained red. Dimethylaminododecyl methacrylate (DMADDM) had less cell cytotoxicity than bisphenol A glycidyl methacrylate (BisGMA). (B) Composite restorations in rat teeth in vivo at 30 d for control restoration without DMADDM and nanoparticles of amorphous calcium phosphate (NACP) versus DMADDM + NACP group. The star indicates an area with inflammatory cells. Blood vessels are indicated by arrows. Control group had slight inflammatory responses. NACP and DMADDM + NACP showed normal pulp tissue without inflammatory response at 30 d and generated greater tertiary dentin thickness. Values are presented as mean ± SD; n = 6. Values indicated by dissimilar letters are significantly different from each other (P < 0.05). (Adapted from Li, Weir, Fouad, et al. 2013 and Li et al. 2014, with permission.) This figure is available in color online.
With in vivo studies, Imazato et al. (2004) performed restorations in dog teeth and examined pulp response histopathologically after 7, 30, and 75 d. They found no pulpal inflammation for experimental primer containing MDPB, in comparison with mild to moderate inflammation for a control group with no MDPB. Antimicrobial adhesive was proposed for pulp care because incorporation of MDPB in adhesives did not adversely affect the biocompatibility of the original material, due to the fact that MDPB had superior biocompatibility to conventional dental monomer BisGMA (Imazato et al. 2012). These results are consistent with a recent study with composite and adhesive containing DMADDM and NACP in rat teeth (Li et al. 2014). DMADDM and NACP groups exhibited milder pulpal inflammation and much greater tertiary dentin formation than did control without DMADDM and NACP (Fig. 4B). These results demonstrate that the bioactive composite and adhesive containing DMADDM and NACP not only can suppress oral pathogens and biofilm acids but also have good biocompatibility and can facilitate the healing of the dentin-pulp complex.
Modulating Biofilm Compositions via Bioactive Resins to Suppress Cariogenic Species
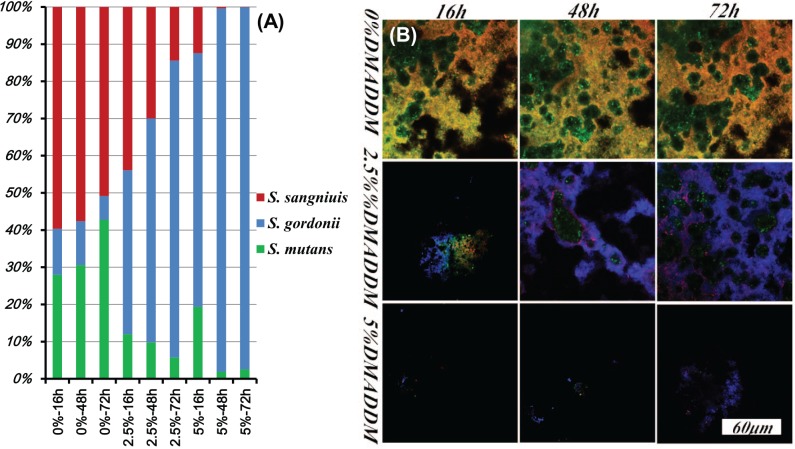
While suppressing biofilm growth and acid production represents one approach to combat caries, another approach aims to modulate the biofilm species toward a healthy composition, instead of killing all bacteria. Several studies investigated how bioactive materials could influence the compositions of biofilm species (Zhang, Wang, et al. 2015; Zheng et al. 2015). Fluoride is a dual agent that can reduce bacterial metabolism and promote remineralization. A 3-species biofilm model showed that fluoride/arginine could prevent the overgrowth of periodontal pathogen Porphyromonas gingivalis and reverse the S. mutans–S. sanguinis equilibrium toward a more ecologically healthy ratio. Although the exact mechanism of this behavior is not clear, it is possibly due to the action of influencing the microbial production of acidic and oxidative metabolites (e.g., H2O2; Zheng et al. 2015). Another study investigated the antibiofilm effects of DMADDM-containing adhesives with a multiple-species model with S. mutans, S. gordonii, and S. sanguinis (Zhang, Wang, et al. 2015). The proportion of S. mutans decreased in DMADDM-containing groups and increased in control group, and the biofilm had a healthier development via the regulation of DMADDM, as shown in Figure 5 (Zhang, Wang, et al. 2015). The proportion of S. mutans in the control group gradually increased with time. In contrast, remarkably, the proportion of S. mutans in the DMADDM groups significantly decreased with time. The S. sanguinis proportion decreased slightly with time in the control group but decreased dramatically with time in the DMADDM groups. S. mutans has been associated with caries due to its acid production and acid tolerance. S. sanguinis and S. gordonii are non-mutans streptococci. S. sanguinis is associated with a low caries risk. S. gordonii is an early colonizer of the dental plaque and is associated with sound enamel. The oral bacteria compete for the limited resources. The mechanism of the competition appears to be that S. mutans can produce antistreptococcal bacteriocins to suppress S. sanguinis and S. gordonii, while S. sanguinis and S. gordonii can produce hydrogen peroxide to compete against S. mutans (Kreth et al. 2008). Figure 5 shows that S. sanguinis was the most sensitive to DMADDM and that S. mutans was more sensitive than S. gordonii. While S. gordonii thrived, S. mutans decreased substantially with increasing DMADDM content in the resin. Although the specific interactions among these 3 species is not clear yet, regarding the mechanisms for modulating biofilm species, it is likely that the dual pressures of 1) bacteria competition and 2) the antibacterial agent led to the proportion shift in the multispecies biofilms. DMADDM greatly decreased the ratio of caries-associated S. mutans in the multispecies biofilm. This could be highly beneficial in modulating biofilm compositions via bioactive resins to suppress cariogenic species and promote benign species. Meanwhile, regarding the effects of antibacterial resins on the cariogenic virulence factors, these results showed that DMADDM-containing adhesive slowed the pH drop and decreased the lactic acid production of the bacteria. Lactic acid production in DMADDM group decreased by 10- to 30-fold as compared with control. Exopolysaccharide was also reduced by 20- to 30-fold via DMADDM. Further studies are needed to investigate the mechanisms and efficacy of antimicrobial and bioactive resins to modulate biofilms to promote benign species and suppress cariogenic/pathogenic species.
Figure 5.
Modulating biofilm compositions via bioactive resin to suppress cariogenic species and promote benign species. (A) The ratio of Streptococcus mutans, Streptococcus gordonii, and Streptococcus sanguinis in biofilms, determined by TaqMan real-time polymerase chain reaction. The percentages on x-axis are for dimethylaminododecyl methacrylate (DMADDM) at 0% (control), 2.5%, and 5%. (B) Fluorescent in situ hybridization images of multispecies biofilms (S. mutans, stained green; S. sanguinis, stained red; S. gordonii, stained blue). (Adapted from Zhang, Wang, et al. 2015, with permission.) This figure is available in color online.
Bacterial Drug Resistance to Antimicrobial QAMS
Although antimicrobial materials have promising clinical benefits, concerns are growing on the overuse of antibiotics in the public and on the antibiotic resistance of bacteria. Drug resistance occurs when microbes acquire mutations that render them immune to certain antibiotics. Microorganisms exhibit resistance to antimicrobials in the following 4 ways: 1) drug inactivation or modification, 2) alteration of target site, 3) alteration of metabolic pathway, and 4) decreasing drug permeability and/or increasing active drug efflux across cell surface (Bayne et al. 2013). The investigation of bacterial resistance to antimicrobial dental resins is in its early stage with few reports. A recent study investigated whether 2 species of oral bacteria developed resistance under the influence of cationic biocides (Kitagawa et al. 2016). The results showed that S. mutans and Enterococcus faecalis did not develop resistance to MDPB after repeated exposures. However, E. faecalis acquired resistance to CHX, as shown in Figure 6 (Kitagawa et al. 2016). While this pilot study indicated no drug resistance of S. mutans and E. faecalis to MDPB, further studies are needed to investigate other QAMs against other oral bacteria.
Figure 6.
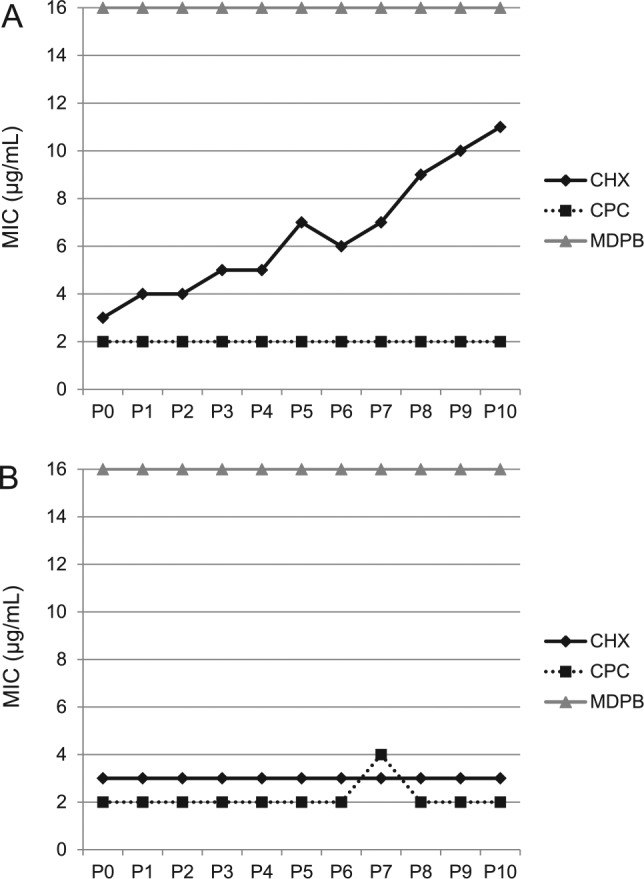
Bacterial drug resistance to cationic biocides. The minimum inhibitory concentrations (MICs) of chlorhexidine (CHX), cetylpyridinium chloride (CPC) and 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) are plotted for (A) Enterococcus faecalis and (B) Streptococcus mutans. MIC determinations were repeatedly performed from passages of 0 to 10 (P0 to P10). MDPB and CPC caused no resistance for E. faecalis and S. mutans. However, repeated exposure of E. faecalis to CHX induced drug resistance. (Adapted from Kitagawa et al. 2016, with permission.)
Another important and related issue is antibiotic tolerance, which is caused by a small subpopulation of microbial cells termed persisters in the biofilms (Lafleur et al. 2010; Lewis 2010). Unlike drug resistance, drug tolerance refers to small fractions of clonal bacterial populations that can survive lethal doses of antimicrobial agents, while the population as a whole remains susceptible. This small fraction of cells can survive in the presence of antibiotics; they can resume growth after the antimicrobial agent is removed; and their progeny is still sensitive to the antimicrobial agents. In addition, the periodic use of antibacterial agents may lead to the selection of strains with a gradually increasing content of persister cells. Indeed, it has been reported that recurrent chronic infections—including dental disease, endocarditis, cystitis, urinary tract infections, and so on—have relationships with persisters or biofilms containing these persisters (Lewis 2010). Studies are needed to investigate whether the use of antibacterial dental materials would induce persister in oral biofilms. A literature search revealed no publications on this topic. In view of extensive research on developing new antimicrobial and bioactive resins, research is needed on whether the long-term use of antimicrobials would induce persisters in oral biofilms.
Conclusions and Future Perspectives
Current restorative materials are mostly bioinert. A new generation of bioactive/therapeutic materials is being developed. They were synthesized by incorporating novel antimicrobial agents—particularly, polymerizable QAMs, remineralizing particles, and protein-repellent compositions. Material designs via tailored bioactive agents yielded novel resins with multiple therapeutic benefits and synergistic effects. With the evolution of caries management shifting to “minimally invasive” techniques, restorative materials are endowed with increasing expectations for therapeutic effects. Most of the studies on developing a new generation of antimicrobial, therapeutic, and bioactive resins are in vitro; in vivo studies are still needed. In addition, how to optimize the microecologic regulative effects of antimicrobial materials has received little attention. Furthermore, studies are needed to determine whether antibacterial resins induce bacterial drug resistance and persister cells. Nonetheless, through multidisciplinary research, the new generation of antimicrobial and bioactive/therapeutic resins is expected to offer tremendous benefits to oral health.
Author Contributions
L. Cheng, K. Zhang, N. Zhang, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M.A.S. Melo, M.D. Weir, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; X.D. Zhou, Y.X. Bai, M.A. Reynolds, contributed to conception and design, critically revised the manuscript; H.H.K. Xu, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Drs. Satoshi Imazato and Haruaki Kitagawa for providing Figure 6. We are grateful to Drs. Lin Wang, Suping Wang, Joseph M. Antonucci, Nancy J. Lin, Sheng Lin-Gibson, Laurence C. Chow, Ashraf F. Fouad, Xianju Xie, Ling Zhang, Fang Li, and others for discussions.
Footnotes
This study was supported by the National Institutes of Health (R01DE17974; H.H.K.X.), Key Program for International S&T Cooperation of Sichuan (L.C.), International S&T Cooperation of China (X.Z.), National Natural Science Foundation of China (81400540; K.Z.), Beijing Administration of Hospitals’ Youth Program (QML20151401; K.Z.), and a seed fund from the University of Maryland School of Dentistry (H.H.K.X.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bayne S, Petersen PE, Piper D, Schmalz G, Meyer D. 2013. The challenge for innovation in direct restorative materials. Adv Dent Res. 25(1):8–17. [DOI] [PubMed] [Google Scholar]
- Beyth N, Domb AJ, Weiss EI. 2007. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 35(3):201–206. [DOI] [PubMed] [Google Scholar]
- Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. 2006. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 27(21):3995–4002. [DOI] [PubMed] [Google Scholar]
- Beyth N, Yudovin-Farber I, Perez-Davidi M, Domb AJ, Weiss EI. 2010. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc Natl Acad Sci U S A. 107(51):22038–22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzistavrou X, Fenno JC, Faulk D, Badylak S, Kasuga T, Boccaccini AR, Papagerakis P. 2014. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 10(8):3723–3732. [DOI] [PubMed] [Google Scholar]
- Chen C, Weir MD, Cheng L, Lin NJ, Lin-Gibson S, Chow LC, Zhou X, Xu HH. 2014. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent Mater. 30(8):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S. 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials. 28(29):4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. 2012. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 28(5):561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang K, Weir MD, Melo MA, Zhou X, Xu HH. 2015. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine (London, England). 10(4):627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang K, Zhou CC, Weir MD, Zhou XD, Xu HH. 2016. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int J Oral Sci. 8(3):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, Lin-Gibson S. 2011. In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res B Appl Biomater. 97(1):124–131. [DOI] [PubMed] [Google Scholar]
- Cocco AR, Rosa WL, Silva AF, Lund RG, Piva E. 2015. A systematic review about antibacterial monomers used in dental adhesive systems: current status and further prospects. Dent Mater. 31(11):1345–1362. [DOI] [PubMed] [Google Scholar]
- Curzon ME, Preston AJ. 2004. Risk groups: nursing bottle caries/caries in the elderly. Caries Res. 38 Suppl 1:24–33. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 15(2):167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. 2011. Development of an antimicrobial resin—a pilot study. Dent Mater. 27(4):322–328. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. 2011. Resin composite—state of the art. Dent Mater. 27(1):29–38. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Giannobile WV. 2014. Novel biomaterials and technologies for the dental, oral, and craniofacial structures. J Dent Res. 93(12):1185–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K, Christensen SK, Lobner-Olesen A. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 3(5):371–382. [DOI] [PubMed] [Google Scholar]
- Imazato S. 2003. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 19(6):449–457. [DOI] [PubMed] [Google Scholar]
- Imazato S, Chen J-H, Ma S, Izutani N, Li F. 2012. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jap Dent Sci Rev. 48(2):115–125. [Google Scholar]
- Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. 1999. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 20(9):899–903. [DOI] [PubMed] [Google Scholar]
- Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. 2004. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 29(4):369–375. [PubMed] [Google Scholar]
- Imazato S, Tarumi H, Ebi N, Ebisu S. 2000. Cytotoxic effects of composite restorations employing self-etching primers or experimental antibacterial primers. J Dent. 28(1):61–67. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. 1998. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res. 39(2):323–330. [DOI] [PubMed] [Google Scholar]
- Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. 2001. Quality of dental restorations: FDI Commission project 2-95. Int Dent J. 51(3):117–158. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Izutani N, Kitagawa R, Maezono H, Yamaguchi M, Imazato S. 2016. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J Dent. 47:18–22. [DOI] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 190(13):4632–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur MD, Qi Q, Lewis K. 2010. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother. 54(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst SE, O’Donnell JN, Skrtic D. 2009. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater. 25(7):884–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AL. 2000. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B Biointerfaces. 18(3–4):261–275. [DOI] [PubMed] [Google Scholar]
- Lewis K. 2010. Persister cells. Annu Rev Microbiol. 64:357–372. [DOI] [PubMed] [Google Scholar]
- Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. 2009. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 37(4):289–296. [DOI] [PubMed] [Google Scholar]
- Li F, Wang P, Weir MD, Fouad AF, Xu HH. 2014. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 10(6):2804–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Weir MD, Fouad AF, Xu HH. 2013. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 41(10):881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Weir MD, Xu HH. 2013. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 92(10):932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaghi S, Jadidi T, Koenderink G, Mashaghi A. 2013. Lipid nanotechnology. Int J Mol Sci. 14(2):4242–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 12(1):147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo MA, Orrego S, Weir MD, Xu HH, Arola DD. 2016. Designing multiagent dental materials for enhanced resistance to biofilm damage at the bonded interface. ACS Appl Mater Interfaces. 8(18):11779–11787. [DOI] [PubMed] [Google Scholar]
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. 16(10):2346–2353. [DOI] [PubMed] [Google Scholar]
- Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. 2009. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 30(28):4921–4929. [DOI] [PubMed] [Google Scholar]
- Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. 2007. Permanent, non-leaching antibacterial surface—2: how high density cationic surfaces kill bacterial cells. Biomaterials. 28(32):4870–4879. [DOI] [PubMed] [Google Scholar]
- Nishida M, Imazato S, Takahashi Y, Ebisu S, Ishimoto T, Nakano T, Yasuda Y, Saito T. 2010. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials. 31(7):1518–1532. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. 2011. State of the art etch-and-rinse adhesives. Dent Mater. 27(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi RL. 2005. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS) June 17-19, 2004, Oregon Health and Science University, Portland, Oregon. Dent Mater. 21(1):3–6. [DOI] [PubMed] [Google Scholar]
- Simoncic B, Tomsic B. 2010. Structures of novel antimicrobial agents for textiles—a review. Text Res J. 80(16):1721–1737. [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, et al. 2010. Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 38(6):1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. 2011. State of the art of self-etch adhesives. Dent Mater. 27(1):17–28. [DOI] [PubMed] [Google Scholar]
- Wang L, Xie X, Imazato S, Weir MD, Reynolds MA, Xu HH. 2016. A protein-repellent and antibacterial nanocomposite for class-V restorations to inhibit periodontitis-related pathogens. Mater Sci Eng C Mater Biol Appl. 67:702–710. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang K, Zhou X, Xu N, Xu H, Weir M, Ge Y, Wang S, Li M, Li Y, et al. 2014. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of Streptococcus mutans biofilm. Int J Mol Sci. 15(7):12791–12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. 2011. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 27(5):487–496. [DOI] [PubMed] [Google Scholar]
- Xu HH, Moreau JL, Sun L, Chow LC. 2011. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 27(8):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wang Y, Liao S, Wen ZT, Fan Y. 2012. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B Appl Biomater. 100(4):1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaura E, ten Cate JM. 2015. Towards understanding oral health. Caries Res. 49 Suppl 1:55–61. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Wu R, Fan Y, Liao S, Wang Y, Wen ZT, Xu X. 2014. Antibacterial dental composites with chlorhexidine and mesoporous silica. J Dent Res. 93(12):1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Cheng L, Weir MD, Bai YX, Xu HH. 2016. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci. 8(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wang S, Zhou X, Xu HH, Weir MD, Ge Y, Li M, Wang S, Li Y, Xu X, et al. 2015. Effect of antibacterial dental adhesive on multispecies biofilms formation. J Dent Res. 94(4):622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Ma J, Melo MA, Weir MD, Bai Y, Xu HH. 2015. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent. 43(2):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Melo MA, Chen C, Liu J, Weir MD, Bai Y, Xu HH. 2015. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent Mater. 31(9):1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Weir MD, Chen C, Melo MA, Bai Y, Xu HH. 2016. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J Dent. 50:51–59. [DOI] [PubMed] [Google Scholar]
- Zheng X, Cheng X, Wang L, Qiu W, Wang S, Zhou Y, Li M, Li Y, Cheng L, Li J, et al. 2015. Combinatorial effects of arginine and fluoride on oral bacteria. J Dent Res. 94(2):344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liu H, Weir MD, Reynolds MA, Zhang K, Xu HH. 2016. Three-dimensional biofilm properties on dental bonding agent with varying quaternary ammonium charge densities. J Dent. 53:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]