Abstract
The current study examines the longitudinal patterns of both cigarette smoking and depressive symptoms as predictors of generalized anxiety disorder (GAD) using data from the Harlem Longitudinal Development Study. There were 674 African American (53%) and Puerto Rican (47%) participants. Among the 674 participants, 60% were females. In the logistic regression analyses, the indicator variables of membership in each of the joint trajectories of cigarette smoking and depressive symptoms from the mid 20s to the mid 30s were used as the independent variables, and the diagnosis of GAD in the mid 30s was used as the dependent variable. The high cigarette smoking with high depressive symptoms group and the low cigarette smoking with high depressive symptoms group were associated with an increased likelihood of having GAD as compared to the no cigarette smoking with low depressive symptoms group. The findings shed light on the prevention and treatment of GAD.
Keywords: generalized anxiety disorder, cigarette smoking, depressive symptoms, trajectory analysis, longitudinal study
Generalized anxiety disorder (GAD) has a number of negative consequences as noted below.1, 2 GAD has a long average duration of symptoms3, 4 and is associated with a significant impairment of quality of life or disability.5 The Christchurch Health and Development Study, a 25-year longitudinal study of over 1,000 participants, found that GAD increased the odds of suicidal ideation by a factor of 8.0 times and increased the rate of suicide attempts by a factor of 5.9 times.1 In addition, exaggerated amygdala activation in response to fearful6 and masked angry facial expressions7 and during the anticipation of aversive photographs8 has been reported in patients with GAD. Also, a study using a clinical sample of 31 women (16 patients with GAD and 15 healthy control participants) reported that patients with GAD had a larger volume of the amygdala and dorsomedial prefrontal cortex,2 both of which have been correlated with psychological symptoms.9
One of the important and common risk factors for GAD is cigarette smoking.10–12 About 15% of the ever-smokers13 compared with about 3% among the general population14 in the United States have had at least one anxiety disorder in their lifetime. The symptoms of general anxiety were more severe in nicotine-dependent smokers than in never-smokers, former smokers, and non-dependent smokers.15 Self-administration of nicotine via smoking may induce anxiety, as dysphoria is one of the observed pharmacologic effects of nicotine.16 The National Comorbidity Survey Replication research found that GAD is significantly associated with smoking behaviors including daily smoking, heavy smoking, and nicotine dependence.17
Comorbidity of anxiety symptoms and depressive symptoms exists.18, 19 The Dunedin birth cohort (N=1,037) study reported 12% of adults experience comorbidity between GAD and major depressive disorders.18 Numerous studies have reported a positive relationship between depressive symptoms and anxiety symptoms.20, 21 Norton21 reported that the correlation between depression and anxiety was higher than 0.7 in a sample of college students. This may be that pathological worry also occurs in depression22 and that active and passive rumination is involved in anxiety.23 Hermans and Evenhuis24 found that increased depressive symptoms were positively associated with increased anxiety symptoms using a cohort living in the Netherlands.
The present study seeks possible precursors of GAD given a number of negative consequences.1–5 Both smoking cigarettes10 and depressive symptoms18 can be the important possible risk factors for GAD. Furthermore, the two possible risk factors (cigarette smoking and depressive symptoms) commonly co-occur.25–28 However, only a few studies have focused on the joint trajectories of cigarette smoking and depressive symptoms as a risk factor for adverse conditions.29 Leventhal and Zvolensky (2015) documented that cigarette smoking, depressive symptoms, and GAD are linked together.30 The current study examines the earlier patterns of cigarette smoking and depressive symptoms simultaneously and their association with later GAD.
Our study is unique in three ways. First, we identify the joint trajectories of cigarette smoking and depressive symptoms occurring simultaneously among the relatively understudied ethnic groups of African Americans and Puerto Ricans living in an urban area. Second, we investigated the association between the earlier joint trajectories of cigarette smoking and depressive symptoms and later GAD. Third, we followed the young adult sample from mean age 14 to mean age 36.31
We hypothesize that there will be at least four joint trajectory groups based on all possible combinations with two levels (high and low) in each of cigarette smoking and depressive symptoms: a) a high level of both cigarette smoking and depressive symptoms trajectory group, b) a high level of cigarette smoking and a low level of depressive symptoms trajectory group, c) a low level of cigarette smoking and a high level of depressive symptoms trajectory group, and d) a low level of both cigarette smoking and depressive symptoms trajectory group. We also hypothesize that the trajectory group with higher levels of both cigarette smoking and depressive symptoms as well as the trajectory groups with a higher level of either cigarette smoking or depressive symptoms will be associated with an increased likelihood of having GAD as compared with a low level of both cigarette smoking and depressive symptoms trajectory group.
Methods
Study Design
Data from the Harlem Longitudinal Development Study were first collected in 1990 (time 1; T1, N=1,332) when the participants were students (grades 7 to 10) attending schools in the East Harlem area of New York City. Data were collected in person or by phone by the National Opinion Research Center at time 2 (T2; 1994–1996; N=1,190). The attrition rate at T2 as compared to T1 was 89%. The Survey Research Center of the University of Michigan collected the data at time 3 (T3; 2000–2001; N=662). At T3, a sub-sample was taken from the T2 sample due to budgetary limitations. The attrition rate at T3 as compared to T2 was 56%. The data were collected by our research group: in person, by phone, or by mailed questionnaire at time 4 (T4; 2004–2006; N=838) and by mailed questionnaire at time 5 (T5; 2011–2013; N=674). The attrition rates at T4 as compared to T3 and at T5 as compared to T4 were 127% and 80%, respectively. For the interview in person or by phone, each participant was interviewed by an interviewer of the same sex and ethnic background. For the sections regarding drug use and possible involvement with the police, the participant marked his or her own responses directly on the questionnaire.
Participants
The present study (N=674; 53% African Americans, 47% Puerto Ricans) is based on time waves 1–5 of the Harlem Longitudinal Development Study. Sixty percent were females (n=405). The mean age of the participants at T1 was 14.1 years (Standard Deviation; SD=1.4 years). The mean age of the participants at this wave was 19.2 years (SD=1.5 years). At T3, the mean age of the participants was 24.4 years (SD=1.3 years). At T4, the mean age of the participants was 29.2 years (SD=1.4 years). At T5, the mean age was 35.9 years (SD= 1.4 years). Among the 674 participants, 88% at T1, 68% at T2, 41% at T3, 54% at T4, and 57% at T5 were non-smokers.
The Institutional Review Board (IRB) of the New York University School of Medicine approved the study for T4 and T5, and the IRBs of the Mount Sinai School of Medicine and New York Medical College (our former affiliations) approved the study’s procedures for earlier waves of data collection. A Certificate of Confidentiality was obtained from the National Institutes of Health for each wave of data collection. Informed consent was obtained from all participants (18 years or older) at each time wave. At T1 and T2, passive consent was obtained from the parents of participants who were minors (< 18 years old).
Measures
Independent Variables
Cigarette use and depressive symptoms were measured from T3–T5 (mean ages 24 to 36 years) since we have only two items for depressive symptoms at T1 and T2. Cigarette smoking (T3–T5) was a single item, i.e., “In the past 5 years, how many cigarettes did you smoke?” The answer options were as follows: none (0), a few cigarettes or less a week (1), 1–5 cigarettes a day (2), about half pack a day (3), about one pack a day (4), about one and a half packs a day (5), and more than one and a half packs a day (6). Depressive symptoms32 (T3–T5) consisted of a 5 item scale, e.g., “Do you sometimes feel unhappy, sad, or, depressed?” The answer options were as follows: completely false (0), false (1), true (2), and completely true (3). The Cronbach’s alphas were 0.74 (T3), 0.77 (T4), and 0.85 (T5).
Control Variables
The participants were asked about their gender (female=1, male=2) and ethnicity (African American=1, Puerto Rican=2). Anxiety symptoms32 (T3) was a 3 item scale, e.g., “Over the past few years, how much were you bothered by feeling nervous?” Answer options were as follows: not at all (0), a little (1), somewhat (2), quite a bit (3), and extremely (4). The Cronbach’s alpha was 0.78. The participants provided their age in years, marital status (live alone=0, married and live together including cohabiting=1), annual income (less than or equal to 10,000=0, between 10,001 and 35,000=1, between 35,001 and 75,000=2, and greater than 75,000=3), and employment status (unemployed=0, employed=1) at T5. The measure socioeconomic status (T5) was a summed score of the marital status, annual income, and employment status which ranged from 0 to 5. Physical disease (T5) was a summed score of diabetes (no=0, yes=1), hypertension (no=0, yes=1), asthma (no=0, yes=1), and obesity (no=0, yes=1). The score ranged from 0 to 4.
Generalized Anxiety Disorder at T5
Generalized Anxiety Disorder (GAD) was assessed using an adaptation of the DSM-5 Generalized Anxiety Disorder measure.33 The participants were asked nine questions: (1) Within the last 5 years, have you had a period of at least 6 months when you worried excessively or were anxious about several things? During this period of 6 months or more: (2) Were these worries present most days? (3) Was it difficult to control the worries or did they interfere with your ability to focus on what you were doing? (4) Did you feel restless, keyed up or on edge? (5) Did you feel tense? (6) Did you feel tired or weak, or were you easily exhausted? (7) Did you have difficulty concentrating or find your mind going blank? (8) Did you feel irritable? and (9) Did you have sleep problems (e.g., difficulty falling asleep, waking up in the middle of the night, early morning awakening, or sleeping excessively)? The internal reliability of this measure was satisfactory (Cronbach’s alpha = 0.94).
If the participants answered “yes” to the first three questions and “yes” to 3 or more of the last six questions, the participant received a score of 1 on the measure of GAD; otherwise, the participant received a score of 0.
Analytic procedure
We used Mplus34 to obtain the joint trajectories of cigarette smoking and depressive symptoms. Cigarette smoking and depressive symptoms at each time point were treated as censored normal variables. We used the Bayesian Information Criterion (BIC) to determine the number of trajectory groups.34, 35 The observed trajectories for each of the groups consisted of the averages of cigarette smoking and depressive symptoms, respectively, at each point in time when the participants were assigned to the group with the largest Bayesian posterior probability (BPP). We applied the full information maximum likelihood approach for missing data.34
Logistic regression analyses were then conducted to examine whether the indicator of the trajectory group membership in either high use of cigarette smoking or a high level of depressive symptoms, compared with the indicator of membership in each of the other trajectory groups from T3 to T5, were associated with GAD at T5. In these analyses, the covariates included gender, ethnicity, anxiety symptoms at T3, age, SES, and physical disease at T5. That is, all pair wise comparisons among the joint trajectory groups were performed.
Results
A four-group model was selected, based on the BIC. The BICs were 8496, 8363, 8224, and 8241 for a 2, 3, 4, and 5-group model, respectively. Since the 4-group model had the smallest BIC score, we selected it. Figure 1 presents the observed trajectories and the percentages of the sample who were members of each of the four trajectory groups. The mean BPP in each trajectory group ranged from 0.86 to 0.96, which indicated an adequate classification.
Figure 1. Joint trajectories of cigarette smoking and depressive symptoms among African American and Puerto Rican adults from the mid-twenties to the mid-thirties (N=674).
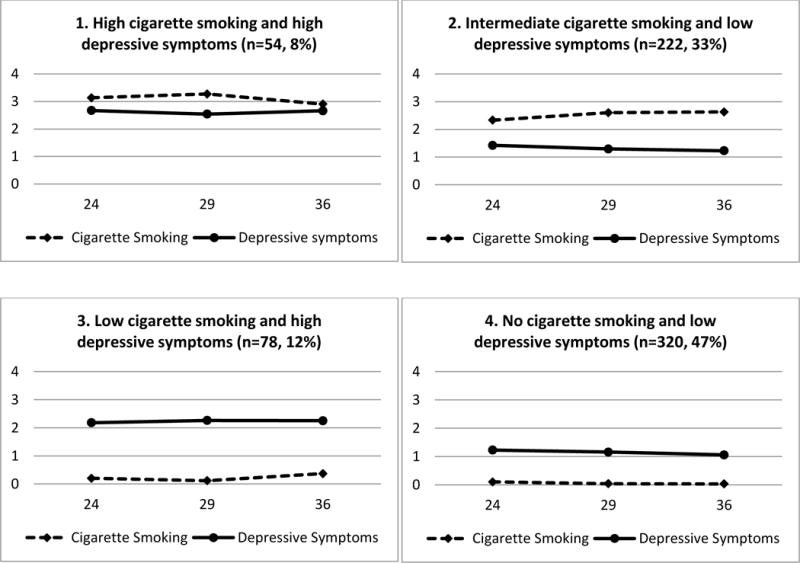
Note. The x-axis and y-axis represent age in years and the level of cigarette smoking and depressive symptoms, respectively.
Answer options for cigarette smoking: none (0); a few cigarettes or less a week (1); 1–5 cigarettes a day (2); about half pack a day (3); about one pack a day (4); about one and half packs a day (5); more than one and half packs a day (6)
Answer options for depressive symptoms: completely false (0); false (1); true (2); completely true (3)
The four trajectory groups were named: 1) high cigarette smoking and high depressive symptoms (prevalence 8%, mean BPP=0.91); 2) intermediate cigarette smoking and low depressive symptoms (prevalence 33%, mean BPP=0.96); 3) low cigarette smoking and high depressive symptoms (prevalence 12%, mean BPP=0.94); and 4) no cigarette smoking and low depressive symptoms (prevalence 47%, mean BPP=0.86). Table 1 presents summary statistics for each of the four trajectory groups. As presented in Table 2, the prevalence of GAD for the whole sample was 12.3%. The high cigarette smoking and high depressive symptoms group had the highest prevalence (50.0%) on the measure of GAD. On the other hand, the no cigarette smoking and low depressive symptoms group had the lowest prevalence (4.7%) on the measure of GAD.
Table 1.
Model fit indexes for the number of joint trajectory groups of cigarette smoking and depressive symptoms among African American and Puerto Rican adults from the mid twenties to the mid thirties (N=674)
| Number of joint trajectory groups | Bayesian Information Criteria | Akaike Information Criteria | p-value for LRT comparing the k class to the k-1 class model |
|---|---|---|---|
| 2 | 8496 | 8411 | <0.0001 |
| 3 | 8363 | 8246 | 0.0593 |
| 4 | 8224 | 8076 | <0.0001 |
| 5 | 8241 | 8061 | 0.4770 |
Table 2.
Summary statistics by the joint trajectory groups of cigarette smoking and depressive symptoms from the mid twenties to the mid thirties among African American and Puerto Rican adults (N=674)
| Joint trajectory groups
|
Whole sample (N=674) | |||||
|---|---|---|---|---|---|---|
| High cigarette smoking and high depressive symptoms (n=54, 8%) | Intermediate cigarette smoking and low depressive symptoms (n=222, 33%) | Low cigarette smoking and high depressive symptoms (n=78, 12%) | No cigarette smoking and low depressive symptoms (n=320, 47%) | F or chi-square test statistics | ||
| Independent variables | ||||||
|
| ||||||
| Cigarette smoking (T3–T5) | T3: 3.14 (1.55) | T3: 2.33 (1.56) | T3: 0.20 (0.52) | T3: 0.10 (0.48) | F=188.6** | T3: 1.20 (1.63) |
| T4: 3.27 (1.18) | T4: 2.60 (1.34) | T4: 0.12 (0.40) | T4: 0.04 (0.20) | F=509.9** | T4: 1.14 (1.58) | |
| T5: 2.91 (1.56) | T5: 2.63 (1.54) | T5: 0.37 (0.83) | T5: 0.03 (0.18) | F=330.7** | T5: 1.18 (1.65) | |
| Depressive symptoms (T3–T5) | T3: 2.67 (0.64) | T3: 1.42 (0.56) | T3: 2.18 (0.64) | T3: 1.23 (0.51) | F=110.9** | T3: 1.55 (0.72) |
| T4: 2.54 (0.68) | T4: 1.29 (0.54) | T4: 2.26 (0.52) | T4: 1.16 (0.46) | F=179.9** | T4: 1.44 (0.70) | |
| T5: 2.66 (0.81) | T5: 1.23 (0.58) | T5: 2.25 (0.73) | T5: 1.06 (0.49) | F=179.0** | T5: 1.38 (0.78) | |
|
| ||||||
| Control variables | ||||||
|
| ||||||
| Female | n=30, 55.6% | n=107, 48.2% | n=55, 70.5% | n=213, 66.6% | x2(3)=22.7** | n=405, 60.1% |
| African American | n=22, 40.7% | n=109, 49.1% | n=36, 46.2% | n=189, 59.1% | x2(3)=10.8* | n=256, 52.8% |
| Anxiety symptoms (T3) | 1.93 (0.96) | 0.84 (0.72) | 1.41 (0.89) | 0.75 (0.63) | F=52.1** | 0.95 (0.80) |
| Age (T5) | 35.83 (1.29) | 35.93 (1.39) | 35.61 (1.36) | 35.99 (1.43) | F=1.6 | 35.91 (1.40) |
| SES (T5) | 1.56 (1.13) | 2.07 (1.32) | 2.24 (1.31) | 2.78 (1.26) | F=22.6** | 2.39 (1.33) |
| Physical disease (T5) | 1.39 (1.06) | 0.98 (0.72) | 1.41 (0.83) | 1.11 (0.83) | F=7.4** | 1.12 (0.83) |
|
| ||||||
| Dependent variable | ||||||
|
| ||||||
| Generalized anxiety disorder (T5) | n=27, 50.0% | n=18, 8.1% | n=23, 29.5% | n=15, 4.7% | x2(3)=** | n=83, 12.3% |
Notes. SES=Socioeconomic status
p<.05,
p<.001
Answer options for cigarette smoking: none (0), a few cigarettes or less a week (1), 1–5 cigarettes a day (2), about half pack a day (3), about one pack a day (4), about one and half packs a day (5), more than one and half packs a day
Answer options for depressive symptoms: completely false (0), false (1), true (2), completely true (3)
Answer options for anxiety symptoms: not at all (0) to extremely (4)
Answer options for SES: lowest (0) to highest (5)
Answer options for physical disease: no disease (0) to 4 diseases (4)
Table 3 shows the adjusted odds ratios (AOR) for GAD in the logistic regression analysis. Memberships in the high cigarette smoking and high depressive symptoms group (AOR=11.2, p <.001) and in the low cigarette smoking and high depressive symptoms group (AOR=5.8, p<.001) were associated with an increased likelihood of having GAD at T5 as compared to membership in the no cigarette smoking and low depressive symptoms group. Additionally, memberships in the high cigarette smoking and high depressive symptoms group (AOR=5.7, p<.001) and in the low cigarette smoking and high depressive symptoms group (AOR=3.8, p<.001) were associated with an increased likelihood of having GAD at T5 as compared with membership in the intermediate cigarette smoking and low depressive symptoms group. Lastly, membership in the high cigarette smoking and high depressive symptoms group was associated with an increased likelihood of having GAD at T5 as compared with membership in the low cigarette smoking and high depressive symptoms group (AOR=2.1, p<.05).
Table 3.
Adjusted odds ratios (95% Confidence Interval): the association of the membership in the joint trajectories of cigarette smoking and depressive symptoms with generalized anxiety disorder (GAD) among African American and Puerto Rican adults (N=674)
| Comparisons of membership in each of the joint trajectory groups | GAD at T5 |
|---|---|
| 1High cigarette smoking and high depressive symptoms vs. No cigarette smoking and low depressive symptoms | 11.2 (4.6, 27.0)*** |
| 2Intermediate cigarette smoking and low depressive symptoms vs. No cigarette smoking and low depressive symptoms | 1.7 (0.8, 3.7) |
| 3Low cigarette smoking and high depressive symptoms vs. No cigarette smoking and low depressive symptoms | 5.8 (2.7, 12.5)*** |
| 4High cigarette smoking and high depressive symptoms vs. Intermediate cigarette smoking and low depressive symptoms | 5.7 (2.4, 13.7)*** |
| 5Low cigarette smoking and high depressive symptoms vs. Intermediate cigarette smoking and low depressive symptoms | 3.8 (1.7, 8.8)*** |
| 6High cigarette smoking and high depressive symptoms vs. Low cigarette smoking and high depressive symptoms | 2.1 (1.0, 4.7)* |
Notes. Two-tailed test:
p<.05,
p<.001
Gender, ethnicity, anxiety symptoms at T3, age at T5, socioeconomic status at T5, and physical disease at T5 were statistically controlled.
In models 1, 2, and 3, female gender and higher anxiety symptoms at T3 were associated with GAD (p<.05).
In model 4, higher anxiety symptoms at T3 were associated with GAD (p<.05).
In model 5, none of the other predictors was associated with GAD.
In model 6, female gender and lower socioeconomic status at T5 were associated with GAD (p<.05).
Discussion
The GAD rate of 12% in our study sample consisting of African Americans and Puerto Ricans is similar to the findings reporting 11% GAD among Blacks and Hispanics obtained in a nationally representative survey.36 The hypotheses were partially supported with one exception. Our results are consistent with the findings of other investigators that cigarette smoking and depressive symptoms are associated with greater symptoms of anxiety or GAD.15, 24, 37 Biological pathways linking cigarette smoking to the risk of GAD may help to explain these findings. Anxiety symptoms may play a role in mitochondrial dysfunction which is also affected by exposure to cigarette smoking.38, 39 Mitochondria can be important sources of oxidative stress, and many abnormalities in mitochondrial function have been found in psychiatric disorders such as GAD.40 Patients exhibiting mitochondrial disorders commonly demonstrate increased anxiety symptoms.41, 42
The association between depressive symptoms and GAD may also be due in part to genetic influences. In adults, the symptoms of depression and anxiety have been linked to the personality trait of neuroticism.43 The role of neuroticism, a known risk factor for common mental disorders including depressive disorder and anxiety disorder,44 may partially explain the linkage between depressive symptoms and GAD. For example, psychological distress (e.g., sadness), core to the neuroticism domain, includes a proneness to irrational ideas, poor impulse control and poor stress management,45 and thus may contribute to GAD.
Our findings regarding the association between joint trajectories of cigarette smoking and depressive symptoms and GAD can also be explained in part from a psychological perspective. Since the comorbidity of chronic/heavy cigarette smoking and chronic/high depressive mood was associated with low self-control, low self-esteem, and low coping,29 individuals who smoke and have depressive symptoms may have narrower social networks and/or poorer relationships with co-workers, friends, and family members.46, 47 This might have an adverse effect on a smooth transition to adulthood including maintaining strong friendships, establishing financial independence, starting a family, and obtaining job security, which may ultimately predict GAD.
An interesting finding is that individuals who smoke very few cigarettes (i.e., less than 1–5 cigarettes a day) but are highly depressed have a 5.8 times greater risk for having GAD as compared with the individuals who do not smoke cigarettes and have low depressive symptoms. Also, individuals who smoke a few cigarettes but are highly depressed have a 3.8 times greater risk for having GAD, as compared with individuals who smoke cigarettes less than a half a pack a day and have low depressive symptoms. Depressive symptoms seem to have a stronger association with GAD than smoking. On the other hand, there is also a significant association between the high cigarette smoking with high depressive symptoms group and GAD as compared with the low cigarette smoking with high depressive symptoms group. Cigarette smoking has some impact on GAD. We also examined the associations between single trajectory groups for cigarette smoking as well as for depressive symptoms and GAD without any other covariates; memberships in the higher level of cigarette smoking trajectory group (p<0.05) and in the higher level of depressive symptoms (p<0.001) trajectory group were associated with GAD (data not shown). These findings indicate that the interaction of cigarette smoking and depressive symptoms increases the likelihood of GAD.
As noted earlier, one of our hypotheses was not supported since there was no significant difference between the intermediate cigarette smoking and low depressive symptoms trajectory group and the no cigarette smoking and low depressive symptoms trajectory group with respect to GAD. The trajectory group smoking less than a half a pack a day with low depressive symptoms, statistically speaking, has the same likelihood of GAD as the trajectory group not smoking with low depressive symptoms.
Limitations-Strengths
Our data are based on self-reports rather than on official records such as medical records. However, studies have shown that use of self-report data yields reliable results.48 We also did not include genetic variables or specific ethnic variables for African Americans and Puerto Ricans. Future studies should include these assessments. In this study, depressive symptoms were used so it is difficult to draw inferences about depressive disorders. Lastly, the sample in this study consisted of African American and Puerto Rican adults residing in an urban area. Consequently, the results may not apply to the general population.
Despite these limitations, the study supports and adds to the literature in a number of important ways. First, unlike most research that focuses on one point in time, we assess cigarette smoking and depressive symptoms simultaneously over a span of up to 12 years. The prospective nature of the data allows us to go beyond a cross-sectional analysis and to consider the temporal sequencing of variables. Second, this community sample varying in SES is unique as it consisted of African American and Puerto Rican inner city adults studied until the mid 30s. Third, a significant contribution of the paper is a set of findings relating different joint trajectories of cigarette smoking and depressive symptoms beginning in the mid 20s to the later occurrence of GAD in the mid 30s. Fourth, the joint trajectory analysis based on a person-centered approach enables one to examine the magnitude, length of time, and the starting point of cigarette smoking and depressive symptoms simultaneously and their associations with later GAD. Therefore, the current study would have important implications for prevention and treatment of smoking and depression among African Americans and Puerto Ricans.
Conclusions
The findings of this study have implications for prevention and treatment, especially, for African American and Puerto Rican adults. Cigarette smoking encourages the brain to switch off its own mechanism for making dopamine, so in the long term the dopamine level decreases, which in turn prompts individuals to smoke more. Prevention strategy may focus on decreasing the frequency of cigarette smoking among individuals who have depressive symptoms. Prevention and treatment programs for GAD may assess the extent of smoking among individuals who are depressed, and may offer treatment for depression as well as for smoking cessation. Cognitive behavioral therapy49 is effective in both the treatment of cigarette smoking and of depression. Acceptance commitment therapy50, 51 and combined pharmacotherapy/psychotherapy52 could be other approaches to consider.
From a public health perspective, our longitudinal study designed to examine the association between earlier joint trajectories of cigarette smoking and depressive symptoms and later GAD suggests that interventions to reduce cigarette smoking and depressive symptoms may lower the rates of GAD in the mid 30s. Federal, state, and/or city governments may consider funding to support programs for treatment including smoking cessation programs focused on reducing the use of cigarettes as well as on the treatment of depressive symptoms.
Future research is necessary to examine the association between the earlier joint trajectories of cigarette smoking and depressive symptoms and later GAD within larger and more diverse samples of individuals at different developmental stages.
Acknowledgments
This research was supported by NIH research grant DA005702, awarded to Dr. Judith S. Brook and by Career Development Award 1K01DA041609, granted to Dr. Jung Yeon Lee from the National Institute on Drug Abuse.
References
- 1.Boden JM, Fergusson DM, Horwood LJ. Anxiety disorders and suicidal behaviours in adolescence and young adulthood: findings from a longitudinal study. Psychological Medicine. 2007;37:431–440. doi: 10.1017/S0033291706009147. [DOI] [PubMed] [Google Scholar]
- 2.Schienle A, Ebner F, Schäfer A. Localized gray matter volume abnormalities in generalized anxiety disorder. European Archives of Psychiatry and Clinical Neuroscience. 2011;261:303–307. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- 3.Keller MB. The long-term clinical course of generalized anxiety disorder. The Journal of Clinical Psychiatry. 2002;63:11–16. [PubMed] [Google Scholar]
- 4.Wittchen H-U, Hoyer J. Generalized anxiety disorder: nature and course. Journal of Clinical Psychiatry. 2001;62:15–21. [PubMed] [Google Scholar]
- 5.Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depression and Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- 6.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJR, Fromm S, Charney DS, Leibenluft E, Ernst M. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 7.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. American Journal of Psychiatry. 2009 doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buss C, Pruessner JC, Mayberg H, Mletzko T, Nemeroff C, Heim C. Larger amygdala volumes after childhood trauma associated with depression and cortisol response to psychosocial stress in adulthood. European Journal of Psychotraumatology. 2012;3 [Google Scholar]
- 10.Mykletun A, Overland S, Aarø LE, Liabø H-M, Stewart R. Smoking in relation to anxiety and depression: evidence from a large population survey: the HUNT study. European Psychiatry. 2008;23:77–84. doi: 10.1016/j.eurpsy.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- 12.Mojtabai R, Crum RM. Cigarette smoking and onset of mood and anxiety disorders. American Journal of Public Health. 2013;103:1656–1665. doi: 10.2105/AJPH.2012.300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClave AK, Dube SR, Strine TW, Kroenke K, Caraballo RS, Mokdad AH. Associations between smoking cessation and anxiety and depression among US adults. Addictive Behaviors. 2009;34:491–497. doi: 10.1016/j.addbeh.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie CS, Reynolds K, Chou K-L, Pagura J, Sareen J. Prevalence and correlates of generalized anxiety disorder in a national sample of older adults. The American Journal of Geriatric Psychiatry. 2011;19:305–315. doi: 10.1097/JGP.0b013e318202bc62. [DOI] [PubMed] [Google Scholar]
- 15.Jamal M, Willem Van der Does A, Cuijpers P, Penninx BW. Association of smoking and nicotine dependence with severity and course of symptoms in patients with depressive or anxiety disorder. Drug and Alcohol Dependence. 2012;126:138–146. doi: 10.1016/j.drugalcdep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics. 1985;234:1–12. [PubMed] [Google Scholar]
- 17.Cougle JR, Zvolensky MJ, Fitch KE, Sachs-Ericsson N. The role of comorbidity in explaining the associations between anxiety disorders and smoking. Nicotine & Tobacco Research. 2010;12:355–364. doi: 10.1093/ntr/ntq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, Goldberg D, Gregory AM, Poulton R. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- 19.Deschênes SS, Burns RJ, Schmitz N. Associations between diabetes, major depressive disorder and generalized anxiety disorder comorbidity, and disability: findings from the 2012 Canadian Community Health Survey—Mental Health (CCHS-MH) Journal of Psychosomatic Research. 2015;78:137–142. doi: 10.1016/j.jpsychores.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, Tennant A. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS) BMC Psychiatry. 2012;12:6. doi: 10.1186/1471-244X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton PJ. Depression anxiety and stress scales (DASS-21): psychometric analysis across four racial groups. Anxiety, Stress, and Coping. 2007;20:253–265. doi: 10.1080/10615800701309279. [DOI] [PubMed] [Google Scholar]
- 22.Starcevic V. Pathological worry in major depression: A preliminary report. Behaviour Research and Therapy. 1995;33:55–56. doi: 10.1016/0005-7967(93)e0028-4. [DOI] [PubMed] [Google Scholar]
- 23.Blagden JC, Craske MG. Effects of active and passive rumination and distraction: A pilot replication with anxious mood. Journal of Anxiety Disorders. 1996;10:243–252. [Google Scholar]
- 24.Hermans H, Evenhuis HM. Factors associated with depression and anxiety in older adults with intellectual disabilities: results of the healthy ageing and intellectual disabilities study. International Journal of Geriatric Psychiatry. 2013;28:691–699. doi: 10.1002/gps.3872. [DOI] [PubMed] [Google Scholar]
- 25.Luger TM, Suls J, Vander Weg MW. How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addictive Behaviors. 2014;39:1418–1429. doi: 10.1016/j.addbeh.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Tjora T, Hetland J, Aarø LE, Wold B, Wiium N, Øverland S. The association between smoking and depression from adolescence to adulthood. Addiction. 2014;109:1022–1030. doi: 10.1111/add.12522. [DOI] [PubMed] [Google Scholar]
- 27.Lopez AD. Disease Control Priorities Project: Global burden of disease and risk factors. New York & Washington DC: Oxford Univeristy Press & World Bank; 2006. [Google Scholar]
- 28.Ouellet K, Bacon SL, Boudreau M, Plourde A, Moullec G, Lavoie KL. Individual and combined impact of cigarette smoking, anxiety, and mood disorders on asthma control. Nicotine & Tobacco Research. 2012;14:961–969. doi: 10.1093/ntr/ntr315. [DOI] [PubMed] [Google Scholar]
- 29.Brook DW, Brook JS, Zhang C. Joint trajectories of smoking and depressive mood: associations with later low perceived self-control and low well-being. Journal of Addictive Diseases. 2014;33:53–64. doi: 10.1080/10550887.2014.882717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion–smoking comorbidity. Psychological Bulletin. 2015;141:176. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angst J, Gamma A, Baldwin DS, Ajdacic-Gross V, Rössler W. The generalized anxiety spectrum: prevalence, onset, course and outcome. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:37–45. doi: 10.1007/s00406-008-0832-9. [DOI] [PubMed] [Google Scholar]
- 32.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral Science. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 33.DSM-5 Diagnostic and Statistical Manual of Mental Disorders. 5th. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 34.Muthé L, Muthén B. Mplus user’s guide. 6th. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 35.Van De Schoot R, Sijbrandij M, Winter SD, Depaoli S, Vermunt JK. The GRoLTS-Checklist: Guidelines for Reporting on Latent Trajectory Studies. Structural Equation Modeling: A Multidisciplinary Journal. 2016:1–17. [Google Scholar]
- 36.Pratt LA, Druss BG, Manderscheid RW, Walker ER. Excess mortality due to depression and anxiety in the United States: results from a nationally representative survey. General Hospital Psychiatry. 2016;39:39–45. doi: 10.1016/j.genhosppsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moylan S, Jacka FN, Pasco JA, Berk M. Cigarette smoking, nicotine dependence and anxiety disorders: a systematic review of population-based, epidemiological studies. BMC Medicine. 2012;10:123. doi: 10.1186/1741-7015-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience & Biobehavioral Reviews. 2012;36:764–785. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, Liu J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-α-lipoic acid. Investigative Ophthalmology & Visual Science. 2007;48:339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nature Reviews Neuroscience. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 41.Miyaoka H, Suzuki Y, Taniyama M, Miyaoka Y, Shishikura K, Kamijima K, Atsumi Y, Matsuoka K. Mental disorders in diabetic patients with mitochondrial transfer RNA (Leu) (UUR) mutation at position 3243. Biological Psychiatry. 1997;42:524–526. doi: 10.1016/S0006-3223(97)00280-1. [DOI] [PubMed] [Google Scholar]
- 42.Ayling E, Aghajani M, Fouche J-P, van der Wee N. Diffusion tensor imaging in anxiety disorders. Current Psychiatry Reports. 2012;14:197–202. doi: 10.1007/s11920-012-0273-z. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and symptoms of depression: same genes, different environments? Archives of General Psychiatry. 1987;44:451–457. doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- 44.Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin. 2010;136:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- 45.McCrae RR, Costa PT. NEO Inventories for the NEO Personality Inventory-3 (NEO PI-3), NEO Five-Factor Inventory-3 (NEO-FFI-3) and NEO Personality Inventory-revised (NEO PI-R): Professional Manual. Lutz, FL: Psychological Assessment Resources; 2010. [Google Scholar]
- 46.de Dios MA, Stanton CA, Caviness CM, Niaura R, Stein M. The social support and social network characteristics of smokers in methadone maintenance treatment. The American Journal of Drug and Alcohol Abuse. 2013;39:50–56. doi: 10.3109/00952990.2011.653424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brook JS, Lee JY, Rubenstone E, Finch SJ, Seltzer N, Brook DW. Longitudinal determinants of substance use disorders. Journal of Urban Health. 2013;90:1130–1150. doi: 10.1007/s11524-013-9827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ledgerwood DM, Goldberger BA, Risk NK, Lewis CE, Price RK. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addictive Behaviors. 2008;33:1131–1139. doi: 10.1016/j.addbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognitive Therapy and Research. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy. New York: Guilford Press; 1999. [Google Scholar]
- 51.Twohig MP. Acceptance and Commitment Therapy. Cognitive and Behavioral Practice. 2012;4:499–507. [Google Scholar]
- 52.Huhn M, Tardy M, Spineli LM, Kissling W, Förstl H, Pitschel-Walz G, Leucht C, Samara M, Dold M, Davis JM. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71:706–715. doi: 10.1001/jamapsychiatry.2014.112. [DOI] [PubMed] [Google Scholar]


