Abstract
Schizophrenia and bipolar disorder are serious neuropsychiatric disorders of uncertain etiology. Recent studies indicate that immune activation may contribute to the etiopathogenesis of these disorders. Numerous studies in animal models indicate that the mucosal microbiome may influence cognition and behavior by altering the functioning of the immune system. It is thus likely that the microbiome plays a role in human psychiatric disorders. The study of immune alterations and the microbiome in schizophrenia and bipolar disorder is in its infancy. Two recent investigations of the oro-pharyngeal microbiota in schizophrenia found differences between cases and controls. Other studies have found increased gastrointestinal inflammation in schizophrenia and bipolar disorder based on measures of microbial translocation. Several studies have also found an association between the receipt of antibiotics and an increased incidence of psychiatric disorders, perhaps due to alterations in the microbiome. Studies to characterize the intestinal microbiome of individuals with these disorders are in progress. The ultimate test of the role of the microbiome and immune-mediated pathology in schizophrenia and bipolar disorder will come from clinical trials of therapeutic agents which alter gut microbiota or gastrointestinal inflammation. The successful development of such modalities would represent a novel strategy to prevent and treat serious psychiatric disorders.
Keywords: immunity, microbiome, schizophrenia, bipolar disorder, gastrointestinal, antibiotics, probiotics
1. Introduction
Schizophrenia is a neuropsychiatric disorder with an onset typically in adolescence or young adulthood and a course which usually persists throughout the lifespan. Characteristic symptoms include hallucinations and delusions as well as apathy and social withdrawal; many affected individuals also have reduced cognitive abilities and impaired social functioning. Because the disorder disrupts multiple life domains and typically persists for decades, the global burden of disease is high (Whiteford, Ferrari, Degenhardt, Feigin, & Vos, 2015). Bipolar disorder is another serious mental illness and shares many features with schizophrenia including some of the characteristic symptoms and the lifelong course.(Dacquino, De Rossi, & Spalletta, 2015; Jobe & Harrow, 2005). Both disorders are categorized by their phenotypic features rather than any biological markers and their etiology is not fully understood. Genome-wide association studies show a great deal of genetic overlap between schizophrenia and bipolar disorder (Lichtenstein et al., 2009; Van Snellenberg & de Candia, 2009) However, while genetic factors are involved in both disorders, risk genes which have been identified account for a small portion of disease risk. For example, a recent genome wide study in schizophrenia found 108 independent loci that account for approximately 7% of the risk of developing schizophrenia from polygenic scores, (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) Of note, many of the genetic loci that were identified are known to modulate inflammation and the immune response.
Previous studies have demonstrated that both schizophrenia and bipolar disorder are associated with alterations of the systemic immune system including low-grade chronic inflammation (increased plasma cytokines, soluble cytokine receptors, chemokines, acute phase reactants) and T-cell activation features; these findings are delineated in previously-published review articles(Anderson & Maes, 2015).(Rosenblat, Cha, Mansur, & McIntyre, 2014) (Leboyer et al., 2016). The immune system provides a two way communication pathway between the gut and the brain via the vagus nerve, short chain fatty acids, and a number of soluble mediators(Erny, de Angelis, & Prinz, 2016; Hyland & Cryan, 2016; Levite, 2016; Sherwin, Sandhu, Dinan, & Cryan, 2016). It has been established that the gut microbiota can influence brain function and thus may play a role in diseases such as schizophrenia and bipolar disorder which are traditionally seen as brain-based (Fond et al., 2015).
The study of the microbiome is relatively new and most of the investigations to date have taken place in animal models. Multiple studies have documented an interaction between the gut microbiome, immunity, cognitive functioning and behavior in a number of models, most of which involve rodents (Desbonnet et al., 2015). Studies linking these findings to human psychiatric disorders are more limited.
2. Scope of review
The purpose of this article is to summarize what is known about immune alterations and the microbiome based on human studies in schizophrenia and bipolar disorder. This field of inquiry is still in its infancy and the number of studies to date is small. However, the groundwork is being laid to better understand immune abnormalities which contribute to the etiology of these major psychiatric disorders and to identify how knowledge of the microbiome might result in novel methods for the treatment of these disorders.
3. Results
Research about immune alterations and the microbiome in schizophrenia and bipolar disorder falls into several categories as described below.
3.1. Studies of the oropharyngeal microbiota in schizophrenia
There have been numerous studies of the fecal microbiome in otherwise healthy children and adults (Collado, Rautava, Isolauri, & Salminen, 2015; Lozupone, Stombaugh, Gordon, Jansson, & Knight, 2012). However the collection and prompt processing of fecal samples from individuals with severe psychiatric disorders is problematic. Published studies analyzing the fecal microbiome of individuals with schizophrenia are currently lacking. The oropharyngeal microbiome can be assessed from throat swab samples which are more easily accessed than samples from the gastrointestinal tract and thus allow potentially for larger sample sizes. Furthermore, while there are many differences in the microbial composition of the fecal and oral microbiome, some studies have documented overlapping metabolic pathways in the different sites (Segata et al., 2012). For this reason many of the studies in our population have focused on the oral microbiome. Furthermore, we have relied on metagenomic sequencing rather than the commonly used 16S sequencing since studies have documented a role for viruses (Houenou et al., 2014), fungi(Severance et al, 2016) and protozoa (Torrey, Bartko, & Yolken, 2012) in the pathogenesis of the psychiatric disorders.
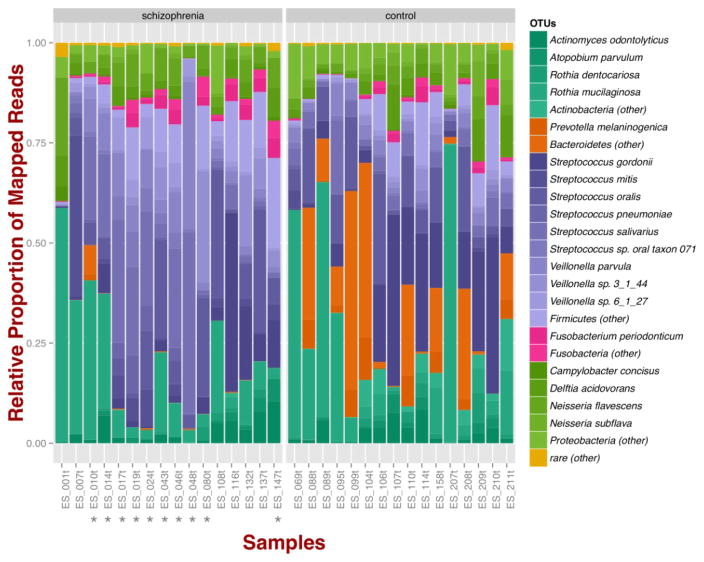
A meta-genomic analysis of the oropharyngeal microbiome in 16 adults with schizophrenia and 16 non-psychiatric controls found differences at both the phylum and the genus levels.(Castro-Nallar et al., 2015) At the phylum level, schizophrenia samples exhibited higher proportions of Firmicutes across samples in comparison to controls; in the controls a higher relative proportion of Badteroidetes and Actinobacteria was observed. Regarding species diversity, controls were richer in the number of species compared to schizophrenia samples but less even in their distribution (Figure 1).
Figure 1. Oropharyngeal microbial composition at phylum and species levels exhibits different patterns for schizophrenia and control samples.
The stacked bar chart shows the most prevalent species present in schizophrenia and controls color-coded by phylum. Green, Actinobacteria; Orange, Bacteroidetes; Blue, Firmicutes; Green, Proteobacteria. The symbol (*) indicates samples from smoker individuals. (Reprinted from Castro-Naller et al PubMed 26336637)
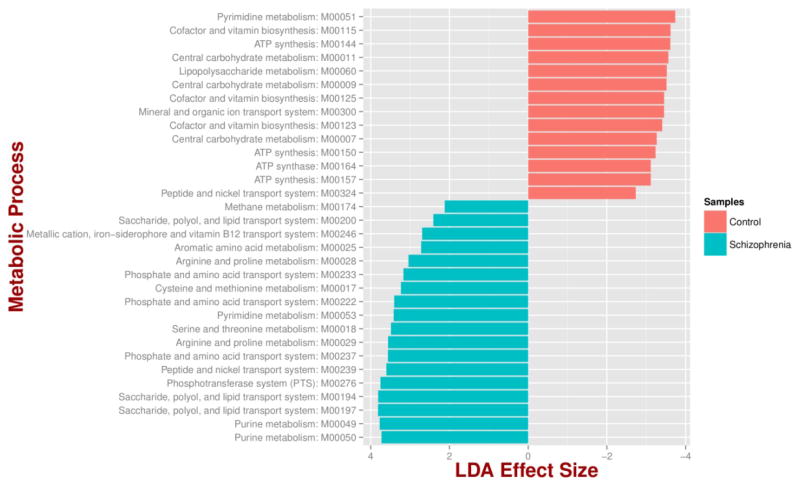
Out of a total of 25 differentially abundant species (bacteria and fungi), 6 microbial species were more abundant in cases than controls after adjusting for relevant covariates. Lactic acid bacteria were relatively more abundant in schizophrenia including Lactobacillus and Bifidobacterium with the largest effect found in Lactobacillus gasseri which appeared to be at least 400 times more abundant in schizophrenia patients than in controls. The study also found that 18 metabolic pathways that were enriched and 14 decreased in schizophrenia relative to controls. Pathways that were significantly altered in schizophrenia were related to environmental information processes such as saccharide, polio, and lipid transport systems (Figure 2).
Figure 2. Microbial metabolic pathways with significantly altered abundances in the schizophrenia oropharyngeal microbiome.
MXXXXX codes correspond to KEGG modules, i.e., a collection of manually defined functional units (genes). LDA, linear discriminant analysis. (Reprinted from Castro-Naller et al. PubMed 26336637)
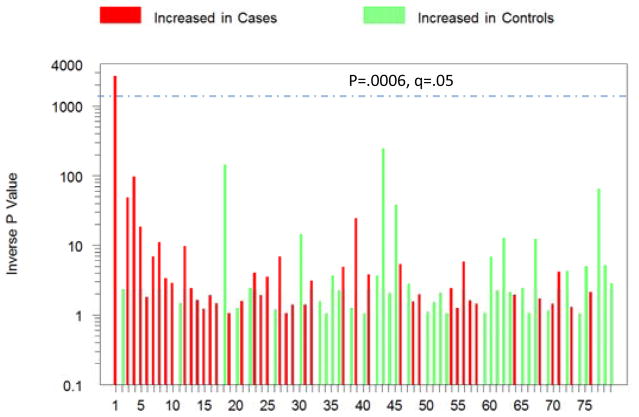
Another study of the oropharyngeal microbiome focused on bacteriophages, viruses that infect bacteria and alter their metabolism and replication, in samples from 41 adults with schizophrenia and 33 non-psychiatric controls.(R. H. Yolken et al., 2015) Of the 79 distinct bacteriophage samples that were identified, one, Lactobacillus phage phiadh, was significantly more abundant in schizophrenia cases than in controls after adjustment for multiple comparisons and demographic covariates (Figure 3). Interestingly the group differences were larger for the phage than for its host bacteria underscoring the importance of examining viral sequences in studies of the microbiome relating to psychiatric disorders.
Figure 3. P values representing different levels of phages in 41 individuals with schizophrenia and 33 controls without a psychiatric disorder.
The red and green colors indicate levels of the individual phages which are increased or decreased in cases, respectively. The dashed line indicates p<.05 corrected for multiple comparisons. (Reprinted from Yolken et al. Pub Med27425597)
Within the schizophrenia group, the level of this phage was significantly associated with the presence of immunological disorders such as diabetes, which are common co-morbid conditions in individuals with schizophrenia (Schoepf, Uppal, Potluri, & Heun, 2014). The level of Lactobacillus phage phiadh was also relatively increased in individuals who were being treated with therapeutic valproate, a medication commonly used for the adjunctive treatment of schizophrenia (Tseng et al., 2016). This finding is of interest since valproate has been shown to modify the microbiome in mouse models of autism in the context of in utero exposure, probably related to us homology with short chain fatty acids(de Theije et al., 2014)). This finding is of note since the mechanisms by which valproate improves the symptoms in some individuals with schizophrenia was not previously known. This finding suggests that other molecules which alter the microbiome may be found that are effective as adjunct therapies for schizophrenia, including ones with less toxicity than valproate (Haddad, Das, Ashfaq, & Wieck, 2009).
3.2. Studies of intestinal inflammation in schizophrenia and bipolar disorder
Gastrointestinal (GI) pathologies are long-standing comorbidities of psychiatric disorders, supporting the centuries old hypotheses that gut and brain physiologies are inter-dependent (Severance, Prandovszky, Castiglione, & Yolken, 2015). In schizophrenia and bipolar disorder, a low-grade inflammatory state is prevalent in a subset of individuals (Bechter, 2013; Fillman, Sinclair, Fung, Webster, & Shannon Weickert, 2014; Miller, Buckley, Seabolt, Mellor, & Kirkpatrick, 2011) The origin of this inflammation is not currently well understood, but recent as well as older studies suggest that it stems from processes related to dysbiosis of the gut microbiome. This dysbiosis provides a mechanism to generate a GI-based inflammatory state through the process of microbial translocation of gut microbes into systemic circulation.
One of the earliest specific documentations of GI inflammation associated with schizophrenia was a post-mortem study of 82 individuals with schizophrenia, where researchers found that 50% had gastritis, 88% enteritis and 92% colitis.(Buscaino, 1953) Interestingly, a converse phenomenon also holds true with reports of psychiatric comorbidities in people with intestinal disorders which have an inflammatory component. Prevalence estimates for any psychiatric comorbidity in patients diagnosed with irritable bowel syndrome (IBS), for example, range from 54–94% (Whitehead, Palsson, & Jones, 2002), and specifically estimates for a schizophrenia comorbidity approach 20%(Gupta, Masand, Kaplan, Bhandary, & Hendricks, 1997). In a large-scale case-control cohort of 4689 IBS patients and 18756 matched controls without IBS, a diagnosis of IBS increased the risk for anxiety and mood disorders as well (Lee et al., 2015). Collectively, these epidemiological studies illustrate that GI inflammation and psychiatric disorders are connected. However the delineation of the magnitude of the correlation is limited by the difficulty in making an accurate diagnosis of intestinal diseases in individuals with psychiatric symptoms and the possible confounding effects of medications.
Numerous biological indices corroborate a role for GI inflammation in schizophrenia and bipolar disorder pathophysiology. GI-derived inflammation is often measured based on biomarkers of the microbial translocation process. The panel of markers used to diagnose Crohn’s Disease for example includes detection of antibodies to the yeast Saccharomyces cerevisiae, an organism that is part of the normal human gut microbiome (Desplat-Jego et al., 2007) The presence of antibodies to this yeast indicates that an immune response has been generated against the organism presumably due to its presence at a potentially compromised gut mucosa-blood vasculature interface. Elevated antibodies to S. cerevisiae were found in individuals with schizophrenia and bipolar disorder and these levels were particularly increased in individuals experiencing a recent onset of their disorder (Severance et al., 2012; Severance, Gressitt, et al., 2014). Furthermore, antibody levels were significantly higher in those with schizophrenia who were antipsychotic-naïve than in those who were medicated suggesting that the relationship between these disorders and gastrointestinal inflammation cannot be attributed solely to the effect of antipsychotic medications. In a follow-up study of a different commensal yeast species, Candida albicans, antibody levels were not only significantly increased in subsets of individuals with schizophrenia, but were particularly elevated in those who had GI symptoms(Severance, Gressitt, et al., 2016). In a similar investigation, markers of bacterial translocation were altered in individuals with schizophrenia and to a certain degree bipolar disorder (Severance et al., 2013).
The theme for translocation of microbial components into circulation via a breached gut barrier can be expanded to include other GI-derived substances such as digested foods. There is a long literature on the anti-milk casein and anti-wheat gluten immune response associated with schizophrenia and a sensitivity to these foods is known to also generate an inflammatory response in the intestinal tract (Severance, Yolken, & Eaton, 2014). Finally, exposure to the neurotropic protozoan pathogen, Toxoplasma gondii, is a well-studied risk factor for the development of schizophrenia(Torrey et al., 2012). Interestingly, T. gondii enters and establishes itself in its host via the intestinal tract and is used in experimental animal models to generate intestinal disorders associated with inflammatory processes (Bereswill et al., 2010). In human studies, antibodies to this parasite were significantly associated with markers of food sensitivity in those with schizophrenia (Severance et al., 2012). Thus, it cannot be ruled out that the association of exposure to this parasite with psychiatric disorders may be a function of its pathological effects in the gut.
3.3. Study of bacterial infections and antimicrobial agents in acute mania
Bacterial infections are a source of immune activation and have been shown to be a risk factor for the subsequent development of schizophrenia and mood disorders(Benros, Mortensen, & Eaton, 2012; Benros et al., 2013; Nielsen, Benros, & Mortensen, 2013) Consistent with some previous population-based studies (Kohler et al., 2014), a recent study employed the prescription of antibiotic agents as a measure of bacterial infections. The study population consisted of 234 individuals hospitalized for acute mania, most diagnosed with bipolar disorder, in either an inpatient unit or a day hospital and also individuals hospitalized for schizophrenia, bipolar depression, major depression, as well as non-psychiatric controls(R. Yolken et al., 2016). The study found that in patients with acute mania, but not those hospitalized for the other conditions, had a substantially increased rate of recent antimicrobial prescription when adjusting for demographic variables. Within the mania group, the prescription of antibiotics was associated with having increased mania symptom severity but not with other clinical ratings. The urinary tract was the most common site of infection in women while the respiratory tract and mucosal surfaces were the most common sites in men. An association between antibiotic exposure and mood disorder has also been found in population based studies performed in the United Kingdom (Lurie, Yang, Haynes, Mamtani, & Boursi, 2015) and Denmark (Kohler et al., 2016).
There are several mechanisms by which antibiotic usage might be associated with episodes of acute mania. One possibility is that the underlying bacterial infections responsible for the antibiotic prescriptions result in immune activation which then leads to the onset of mania. A second possibility is that the higher rate of presumed bacterial infections in individuals with mania is reflective of decreased levels of ability of the immune system to prevent infections in this population. A third possibility is that the administration of antibiotics can result in changes in the microbiome which themselves increased the risk of altered mood states. This finding is consistent with a number of studies in animal models linking the microbiome to altered behavior and cognition. However, the possible role of alterations in the microbiome in the onset of mania in this study population was rendered less likely by the fact that, in many cases, antibiotics were not administered to patients until after they had been admitted to the hospital. However, the possibility that the microbiome may have been altered in these individuals by past administration of antibiotics cannot be excluded. It is of note that these proposed mechanisms of action are not mutually exclusive but might be interacting in different degrees to result in mania or other psychiatric symptoms in different individuals.
3.4. Trials of probiotic compounds in schizophrenia and bipolar disorder
Another research strategy to probe the role of the microbiome in schizophrenia and bipolar disorder involves clinical trials with compounds that may alter the gut microbiome and modulate the immune response and thus potentially have an effect on psychiatric illness symptoms. Probiotic compounds provide a safe and well tolerated means for the modulation of the immune response to harmful antigens such as food-derived proteins. Probiotics have been studied in animal models and have shown benefits in trials of individuals with some gastrointestinal disorders and allergic conditions (De Angelis et al., 2006; Guerra et al., 2011; Saulnier, Kolida, & Gibson, 2009).
One of the first trials of probiotic compounds in schizophrenia involved an add-on probiotic compound (combined Lactobacillus rhammosus strain GG and Bifidobacterium animals subsp. Lactis strain Bb12 (F. B. Dickerson et al., 2014). Results showed no significant difference in psychiatric symptom severity between probiotic and placebo supplementation at the end of the trial thought those patients who received the probiotic compound vs. the placebo were less likely to develop severe bowel difficulty over the course of the trial, consistent with an effect of probiotics on the gastrointestinal tract. In addition, the probiotic supplementation did significantly alter the levels of several serum proteins assessed before and after the trial including von Willebrand factor and brain-derived neurotrophic factor (Tomasik, Yolken, Bahn, & Dickerson, 2015). Probiotic treatment also lowered the level of antibodies to the fungus Candida albicans and associated gastrointestinal symptoms in male individuals in the trial (Severance, Gressit, et al., 2016).
Another trial of probiotic supplementation is underway in psychiatric patients, this one in mania. This trial is based on a longitudinal observational study of individuals with acute mania, which found that levels of immune makers, IgG antibodies to gliadin, a measure of gluten sensitivity and levels of antibodies to the NR2 peptide of the NMDA receptor, were significantly increased during the acute manic episode but did not differ from the levels of controls at a 6 month follow up(F. Dickerson, Stallings, Origoni, et al., 2012; F. Dickerson, Stallings, Vaughan, et al., 2012) (F. Dickerson et al., 2013) In addition, a combined inflammation score, calculated by factor analysis of the levels of class specific antibodies to the NR peptide of the NMDA receptor, gliadin, Mason-Pfizer monkey virus protein 24, and Toxoplasma gondii, differed from the controls during the acute episode but not at the 6 month follow up(F. Dickerson et al., 2013). In addition, within the mania group, an elevated inflammation score during the acute episode predicted rehospitalization for a new mood episode during the follow-up period. Clinical trials of therapeutic agents which alter gut microbiota or gastrointestinal inflammation will be the ultimate test of the role of the microbiome and immune-mediated pathology in schizophrenia and bipolar disorder
4. Limitations in Current Knowledge
There are a number of limitations in the current state of knowledge regarding the role of the microbiome in etiopathogenesis of schizophrenia and bipolar disorder. These include the following
There are as yet no published descriptions of the fecal or intestinal microbiome of individuals with schizophrenia or bipolar disorder. This limitation is undoubtedly related to the difficulty of obtaining relevant sample in this study population. Of particular importance in this regard would be longitudinal studies in which changes in the fecal or intestine microbiome could be studies over time. Such studies could also address the effects of environmental factors such as medications (Bahra et al., 2015), hospitalization, cigarette smoking and living conditions on the composition of the microbiome. Since there are also differences in the microbiome at different levels of the human gastrointestinal tract, studies of the microbial composition of the small bowel and colon in individuals with psychiatric disorders might provide information supplementary to that obtained from analyses of fecal samples in this population.
It is not known whether changes in the microbiota associated with schizophrenia and bipolar disorder are state or trait related and how the microbiome may be involved in mood switching in bipolar disorder and in psychotic exacerbations in schizophrenia.
Animal studies directed specifically at models of serious psychiatric disorders are limited. Of particular importance would be the interaction of immune related risk genes such as those involved in the complement system (Xiao et al., 2016) with the composition of the microbiome. Animal models would also be useful to better define the effects of psychiatric medications on the composition of the microbiome in terms of both psychiatric activity and side effects such as weigh gain (Bahr et al., 2015).
Conclusions and Perspectives
Studies to date indicate a role for the microbiome in the etiopathogenesis of serious human psychiatric disorders such as schizophrenia and bipolar disorder. While animal models focus on the bacterial composition of the intestinal tract, studies to date in individuals with psychiatric disorders also point to the possible role of viruses and fungi as well as the involvement of other mucosal body sites such as the nasopharynx. Critical research needs include prospective studies to define risk prior to the onset of therapeutic interventions as well as methods for the accurate assessment of the microbiome at different mucosal sites in a practical manner. There is also a need for effective modalities for the modulation of the microbiome and the control of inflammation in a population of individuals who are taking a range of psychiatric medications. Despite these limitations the analysis of the microbiome in individuals with psychiatric disorders and the development of methods for its modulation offer great promise in terms of developing new methods for the prevention and treatment of these devastating disorders.
Acknowledgments
Funding
This work was supported by the Stanley Medical Research Institute (grant #7R-1690) and by the NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant #MH-94268).
References
- Anderson G, Maes M. Bipolar disorder: role of immune-inflammatory cytokines, oxidative and nitrosative stress and tryptophan catabolites. Curr Psychiatry Rep. 2015;17(2):8. doi: 10.1007/s11920-014-0541-1. [DOI] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, … Calarge CA. Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry. 2015;5:e652. doi: 10.1038/tp.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahra SM, Weidemann BJ, Castro AN, Walsh JW, deLeon O, Burnett CM, … Kirby JR. Risperidone-induced weight gain is mediated through shifts in the gut microbiome and suppression of energy expenditure. EBioMedicine. 2015;2(11):1725–1734. doi: 10.1016/j.ebiom.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:71–91. doi: 10.1016/j.pnpbp.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, … Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaino Patologia extraneurale della schizofrenia. Fegato, tubo digerente, sistema reticolo-endoteliale. Acta neurologica VIII. 1953:1–60. [Google Scholar]
- Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, … Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Isolauri E, Salminen S. Gut microbiota: a source of novel tools to reduce the risk of human disease? Pediatr Res. 2015;77(1–2):182–188. doi: 10.1038/pr.2014.173. [DOI] [PubMed] [Google Scholar]
- Dacquino C, De Rossi P, Spalletta G. Schizophrenia and bipolar disorder: The road from similarities and clinical heterogeneity to neurobiological types. Clin Chim Acta. 2015 doi: 10.1016/j.cca.2015.02.029. [DOI] [PubMed] [Google Scholar]
- De Angelis M, Rizzello CG, Fasano A, Clemente MG, De Simone C, Silano M, … Gobbetti M. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for Celiac Sprue. Biochim Biophys Acta. 2006;1762(1):80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, … Oozeer R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun. 2014;37:197–206. doi: 10.1016/j.bbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, … Cryan JF. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Johanet C, Escande A, Goetz J, Fabien N, Olsson N, … Humbel RL. Update on Anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: results of a multicenter study. World J Gastroenterol. 2007;13(16):2312–2318. doi: 10.3748/wjg.v13.i16.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, … Yolken RH. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;16(1) doi: 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Katsafanas E, Khushalani S, Yolken R. A combined marker of inflammation in individuals with mania. PLoS One. 2013;8(9):e73520. doi: 10.1371/journal.pone.0073520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Markers of gluten sensitivity in acute mania: a longitudinal study. Psychiatry Res. 2012;196(1):68–71. doi: 10.1016/j.psychres.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Vaughan C, Origoni A, Khushalani S, Yolken R. Antibodies to the glutamate receptor in mania. Bipolar Disord. 2012;14(5):547–553. doi: 10.1111/j.1399-5618.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- Erny D, de Angelis AL, Prinz M. Communicating systems in the body: how microbiota and microglia cooperate. Immunology. 2016 doi: 10.1111/imm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry. 2014;4:e365. doi: 10.1038/tp.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, … Leboyer M. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris) 2015;63(1):35–42. doi: 10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Guerra PV, Lima LN, Souza TC, Mazochi V, Penna FJ, Silva AM, … Guimaraes EV. Pediatric functional constipation treatment with Bifidobacterium-containing yogurt: a crossover, double-blind, controlled trial. World J Gastroenterol. 2011;17(34):3916–3921. doi: 10.3748/wjg.v17.i34.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Masand PS, Kaplan D, Bhandary A, Hendricks S. The relationship between schizophrenia and irritable bowel syndrome (IBS) Schizophr Res. 1997;23(3):265–268. doi: 10.1016/s0920-9964(96)00099-0. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Das A, Ashfaq M, Wieck A. A review of valproate in psychiatric practice. Expert Opin Drug Metab Toxicol. 2009;5(5):539–551. doi: 10.1517/17425250902911455. [DOI] [PubMed] [Google Scholar]
- Houenou J, d’Albis MA, Daban C, Hamdani N, Delavest M, Lepine JP, … Leboyer M. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:142–148. doi: 10.1016/j.pnpbp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Hyland NP, Cryan JF. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016 doi: 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Jobe TH, Harrow M. Long-term outcome of patients with schizophrenia: a review. Can J Psychiatry. 2005;50(14):892–900. doi: 10.1177/070674370505001403. [DOI] [PubMed] [Google Scholar]
- Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12):1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kohler O, Petersen L, Mors O, Mortensen PB, Yolken R, Gasse C, Benros ME. Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study. Acta Psychiatrica Scandinavica. 2016 doi: 10.1111/acps.12671. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D, Groc L. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med. 2016;14(1):173. doi: 10.1186/s12916-016-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YT, Hu LY, Shen CC, Huang MW, Tsai SJ, Yang AC, … Hung JH. Risk of Psychiatric Disorders following Irritable Bowel Syndrome: A Nationwide Population-Based Cohort Study. PLoS One. 2015;10(7):e0133283. doi: 10.1371/journal.pone.0133283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M. Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases. Acta Physiol (Oxf) 2016;216(1):42–89. doi: 10.1111/apha.12476. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/s0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry. 2015;76(11):1522–1528. doi: 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PR, Benros ME, Mortensen PB. Hospital Contacts With Infection and Risk of Schizophrenia: A Population-Based Cohort Study With Linkage of Danish National Registers. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23–34. doi: 10.1016/j.pnpbp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Saulnier DM, Kolida S, Gibson GR. Microbiology of the human intestinal tract and approaches for its dietary modulation. Curr Pharm Des. 2009;15(13):1403–1414. doi: 10.2174/138161209788168128. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepf D, Uppal H, Potluri R, Heun R. Physical comorbidity and its relevance on mortality in schizophrenia: a naturalistic 12-year follow-up in general hospital admissions. Eur Arch Psychiatry Clin Neurosci. 2014;264(1):3–28. doi: 10.1007/s00406-013-0436-x. [DOI] [PubMed] [Google Scholar]
- Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, … Izard J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13(6):R42. doi: 10.1186/gb-2012-13-6-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, … Yolken RH. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressit KL, Stallings C, Katsafanas E, Savage C, Schweinfurth L, … Yolken R. Probioitc normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain, Behavior, and Immunity. 2016 doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, … Yolken RH. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016;2:16018. doi: 10.1038/npjschz.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, … Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148(1–3):130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Yang S, Stallings CR, Origoni AE, Vaughan C, … Yolken RH. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disord. 2014;16(3):230–240. doi: 10.1111/bdi.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17(5):27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the Force Be With You: The Light and Dark Sides of the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs. 2016 doi: 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights. 2015;10:47–54. doi: 10.4137/bmi.s22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38(3):642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng PT, Chen YW, Chung W, Tu KY, Wang HY, Wu CK, Lin PY. Significant Effect of Valproate Augmentation Therapy in Patients With Schizophrenia: A Meta-analysis Study. Medicine (Baltimore) 2016;95(4):e2475. doi: 10.1097/md.0000000000002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2009;66(7):748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820. doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- Xiao J, Li Y, Gressitt KL, He H, Kannan G, Schultz TL, … Severance EG. Cerebral complement C1q activation in chronic Toxoplasma infection. Brain Behav Immun. 2016;58:52–56. doi: 10.1016/j.bbi.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R, Adamos M, Katsafanas E, Khushalani S, Origoni A, Savage C, … Dickerson F. Individuals hospitalized with acute mania have increased exposure to antimicrobial medications. Bipolar Disord. 2016 doi: 10.1111/bdi.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, … Dickerson FB. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull. 2015;41(5):1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]