Abstract
A revolution in the analysis of seven transmembrane (7TM) receptors has provided detailed information about how these physiologically important signalling proteins interact with extracellular cues. However, it has proven much more challenging to understand how they convey information to their principal intracellular targets: heterotrimeric G proteins, G protein-coupled receptor kinases, and arrestins. Recent structures now suggest a common mechanism that enables structurally diverse cytoplasmic proteins to hitch a ride on hundreds of different activated 7TM receptors in order to instigate physiological change.
Introduction
Seven transmembrane domain (7TM) receptors are integral membrane proteins capable of recognizing an extraordinary variety of ligands (Figure 1) and relaying this event across lipid bilayers to a much smaller set of signalling proteins inside the cell. Because many 7TM receptors transduce signals to heterotrimeric G proteins, they are commonly referred to as G protein-coupled receptors (GPCRs). However, this term is misleading because these receptors also interact with at least two other protein families in an activation dependent manner, the GPCR kinases (GRKs) and arrestins, which are responsible for cellular responses distinct from those of heterotrimeric G proteins 1, 2 (Box 1). The physiological importance of 7TM receptors is evidenced by their great expansion in the genomes of complex eukaryotes, with around 1000 different members typically found in mammalian species (humans have around 800 members) 3. In general, 7TM receptors control strikingly diverse physiological phenomena ranging from glucose metabolism to blood pressure to neurotransmission, but a large fraction are involved in sensory perception, where individual genes have evolved to encode receptors recognizing specific classes of odorants, tastants, or wavelengths of light. Most mammalian 7TM receptors can be classified by sequence homology in their transmembrane spans into one of five families 3, 4: glutamate, rhodopsin, adhesion, frizzled, and secretin (Figure 1). The rhodopsin family (also known as Class A) is the largest and most diverse in terms of its ligand binding repertoire, and is further subdivided into α, β, δ, and γ subfamilies. The α subfamily contains receptors responsive to most of the biogenic amines (e.g. epinephrine and acetylcholine), and, because it includes the photoreceptor rhodopsin, is the best characterized functionally and structurally 5. The other rhodopsin subfamilies are primarily peptide or odorant receptors. Although signalling by all five 7TM receptor families has been shown to be dependent on heterotrimeric G proteins, direct coupling still remains to be definitively shown for the frizzled family 6. The ability of their extracellular domains to interact with small molecules and the profound physiological consequences of these interactions have rendered 7TM receptors the targets of a substantial fraction of currently prescribed drugs 7. Understanding the molecular basis of their function is thus an important step towards understanding human physiology and disease.
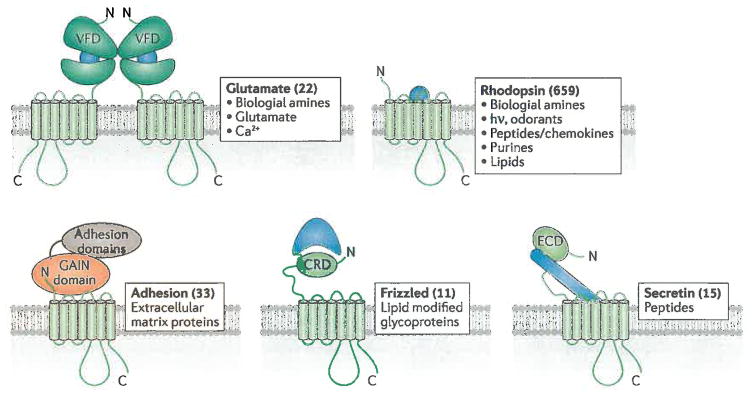
Figure 1.
7TM receptor families3 and their ligands. 7TM receptor families all possess a characteristic arrangement of 7 transmembrane spans, but often diverse extracellular domains. Numbers in parentheses correspond to the number of receptors identified in each family in humans 4 and some representative activating lignads are listed for each group. Odorant receptors (388 in number) are included in the rhodopsin family. Not shown are the taste2 receptors (25 in number), whose transmembrane domains most closely resemble those of the frizzled family, but lack analogous extracellular domains. Glutamate family receptors dimerize through their VFD domains. The GAIN domain of adhesion receptors has autoproteolytic activity that cleaves its extracellular domain such that the mature protein exists as two non-covalently associated subunits. Blue shapes represent the mode by which agonists interact with the extracellular regions in each receptor family. The frizzled ligand is covalently modified by a palmitoyl group (jagged line), which forms part of the interface with the CRD domain. VFD, Venus fly-trap domain; CRD, cysteine-rich domain; ECD, extracellular domain; GAIN, GPCR autoproteolysis inducing.
Box 1. Interaction of 7TM receptors with downstream signalling partners.
7TM receptors interact, in an agonist dependent manner (R* indicates agonist bound receptor), with at least three families of intracellular proteins: heterotrimeric G proteins, G protein-coupled receptor kinases (GRKs), and arrestins. In each case, the activated receptor, in collaboration with the local membrane environment, drives a conformational change in the signalling partner that instigates downstream signalling events. For heterotrimeric G proteins (a), it is the exchange of bound GDP for GTP on Gα, which reorganizes a structural element known as Switch II (red helix) in its Ras-like domain that interacts directly with effector enzymes, which include adenylyl cyclase, phospholipase Cβ (PLCβ), cGMP phosphodiesterase and Rho family guanine exchange factors (GEFs). This nucleotide exchange also results in the dissociation of the Gβγ subunit from Gα thereby enabling its own signalling pathways, including the regulation of G protein-coupled inwardly-rectifying potassium (GIRK) channels, GRK 2 and 3, phosphoinositide 3-kinase γ (PI3Kγ), phosphatidylinositol 3,4,5-trisphosphate-dependent Rac exchanger 1 protein (P-Rex1) and PLCβ and ε. For GRKs (b), this conformational change involves stabilization of the active conformation of its kinase domain which uses ATP to phosphorylate multiple serine and threonine residues on the receptor itself, but also potentially on nearby proteins including non-active receptors in a process called. high gain phosphorylation, which inhibits nearby receptors from signalling in response to future agonist exposure 83. 7TM receptor phosphorylation then facilitates arrestin binding to the receptor, which results in conformational changes in arrestin (c), including rearrangement of its polar core, a change in orientation of its N and C domains, and release of its C-terminal tail. These changes provide binding sites for endocytotic machinery on its C-terminus and grants access to other signalling proteins such as MAP kinases on its N and C domains. The C domain has also been proposed to interact with the lipid bilayer in the receptor-bound state, but the importance of such an interaction is as of now unknown.

This review discusses recent insights provided by X-ray crystallography and complementary techniques into how activated 7TM receptors interact with and transmit extracellular signals to their cytoplasmic signalling partners. Focusing on the interactions with heterotrimeric G proteins and arrestins, this review presents a common mechanism by which intracellular signalling components ‘hitch a ride’ on activated receptors by extending a structural ‘thumb’ that interacts with an exposed pocket in the cytoplasmic domain of the receptor. In each case, receptor engagement stabilizes an allosteric change in the hitchhiking protein that instigates intracellular signalling cascades.
Signalling by 7TM receptors
The most well-known property of 7TM receptors is to catalyse the exchange of GDP bound to the heterotrimeric G protein α subunit (Gα)–for GTP. Subsequently, Gα–GTP dissociates from the heterotrimeric G protein βγ subunits (Gβγ) and both components can then interact with downstream effector targets (Box 1). Hydrolysis of GTP on Gα returns the subunit to a state with high affinity for Gβγ, ultimately regenerating the inactive Gαβγ heterotrimer. A single activated receptor can turnover multiple heterotrimeric G protein complexes. For example, each photoactivated molecule of rhodopsin is thought to turn over hundreds of transducin (Gt) heterotrimers to instigate the visual response 8. GRKs and arrestins also interact preferentially with the activated form of 7TM receptors, and together orchestrate receptor uncoupling from heterotrimeric G proteins and receptor internalization in a process called homologous desensitization 9. GRKs phosphorylate most 7TM receptors at multiple serine and threonine residues, primarily in their extended C-terminal tails or third intracellular loops10, which enhances the affinity of arrestins. Arrestin binding then blocks access of heterotrimeric G proteins 11, targets the receptor for clathrin-mediated endocytosis 12, 13, and instigates unique signalling cascades including those of MAP kinases 14 and Src 15 (Box 1).
Understanding the mechanisms by which agonists promote the activated state of a 7TM receptor remains one of the most important holy grails of modern molecular pharmacology. This quest has taken on new importance with the recent discovery that some agonists can preferentially promote recruitment of one cytoplasmic partner over another, and hence differentially modulate downstream signalling via the same receptor–a concept known as biased agonism or functional selectivity 16, 17. For example, morphine, which acts at opioid receptors, not only provides pain relief (analgesia) but also causes constipation and dependency, which are undesirable side effects. However, mice lacking arrestin3 exhibit analgesia in response to morphine but reduced constipation and tolerance.18 Thus, there is great interest in developing analgesics that activate G protein signalling without recruiting arrestin319. The simplest molecular explanation for the phenomenon of biased signalling is that some ligand-stabilized receptor conformations are more compatible with heterotrimeric G proteins than other signalling partners, and vice versa.
The 7TM receptor structural revolution
Rhodopsin is an unusual member of the 7TM family in that it is covalently attached to a light-reactive ligand, 11-cis-retinal, which effectively serves as an inverse agonist that keeps basal signalling very low in the absence of light. Conformational stabilization of rhodopsin by 11-cis-retinal, along with its natural abundance in vertebrate retinas, facilitated its structural characterization long before other, more conformationally labile 7TM receptors (Supplementary information S1(Figure))20. In 1993, rhodopsin isolated from bovine and frog retinas was crystallized in thin, ordered sheets, and then analysed by electron crystallography to reveal electron density for each of the seven helical transmembrane spans (referred to as TM1–TM7), which confirmed the predicted topology of a 7TM receptor 21, 22. It took seven more years to obtain a three-dimensional structure, which revealed the presence of a short eighth cytoplasmic helix (α8) that bears palmitoylation sites in rhodopsin and many other 7TM receptors 23. Another seven years passed before the atomic structure of another 7TM receptor, the hormone responsive β2-adrenergic receptor (β2AR), was reported 24, 25. At the time of submission, 34 unique 7TM receptors have been characterized, described by 138 unique entries within the Protein Data Bank (Supplementary information S1 (Figure), Supplementary information S2 (Table)). There are now representative crystal structures for the transmembrane domains for every 7TM receptor family except the adhesion class, likely because the functional anaylsis of this family lags behind the others. All confirm the same underlying heptahelical fold, yet great structural diversity in their extracellular ligand binding pockets, as would befit the great variety of ligands that interact with 7TM receptors 5(Figure 1). Some technological advances that helped catalyse the expansion in the number of 7TM receptor crystal structures are summarized in Box 2.
Box 2. Crystallographic technologies driving the 7TM receptor revolution.
Successful structural analysis of hormone responsive 7TM receptors required a deep understanding of which parts of the receptor were conformationally labile (for example C-terminal tail and third cytoplasmic loop) and then either truncating and/or substituting them with more rigid, crystallisable exogenous domains, such as, lysozyme, which has been used to replace the third cytoplasmic loop of some receptors25, and was fused to the N-terminus of rhodopsin in the arrestin1 complex38. Additionally, conformationally selective antibody fragments have been developed to stabilize specific receptor conformations and, like lysozyme, provide additional surface area for the formation of crystal contacts. For example, Nb80 is a cameloid nanobody specific for the active conformation of the β2AR and not only facilitates crystallization30 but was also engineered to detect active internalized receptors in cells84. Thermostabilization of 7TM receptors via mutagenesis has also been used to dampen conformational heterogeneity and improve expression. These and other biochemical breakthroughs have been extensively reviewed elsewhere 85,86.
Advances in receptor engineering have been accompanied by parallel advances in X-ray diffraction data collection technologies developed at synchrotron sources. The General Medical Sciences and Cancer Institutes Structural Biology Facility at the Advanced Photon Source (GM/CA) has been particularly successful, as it is responsible for and/or contributed to the largest fraction of 7TM receptor structures currently deposited in the Protein Data Bank (Supplementary information S1 (Figure), Supplementary information S2 (Table)). They were the first to bring together raster scanning of samples, the use of an intense micro-beam, and stable software 87,88 which together facilitated data collection from small receptor crystals that are all but invisible when grown in lipid cubic phase89.
An exciting new development is the arrival of serial femtosecond crystallography (SFX) 90, which involves microcrystals of receptors or receptor complexes grown in lipid cubic phase being passed through an X-ray free-electron laser in a stream with the consistency of toothpaste. When the laser hits a microcrystal, the crystal is obliterated but persists long enough to emit a diffraction pattern. Thus, the technique relies on gathering data from tens of thousands of crystals in random orientations and then stitching the data back together to create integrated diffraction data akin to those collected in a conventional single crystal experiment. An advantage of the technique is its ability to rapidly collect data from microcrystals with a high signal to noise ratio and in a manner essentially free from radiation damage, which limits the resolution and completeness of traditional crystallographic experiments. SFX was essential for determining the crystal structure of the rhodopsin–arrestin complex38, but has also been used to determine several other 7TM receptor structures91–93. The high rate of data collection in this technique also opens the door to ultrafast time-resolved studies that can be used to measure conformational changes and chemical reactions in crystals94.
From structure to function
But what can we learn from all these new 7TM receptor structures about how they interact and activate their downstream partners, namely heterotrimeric G proteins, GRKs and arrestins? To begin addressing this question mechanistically, one ideally would compare structures of both active and inactive states, preferably of the same receptor. However, this is much more challenging than it sounds, in part because most receptors have only been resolved in complex with an inverse agonist or an antagonist, that is in their inactive (inhibited) states, or have had functionally important loops truncated or replaced by rigid domains to facilitate their crystallization (Box 2). Additionally, comparison is confounded by the fact that it is not always clear what is meant by an ‘active’ structure because simple addition of agonists only increases the probability of achieving an active conformation. For example, crystal structures of the β1AR, β2AR, and A2A adenosine receptor (A2AAR) were each determined in complex with an agonist, but their transmembrane domains seem to adopt inactive or intermediate conformations 26–29. This result could be a consequence of the protein engineering employed to achieve the structures (thermostabilization in β1AR or insertion of lysozyme in the third intracellular loops of the β2AR and A2AAR), or of the fact that, in the absence of cytoplasmic partners, agonist-bound receptors still frequently relax into inactive conformations 26. Hereafter, this review considers an “active” conformation to be one that has been determined in complex with proteins that recognize the receptor’s activated state, such as heterotrimeric G proteins, arrestins, and conformationally sensitive nanobodies 30–33. Notably, rhodopsin seems to be a special case, because its retinal-free form (opsin) can crystallize in what seems to be an activated configuration whether or not all-trans-retinal, the light-activated conformation of its chromophore, is bound34. This characteristic has enabled rhodopsin to be visualized in complex with peptide fragments derived from the Gα subunit of transducin (Gαt) 35 and arrestin136. Of course the most definitive examples of active 7TM receptors are those in complex with intact cytoplasmic targets, of which there are currently only two examples: β2AR in complex with the stimulatory Gαsβγ heterotrimer (referred to as Gs) and rhodopsin in complex with arrestin137, 38. After a brief overview of how agonists influence the conformation of 7TM receptors, these two complexes will serve as the basis for discussing how activated receptors might generally interact with their intracellular partners to ensure regulated intracellular signal transduction.
Agonist-induced conformational changes in 7TM receptors
Currently, structures representing the inactive and active states of the same 7TM receptor are only available for rhodopsin, the β2AR, the μ opioid receptor, and the M2 muscarinic receptor (Figure 2). In each case the transmembrane domain of the receptor forms a twisted helical barrel with an up-and-down fold. A deep pocket or cavity in the extracellular end of each of the barrels features specific residues and/or surfaces that complement the chemical properties of their diverse ligands (Figure 1, 2a). Ligand binding promotes the generation of a shallower pocket on the cytoplasmic end of the receptor, achieved by a large motion of the ends of TM5 and, in particular, TM6 away from the transmembrane core, and usually an inward shift of the end of TM7 and the α8 helix (Figure 2b). This conformational change was first proposed based on light-induced changes in the electron paramagnetic resonance of spin-labelled rhodopsin 39 (Box 3). Relative to the antagonist-bound conformation, the extracellular pocket can either expand, contract, or remain essentially unchanged upon agonist binding (Figure 2a). In comparison, the cytoplasmic pockets of these receptors exhibit dramatic but consistent changes to achieve their activated conformation (Figure 2b). In other words, 7TM receptors seem to act as ‘allosteric funnels’, wherein diverse ligand-induced structural changes in the extracellular pocket trigger allosteric pathways that converge on specific transitions in the cytoplasmic domain. Experimental support for this model was recently provided by nuclear magnetic resonance (NMR) studies (Box 3) of thermostabilized β1AR incorporated with 15N-valine, wherein the backbone conformation of specific amino acids in the cytoplasmic domain altered their environment in a unified way, indicating a common conformational change. The degree of change correlated strongly with the efficacy of the bound ligand40. Facilitating conformational transitions in 7TM receptors is a loose network of water molecules and polar interactions inside the transmembrane barrel, which thereby are thought to contribute to allosteric coupling between the extracellular and cytoplasmic pockets 41. The binding of ions within these internal cavities and/or amino acid substitutions that perturb the polar interactions can significantly affect the ability of receptors to respond to extracellular cues 42, 43.
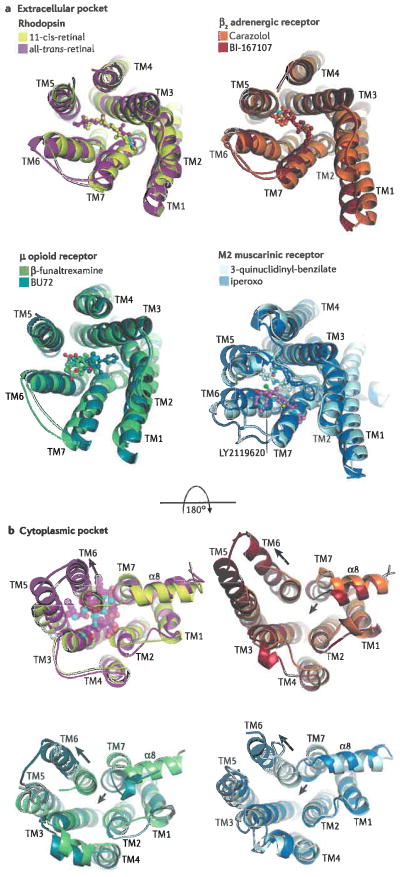
Figure 2.
Extracellular (a) and cytoplasmic (b) pockets of four different 7TM receptors: rhodopsin (PDB entries 1F8823, yellow, and 3PQR35, magenta), the β2AR (PDB entries 2RH125, orange, and 3SN637, brick), the μ-opioid receptor (PDB entries 4DKL108, chartreuse, and 5C1M31, teal), and the M2 muscarinic receptor (PDB entries 3UON109, pale cyan, and 4MQT32, blue). In each overlay, the darker colour corresponds to an active, agonist-bound form of the receptor, whereas the lighter colour corresponds to an inactive form. Panel a illustrates the extracellular pockets bound to various ligands (ball and stick models, with carbons coloured the same as their receptor). N-termini and the second extracellular loop (the loop connecting TM4 and TM5) were removed when necessary for clarity. Photoactivation or the binding of ligands leads to relatively subtle and inconsistent changes in the helices and loops that form the extracellular pocket (rhodopsin expands, β2AR is relatively unchanged, the μ-opioid and M2 receptors contract). LY2119620 (ball and stick model with pink carbons; see muscarinic receptor) is a positive allosteric modulator that binds to the ‘vestibule’ of the M2 muscarinic receptor, formed by the three extracellular loops of the receptor, and thereby increases the effective affinity of the agonist iperoxo. Receptor activation promotes more dramatic yet consistent changes in the cytoplasmic pockets (illustrated in panel b), where the end of TM6 swings away from the core of the transmembrane domain (long arrows), and TM7 and the α8 helix tend to push inward (short arrows) except in the case of rhodopsin. This transition creates a binding site for cytoplasmic proteins, as represented by the transducin peptide bound to opsin35 (transparent spheres in the cytoplasmic pocket of rhodopsin).
Box 3. Spectroscopic methods used to complement crystal structures of 7TM receptors.
Deuterium exchange mass spectrometry. (DXMS)
In DXMS, also known as hydrogen-deuterium exchange (HDX), a receptor complex is placed in D2O and over time amide hydrogens in the peptide backbone of the complex will exchange for deuterium. Amides in less solvent exposed regions, such as in the hydrophobic core of the protein or in protein-protein interfaces, exchange more slowly than when the proteins are dissociated. Thus the experiment can be used to map which regions of a protein experience a change in solvent exposure and/or dynamics as a consequence of signalling events and/or complex formation. Data are collected at various times by acidifying the protein sample to quench further exchange, followed by proteolysis with an acid stable protease. Tandem mass spectrometry then identifies the sequence and degree of deuteration of each proteolytic fragment. The resolution of a DXMS experiment is however limited by the size of the peptides generated and which peptide fragments can be identified by the spectrometer. For example, in a DXMS study of the β2AR-Gs complex, peptides from strands adjacent to β1 in Ras-like domain of Gα were not observed, and thus could not be used to confirm the unexpectedly high mobility of this internal strand (Figure 3a) 49.
Double electron-electron resonance (DEER) distance measurements
DEER provides information on the distance between paramagnetic centers (chemical moieties with unpaired electrons) in a protein and their relative orientation95. Samples are first frozen in vitrified ice, thus emulating a solution environment, and there is no limit on the size of the macromolecular assembly, although the centers typically need to be between 20–60 Å apart from each other for their interactions to be measured. Because most proteins lack such centers, nitroxide modifications are installed at discrete locations by chemical modification of specific cysteine side chains. This requires the creation of mutant proteins with only one or two reactive cysteine side chains per protein complex in a process called site-directed spin-labeling (SDSL). If a nitroxide modification is introduced at a specific position in a receptor, such as rhodopsin, and another one in a cytoplasmic target, such as arrestin, then DEER can be used to estimate the distance between their nitroxide groups in the resulting complex 38 If enough unique pairs of SDSLs can be analyzed, a low resolution model of the complex can be assembled, provided the structure of each protein is known. DEER is also useful for monitoring allosteric changes within a protein. For example, DEER successfully predicted a receptor-induced rigid body change in the α5 helix of Gα (Figure 3a) years before the crystal structure of a receptor–Gα complex was known 52.
Nuclear magnetic resonance (NMR)
Unlike DXMS, NMR can assess the dynamics of a protein on a single residue basis. However, it has several disadvantages: it requires isotopic labelling, assignment of peaks in the resulting spectra to specific amino acids (which can be arduous), and generally requires proteins of relatively small molecular weight (typically < 40 kDa). Even so, this technique was recently used with striking success to determine allosteric mechanisms for activation based on >20 backbone positions in a 15N-valine labelled 7TM receptor 40. It is generally easier to measure signals from the more flexible side chains, such as from lysines reductively dimethylated with 13C-formaldehye 96,97, or cysteine residues labelled with either 3-bromo-19F-trifluoroacetate20,98 or 19F-trifluoroethanethiol99, or by incorporation of 13CH3ε-methionine 100,101. Although these labels generate a more limited number of peaks, they can still provide evidence for distinct conformations as a function of ligand complex and their spectra are typically easier to interpret. Of course, 15N-valine and 13C-methionine incorporated receptors have the advantage of being free from artifacts that could arise from covalent modification.
Activation of heterotrimeric G proteins
As discussed above, activation of heterotrimeric G proteins occurs through the exchange of nucleotide on the Gα subunits, and the two receptors that have provided the most insight into this process are rhodopsin and the β2AR. In 2008, opsin was shown to adopt what seems to be an active configuration because it exhibits an outward swing of TM5 and 6 relative to inactive, dark-adapted rhodopsin, leading to an opening of the cytoplasmic pocket 34. In a follow-up structure, a peptide derived from the C-terminal end of the α5 helix of Gαt, a region known to underlie heterotrimeric G protein specificity for receptors 44, was shown to bind in the open cytoplasmic pocket (Figure 2b) 35. Later, the structure was re-determined in the presence of all-trans-retinal, reconstituting what was described as an agonist-activated receptor bound to a G protein surrogate45. However, these structures all utilized opsin that was crystallized in the same way, and thus crystal lattice contacts may limit conformational changes that could further distinguish among the three structures. A further complication is that if one models a Gαβγ heterotrimer bound to the opsin– Gαt peptide structure by overlaying the C-terminus of Gα with the peptide, the resulting heterotrimeric G protein would collide with the membrane surface 46, suggesting that there are additional conformational changes when a 7TM receptor interacts with an intact heterotrimeric G protein.
In 2011, a consortium of labs reported the first such structure: the β2AR bound to a nucleotide free Gs heterotrimer 37. The structure determination required a high affinity ‘super’ agonist (BI-167107), an N-terminal T4 lysozyme fusion of a C-terminally truncated β2AR, novel detergents, and a cameloid antibody fragment known as nanobody 35 (Nb35; isolated from a llama immunized with a β2AR–Gs complex stabilized via crosslinking with bis(sulfosuccinimidyl)glutarate). Comparison with inactive β2AR structures revealed a conformational transition in the cytoplasmic pocket analogous to that of opsin described above (Figure 2) 25, 47. The structure also revealed that the nucleotide-free, receptor-bound Gα subunit adopts an unusual configuration as compared to all previously reported structures of Gα, with its α-helical domain swung more than 130° degrees away from the Ras-like domain, and with a markedly rearranged α5 helix at the C-terminus of the Ras-like domain that docks into the cytoplasmic pocket of the receptor (Figure 3a, b). But which of these differences is responsible for receptor-mediated nucleotide exchange? In an accompanying study, single particle electron microscopy studies (Box 4) of the complex showed that the α-helical domain is in fact highly mobile when nucleotide-free heterotrimers are bound to the β2AR 48 (Figure 3a), indicating that the α-helical domain in the β2AR complex structure is trapped in its unusual configuration by strong crystal contacts mediated by Nb35. Regardless, both studies suggest that exposure of the nucleotide binding site on Gα to solvent could be an important component of receptor-mediated nucleotide exchange.
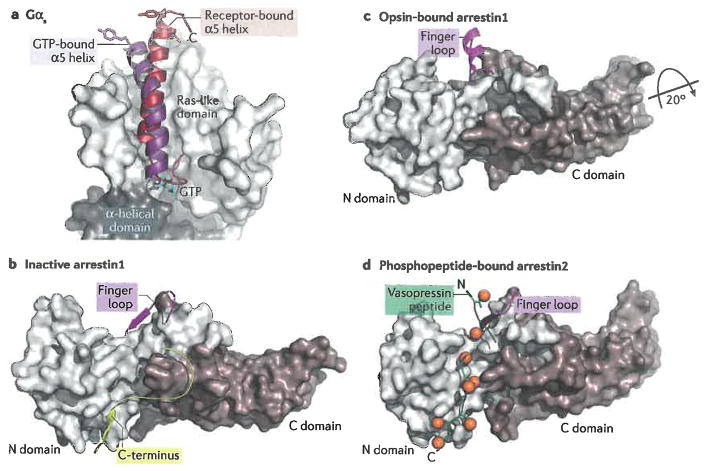
Figure 3.
Interactions of heterotrimeric G proteins and arrestins with 7TM receptors. a) Schematic summarizing how Gα subunits bind to activated receptors. The most prominent interaction is that of its C-terminus (α5) with the cytoplasmic pocket of the receptor. This results in lowering affinity for bound nucleotides and allows the α helical domain to swing away from the Ras-like domain, facilitating nucleotide exchange 50. Arrows represent the β-strands that form the core sheet of the Ras-like domain. The β1 strand, which precedes the P loop that binds phosphate moieties of guanine nucleotides (circles marked with “P”), has also been implicated in become more solvent accessible in the nucleotide free state49. The termini of Gα are marked N and C. b) The C-terminal α5 helix of Ras-like domain of Gαs features a markedly different position in the receptor bound conformation (firebrick, PDB entry 3SN637) as compared to its conformation in its GTP-bound state (purple, PDB entry 1AZT110). The side chains of two hydrophobic residues (Leu388 and Tyr391) that make extensive interactions with TM3, TM5 and TM6 of the receptor (in this case β2AR) are shown as sticks to emphasize the change in the position of α5 helix. c) Schematic of the interactions of arrestin with an activated receptor. Most contacts are formed by the N domain, most prominently by the finger loop which projects into the cytoplasmic pocket. The N domain also forms most of the interactions with phosphorylated residues from the receptor. Together these interactions help to rearrange the polar core between the N and C domains and release the C-terminus of arrestin from the N domain. d) Inactive structure of arrestin1 (PDB entry 1CF164). Its C-terminus (yellow) binds to the N-domain, thereby blocking interactions with MAP kinases and with the endocytotic machinery. e) When arrestin binds to an activated receptor, the C-domain twists ~20° relative to the N-domain and the conformation of the finger loop (magenta)is altered so that it can directly interact with the transmembrane domains of 7TM receptors (PDB entry 4ZWJ 38). f) Arrestins also bind to the phosphorylated C-terminal tails of 7TM receptors, as exemplified here by the arrestin2-vasopressin phosphopeptide complex (PDB entry 4JQI 67). Phosphates are depicted as orange spheres. Note that the C-terminal end of the vasopressin peptide would displace the C-terminus of arrestin shown in panel b indicating that this interaction can activate the signalling function of arrestins by unblocking the sites on the N domain responsible for interactions with other cellular components. It remains to be determined which of the “activated” finger loop models shown, if any, are physiologically relevant. The conformation observed in 4JQI could, however, reflect how arrestin2 binds to a phosphorylated C-terminal tail when it is not also engaged with the transmembrane domain of a 7TM receptor.
Box 4. Other complementary structural techniques.
Molecular dynamics
Detailed information on processes including ligand binding and conformational change, such as nucleotide exchange on Gα, cannot be obtained from static crystal structures. Direct measurement of these events is also beyond the range of most experimental techniques because they occur on μs time scales. However, crystallographic models do provide a framework for computational simulations that can generate novel hypotheses about these physiologically important processes. In such in silico experiments, atoms of a protein model are allowed to interact with each other and with solvent molecules after some kinetic energy is imparted, and the resulting trajectories are analyzed to learn about protein or ligand binding behavior. Recently, specialized microprocessors engineered specifically for molecular dynamics and new physical models for interatomic interactions have extended the range of these computations by orders of magnitude so they can sample μs time scales 102. For example, simulations were used to monitor the binding and release of tiotropium to muscarinic acetylcholine receptors, and provided evidence for the presence of an allosteric ligand binding site in the ‘vestibule’ of the receptor (Figure 2a, bottom right) 103.
Single particle electron microscopy reconstructions
Recent technological advances, such as the direct electron detector, have enabled ever higher resolution three-dimensional single particle reconstructions of samples imaged by electron microscopy 104. Small targets (< 200 kDa) are usually imaged by staining with chemicals such as uranyl acetate, which improves contrast, but ideally samples are embedded in vitreous ice for a cryo-electron microscopy study, thereby preserving them in a more native environment. In this technique, tens of thousands of individual measured projections of the particles are aligned and then integrated into a consistent three-dimensional structure. Asymmetric protein assemblies are better candidates for this approach than globular because they allow more accurate alignment. Different conformational states present in a single sample can also be differentiated and independently reconstructed. Recently, the structure of a 500 kDa enzyme 105, as well as membrane proteins such as the 400 kDa TrpV1 channel 106 were reported at 3–4 Å resolution, which was sufficient to trace the backbone, model side chains, and identify ligand binding sites. Thus, single particle electron microscopy is increasingly viewed as an alternative technique to X-ray crystallography. In practice, however, crystallographic models are still required for validation of electron microscopy derived data, as in the case of single particle electron microscopy reconstructions of receptor–heterotrimeric G protein48,107 and arrestin38,57 complexes.
In another accompanying study, deuterium exchange mass spectrometry (DXMS) (Box 3) was used to map which regions of Gαs change their solvent accessibility and/or dynamics when bound to the β2AR, this time in the absence of Nb3549. The results were mostly consistent with those predicted by the crystal structure, except that it was found that regions of Gα that contributed to the nucleotide binding pocket are highly mobile, as one might expect if both GDP ligand and nanobody were dissociated from the α subunit. Surprisingly, a central (β1) strand in the Ras-like domain of Gα also exhibited higher mobility. Based on this result and the crystal structure of the complex, it was proposed that that binding to the β2AR reorganizes the C-terminal α5 helix and destabilizes the structure of the internal β1 strand of Gαs. As these elements are structurally tethered to bound nucleotides (to the base and phosphates, respectively, Figure 3a), these conformational changes would explain loss of affinity for guanine nucleotides.
Recently, state-of-the-art molecular dynamics simulations were used to further elucidate the nucleotide exchange mechanism (Box 4). Simulations up to 50 μs in duration revealed spontaneous opening of the nucleotide binding pocket of Gαs, even in the absence of receptor 50, which is not surprising given that Gα subunits can readily exchange nucleotides in solution 51. Although this opening was necessary, it was not sufficient for nucleotide release in the simulations. Instead, the movement of the α5 helix, which, as discussed above, undergoes a rigid body rotation in the Ras-like domain of Gαs when it docks with the receptor (Figure 3b), seems to be the key conformational change. The results suggest a model for receptor mediated nucleotide exchange on Gs wherein the receptor pulls and rotates the α5 helix, thereby perturbing the guanine ring binding site. This transition is expected to weaken the affinity of Gαs for the bound nucleotide, and, in combination with dislodging the α-helical domain from the Ras-like domain, facilitates nucleotide exchange (Figure 3a). A similar model of nucleotide exchange was also proposed for rhodopsin based on energetic analysis and site-directed spin labelling (SDSL)/double electron-electron resonance (DEER) distance measurements 52, 53 (Box 3). The role of the β1 strand of the Ras-like domain in nucleotide exchange as of now remains unclear.
However, this is unlikely to be the full story. Simulations, even atomistic ones, are at best low-resolution techniques because they are often difficult to confirm experimentally and can seldom be run over time scales long enough to sample major conformational change. They are also reliant on high resolution crystal structures, which, as noted above, have their own limitations. NMR, however, is well adapted to study the structure and dynamics of nucleotide-free Gα subunits in solution (Box 3). In well-folded proteins, NMR 15N-1H heteronuclear single quantum coherence spectra give well-dispersed, sharp peaks corresponding to all backbone and side chain amide protons. However, the spectra from a nucleotide-free 15N-labeled Gα bound to activated rhodopsin suggested extensive conformational heterogeneity 54; in other words, receptor-bound Gα subunits are very dynamic. The same conclusion was reached for nucleotide-free inhibitory Gα subunit (Gαi) bound to Ric8A, a soluble protein that, like 7TM receptors, promotes nucleotide exchange on some classes of Gα subunits in the absence of Gβγ 55. Interestingly, Ric8A is also thought to interact primarily with the α5 helix of Gα, implying a common mechanism for nucleotide release. These results could be interpreted as a consequence of dissociation of a stabilizing ligand (in this case a guanine nucleotide), but do not exclude the possibility that receptor-mediated disruption of the fold of the Ras-like domain is a part of the nucleotide exchange process.
Activation of arrestins
Of the four arrestins found in humans, two are primarily expressed in visual neurons (arrestin1 and 4), and the other two (arrestin2 and 3, also known as β-arrestin1 and 2) are ubiquitously expressed and thus responsible for uncoupling G proteins from hundreds of different 7TM receptors. Two independent structural cues can be used by arrestins for recruitment to activated receptors. The first is a transmembrane domain in its active conformation, and the second is a cluster of phosphorylated residues on the cytoplasmic loops or tails of the receptors, typically introduced by a GRK (Figure 3c). Which localization cue plays a dominant role seems to vary from receptor to receptor, but use of both can be synergistic 56. Negative stain single particle electron microscopy analysis (Box 4) of a chimera between the β2AR and the vasopressin receptor in complex with arrestin2 revealed two distinct regions of the receptor involved in the interaction: the phosphorylated C-tail of the receptor, serving as the dominant site, and the transmembrane core of the receptor (which became more prominently bound by the arrestin after treatment with a chemical crosslinker). The latter structure was supported by DXMS studies (Box 3), which revealed loss of mobility in the regions of arrestin expected to interact with the transmembrane core of the receptor (see below) 57. In a subsequent electron microscopy study, the complex between a constitutively active mutant of opsin and a ‘pre-activated’ variant of arrestin1 (having three point mutations that induce its active conformation) only yielded reconstructions with the transmembrane core-bound configuration 38, likely because the receptor used in this study was not phosphorylated.
How does arrestin interact with the transmembrane domain of the receptor? Arrestin is composed of two immunoglobulin-like domains, referred to as the N and C domains (Figure 3c,d, Box 1). Biophysical measurements showed that a so-called “finger loop” in the N domain becomes immobilized upon receptor binding 58, consistent with other studies indicating that this region undergoes a major conformational change upon formation of the complex 59, 60. In the crystal structure of opsin in complex with a peptide derived from the arrestin1 finger loop, the peptide was modelled as binding in the cytoplasmic pocket in a conformation similar to that of the C-terminus of Gα (Figure 3b) 36. However, the crystal form was the same as that used for other opsin structures, and thus this structure may not provide an accurate picture of the rhodopsin-arrestin interaction.
Most recently, the atomic structure of a constitutively active variant of rhodopsin in complex with the pre-activated variant of arrestin1 was reported 38. The two proteins were fused together with a 15 amino acid linker to ensure 1:1 stoichiometry and facilitate crystallization. The structure determination was exceptionally challenging because of the extraordinarily small size of the crystals (~10 μm) and their poor diffraction behaviour. To get around this, the authors turned to serial femtosecond crystallography (SFX) (Box 2) 61, 62. Although ultimately many amino side chains, including those in the opsin–arrestin interface, were not resolved in the final maps, the structure does provide a reasonably high resolution electron density envelope for a 7TM receptor–arrestin complex that is consistent with those of electron microscopy single particle reconstructions, and in the future can be used as a starting point for modelling interactions between the arrestin N and C domains and other signalling partners 63. The crystal structure was further validated by DXMS, cross-linking, and DEER distance measurements (Box 3). As expected, the model revealed that the finger loop of arrestin1 is inserted into the cytoplasmic pocket of the activated receptor, but its conformation was different from that modelled for the peptide in the opsin–finger loop peptide complex (Figure 3e) 36. Unfortunately, unbiased electron density maps for the finger loops in both of these crystal structures are relatively featureless, rendering modelling difficult. For example, there are many chemically implausible intra- and intermolecular contacts formed by the finger loop in the SFX structure. The consistently poor quality of the finger loop density in these structures may however indicate that the loop can adopt multiple configurations within the cytoplasmic pocket of the receptor. This protean quality may underlie the ability of arrestin to recognize the activated conformation of hundreds of different 7TM receptors. Whereas exposed loops of the arrestin1 N domain underlie the bulk of the interactions with rhodopsin, the C domain is rotated upwards 20° relative to that of inactive arrestin164 such that it could interact with the membrane surface through the hydrophobic residues presented at the tip of the C domain (cf. Figure 3d,e). This would be consistent with the existence of a potential phosphoinositide head group binding site previously observed in the C domain of arrestin265, and would further support the idea that some arrestins, like heterotrimeric G proteins, require coordinate interactions with both receptor and membrane for full activity 66.
Interaction of arrestins with phosphorylated receptor peptides has also been investigated. The crystal structure of an antibody-stabilized complex between an octa-phosphorylated peptide derived from the C-terminus of the vasopressin receptor and C-terminally truncated arrestin2 showed how phosphorylated residues interact with basic residues in the N domain (Figure 3f). These interactions seem to disrupt the polar core of arrestin2, thereby inducing a conformational change between its N and C domains and reorganizing exposed loops that could then interact with receptors 67. Caveats from this experiment are the extent of phosphorylation of the peptide (because it is unclear how many sites need to be phosphorylated for arrestin to functionally interact with receptors in vivo 68) and the fact that the antibody interacts with both arrestin and the phosphopeptide, and thus may be in part responsible for the observed arrestin conformation. However, the N and C lobes of arrestin2 in this complex superimpose well with those of the pre-activated variant of arrestin1 whose structure was determined in complex with rhodopsin 38 (Figure 3e)
Taken together, these studies support a model wherein the binding of arrestin to phosphorylated sites on 7TM receptors disrupts the interaction of its C-terminal tail with its N-terminal strand and helix I in the N domain (the so-called ‘three-element interaction’69), thereby allowing the N and C domains to reorganize and create binding sites for other signalling molecules 63 as well as the transmembrane core of the receptor. Multivalent interactions between arrestin and the receptor help to increase their affinity and thereby more effectively block heterotrimeric proteins from the receptor. In the case of arrestin2 and 3, the released C-terminal tail has binding sites for the endocytotic machinery 70, 71, which targets the complex for internalization and instigates other signalling events (Box 1).
Activation of GRKs
Less well understood is the molecular basis for how GRKs interact with 7TM receptors. These complexes present additional challenges due to the larger size of GRKs relative to arrestins, their conformational flexibility, their low affinity for activated receptors, and their requirement for phospholipids for full activity. There are also no known constitutively active mutants of GRKs that could be exploited to gain insight into receptor-mediated activation mechanisms. The available studies suggest that GRKs target activated receptors via an intrinsically disordered N-terminus that becomes helical when it encounters an activated receptor or, alternatively, an appropriate lipid environment because lipids can activate GRK phosphorylation of non-receptor targets 72–74. This helical region is predicted to form a bridge between the cytoplasmic pocket of the receptor and the GRK that concomitantly stabilizes a catalytically competent configuration of the kinase domain 73. As in the C-termini of Gα subunits and the finger loops of arrestins, the N-terminal helix of GRKs harbours both conserved acidic and hydrophobic residues that complement the general surface properties of the cytoplasmic pocket in activated 7TM receptors.
Conclusions and outlook
The available structural data has revealed that the process by which 7TM receptors transfer signals to heterotrimeric G proteins, GRKs, and arrestins is analogous to thumbing a ride. In their receptor interacting conformations, Gα subunits rotate and extend their C-terminal α5 helix, arrestins reorient a centrally projecting finger loop and GRKs offer an N-terminal helix. In each case, the extended structure is accompanied by an activating conformational change of the cytoplasmic partner, which is stabilized not only by the receptors, but also by interactions with the surrounding phospholipid bilayer (Box 1). Such ‘hitchhiking’ on activated 7TM receptors by these diverse proteins seems to involve common principles: an open cytoplasmic ‘door’ on the receptor (achieved by receptor activation), a patch of negatively charged membrane surrounding the receptor, and physicochemical complementarity of the extended thumbs with the cytoplasmic pocket of the receptor. This is consistent with the central metaphor of this review: like hitchhikers, who accept rides from diverse vehicles, heterotrimeric G proteins, GRKs, and arrestins have to regulate signalling from diverse receptors by taking advantage of general features of an activated 7TM receptor, rather than specific interactions.
Similarities in the interaction of 7TM receptors with their various intracellular partners extend beyond the ‘thumb’. Whereas DXMS indicates that the receptor interacting regions of Gα subunits and arrestins become more ordered upon complex formation38, 49, both NMR and DXMS indicate that the rest of their domains become more dynamic 49, 54, 57, 75. In other words, free energy gained by complex formation is used to leverage energetically unfavourable structural changes necessary for signal propagation, such as ejection of the guanine nucleotide from Gα or reorganization of the N and C domains of arrestin. Interestingly, the current structural models suggest that heterotrimeric G proteins and arrestins establish distinct contacts with 7TM receptors in and around the cytoplasmic pocket, and these differences could provide the molecular basis for biased agonism: different ligands binding at the same receptor can potentially influence these structural elements in unique ways, thereby leading to differential recruitment and/or interaction with G proteins, GRKs, or arrestins, and consequently distinct cellular responses.
Not covered in this review, but of possible importance for some 7TM receptors is the prospect of the regulation of signalling through oligomerization (glutamate-family 7TM receptors are already constitutive dimers via interaction of their extracellular domains, see Figure 1), which could influence their ligand-stabilized conformations and how they interact with cytoplasmic partners. Although parallel (hence physiologically reasonable) packing interactions have been observed between transmembrane helical bundles in some crystal structures of 7TM receptors implicated to be dimeric in vivo 76, it is not yet clear whether these lattice contacts are functionally relevant or represent fortuitous crystal packing. Current in vitro evidence seems to disfavour the idea of dimerization as a general feature of 7TM receptors, contributing to their interactions with cytoplasmic targets: when constrained to be monomeric in artificial nanodisc particles, 7TM receptors are fully functional with respect to G protein coupling77–79, GRK phosphorylation80, 81, and arrestin binding80, 82.
There are also some overarching general conclusions regarding the structural analyses of 7TM receptor complexes. Membrane proteins are already challenging targets, and complexes between 7TM receptors and their signalling partners adds an additional layer of difficulty because they are often transient and involve various conformational transitions as part of their function. It is notable, and not accidental, that the β2AR-Gs and rhodopsin–arrestin crystal structures were extensively supplemented by companion studies including electron microscopy, DXMS, DEER, and molecular dynamics (Boxes 3 and 4). This at first glance may suggest that the approaches used to generate these atomic models impose enough caveats that other methods are required for validation. Alternatively, it may simply reflect the fact that crystal structures on their own are just snapshots–very important ones–but snapshots nevertheless, and as such they could never provide a full molecular description of a dynamic biological process. Big structural problems, such as receptor–G protein or –arrestin complexes, require networks of complementary expertise not often found in a single lab.
Finally, no one likes n=1 experiments, and thus there is an immense need for additional structures of 7TM receptors in complex with their cytoplasmic signalling partners to provide a broader and more balanced view of their interactions. The different ways in which various labs tackle these complex problems will iron out any artefacts resulting from any specific experimental approach. Additional structures are also expected to provide further insights into the molecular basis for selectivity (for instance how receptors determine which Gα subunits they couple with) and for biased signalling. Determining these important structures will require not only great fortitude but also funding for projects that are long-term, high-risk, and highly collaborative endeavours. But the potential payoffs are huge, as these structural studies immensely contribute to revealing molecular mechanisms underlying cellular responses to extracellular stimuli, thereby opening the prospects of controlling cellular physiology and curing numerous diseases.
Supplementary Material
Online summary.
Since 2007, the number of high resolution studies of 7TM receptors (including G protein-coupled receptors) has greatly expanded, revealing new insights into signaling by a formerly intractible family of integral membrane proteins.
Although new structures have revealed many important pharmacological details about specific ligand binding sites in different receptor classes, it is still poorly understood how signals are transduced by 7TM receptors from their ligand binding pockets to their cytoplasmic partners.
Structures of the β2-adrenergic receptor in complex with the heterotrimeric G protein Gs and of rhodopsin in complex with arrestin, along with complementary biophysical studies, provide the most detailed views of how such signaling might occur.
Both heterotrimeric G proteins and arrestins, and likely G protein-coupled receptor kinases (GRKs), recognize activated 7TM receptors via a common mechanism involving a structural projection that packs into the cytoplasmic pockets of activated receptors. The projection is allosterically coupled such that the binding event translates into functional consequences inside the cell.
Interactions with activated receptors seem to have relatively few specific contacts, which likely explains how a relatively small number of G proteins, arrestins and GRKs can recognize the activated state of hundreds of different 7TM receptors.
Acknowledgments
Research in the Tesmer laboratory is supported by NIH grants HL086865, HL122416, and HL071818 and fellowships from the American Cancer Society and the American Heart Association. The author thanks Melinda Mackey for technical assistance.
Glossary
- transducin
The heterotrimeric G protein (also known as Gt), which is responsible for the propagation of visual signals from light-activated rhodopsin.
- homologous desensitization
The process by which receptors that recognize a specific agonist or signal become gradually resistant to further stimulation, often by downregulation of the number of receptors on the cell surface through endocytosis.
- clathrin-mediated endocytosis
A process by which membrane proteins are sequestered into distinct regions of the cell membrane which are then internalized with the help of the protein clathrin to form intracellular vesicles.
- agonist
A molecule that promotes a conformation (or perhaps more accurately an ensemble of conformations) of a receptor that is more capable of binding and activating a downstream signalling protein.
- biased agonism
A phenomenon in which an agonist (known as a biased agonist) promotes interactions with one downstream signalling partner or cascade over another.
- inverse agonist
A molecule that reduces signalling by a receptor to a level below that observed in its basal, unliganded state.
- antagonist
A molecule that blocks the binding of other molecules to a receptor, but on its own has no effect on signalling.
- nanobodies
Single domain, ~15 kDa fragments produced from single heavy chain antibodies found in cameloid species (e.g. llamas and alpacas). They are now also generated by in vitro methodologies such as directed evolution.
- α-helical domain
A helical domain unique to heterotrimeric G proteins inserted into a loop of the Ras-like domain. It contributes to the rate of GTP hydrolysis and also can modulate interactions between the Ras-like domain of Gα and its signalling partners.
- Ras-like domain
The nucleotide binding domain of heterotrimeric G protein α subunits, which also contains binding sites for 7TM receptors, Gβγ subunits, and downstream effector enzymes. It contains three switch regions that are conformationally responsive to the identity of the bound nucleotide.
- allosteric change
An alteration in the structure of a protein in a specific region that results from an event that occurs at a remote site.
Footnotes
Competing interests statement
The author declares no competing interests.
Dr. Tesmer received a B.A. in Biochemistry and English from Rice University in 1990, and a Ph.D. in Biological Sciences from Purdue University in 1995. Since 1996, he has worked primarily on the structural basis of heterotrimeric G protein signaling, first as a post-doctoral fellow at University of Texas Southwestern Medical Center at Dallas, then as a faculty member at the University of Texas at Austin, and currently the University of Michigan.
References
- 1.Hall RA, Premont RT, Lefkowitz RJ. Heptahelical receptor signaling: beyond the G protein paradigm. J Cell Biol. 1999;145:927–32. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–30. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnadottir TK, et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–73. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Katritch V, Cherezov V, Stevens RC. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol Sci. 2012;33:17–27. doi: 10.1016/j.tips.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dijksterhuis JP, Petersen J, Schulte G. WNT/Frizzled signalling: receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR Review 3. Br J Pharmacol. 2014;171:1195–209. doi: 10.1111/bph.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discov Today. 2002;7:235–46. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- 8.Kahlert M, Hofmann KP. Reaction rate and collisional efficiency of the rhodopsin-transducin system in intact retinal rods. Biophys J. 1991;59:375–86. doi: 10.1016/S0006-3495(91)82231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin AB, Butcher AJ, Kong KC. Location, location, location…site-specific GPCR phosphorylation offers a mechanism for cell-type-specific signalling. Trends Pharmacol Sci. 2008;29:413–20. doi: 10.1016/j.tips.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krupnick JG, Gurevich VV, Benovic JL. Mechanism of quenching of phototransduction. Binding competition between arrestin and transducin for phosphorhodopsin. J Biol Chem. 1997;272:18125–31. doi: 10.1074/jbc.272.29.18125. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–6. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 13.Goodman OB, Jr, et al. Nature. 1996;383:447–50. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 14.Daaka Y, et al. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–8. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 15.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 16.Kenakin T, Miller LJ. Seven Transmembrane Receptors as Shapeshifting Proteins: The Impact of Allosteric Modulation and Functional Selectivity on New Drug Discovery. Pharmacol Rev. 2010;62 doi: 10.1124/pr.108.000992. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–97. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 19.DeWire SM, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–17. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 20.Kim TH, et al. The role of ligands on the equilibria between functional states of a G protein-coupled receptor. J Am Chem Soc. 2013;135:9465–74. doi: 10.1021/ja404305k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schertler GF, Villa C, Henderson R. Projection structure of rhodopsin. Nature. 1993;362:770–2. doi: 10.1038/362770a0. [DOI] [PubMed] [Google Scholar]
- 22.Unger VM, Hargrave PA, Baldwin JM, Schertler GF. Arrangement of rhodopsin transmembrane alpha-helices. Nature. 1997;389:203–6. doi: 10.1038/38316. [DOI] [PubMed] [Google Scholar]
- 23.Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. The first X-ray crystallographic structure of a 7TM receptor. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen SG, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–7. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 25.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum DM, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–40. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warne T, et al. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011;469:241–4. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebon G, et al. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–5. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu F, et al. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–7. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–80. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, et al. Structural insights into micro-opioid receptor activation. Nature. 2015;524:315–21. doi: 10.1038/nature14886. This structure, along with that of an antagonist-bound μ-opioid receptor (Manglik et al. 2012), provides a third pair of models describing the transition from inactive to active receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruse AC, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–6. doi: 10.1038/nature12735. This structure, along with that of an antagonist-bound M2 muscarinic receptor (Haga et al. 2012), provides a fourth pair of models describing the transition from inactive to active receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ring AM, et al. Adrenaline-activated structure of beta2-adrenoceptor stabilized by an engineered nanobody. Nature. 2013;502:575–9. doi: 10.1038/nature12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–7. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 35.Scheerer P, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 36.Szczepek M, et al. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat Commun. 2014;5:4801. doi: 10.1038/ncomms5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–55. doi: 10.1038/nature10361. The first crystal structure of an activated heptahelical receptor in complex with an intact cytoplasmic target, providing a high resolution glimpse at conformational changes involved in nucelotide exchange. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y, et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523:561–7. doi: 10.1038/nature14656. SFX crystal structure of the rhodopsin-arrestin1 complex, along with confirmatory EM, DXMS, and DEER studies. The results were consistent with those of Shukla et al. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–70. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 40.Isogai S, et al. Backbone NMR reveals allosteric signal transduction networks in the beta-adrenergic receptor. Nature. 2016 doi: 10.1038/nature16577. Uses a 15N labeled 7TM receptor to map allosteric pathways induced by a series of different ligands with a broad range of efficacy. [DOI] [PubMed] [Google Scholar]
- 41.Blankenship E, Lodowski DT. Rhodopsin purification from dark-adapted bovine retina. Methods Mol Biol. 2015;1271:21–38. doi: 10.1007/978-1-4939-2330-4_2. [DOI] [PubMed] [Google Scholar]
- 42.Fenalti G, et al. Molecular control of delta-opioid receptor signalling. Nature. 2014;506:191–6. doi: 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, et al. Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J Biol Chem. 2015 doi: 10.1074/jbc.M115.689000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slessareva JE, et al. Closely related G-protein-coupled receptors use multiple and distinct domains on G-protein alpha-subunits for selective coupling. J Biol Chem. 2003;278:50530–6. doi: 10.1074/jbc.M304417200. [DOI] [PubMed] [Google Scholar]
- 45.Choe HW, et al. Crystal structure of metarhodopsin II. Nature. 2011;471:651–5. doi: 10.1038/nature09789. Provided a high resolution snapshot of rhodopsin in its activated state bound to a Gα-derived peptide. [DOI] [PubMed] [Google Scholar]
- 46.Huang CC, Tesmer JJ. Recognition in the face of diversity: interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. J Biol Chem. 2011;286:7715–21. doi: 10.1074/jbc.R109.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–73. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 48.Westfield GH, et al. Structural flexibility of the Gαs α-helical domain in the β2-adrenoceptor Gs complex. Proc Natl Acad Sci U S A. 2011;108:16086–91. doi: 10.1073/pnas.1113645108. Complements the Rasmussen (2011) structure by providing low resolution glimpses of the β2AR–Gs complex in single particle EM reconstructions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung KY, et al. Conformational changes in the G protein Gs induced by the beta2 adrenergic receptor. Nature. 2011;477:611–5. doi: 10.1038/nature10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dror RO, et al. SIGNAL TRANSDUCTION. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science. 2015;348:1361–5. doi: 10.1126/science.aaa5264. Atomistic molecular dynamics simulations at microsecond time scales demonstrating conformational transitions in Gα that may underlie nucleotide release. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sternweis P, Robishaw J. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984;259:13806–13. [PubMed] [Google Scholar]
- 52.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–7. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 53.Alexander NS, et al. Energetic analysis of the rhodopsin-G-protein complex links the alpha5 helix to GDP release. Nat Struct Mol Biol. 2014;21:56–63. doi: 10.1038/nsmb.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdulaev NG, et al. The receptor-bound “empty pocket” state of the heterotrimeric G-protein alpha-subunit is conformationally dynamic. Biochemistry. 2006;45:12986–97. doi: 10.1021/bi061088h. [DOI] [PubMed] [Google Scholar]
- 55.Thomas CJ, et al. A specific interaction of small molecule entry inhibitors with the envelope glycoprotein complex of the Junin hemorrhagic fever arenavirus. J Biol Chem. 2011;286:6192–200. doi: 10.1074/jbc.M110.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shukla AK, et al. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature. 2014;512:218–22. doi: 10.1038/nature13430. Structure of a receptor-arrestin complex by single particle EM analysis, suggesting two modes of interaction between the two proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanson SM, et al. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci U S A. 2006;103:4900–5. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sommer ME, Farrens DL, McDowell JH, Weber LA, Smith WC. Dynamics of arrestin-rhodopsin interactions: loop movement is involved in arrestin activation and receptor binding. J Biol Chem. 2007;282:25560–8. doi: 10.1074/jbc.M702155200. [DOI] [PubMed] [Google Scholar]
- 60.Feuerstein SE, et al. Helix formation in arrestin accompanies recognition of photoactivated rhodopsin. Biochemistry. 2009;48:10733–42. doi: 10.1021/bi900544p. [DOI] [PubMed] [Google Scholar]
- 61.Neutze R, Branden G, Schertler GF. Membrane protein structural biology using X-ray free electron lasers. Curr Opin Struct Biol. 2015;33:115–125. doi: 10.1016/j.sbi.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Liu W, Wacker D, Wang C, Abola E, Cherezov V. Femtosecond crystallography of membrane proteins in the lipidic cubic phase. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130314. doi: 10.1098/rstb.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song X, Coffa S, Fu H, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem. 2009;284:685–95. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–69. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 65.Milano SK, Kim YM, Stefano FP, Benovic JL, Brenner C. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J Biol Chem. 2006;281:9812–23. doi: 10.1074/jbc.M512703200. [DOI] [PubMed] [Google Scholar]
- 66.Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J Cell Sci. 2005;118:2093–104. doi: 10.1242/jcs.02387. [DOI] [PubMed] [Google Scholar]
- 67.Shukla AK, et al. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–41. doi: 10.1038/nature12120. Provides a glimpse of how phosphorylated loops or tails of heptahelical receptors bind to and help activate arrestins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tobin AB. G-protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol. 2008;153(Suppl 1):S167–76. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurevich VV. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J Biol Chem. 1998;273:15501–6. doi: 10.1074/jbc.273.25.15501. [DOI] [PubMed] [Google Scholar]
- 70.Kern RC, Kang DS, Benovic JL. Arrestin2/clathrin interaction is regulated by key N- and C-terminal regions in arrestin2. Biochemistry. 2009;48:7190–200. doi: 10.1021/bi900369c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krupnick JG, Santini F, Gagnon AW, Keen JH, Benovic JL. Modulation of the arrestin-clathrin interaction in cells. Characterization of beta-arrestin dominant-negative mutants. J Biol Chem. 1997;272:32507–12. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- 72.Pao CS, Barker BL, Benovic JL. Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry. 2009;48:7325–33. doi: 10.1021/bi900408g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boguth CA, Singh P, Huang CC, Tesmer JJ. Molecular basis for activation of G protein-coupled receptor kinases. EMBO J. 2010;29:3249–59. doi: 10.1038/emboj.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noble B, Kallal LA, Pausch MH, Benovic JL. Development of a yeast bioassay to characterize G protein-coupled receptor kinases. Identification of an NH2-terminal region essential for receptor phosphorylation. J Biol Chem. 2003;278:47466–76. doi: 10.1074/jbc.M308257200. [DOI] [PubMed] [Google Scholar]
- 75.Zhuang T, et al. Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci U S A. 2013;110:942–7. doi: 10.1073/pnas.1215176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shang Y, Filizola M. Opioid receptors: Structural and mechanistic insights into pharmacology and signaling. Eur J Pharmacol. 2015;763:206–13. doi: 10.1016/j.ejphar.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whorton MR, et al. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387–94. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282:14875–81. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 80.Bayburt TH, et al. Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem. 2011;286:1420–8. doi: 10.1074/jbc.M110.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vishnivetskiy SA, et al. Constitutively active rhodopsin mutants causing night blindness are effectively phosphorylated by GRKs but differ in arrestin-1 binding. Cell Signal. 2013;25:2155–62. doi: 10.1016/j.cellsig.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsukamoto H, Sinha A, DeWitt M, Farrens DL. Monomeric rhodopsin is the minimal functional unit required for arrestin binding. J Mol Biol. 2010;399:501–11. doi: 10.1016/j.jmb.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi GW, et al. Light causes phosphorylation of nonactivated visual pigments in intact mouse rod photoreceptor cells. J Biol Chem. 2005;280:41184–91. doi: 10.1074/jbc.M506935200. [DOI] [PubMed] [Google Scholar]
- 84.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–8. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghosh E, Kumari P, Jaiman D, Shukla AK. Methodological advances: the unsung heroes of the GPCR structural revolution. Nat Rev Mol Cell Biol. 2015;16:69–81. doi: 10.1038/nrm3933. [DOI] [PubMed] [Google Scholar]
- 86.Maeda S, Schertler GF. Production of GPCR and GPCR complexes for structure determination. Curr Opin Struct Biol. 2013;23:381–92. doi: 10.1016/j.sbi.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Hilgart MC, et al. Automated sample-scanning methods for radiation damage mitigation and diffraction-based centering of macromolecular crystals. J Synchrotron Radiat. 2011;18:717–22. doi: 10.1107/S0909049511029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith JL, Fischetti RF, Yamamoto M. Micro-crystallography comes of age. Curr Opin Struct Biol. 2012;22:602–12. doi: 10.1016/j.sbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cherezov V, et al. Rastering strategy for screening and centring of microcrystal samples of human membrane proteins with a sub-10 microm size X-ray synchrotron beam. J R Soc Interface. 2009;6(Suppl 5):S587–97. doi: 10.1098/rsif.2009.0142.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boutet S, et al. High-resolution protein structure determination by serial femtosecond crystallography. Science. 2012;337:362–4. doi: 10.1126/science.1217737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu W, et al. Serial femtosecond crystallography of G protein-coupled receptors. Science. 2013;342:1521–4. doi: 10.1126/science.1244142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fenalti G, et al. Structural basis for bifunctional peptide recognition at human delta-opioid receptor. Nat Struct Mol Biol. 2015;22:265–8. doi: 10.1038/nsmb.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H, et al. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161:833–44. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fromme P. XFELs open a new era in structural chemical biology. Nat Chem Biol. 2015;11:895–9. doi: 10.1038/nchembio.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeschke G. DEER distance measurements on proteins. Annu Rev Phys Chem. 2012;63:419–46. doi: 10.1146/annurev-physchem-032511-143716. [DOI] [PubMed] [Google Scholar]
- 96.Bokoch MP, et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature. 2010;463:108–12. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sounier R, et al. Propagation of conformational changes during mu-opioid receptor activation. Nature. 2015;524:375–8. doi: 10.1038/nature14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manglik A, et al. Structural Insights into the Dynamic Process of beta2-Adrenergic Receptor Signaling. Cell. 2015;161:1101–11. doi: 10.1016/j.cell.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Biased signaling pathways in beta2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–10. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kofuku Y, et al. Efficacy of the beta(2)-adrenergic receptor is determined by conformational equilibrium in the transmembrane region. Nat Commun. 2012;3:1045. doi: 10.1038/ncomms2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nygaard R, et al. The dynamic process of beta(2)-adrenergic receptor activation. Cell. 2013;152:532–42. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dror RO, Dirks RM, Grossman JP, Xu H, Shaw DE. Biomolecular simulation: a computational microscope for molecular biology. Annu Rev Biophys. 2012;41:429–52. doi: 10.1146/annurev-biophys-042910-155245. [DOI] [PubMed] [Google Scholar]
- 103.Kruse AC, et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–6. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Zorzi R, Mi W, Liao M, Walz T. Single-particle electron microscopy in the study of membrane protein structure. Microscopy (Oxf) 2015 doi: 10.1093/jmicro/dfv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartesaghi A, Matthies D, Banerjee S, Merk A, Subramaniam S. Structure of beta-galactosidase at 3.2-A resolution obtained by cryo-electron microscopy. Proc Natl Acad Sci U S A. 2014;111:11709–14. doi: 10.1073/pnas.1402809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–12. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jastrzebska B, et al. Rhodopsin-transducin heteropentamer: three-dimensional structure and biochemical characterization. J Struct Biol. 2011;176:387–94. doi: 10.1016/j.jsb.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manglik A, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–6. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haga K, et al. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–51. doi: 10.1038/nature10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. Crystal structure of the adenylyl cyclase activator Gsa. Science. 1997;278:1943–1947. doi: 10.1126/science.278.5345.1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.