Abstract
The mammalian forebrain is characterized by the presence of several parallel cortico-basal ganglia circuits that shape the learning and control of actions. Among these are the associative, limbic and sensorimotor circuits. The function of all of these circuits has now been implicated in responses to drugs of abuse, as well as drug seeking and drug taking. While the limbic circuit has been most widely examined, key roles for the other two circuits in control of goal-directed and habitual instrumental actions related to drugs of abuse have been shown. In this review we describe the three circuits and effects of acute and chronic drug exposure on circuit physiology. Our main emphasis is on drug actions in dorsal striatal components of the associative and sensorimotor circuits. We then review key findings that have implicated these circuits in drug seeking and taking behaviors, as well as drug use disorders. Finally, we consider different models describing how the three cortico-basal ganglia circuits become involved in drug-related behaviors. This topic has implications for drug use disorders and addiction, as treatments that target the balance between the different circuits may be useful for reducing excessive substance use.
Circuitry including the cortex, basal ganglia and thalamus comprises a large portion of the vertebrate brain that is greatly expanded in mammals. Indeed, every cortical region innervates a corresponding region of the striatum, with parallel projections running from striatum to other basal ganglia areas, and ultimately to thalamus and back to cortex. These parallel loop circuits modulate cortical output to brainstem and spinal cord to control the performance and learning of actions. The role of cortico-basal ganglia circuitry in behavior includes contributions to both elicited or Pavlovian behaviors as well as self-controlled learning of new actions. Forebrain areas modify existing behaviors and generate new behavioral patterns through associations with specific stimuli, external and internal environments, and outcomes generated by behavior. The evolution of this brain network is most likely the key to the combined use of fixed and flexible action patterns that is the hallmark of vertebrate behavioral adaptation. It is often these same fixed and flexible action patters that are disrupted in various addiction phenotypes, suggesting a strong contribution of addiction-induced alterations to cortico-basal ganglia circuits to the addiction pathology.
The evolution of the forebrain in vertebrates highlights the parallel development of components of the cortico-basal ganglia circuit (Reiner, 2010). As allocortical and neocortical structures became more specialized through mammalian evolution, striatal and other basal ganglia regions have expanded to process this ever more diverse cortical input. One result has been a gradual separation of striatal and downstream basal ganglia regions from the close interaction with extended amygdala circuitry that is characteristic of many non-mammalian vertebrates. Another result has been increasing specialization of striatum and other basal ganlgia nuclei that communicate with the increasingly specialized cortex, neocortex in particular. Overall, the mammalian forebrain can now be divided into several cortical-basal ganglia-thalamic-cortical loops that can be subdivided in many ways based on anatomical connectivity and cortical function (Alexander & Crutcher 1990).
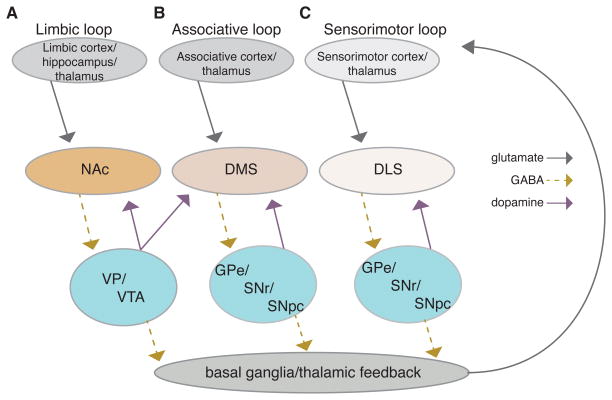
For the purposes of this review, we will concentrate on one of the simplest division schemas, separating the circuitry into associative, sensorimotor and limbic loops in the basal ganglia (Fig. 1). Before we continue, we want to emphasize that the loop segregation is not complete. There is a gradient of cortical inputs across associative, sensorimotor, and limbic loops, and cortical projection neurons have multiple collaterals that extend across loops and to numerous downstream structures (Haber et al. 2006; Pan et al. 2010; Harris & Shepherd 2015; Heilbronner et al., 2016; Hintiryan et al., 2016;). In addition, intra-cortical connectivity provides interconnectedness to the loops, such that segregation of information is not strict (for review see Harris & Shepherd, 2015). That being said, to lay a framework for exploring addiction-related changes, we apply the following commonly used descriptors. The associative circuit (Figure 1B) consists of glutamatergic projections from both prefrontal cortices and neocortical (e.g. entorhinal, EC, and posterior parietal cortices) structures to the dorsal and ventral portions of the medial striatum (roughly equivalent to the primate caudate nucleus). Dopaminergic projections from the medial portions of the substantia nigra pars compacta (SNc) and VTA heavily innervate these striatal subregions (Haber et al. 2000; Pan et al. 2010; Ikemoto 2007), while the thalamic nuclei provide a second source of glutamatergic input. GABAergic efferent projections from the striatum innervate downstream BG nuclei (globus pallidus external segment, GPe, and substantia nigra pars reticulata, SNr) in parallel streams (although there is a small degree of overlap) sometimes called the direct and indirect pathways (discussed in more detail later in this review). Ultimately, the BG output nuclei project to corticothalamic projection neurons that help drive cortical output neurons, as well as to downstream brainstem nuclei that can stimulate actions more directly. Findings suggest this circuit influences actions using information that has been “integrated” across sensory and motor modalities (e.g. spatial information from the EC) as well as information about the consequences of future and past actions.
Figure 1.
Simplified schematic diagram of the three cortico-basal-ganglia loops, including indications of glutamate, GABA, and dopamine neuron projections; A) limbic loop, B) associative loop, C) sensorimotor loop. Dorsal Medial Striatum (DMS), Dorsal Lateral Striatum (DLS), Globus Pallidus external segment (GPe), Nucleus Accumbens (NAc) Substantia Nigra pars reticulata (SNr), Substantia Nigra pars compacta (SNpc), Ventral Pallidum (VP), and Ventral Tegmental Area (VTA).
The parallel sensorimotor circuit (Figure 1C) includes primary and secondary sensory and motor cortices, the dorsolateral striatum in rodents (roughly equivalent to the primate putamen), as well as GPe and SNr subregions. Dopaminergic innervation of the sensorimotor striatum comes from lateral portions of the SNc (Haber et al. 2000; Pan et al. 2010; Ikemoto 2007), and glutamatergic thalamic input comes largely from the intralaminar nuclei, although there seem to be limited projections from lateral and posterior thalamic nuclei (Smith et al. 2004; Pan et al. 2010). This circuit modulates output from motor and sensory cortices to influence actions including well-learned movement patterns that rely on information about external and internal environmental contexts as well as the history of context-reinforcement relationships.
The major proposed role of the associative and sensorimotor circuits is to allow for development and control of novel or non-innate actions and action sequences (commonly referred to as instrumental or operant learning) (Graybiel & Grafton 2015; Costa 2011; Yin & Knowlton 2006), as opposed to modification of reflexes and other innate behavioral patterns. The associative and sensorimotor circuits appear to compete for control of instrumental behaviors that converge on the same motor components, but do so based on different types of information. Importantly, the neurobiological evidence at present suggests that these two cortico-basal ganglia circuits work in parallel from the onset of learning a new action or action sequence and they compete for action control. For example, lesions or manipulations to the associative circuit prior to novel action training do not prevent action learning, but instead induce a reliance on the sensorimotor circuit (Yin et al. 2005; Hilario et al. 2012; Gremel & Costa 2013; Gremel et al., 2016). Furthermore, concurrent encoding of the same action is seen in associative and sensorimotor circuits, even early in training (Gremel & Costa 2013; Thorn et al. 2010; Stalnaker et al. 2010). More recent work with computations models have raised interesting hypotheses about a potential hierarchical organization in the transition of initially goal-directed actions into action sequences insensitive to outcome value changes (Dezfouli & Balleine, 2012; 2013, Dezfouli et al., 2014). At present, it has not been shown that for actions to be under habitual control, they need to be organized within sequences. Moreover, the neurobiological findings show that lesions to one system leaves the other intact and capable of learning and executing action control, thereby suggesting at least some parallel processing. More work investigating neural mechanisms of hierarchal control is needed. Thus, while there has been a recent emphasis on the gradual transition from associative circuit (goal-directed) to sensorimotor circuit (habitual) control of actions, this learning order is not obligatory. As we will discuss further in this review, goal-directed control over behavior is often observed at an earlier time point than habitual control, but this is probably due to early engagement of the associative circuit and its ability to link actions with outcomes versus the more gradual buildup of reinforcement history that leads to habit learning.
In this schema, the “limbic” circuit (Figure 1A) is the third major division. This circuit is composed of prefrontal and mesocortical structures (and lateral amygdala and hippocampus) that project glutamatergic afferents to the ventral striatum (aka Nucleus accumbens, NAc), with downstream processing by the ventral pallidum and more ventromedial output nuclei (Haber, 2011). The ventral tegmental area (VTA) provides the large majority of dopaminergic innervation to the ventral striatum, but areas of VTA also innervate central striatum (Haber et al. 2000; Ikemoto 2007; Haber 2014). Of course, the limbic circuit can be further subdivided based on cortical inputs and different ventral striatal subregions (e.g. the NAc core and shell). However, this will not be discussed in the present review given our focus on the associative and sensorimotor circuits. Ultimately however, the BG output influences the function of downstream brainstem areas, and through thalamus influences the prefrontal cortex output. Hence, the recurrent loops within the corticobasal ganglia circuits provide the potential for information from the limbic circuit to influence functions of the associative and sensorimotor circuits, and vice versa.
In relation to drug abuse and addiction, the limbic circuit has received the lion’s share of attention, and there are clearly important roles for this circuit and its interactions with extended amygdala in many aspects of drug seeking and taking, withdrawal and relapse. However, it is not so clear why and when consideration of the two other circuits lost favor in this field. While the limbic circuit is often characterized as “the brain reward circuit”, there is every bit as much evidence that the associative circuit supports brain substrates of reward or reinforcement. Early studies by Wise, Routtenberg (Routtenberg and Malsbury, 1969; Corbett and Wise, 1980) and others indicated that electrical stimulation of the SNc supports intracranial self-stimulation (ICSS), one of the key behavioral indices of reward (nicely reviewed in Wise 2009). This idea has been resurrected in recent studies showing that specific optogenetic activation of SNc dopaminergic neurons has the same rewarding effect (Rossi et al. 2013; Ilango et al. 2014). The DS has also been shown to contain sites that support ICSS (Phillips et al., 1976), and it has been suggested that striosome compartments are the major contributors to this effect (White & Hiroi 1998). Further, ICSS targeted at dopamine type 1 (D1) receptor expressing and dopamine type 2 (D2) receptor expressing medium spiny neurons (MSNs) via optogenetic stimulation in DS has been observed (Kravitz et al. 2012; Vicente et al. 2016). There is also a large and growing literature indicating that dorsal striatum has key roles in reward-based learning (Reynolds et al. 2001). In addition, there is increasing realization that drugs of abuse alter the associative and sensorimotor circuits, both following acute and more prolonged drug exposure (Everitt & Robbins 2016). Finally, studies from a variety of investigators indicate roles for the associative and sensorimotor circuits in responses to drugs of abuse, drug seeking and taking, and addiction. This review will focus on the drug-induced associative and sensorimotor circuit changes and roles in addiction. This field has grown to the extent that not all findings in this area can be covered, but we will try to point out key examples for each of the topics we discuss, and we will cite other reviews that cover some of the topics in more detail.
Acute Drug Actions in Associative and Sensorimotor Circuits
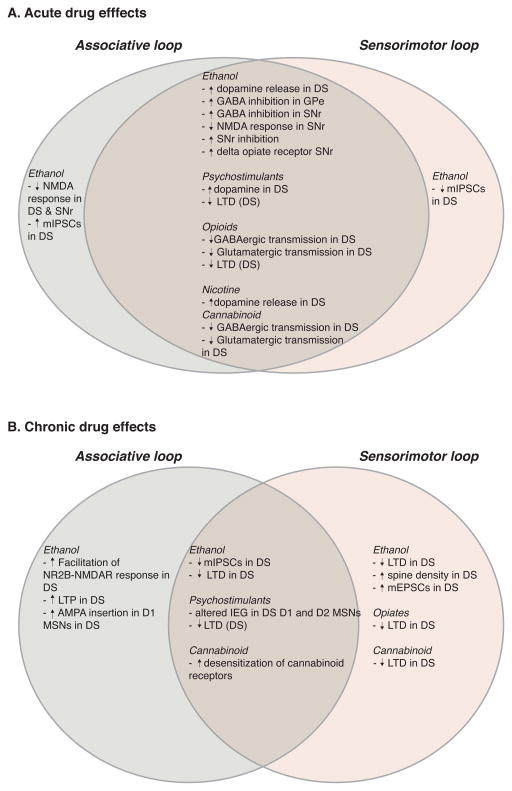
It is well known that dopamine has key roles in responses to drugs of abuse. Indeed, a common trope in the drug abuse research field is that every addictive drug increases dopamine (usually followed by “in the nucleus accumbens”). This assertion appears to be true, and indeed it extends to the dorsal striatum as well (Figure 2A). Past studies indicated that drugs of abuse administered in vivo can alter dopamine, dopaminergic markers, dopamine-related immediate-early gene expression, and neuronal activity in dorsal striatum (Chang et al. 1988; Reggiani et al. 1980; Vezina et al. 1992; Nye & Nestler 1996; Ferguson & Thomas 2004; Valjent et al. 2004) (Marshall and Smith, 1974; Fadda et al., 1980; Kiba and Jayaraman, 1994; Curran et al., 1996; Fadda et al., 2005; Mathews et al., 2006). Measurements of dorsal striatal dopamine with microdialysis have revealed increased dopamine following injection of alcohol, cocaine, nicotine and amphetamine (Benwell & Balfour 1997; Di Chiara & Imperato 1988; Mathews et al., 2006). The cocaine and amphetamine actions are hardly surprising given that these drugs have direct effects on dopaminergic terminals. Other drugs, such as ethanol and nicotine, appear to increase striatal dopamine mainly through mechanisms that increase the firing of midbrain dopaminergic neurons (Morikawa & Morrisett 2010). When investigators have bothered to examine effects of these drugs on firing of SNc (as opposed to VTA) neurons, they have indeed observed increased firing (Grenhoff et al. 1986; Matsubayashi et al. 2003; Morikawa & Morrisett 2010) (Mereu et al., 1984; Lichtensteiger et al., 1982; Mercuri et al., 1992). The rationale for the strong focus on DA increases in NAc is not entirely clear, but it appears to be due, at least in part, to early studies (Benwell & Balfour 1997; Di Chiara & Imperato 1988) that showed larger DA increases induced by peripheral administration of a variety of drugs (amphetamine, cocaine, morphine, alcohol and nicotine) in the NAc, compared to what they call striatum or “dorsal caudate” using older microdialysis technology. However, increases induced by all drugs were clearly seen in the DS in these studies, and thus the interpretation rests on the degree to which the magnitude of the DA effect reflects the impact on the circuit and behavior. Furthermore, the magnitude of the DA increase in response to amphetamine is not always larger in NAc (e.g., (Kuczenski & Segal 1997). In these early studies, no attempt was made to parse out DA increases in different DS subregions, leaving a lack of information as to whether the associative and sensorimotor striatum are preferentially affected by these drugs. Thus, the dorsal striatum certainly experiences increased dopaminergic signaling in the presence of drugs of abuse. The focus on the NAc with regard to DA and abused drugs is the product of outdated studies using early technology, that certainly needs revisiting.
Figure 2.
Schematic representation of reported drug-related findings in Associative (light green) and Sensorimotor (tan) loops following acute (A) or chronic (B) drug exposure. Overlap between loops is indicative of general effects with no differentiation between Associative and Sensorimotor loops. Dopamine type 1-expressing neurons (D1), dopamine type 2-expressing neurons (D2), Dorsal Striatum (DS), Globus Pallidus external segment (GPe), intermediate early gene expression (IEG), long term depression (LTD), long term potentiation (LTP), medium spiny neurons (MSNs), miniature excitatory post-synaptic currents (mEPSCs), miniature inhibitory post-synaptic currents (mIPSCs), SNr (Substantia pars reticulata).
Acute exposure to the psychostimulant drugs (e.g. amphetamine, methamphetamine, methylphenidate and cocaine) that directly increase extracellular DA through effects on the dopamine transporter (DAT) produces increased locomotion and motor stereoptypies. Early studies indicated involvement of DS in drug-induced stereotypies (Staton & Solomon 1984; Kelley et al, 1989), while the NAc was implicated in the increase in locomotion (Carr & White, 1987; Staton & Solomon, 1984; Kelley et al., 1989), although this pattern appears to vary for different psychostimulants and different DS subregions (e.g. Carr and White, 1987; Kelley et al., 1989; Delfs et al., 1990).
There are several other acute drug actions in the DS itself that could contribute to intoxication and later-developing neuronal responses (Figure 2A). For example, nicotine can alter the influence of cholinergic neurons on dopamine release via actions at nicotinic receptors on dopaminergic terminals (Rice & Cragg, 2004). Ethanol inhibits NMDA receptor-mediated synaptic responses in DMS, and this inhibition is followed by a rebound “long-term facilitation” of this response (Wang et al., 2007a). This facilitation requires ethanol effects on the Fyn protein tyrosine kinase, consistent with the observation that this effect is most pronounced when a tyrosine phosphatase inhibitor is included in the slice recording solution (Wang et al., 2007a). Ethanol has mixed effects on GABAergic synaptic transmission in dorsal striatum. GABAergic miniature inhibitory synaptic currents (mIPSCs) are potentiated in DMS, but inhibited in DLS (Wilcox et al. 2014). Transmission at GABAergic synapses between fast-spiking interneurons and SPNs are inhibited by acute ethanol exposure, and this effect appears to involve activation of delta opiate receptors (Patton et al., 2016). Acute ethanol administration also appears to activate delta opiate receptors in the nigrostriatal pathway (Méndez et al. 2004). Opiate and cannabinoid receptor agonists inhibit glutamatergic and GABAergic synaptic transmission in striatum ( Jiang and North, 1992; Szabo et al., 1998; Gerdeman and Lovinger, 2001; Gerdeman et al., 2002; Atwood et al. 2014; Banghart et al., 2015). However, effects of THC and abused opiates have not been examined in any detail in the DS.
Information about acute drug effects becomes even sparser when considering other basal ganglia regions within the associative and sensorimotor circuits (Figure 2A). Ethanol enhances GABA inhibition of neuronal firing in the globus pallidus external segment (GPe) (Criswell et al. 1995). We have also recently found that ethanol inhibits the firing of a subclass of GPe neurons (unpublished data). These neurons exhibit low frequency firing, and express the transcription factors LHX6 and/or NPas1. The majority of the neurons in GPe are GABAergic, and thus this ethanol effect would likely disinhibit neurons that are projection targets of this GPe neuronal subclass. Inhibition of the firing of SNr neurons by ethanol was shown in vivo (Mereu et al. 1984), but this effect has not been examined in freely-moving animals. Ethanol also potentiates inhibitory GABA effects in SNr (Yang et al. 1996; Criswell et al. 1995), and inhibition of NMDA receptor-mediated responses has also been observed in this BG region (Criswell et al., 2003). These studies indicate selective effects on subsets of SNr neurons. In light of our expanding knowledge of SNr neuronal subtypes (Zhou & Lee, 2011; Higgs & Wilson, 2016), this is an area that deserves further study. There is very little information on acute ethanol actions in the subthalamic nucleus, but changes in cFos expression in this region have been associated with acute withdrawal following ethanol exposure (Kozell et al. 2005). Clearly there is more work to be done to sort out the effects of acute exposure to drugs of abuse alters the function of the associative and sensorimotor circuits. There is also very little information on the contribution of these circuits to acute drug intoxication. Most of the rewarding or reinforcing effects of these drugs have been attributed to effects in ventral striatum, but this idea needs to be revisited.
Chronic Drug Actions in Associative and Sensorimotor Circuits
Chronic exposure to ethanol and chronic ethanol consumption produce a variety of changes in associative and sensorimotor circuitry, particularly in the striatum (Figure 2B). As mentioned above, long-term facilitation of NR2B-NMDAR mediated synaptic responses begins just after the cessation of acute ethanol administration in brain slices in DMS (Wang et al., 2007a). Following chronic ethanol administration of drinking this increase appears to persist in the DMS for days to weeks (Wang et al., 2010), at least when recordings conditions include tyrosine phosphatase inhibitor. One consequence of this increase in NMDAR function is to activate mechanisms that result in a long-term potentiation (LTP), given that activation of this receptor is a key step in induction of this form of synaptic plasticity. Following acute exposure to ethanol in slices, or 7 days of ethanol treatment in vivo, Wang et al. (2010), found that induction of LTP by repetitive afferent stimulation was facilitated (Wang et al. 2010). This LTP induction was largely dependent on activation of NR2B-containing NMDARs, consistent with the increase in function of this receptor subtype previously shown following chronic ethanol exposure. Expression of LTP involves increased synaptic insertion and function of AMPA-type glutamate receptors (Granger & Nicoll, 2013). Increased synaptic insertion of AMPARs was observed following the 7 day ethanol administration procedure or repeated alcohol consumption/withdrawal cycles (Wang et al., 2010). A recent study from this group indicates that increased AMPAR function is enriched in direct pathway MSNs, and not indirect pathway neurons, in DMS (Wang et al. 2015).
Long-lasting changes in GABAergic synaptic transmission have also been observed in DS following chronic ethanol exposure or intake (Figure 2B). Examination of spontaneous miniature inhibitory synaptic currents (mIPSCs) in MSNs within the associative striatum and sensorimotor striatum indicate that presynaptic GABA release is decreased following either forced ethanol exposure or chronic ethanol drinking(Wilcox et al. 2014). A similar decrease in GABAergic transmission was also observed in the putamen nucleus of macaque monkeys that consumed alcohol for more than three years, and the extent of the decrease in GABA release was correlated with average ethanol consumption in these non-human primates (Cuzon Carlson et al. 2011). This decrease was observed 22 days after the last ethanol-drinking day, indicating that the synaptic change is persistent. Thus, one consequence of long-term ethanol exposure is to disinhibit striatal output. Within the sensorimotor circuit this could contribute to the increase in habit formation and habitual alcohol seeking induced by chronic ethanol exposure (Corbit et al. 2012; Dickinson et al. 2002).
Drugs of abuse also alter long-term synaptic depression (LTD) at striatal synapses (Figure 2B). The most common form of striatal LTD synaptic plasticity occurs at corticostriatal synapses. This form of plasticity is dependent on dopamine release and activation of D2 dopamine receptors, and involves retrograde signaling by endocannabinoids that act on presynaptic CB1 cannabinoid receptors to induce a long-lasting decrease in glutamate release (Gerdeman et al., 2002). In vivo administration of a variety of different drugs of abuse including alcohol, THC, and opiates results in a loss of the ability to induce LTD at these synapses, particularly in DLS (DePoy et al. 2013; Nazzaro et al. 2012; Atwood et al. 2014). It is not yet clear what mechanisms account for the drug-induced loss of LTD, or even if there is a common mechanism through which all the different drugs produce this action. In the case of in vivo exposure to THC, studies have shown that desensitization of responses to CB1 agonists occurs after acute or prolonged in vivo exposure to this phytocannabinoid partial agonist (Lazenka et al., 2013; Mato et al., 2004; Hoffman et al., 2003). Internalization of CB1 receptors after THC treatment has also been observed, but mostly in heterologous expression systems or in cell culture, and not in presynaptic terminals (Hsieh et al., 1999; Laprairie et al., 2014). Thus, it is unclear if loss of CB1 LTD in DS is due to this desensitization. This form of LTD is also lost in striatum following a single in vivo exposures to cocaine or the opiate drug oxycodone (Fourgeaud et al. 2004; Atwood et al. 2014), and following days-weeks of ethanol exposure (DePoy et al. 2013; Xia et al. 2006; Adermark et al. 2011). Loss of LTD after exposure to these drugs could be due to CB1 receptor desensitization, and indeed the response to a CB1 agonist is decreased following oxycodone treatment (Atwood et al. 2014). However, the fact that other presynaptic forms of LTD are also impaired by drugs, such as oxycodone, suggests a more general impairment of presynaptic signaling activated by Gi/o-coupled GPCRs. Clearly, more work is needed to determine the mechanisms involved in drug actions on presynaptic terminals and LTD. While it is difficult to specifically manipulate intra-terminal signaling in presynaptic elements, new molecular and imaging tools should aid in this effort.
Chronic exposure to drugs of abuse is known to alter the morphology of neurons in DS (Figure 2B). Robinson and coworkers showed that amphetamine treatment that induced sensitization increased spine density of distal dendrites of MSNs in lateral striatum. Using a 3 month methamphetamine exposure regimen, the Robinson group also found increases in mushroom-shaped and thin spines in DLS, with a decreases in the presumed mature mushroom-shaped spines in DMS (Jedynak et al. 2007). This finding does fit with the idea that prolonged drug exposure may foster plasticity that increases sensorimotor circuit function, while decreasing associative circuit activity. These sorts of changes could contribute to habitual drug seeking and taking. Indeed, both acute and chronic psychostimulants induce changes in the immediate early gene expression in dorsal striatum (e.g., (Graybiel et al. 1990; Curran et al., 1996). More recently, changes in immediate early gene expression have been seen following both acute and prolonged methamphetamine abstinence and subsequent incubation of craving in D1 type and D2 type MSNs in dorsal striatum (Li et al. 2015). Alcohol also induces changes in SPN morphology in a non-human primate model (Cuzon Carlson et al. 2011). In this case, SPNs in monkey putamen were examined after more than 3 years of alcohol consumption. Increased density of dendritic spines was observed in the alcohol drinking monkey putamen, and this was associated with an increase in the frequency of glutamatergic miniature excitatory postsynaptic currents (mEPSCs). Thus, increased glutamatergic synaptic transmission may reflect greater numbers of functional synapses on dendritic spines induced by long-term alcohol consumption. Other drugs of abuse have been suggested to alter dendritic morphology and spine number in the DS (c.f. Li et al., 2015), but the major emphasis has been on morphological changes in NAc.
Associative and sensorimotor loops and drug-related reward and reinforcement
Conflation of the terms “reward’ and “reinforcement” is one source of confusion that contributes to misconceptions about the roles of different cortico-basal ganglia circuits in behavioral control and drug actions. As described by White (1989) and others (Dezfouli & Balleine, 2012; Yin et al., 2006) we believe these terms are best used to describe different neural processes that can be operationally defined, and are controlled by largely different neuronal circuits. In this conceptualization, the broad definition of reward refers to an outcome that is subjectively pleasurable or valuable to an organism in a given context. Reward will drive instrumental behavior as long as the value (either immediate expected value or historical value) to the organism is intact at the time that behavior is examined and the reward is contingent on that behavior. However, if that reward value is diminished (i.e. through satiety or other devaluation procedures), or uncoupled from action performance (as in contingency degradation), then instrumental performance will slow over time, or stop in a relatively rapid timeframe. In the context of Pavlovian conditioning, the rewarding effects of a drug can, through repeated pairings with an initially neutral stimulus, impart rewarding properties to an environmental stimulus such that this “conditioned stimulus” can elicit a conditioned response reflective of the conditioned rewarding effects. Depending on the conditioning paradigm, it can be measured as a cue-elicited seeking behavior. Importantly drug reward can be examined through instrumental and Pavlovian conditioning paradigms.
Skinner initially coined the term reinforcement to describe what he believed was stimulus-response learning (S-R), the process by which exposure to an outcome following a behavior leads to repetition of that behavior (in a particular context) (Skinner, 1938). We would like to emphasize the view that learned actions are initiated in the organism and selected by the environment (for review see; Costa 2011). While reinforcement has similarities to reward (i.e. both increase instrumental behavior), reinforcement is often a gradual process occurring through a trial and selection experience, in which a subject learns an association between a particular response and the outcome it produces. Under conditions of uncertainty or with continued trial and selection experience, actions may come to be elicited by particular stimuli or situations as in S-R learning otherwise known as habitual action control. However, work over the last three decades has shown that the expected outcome can also control actions. This has been termed action-outcome (A-O) learning and is also known as goal-directed control. Psychological research has shown that goal-directed control over actions depends in part on the expected outcome value, and the current knowledge that a particular action produces the outcome (Adams, 1982; Adams and Dickinson, 1981; Colwill and Rescorla, 1985; Dickinson, 1985). That is, goal-directed actions are performed with the expectation of receiving an outcome of a particular value. If that outcome is devalued (e.g. using a sensory-specific satiety procedures) or the relationship between action and outcome altered (e.g. with a contingency degradation procedure), then performance will decrease. In contrast, S-R or habitual control has been operationally defined as behavior that is less sensitive to immediate reward value and immediate action-outcome contingency (Adams, 1982; Adams and Dickinson, 1981; Colwill and Rescorla, 1985; Dickinson, 1985) Initial instrumental learning processes may rely more on goal-directed control, with the expected outcome controlling the process. However, with continued experience or in uncertain circumstances, habitual control over instrumental behaviors may develop (Dickinson & Balleine 1994; Balleine & Ostlund 2007; Balleine & O’Doherty 2009; DeRusso et al., 2011). Habitual control, while still a motivated self-initiated action process, is more dependent on the historical reward value and historical action-outcome relationship. Habitual behavior is still sensitive to value changes when assessed independently of action control (i.e. reduced consumption following devaluation), with the distinction being that with habits there is an inability to use that updated outcome value information to control action selection. Hence, immediate reward value may influence goal-directed control to a greater degree than it does habitual control.
This distinction is critical for the greater addiction field; the fact that habitual drug-related responding may not be sensitive to devaluation does not mean that changes to the expected outcome value have not been updated. Using these definitions, a subject could still update the value of a devalued drug; however, that updated value is no longer controlling the action. To put this in lay terms, an addict may be aware of the negative consequences of taking a drug, but that knowledge is not controlling particular drug seeking or taking behaviors. Indeed, sensitivity to the changed outcome value may be perceived differently under varying conditions, such as a subjective assessment of the drug’s effects or the subjective assessment of a desired effect. Furthermore, it may be hard to target different individual components of value (e.g., taste processing versus motivational) that could differentially contribute to behavioral control.
The distinction between goal-directed and habitual action processes might have remained largely in semantic arguments among experimental psychologists. However, with the discovery that different cortico-basal ganglia circuits control behavior through the two processes (for review see; (Yin and Knowlton, 2006; Balleine and O’Doherty 2009), there is neurobiological evidence that the separation of these concepts is useful for understanding neural mechanisms of action control. To summarize the neurobiology related to these processes, regions within the associative circuit including the medial striatum contribute to behavior that is goal-directed (e.g. driven by reward in the case of increased instrumental actions) (Figure 1B). In contrast, regions within the sensorimotor circuit including the dorsal lateral striatum contribute to actions that depend on past training history but are independent of immediate outcome value (habitual behaviors) (Figure 1C). This implies a fundamental role for both associative and sensorimotor circuits in action control, with the potential for drugs of abuse and addiction-related processes to alter the function of these circuits, and thereby contribute to associated pathological behaviors. Viewed another way, tasks probing these different action strategies may be used as a tool to examine drug-induced changes to the associative and sensorimotor striatum. With an eye to these dissociations in reward and reinforcement-related behaviors and functional differentiation of circuits, we will next review behavioral procedures and associated data designed to assess associative and sensorimotor circuit contribution to drug-related behaviors. As expected, the recent growth of interest in this topic precludes us from providing an exhaustive review of all the literature. Instead, we will focus largely on reviewing examples of studies that provide functional evidence of associative and sensorimotor striatal involvement in drug reward and drug reinforcement.
The conditioned place preference (CPP) procedure is an appropriately popular task often used to evaluate drug reward and cue-induced drug-seeking. CPP utilizes a Pavlovian form of associative conditioning where a distinctive conditioned stimulus (CS+) is paired with a drug (unconditioned stimulus or US) (Cunningham et al., 2006; Tzschentke 2007). An association between the CS+ and US develops across repeated pairings, such that the CS+ is thought to be able to elicit a conditioned rewarding response similar to the drug itself. CPP is then measured as the amount of time spent in the presence of the CS+ compared to an unpaired stimulus (CS−), with the interpretation that an increase in CPP reflects an increase in the conditioned rewarding properties of the drug. Hence, the assumption is that the more rewarding a drug is, the stronger the CPP will be. Used extensively in rodents to assess a wide-array of drugs for abuse potential, researchers have combined it with intracranial targeting measures to identify neural circuits mediating drug-reward. Not surprisingly given the strong influence of Pavlovian learning in CPP, the majority of work has identified limbic circuit involvement in a drug-induced CPP (Tzschentke 2007). Indeed, lesions of the sensorimotor striatum had no effect on a food-CPP (Featherstone & Macdonald, 2005). However, there are findings that suggest the dorsal striatum is recruited when place preference expression is under control of instrumental processes. In particular, mice show a place preference when locomotion within a context was paired with optogenetic stimulation of D1 MSNs in the dorsal striatum (Kravitz et al., 2012). The lack of effect observed in CPP procedures may be reflective of the limited studies examining the dorsal striatal contribution to this behavior. More likely, it may be because the neurobiology recruited during CPP learning and expression and cue-related learning in general have been shown to be more dependent on ventral striatum processing (for review see Tzschentke 2007).
The purported gold standard that is the focus of most addiction models has long been the instrumental self-administration procedure. Indeed, an ever-increasing body of literature points to a role for dorsal striatum in drug self-administration and related behaviors where actions are necessary for drug reward or reinforcement. Generally, these procedures involve the subject learning to execute a novel action at a manipulandum (e.g., lever) that results in the delivery of a drug in an operant box setting, although sometimes drug self-administration in the home-cage is employed (for review see Shippenberg & Koob, 2002). Of course, multiple variations exist, including different lever-press requirements such as a fixed ratio of responses (FR), or the presence of discriminative stimuli or contextual information that signal drug availability. Chain schedules of reinforcement are often used to examine drug-seeking or drug-taking behaviors, where an initial response requirement (i.e. seeking response) needs to be completed prior to the availability to perform the second drug-taking response requirement (for review see: Everitt & Robbins, 2005). Additionally, the majority of procedures employ Pavlovian conditioning and pair initially neutral cues with an aspect of instrumental drug self-administration. While this may offer advantages in modeling human use, it complicates isolating neurobiological mechanisms that contribute to either instrumental or Pavlovian processes and the corresponding interpretations, as discussed later. A cue may serve as a discriminative stimulus and signal the availability of drug. A direct measure of the ability of classical or Pavlovian conditioning to influence self-administration is often examined through the use of Pavlovian to Instrumental Transfer paradigms that assess the degree to which a previously neutral cue paired with a drug’s effect can increase or decrease drug self-administration (e.g., LeBlanc et a., 2012). Further, in second order schedules a cue paired with drug delivery may act as a conditioned reinforcer, supporting seeking responses and helping to bridge delays until drug delivery (e.g., Everitt & Robbins, 2000). In some cases, actions aimed at gaining access to the cue in the absence of drug delivery are assessed as a measure of drug-seeking (e.g., Di Ciano & Everitt, 2004). Additionally, some paradigms examine effects of stress on drug self-administration or pair aversive events (e.g., punishing floor shocks) with drug-seeking or drug-taking behaviors (for reviews see: Lu et al, 2003; Logrip et al., 2012). Across all versions of the task, one core component of drug self-administration is that drug outcome delivery depends upon the animal’s action at the manipulandum. The use of self-administration procedures has proven successful for examining critical components of the addiction cycle; specifically, it is useful for investigating the neural mechanisms related to acquisition, maintenance, and escalation of drug reinforcement, extinction of drug-related actions, as well as the phenomena of relapse and reinstatement of drug-seeking behaviors (for reviews see: Wise & Koob, 2014; Edwards & Koob, 2013; Crombag et al., 2008; Bossert et al., 2013; Marchant et al., 2013).
Hedonic aspects of drug reward have also been examined, but separating effects on reward from effects on reinforcement are often difficult. One distinction of drug self-administration studies versus CPP is that experience with the drug is gained through self-administration. That is, self-administration behavior is dependent on the ability of the animal to learn the association that a response produces a drug effect. The time course of experienced drug effects is largely determined by the pattern of responding. The animal can to variable degrees decide when to administer drug and at what rate to achieve an effect. Of course, this lends to the face validity of the model, but an implication too often overlooked is that differing self-administration patterns can produce varied results in terms of subjective reinforcement or reward assessment and corresponding neurobiological effects and/or long-term changes. One way investigators have circumvented this issue is to examine the direct effects of prior drug exposure on the ability to control food-related or drug-related behaviors (Nelson 2006; Corbit et al. 2012; DePoy et al., 2013; Vendruscolo & Roberts, 2014; Corbit, et al. 2014; Schmitzer-Torbert et al. 2015). Then it may be possible to compare changes in neural circuits induced by the direct effects of drug-exposure with neural circuit changes seen following chronic drug-self administration.
Intriguingly, it appears that exposure to drugs independent of self-administration can produce long-lasting changes in the function of associative and sensorimotor circuits. Cocaine exposure immediately following food self-administration sessions biased use of habitual action strategies through mechanisms dependent on sensorimotor striatum (Schmitzer-Torbert et al. 2015). This drug exposure-induced control by sensorimotor striatum is seen with alcohol as well. Chronic alcohol vapor exposure been shown to bias mice towards behaviors mediated by sensorimotor striatum, and induced changes to the endocannabinoid system in sensorimotor striatum (DePoy et al. 2013). Although under the control of the subject, alcohol drinking in the home cage during instrumental training for a food outcome resulted in a reliance on habitual action control shown to be dependent on sensorimotor striatum, with a negative correlation between how much an individual animal drank and the degree to which the action was goal-directed (Corbit et al. 2012). Together these findings suggest that chronic drug exposure can favor behaviors controlled by sensorimotor circuits, and there is now a focus on understanding mechanisms underlying these behavioral changes. Previous work has shown the necessity of dopamine neurons that send projections to sensorimotor striatum for habit control (Faure et al. 2005). This suggests the hypothesis that chronic drug-induced changes to dopamine neuron function and modulation may underlie the bias towards reliance on habitual strategies. Indeed, exposure to drugs that act directly on dopaminergic transmission, including both cocaine and amphetamine, biased animals toward habitual actions when food was the reinforcer even when training was initiated after the end of drug exposure (Nelson 2006; Corbit et al. 2014). In addition to chronic drug effects in dorsal striatum, chronic drug exposure-induced changes in cortical and thalamic function most likely plays a role, as well as drug-induced changes to subcortical circuits, highlighting the need for a greater understanding of drug-induced changes to cortical function and flow of information through associative and sensorimotor striatum. Obviously more work is necessary to gain a greater understanding of the mechanisms that induce a drug-induced reliance of actions on control by sensorimotor striatum. These mechanisms may include disruptions to associative and limbic loops that bias towards a reliance on sensorimotor control, or a strengthening of sensorimotor circuit control over behavioral output, hypotheses that will be discussed later in the review.
As we noted earlier, the associative and sensorimotor striatum have been functionally implicated in the regulation of drug self-administration. Functional evidence suggests associative striatum is involved in the early stages of ethanol and cocaine self-administration A home-cage alcohol drinking paradigm in mice found depressed GABAergic transmission in the associative and sensorimotor striatum; however, no significant correlations with blood alcohol concentrations were observed (Wilcox et al., 2013). Disruption of NMDA receptor signaling involving receptors containing the NR2B subunit, specifically within the associative striatum, appears to regulate moderate levels of alcohol self administration as well as relapse of alcohol seeking behaviors (Wang et al., 2007b; Wang et al. 2010; Wang et al. 2015). Similar results were observed with inhibition of dopamine D1 receptor function (Wang et al., 2015). Cued drug seeking but not drug taking under a second order schedule cocaine self-administration task was found to be dependent on dopamine receptors (non-specific targeting of D1 and D2 type receptors) and AMPA/KA (but not NMDA) receptors in associative striatum (Vanderschuren et al. 2005). Similar findings were observed with blockade of dopamine receptors during early acquisition in a cued cocaine self-administration procedure, with broad dopamine receptor antagonism in associative striatum reducing responding for the cue previously paired with drug-delivery (Murray et al. 2012).
Similarly, the sensorimotor striatum has also been implicated in drug self-administration, although generally it has been suggested to be involved in mediating chronic drug self-administration. It has been shown that the sensorimotor striatum is necessary for habitual alcohol seeking that emerges with continued self-administration training, an effect that depends on AMPA and D2 receptors (Corbit et al. 2012; Corbit, et al. 2014). Further, it appears that long-term alcohol self-administration may be attenuated through BDNF activation of the map kinase pathway in sensorimotor striatum (Jeanblanc et al. 2009; Jeanblanc et al. 2012). In addition, the opioid system in sensorimotor striatum has been implicated in intermittent alcohol self-administration across development, with delta opioid peptide (DOP) receptor antagonism inducing a long-lasting reduction in alcohol consumption (Nielsen et al. 2012). Cued-cocaine self-administration and chronic cocaine seeking behaviors have also shown a dependency on the sensorimotor striatum. Using a second order schedule to separately evaluate drug-seeking from drug-taking components, inactivation of sensorimotor striatum disrupted well-established cocaine-seeking (Zapata et al. 2010). Furthermore, a broad-spectrum dopamine receptor antagonist delivered to sensorimotor striatum disrupted well established cocaine seeking in a chronic cued cocaine self-administration procedure (Murray et al. 2012). This follows previous work showing an increase in dopamine release in dorsal striatum during cue-induced cocaine seeking (Ito et al. 2002) With more chronic cocaine self-administration, increased radioligand binding to the of dopamine transporter (DAT) (Letchworth et al. 2001), and decreased density of D2 receptor binding in dorsal striatum have been observed (Nader et al. 2002). Relapse to drug self-administration also seems to require the dorsal striatum. Inactivation of sensorimotor striatum, as well as substantia nigra, attenuated cocaine-seeking after abstinence (Fuchs et al. 2006; See et al. 2007). Additionally, inactivation of sensorimotor but not associative striatum disrupted context-induced reinstatement of methamphetamine self-administration (Rubio et al. 2015), and antagonism of sensorimotor striatum D1 receptors attenuates a similar context-induced heroin seeking behavior (Bossert et al. 2009). Evidence also comes from studies examining transcription and translation processes within dorsal striatum following drug-self-administration. The transcriptional repressor MeCP2 has been implicated in controlling cocaine intake via alterations in BDNF expression (Im et al. 2010). In addition, microRNA control over CREB signaling in dorsal striatum contributes to the motivational properties of cocaine (Hollander et al. 2010).
While these findings suggest involvement of associative and sensorimotor striatum in drug seeking and taking behaviors, as well as some similarities in the molecular mechanisms (mainly dopamine and dopamine receptor involvement), they also underscore a hypothesis put forth by many in the addiction field; control over drug-self-administration initially depends on goal-directed processes, and with continued use habitual processes are recruited that are more dependent on sensorimotor striatum (for reviews see: Hogarth et al., 2012; O’Tousa & Grahame, 2014; Barker et al., 2015). For example, Corbit and coworkers have shown that moderate alcohol self administration is initially goal-directed and under the control of associative striatum, but with continued use and self-administration experience it transitions to sensorimotor circuit control that is dependent on D2 and AMPA receptor activation (Corbit et al. 2012; Corbit et al. 2014). Similar findings have been seen with cocaine seeking behaviors, with initial behavioral control more dependent on more limbic circuits including ventral striatum that with prolonged cocaine self-administration, come to be dependent on sensorimotor striatum (Murray et al. 2012; Murray et al. 2014; Murray et al. 2015).
Circuit Changes Underlying Involvement of Habitual Drug Seeking and Intake
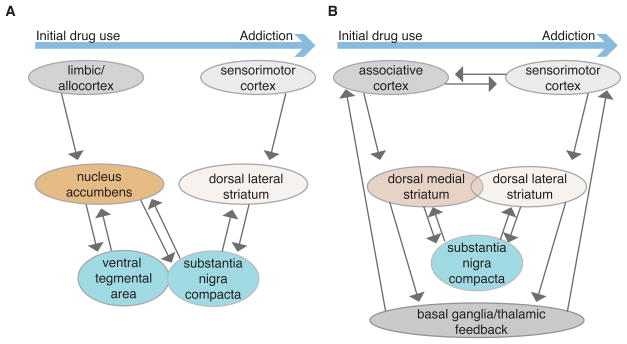
One popular model of the transition to habitual drug seeking and taking comes from the work of Everitt, Robbins and colleagues (for recent reviews see: Everitt & Robbins, 2016; Belin et al., 2009) (Figure 3A). Given the well-known effects of drugs of abuse in the NAc in many paradigms, these investigators postulate that initial drug actions that support drug-seeking and drug-taking involve changes in this limbic area. With continued drug usage, gradually developing changes in the sensorimotor circuit within the striatum are induced, leading to habitual responding for drug access (usually assessed with a second-order schedule involving a response made to gain access to a drug-associated conditioned stimulus). The connection between limbic and sensorimotor circuits is postulated to involve long-loops through midbrain dopaminergic regions (Figure 3A). Drug-induced changes in output from NAc are thought to influence not only the VTA, but also dopaminergic neurons within the SNc. This alters the dopaminergic input to the DLS, perhaps setting into motion the reinforcement process underlying habitual drug seeking.
Figure 3.
Example simplified schematic of differing models showing brain areas involved in the transition from initial drug use to addiction. A. Model where parallel striatal-midbrain loops link limbic and sensorimotor circuits, leading to gradual habitual control of actions involving Pavlovian-Instrumental transfer. B) Model where parallel associative and sensorimotor circuits mediate competing goal-directed and habitual instrumental actions, with multiple sites of circuit modulation & interaction (e.g. corticostriatal efficacy, midbrain, and basal ganglia/thalamic loops).
This attractive model has received considerable experimental support, including disconnection studies in which drug-related responding is altered by disruption of key points in the limbic circuit in one brain hemisphere are combined with disruption of sensorimotor circuitry in the other hemisphere. However, it is important to point out key features of the experiments that influence the findings. The assessment of seeking through use of a second-order schedule necessarily involves development of PIT, a type of learning well known to involve the amygdala, NAc and ventral pallidum ( Blundell et al. 2001; Corbit et al. 2001; Cardinal et al. 2002; Holland & Gallagher 2003; Shiflett & Balleine 2010; Corbit & Balleine 2011; Root 2013). Indeed, using a second order schedule, it has been shown that progression to sensorimotor dependence involves transitions in NAc (Belin & Everitt 2008) and amygdala control (Murray et al. 2015). Thus, there is little surprise that the NAc has an important role in the initial learning of the drug association, and the subsequent PIT-driven drug seeking behavior. Recent work has also shown that cued-ICSS via optogenetic stimulation of ventral tegmental dopaminergic neurons was sufficient to induce long-term plasticity changes in downstream NAc and mice that received this regimen showed cue-induced relapse behavior (Pascoli et al., 2015). While this suggests that ICSS behavior may mimic one set of behaviors often observed in chronic drug self-administration models, it is still a behavior strongly controlled by previous Pavlovian learning. It is not so clear to us that the limbic circuit would have as prominent a role when drug seeking is assessed using a self-paced instrumental task in the absence of predictive cues. Previous work using a non-cued cocaine self-administration task does suggest recruitment of the sensorimotor striatum in context-induced relapse, independent of any recruitment by cue-related processes (Fuchs et al. 2006; See et al. 2007). The focus on how limbic information may be more likely to influence sensorimotor circuitry in drug-related processes unfortunately downplays the role of sensorimotor and associative striatum in both initial drug use as well as in more chronic drug-use situations (Figure 3B).
This distinction is important, and neglect of it may result in overlooking fundamental involvement and addiction-related changes that happen to both associative and sensorimotor striatum. While the importance of predictive (Pavlovian) information in our environment in supporting ongoing seeking and taking behaviors should not be ignored and does contribute greatly to drug use and addiction, action control is often generated within the individual or at least influenced strongly by the individual’s trial and selection experience. Either associative or sensorimotor striatum is sufficient for initial action learning and can support acquisition of self-administration in the absence of discreet predictive stimuli (Yin et al. 2004; Yin et al. 2005; Hilario et al. 2012; Gremel & Costa 2013). Indeed, experiments that use procedures to bias toward the development of goal-directed or habitual control and then inhibit the associative or sensorimotor striatum, respectively, have shown the remaining system is capable of taking over and controlling action execution ( Yin et al. 2005; Yin et al. 2006). Importantly, the ability to use associative striatum does not appear to be fully impaired following chronic alcohol self-administration, with inactivation of sensorimotor striatum resulting in goal-directed control that is mediated by associative striatum (Corbit et al. 2012; Corbit, et al. 2014). These initial findings suggest three things; first, while chronic drug self-administration may induce greater reliance on sensorimotor striatum, the sensorimotor striatum can support early aspects of the behavior as well. Second, the underlying competition with associative striatum is still intact. Lastly, goal-directed processes can still be recruited to control the behavior following chronic drug self-administration. So while many behavioral procedures currently used to probe the development of habitual drug self-administration often produce a reliance on sensorimotor action control, the evidence to date does not show an inability for behavioral control by the associative circuit (Figure 3B).
One criticism of the hypothesis that habits contribute to addiction is that often an addict’s behavior may appear to be under goal-directed control. One example comes from another prominent contributing factor to the addiction phenotype, negative reinforcement, where actions are aimed at alleviating a particular aversive state (i.e. drug withdrawal) (Koob & Le Moal 1997; Koob, 2013). Whether associative and sensorimotor striatum mediate negative reinforcement are an open avenue for exploration, but a growing literature suggests roles of these brain areas in aversive conditioning (White & Salinas, 2003; Ferreira et al., 2003; Medina et al., 2007; Darvas et al., 2011). Clearly, the involvement of dorsal striatum and associated circuitry in withdrawal and relapse to drug use is an area that deserves increased study. It is also possible that associative and sensorimotor circuits have less involvement than limbic circuits in negative reinforcement-driven relapse. However, the circuits involving dorsal striatum are still likely to be involved in other aspects of substance abuse disorders, such as excessive drug intake after relapse.
It is not always clear that insensitivity to changes in the post-ingestive/absorption effects (presumably the pharmacologically reinforcing properties) is responsible for the greater reliance on sensorimotor circuits. For example, when the post-ingestive effects of alcohol were devalued, the rats showed goal-directed control and reduced responding (Samson et al. 2004). It may be that the habitual responding for alcohol previously observed is due to insensitivity or tolerance to changes in sensory processes (i.e. taste) that are more proximal to alcohol self-administration and therefore play a greater role in controlling the behavior (Corbit et al. 2012; Corbit, Nie, et al. 2014). The role associative and sensorimotor circuits play in the hedonic aspect of updating drug reward through incentive learning processes needs more investigation. Certainly, there are multiple forms of learning involving actions, cues, and outcomes that may differentially contribute to behavioral control depending on the behavioral task used (Hogarth et al. 2012). It would benefit the addiction field to not discount the early and potentially late involvement of associative and sensorimotor circuits in the control over drug-seeking and drug-taking behaviors.
It bears reinforcing that the evidence discussed above indicates that the sensorimotor circuit is engaged very early during instrumental learning for food reward. Thus this circuit is constantly storing information supporting reinforcement learning, and this should apply to drugs of abuse as well as any other reward. Indeed, we now know that drug-promoted habit formation begins to develop within days to weeks of training/drug exposure (e.g., Corbit et al., 2012). The development of habitual behavioral control is not a slow and gradual process, but one that begins very early in learning and could be influenced even by acute effects of drugs of abuse (as suggested in Figure 3B). Ultimately, there are likely to be several mechanisms underlying the shifting control of drug responses by the three parallel cortico-basal ganglia circuits. Examination of drug and training effects on many components of each circuit at all stages of drug exposure and seeking/taking will be needed to fully understand these control mechanisms. Indeed all three circuits are likely recruited in addiction, although perhaps to varying degrees, during drug-related behaviors. One goal of the addiction field should be to gain an understanding of how the output from these three circuits (Figure 3A and B) is used to produce ongoing behavior and neural feedback in the support of future drug-related behaviors
We have used this review to give a broad overview of the effects of acute and chronic drug exposure on associative and sensorimotor circuits, and in turn the involvement of these circuits in behavioral phenotypes of addiction. Overall, we would like to emphasize the importance of these circuits in control of drug-related behaviors, working in parallel with each other and the limbic circuit. The emerging evidence that drugs act on all three circuits from initial acute exposure through chronic use indicates that excessive drug use, drug use disorders, and addiction may be prevented or curtailed by targeting components of each circuit. For example, strengthening associative circuit, as opposed to sensorimotor circuit, control of decisions and actions related to drugs of abuse has the potential to reduce relapse and may also allow for more conscious control that could limit drug intake. Clearly more work is needed to fully understand the different drug effects and circuit roles, but we believe this circuit-inclusive model of addiction will be useful in understanding drug-related disorders, and developing new therapies for drug use disorders.
Acknowledgments
This research was supported by the NIAAA Division of Intramural Clinical and Biological Research, and a Pathway to Independence Award R00 AA021780 and NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation to C.M.G.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. The Quarterly journal of experimental psychology B, Comparative and physiological psychology. 1982;34:77–98. [Google Scholar]
- Adams CD, Dickinson A. Instrumental Responding Following Reinforcer Devaluation. Q J Exp Psychol-B. 1981;33:109–121. [Google Scholar]
- Adermark L, Jonsson S, Ericson M, Soderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61(7):1160–1165. doi: 10.1016/j.neuropharm.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in neurosciences. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Kupferschmidt DA, Lovinger DM. Opioids induce dissociable forms of long-term depression of excitatory inputs to the dorsal striatum. Nature neuroscience. 2014;17(4):540–548. doi: 10.1038/nn.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;35(1):48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Annals of the New York Academy of Sciences. 2007;1104:147–171. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Banghart MR, Neufeld SQ, Wong NC, Sabatini BL. Enkephalin Disinhibits Mu Opioid Receptor-Rich Striatal Patches via Delta Opioid Receptors. 2015;88(6):1227–1239. doi: 10.1016/j.neuron.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barker JM, Corbit LH, Robinson DL, Gremel CM, Gonzales RA, Chandler LJ. Corticostriatal circuitry and habitual ethanol seeking. Alcohol. 2015;49:817–824. doi: 10.1016/j.alcohol.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and incentive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199(1):89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. Regional variation in the effects of nicotine on catecholamine overflow in rat brain. European journal of pharmacology. 1997;325(1):13–20. doi: 10.1016/s0014-2999(97)00101-5. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. Journal of Neuroscience. 2001;21(22):9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, et al. Role of dopamine D1-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206(1):51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229(3):453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. In: The Neuropharmacological Basis of Reward. Copper JMLSJ, editor. Clarendon Press; Oxford: 1989. pp. 264–319. [Google Scholar]
- Carr GD, White NM. Effects of systemic and intracranial amphetamine injections behavior in the open field: a detailed analysis. Pharm Biochem Behav. 1987;27:113–122. doi: 10.1016/0091-3057(87)90485-0. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson Ja, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, Morrison CH, Howes SR, Robbins TW, Everitt BJ. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behavioral Neuroscience. 2002;116(4):553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- Chang SL, Squinto SP, Harlan RE. Morphine activation of c-fos expression in rat brain. Biochemical and biophysical research. 1988;157(2):698–704. doi: 10.1016/s0006-291x(88)80306-1. [DOI] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: A moveable electrode mapping study. Brain research. 1980;185:1–15. doi: 10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA. Postconditioning devaluation of a reinforcer affects instrumental responding. J Exp Psychol Anim Behav Process. 1985;11:120–132. doi: 10.1037/0097-7403.11.1.120. [DOI] [Google Scholar]
- Corbit LH, Balleine BW. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. Journal of Neuroscience. 2011;31(33):11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39(8):1893–1901. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. Journal of Neuroscience. 2001;21(9):3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry. 2012;72(5):389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Frontiers in Behavioral Neuroscience. 2014;8:301. doi: 10.3389/fnbeh.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM. A selectionist account of de novo action learning. Current opinion in neurobiology. 2011;21:1–8. doi: 10.1016/j.conb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Simson PE, Knapp DJ, Devaud LL, McCown Tj, Duncan GE, Morrow AL, Breese GR. Effect of zolpidem on gamma-aminobutyric acid (GABA)-induced inhibition predicts the interaction of ethanol with GABA on individual neurons in several rat brain regions. The Journal of pharmacology and experimental therapeutics. 1995;273(1):526–536. [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther. 2003;304(1):192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koye E, Shaham Y. Review. Context-induced relaspe to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature protocols. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Curran EJ, Akil H, Watson SJ. Psychomotor stimulant- and opiate-induced c-fos mRNA expression patterns in the rat forebrain: comparisons between acute drug treatment and a drug challenge in sensitized animals. Neurochem Res. 1996;21(11):1425–1435. doi: 10.1007/BF02532384. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odarigi M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology. 2011;36(12):2513–2528. doi: 10.1038/npp.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RL. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learning and Memory. 2011;18(3):136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. The Journal of Neuroscience. 1990;10(1):303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences. 2013;110(36):14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli A, Balleine BW. Habits, action sequences and reinforcement learning. Eur J Neurosci. 2012;35(7):1036–1051. doi: 10.1111/j.1460-9568.2012.08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli A, Balleine BW. Actions, Action Sequences and Habits: evidence that goal-directed and habitual action control are hierarchically organized. PLOS Comp Biology. 2013;9(12):1–14. doi: 10.1371/journal.pcbi.1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfouli A, Lingawi NW, Balleine BW. Habits as action sequences: hierarchical action control and changes in outcome value. Phil Trans R Soc B. 2014;369:20130482. doi: 10.1098/rstb.2013.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47(Suppl):202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Actions and Habits: The Development of Behavioural Autonomy. Philos Trans R Soc Lond, B, Biol Sci. 1985;308:67–78. doi: 10.1098/rstb.1985.0010. [DOI] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Animal Learning & Behavior. 1994;22(1):1–18. [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? The Quarterly journal of experimental psychology B, Comparative and physiological psychology. 2002;55(4):331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24(5–6):356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Ann Rev Psych. 2016;67(1):23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Fadda F, Argiolas A, Melis MR, Serra G. Effect of acute and chronic ethanol on dopamine synthesis in the caudate nucleus, substantia nigra and frontal cortex. Boll Soc Ital Biol Sper. 1980;56(24):2553–2558. [PubMed] [Google Scholar]
- Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Dopamine and serotonin release in dorsal striatum and nucleus accumbens is differentially modulated by morphine in DBA/2J and C57BL/6J mice. Synapse. 2005;56(1):29–38. doi: 10.1002/syn.20122. [DOI] [PubMed] [Google Scholar]
- Faure A, et al. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. Journal of Neuroscience. 2005;25(11):2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Lesions of the dorsolateral or dorsomedial striatum impair performance of a previously acquired simple discrimination task. Neurobiol Learn Mem. 2005;84:159–167. doi: 10.1016/j.nlm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Thomas MJ, Robinson TE. Morphine-induced c-fos mRNA expression in striatofugal circuits: modulation by dose, environmental context, and drug history. Neuropsychopharmacology. 2004;29:1664–1674. doi: 10.1038/sj.npp.1300465. [DOI] [PubMed] [Google Scholar]
- Ferreira TL, Moreira KM, Ikeda DC, Bueno OF, Oliveira MG. Effects of dorsal striatum lesions in tone fear conditioning and contextual fear conditioning. Brain Research. 2003;987(1):17–24. doi: 10.1016/s0006-8993(03)03217-7. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. Journal of Neuroscience. 2004;24(31):6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. Journal of Neuroscience. 2006;26(13):3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85(1):468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5(5):446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Granger AJ, Nicoll RA. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos Trans R Soc Lond B Biol Sci. 2013;369(1633):20130136. doi: 10.1098/rstb.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Grafton ST. The striatum: where skills and habits meet. Cold Spring Harbor Perspectives in Biology. 2015;7(8):1–13. doi: 10.1101/cshperspect.a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(17):6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nature communications. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM. Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron. 2016;90:132–1324. doi: 10.1016/j.neuron.2016.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta physiologica Scandinavica. 1986;128(3):351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Haber SN. Integrative Networks across Basal Ganglia Circuits. In Handbook of Basal Ganglia Structure and Function. (1) 2010;Chapter 24 [Google Scholar]
- Haber SN. Neuroanatomy of Reward: A view from the Ventral Striatum. In: Gottfried JA, editor. In Neurobiology of Sensation and Reward. Boca Raton (FL): 2011. [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim K, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. Journal of Neuroscience. 2000;20(6):2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Shepherd GMG. The neocortical circuit: themes and variations. Nature neuroscience. 2015;18(2):170–181. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: The distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiology of Learning and Memory. 2014;108:104–118. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner Sarah R, Rodriguez-Romaguera Jose, Quirk Gregory J, Groenewegen Henk J, Haber Suzanne N. Circuit Based Cortico-Striatal Homologies between Rat and PrimateRat-Primate Cortico-Striatal Homologies. Biological Psychiatry. doi: 10.1016/j.biopsych.2016.05.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs MH, Wilson CJ. Unitary synaptic connections among substantia nigra pars reticulata neurons. J Neurophysiology. 2016;115(6):2814–2829. doi: 10.1152/jn.00094.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario M, Holloway T, Jin X, Costa RM. Different dorsal striatum circuits mediate action discrimination and action generalization. The European journal of neuroscience. 2012;35(7):1105–1114. doi: 10.1111/j.1460-9568.2012.08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neuroscience. 2003;23(12):4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Annals of the New York Academy of Sciences. 2012;1282(1):12–24. doi: 10.1111/j.1749-6632.2012.06768.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Im H, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466(7303):197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73(2):493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56(1):27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, Ikemoto S. Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. Journal of Neuroscience. 2014;34(3):817–822. doi: 10.1523/JNEUROSCI.1703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H-I, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nature neuroscience. 2010;13(9):1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience. 2002;22(14):6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He D-Y, Carnicella S, Kharazia V, Janak P, Ron D. Endogenous BDNF in the Dorsolateral Striatum Gates Alcohol Drinking. Journal of Neuroscience. 2009;29(43):13494. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, Ron D. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. The European journal of neuroscience. 2012;37(4):607–612. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA. Methamphetamine-induced structural plasticity in the dorsal striatum. The European journal of neuroscience. 2007;25(3):847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. Journal of Neuroscience. 1992;12(1):356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. Journal of Neuroscience. 2012;32(13):4645–4650. doi: 10.1523/JNEUROSCI.0348-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res Journal of Neuroscience. 1989:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine D1 receptor and is dependent on NMDA stimulation. Brain Res Mol Brain Res. 1994;23(1–2):1–13. doi: 10.1016/0169-328x(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Current opinion in neurobiology. 2013;23(4):559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kozell LB, Hitzemann R, Buck KJ. Acute Alcohol Withdrawal is Associated with c-Fos Expression in the Basal Ganglia and Associated Circuitry: C57BL/6J and DBA/2J Inbred Mouse Strain Analyses. Alcoholism: Clinical and Research. 2005;29(11):1939–1948. doi: 10.1097/01.alc.0000187592.57853.12. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature neuroscience. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. An escalating dose/multiple high-dose binge pattern of amphetamine administration results in differential changes in the extracellular dopamine response profiles in caudate-putamen and nucleus accumbens. The Journal of Neuroscience. 1997;17(11):4441–4447. doi: 10.1523/JNEUROSCI.17-11-04441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Dupré DJ, Denovan-Wright EM. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J Biol Chem. 2014;289(36):24845–24862. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci. 2013;92(8–9):446–452. doi: 10.1016/j.lfs.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. Pavlovian -to-instrumental transfer in cocaine seeking rats. Behavioral Neuroscience. 2012;126(5):681–689. doi: 10.1037/a0029534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. Journal of Neuroscience. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger W, Hefti F, Felix D, Huwyler T, Melamed E, Schlumpf M. Stimulation of nigrostriatal dopamine neurones by nicotine. Neuropharmacology. 1982;21(10):963–968. doi: 10.1016/0028-3908(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, Kennedy PJ, Liu QR, Cimbro R, Hope BT, Nestler EJ, Shaham Y. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. Journal of Neuroscience. 2015;35(21):8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2012;62(2):552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement, and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27(5):457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent development in animal models of drug relapse. Current Opinion in Neurobiology. 2013;23(4):675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I, Smith CB. Acute and chronic morphine treatment and the hydroxylation of (1–14C)-L-tyrosine in the mouse brain. Br J Pharmacol. 1974;50(3):428–430. doi: 10.1111/j.1476-5381.1974.tb09620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics. Synapse. 2006;60(4):288–294. doi: 10.1002/syn.20301. [DOI] [PubMed] [Google Scholar]
- Mato S, Chevaleyre V, Robbe D, Pazoa A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nature Neuroscience. 2004;7(6):585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- Matsubayashi H, Amano T, Seki T, Sasa M. Electrophysiological characterization of nicotine-induced excitation of dopaminergic neurons in the rat substantia nigra. Journal of Pharmacological Sciences. 2003;93:143–148. doi: 10.1254/jphs.93.143. [DOI] [PubMed] [Google Scholar]
- Medina AC, Charles JR, Espinoza-Gonzalez Vl, Sanchez-Resendis O, Prado-Alcala RA, Roozendall B, Quirarte GL. Glucocorticoid administration into the dorsal striatum facilitates memory consolidation of inhibitory avoidance training but not of the context or footshock components. Learning and Memory. 2007;14(10):673–677. doi: 10.1101/lm.654407. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Bernardi G. The electrophysiological actions of dopamine and dopaminergic drugs on neurons of the substantia nigra pars compacta and ventral tegmental area. Life Sciences. 1992;51(10):711–718. doi: 10.1016/0024-3205(92)90479-9. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain research. 1984;292(1):63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- Méndez M, Morales-Mulia M, Leriche M. [3H]DPDPE binding to delta opioid receptors in the rat mesocorticolimbic and nigrostriatal pathways is transiently increased by acute ethanol administration. Brain research. 2004;1028(2):180–190. doi: 10.1016/j.brainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. International review of neurobiology. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37(11):2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin-Rauscent A, Simon M, Giuliano C, Benoit-Marand M, Everitt BJ, Belin D. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nature communications. 2015;6:10088. doi: 10.1038/ncomms10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D, Everitt BJ. Increased Impulsivity Retards the Transition to Dorsolateral Striatal Dopamine Control of Cocaine Seeking. Biological Psychiatry. 2014;76(1):15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, Tonini R. SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nature neuroscience. 2012;15(2):284–293. doi: 10.1038/nn.3022. [DOI] [PubMed] [Google Scholar]
- Nelson A. Amphetamine Exposure Enhances Habit Formation. Journal of Neuroscience. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, Santos N, Bartlett SE. δ-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats. Journal of Neuroscience. 2012;32(13):4540–4552. doi: 10.1523/JNEUROSCI.5345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Molecular pharmacology. 1996;49(4):636–645. [PubMed] [Google Scholar]
- O’Tousa D, Grahame N. Habit formation: Implications for alcoholism research. Alcohol. 2014;48:327–335. doi: 10.1016/j.alcohol.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Mao T, Dudman JT. Inputs to the Dorsal Striatum of the Mouse Reflect the Parallel Circuit Architecture of the Forebrain. Frontiers in neuroanatomy. 2010;4:147. doi: 10.3389/fnana.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Luscher C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron. 2015;88:1–13. doi: 10.1016/j.neuron.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Carter DA, Fibiger HC. Dopaminergic substrates of intracranial self-stimulation in the caudate-putamen. Brain Res. 1976;104(2):221–232. doi: 10.1016/0006-8993(76)90615-6. [DOI] [PubMed] [Google Scholar]
- Reggiani A, Barbaccia ML, Spano PF, Trabucchi M. Dopamine Metabolism and Receptor Function After Acute and Chronic Ethanol. Journal of neurochemistry. 1980;35(1):34–37. doi: 10.1111/j.1471-4159.1980.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Reiner A. The Conservative Evolution of the Vertebrate Basal Ganglia. In Handbook of Basal Ganglia Structure and Function. (1) 2010;Chapter 2 [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413(6851):67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Root DH. The ventromedial ventral pallidum subregion is necessary for outcome-specific Pavlovian-instrumental transfer. Journal of Neuroscience. 2013;33(48):18707–18709. doi: 10.1523/JNEUROSCI.4021-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH. Operant Self-Stimulation of Dopamine Neurons in the Substantia Nigra. PLoS ONE. 2013;8(6):e65799. doi: 10.1371/journal.pone.0065799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio FJ, Liu QR, Li X, Cruz FC, Leão RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y, Hope BT. Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. Journal of Neuroscience. 2015;35(14):5625–5639. doi: 10.1523/JNEUROSCI.4997-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Malsbury C. Brainstem pathways of reward. J Comp Physiol Psychol. 1969;68:22–30. doi: 10.1037/h0027655. [DOI] [PubMed] [Google Scholar]
- Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32(3):203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Apostolidis S, Amoa R, O’Rear C, Kaster M, Stowers J, Ritz R. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiology of Learning and Memory. 2015;118:105–112. doi: 10.1016/j.nlm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior following prolonged abstinence in rats. Psychopharmacology. 2007;194(3):321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. The European journal of neuroscience. 2010;32(10):1735–1743. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Koob GF. Recent advances in animal models of drug addiction. In: Davis, Charney, Coyle, Nemeroff, editors. Neuropsychopharmacology-5th generation of progress. Lippincott, Williams, & Wilkins; Philadelphia, Pennsylvania: 2002. [Google Scholar]
- Skinner BF. The Behavior of organisms: An experimental analysis. New York: Appleton-Century; 1938. [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Neural correlates of stimulus-response and response-outcome associations in dorsolateral versus dorsomedial striatum. Frontiers in integrative neuroscience. 2010;4:12. doi: 10.3389/fnint.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton DM, Solomon PR. Microinjections of d-amphetamine into the nucleus accumbens and caudate-putamen differentially affect stereotypy and locomotion in the rat. Physiological Psychology. 1984;12(2):159–162. [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM. Differential Dynamics of Activity Changes in Dorsolateral and Dorsomedial Striatal Loops during Learning. Neuron. 2010;66(5):781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. REVIEW ON CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction biology. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pagès C, Hervé D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. European Journal of Neuroscience. 2004;19(7):1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. Journal of Neuroscience. 2005;25(38):8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-adminsitration in dependent rats: focus on the vapor model. Alcohol. 2014;48(3):277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. The Journal of pharmacology and experimental therapeutics. 1992;261(2):484–490. [PubMed] [Google Scholar]
- Vicente AM, Galvão-Ferreira P, Tecuapetla F, Costa RM. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Current Biology. 2016;26(7):267–269. doi: 10.1016/j.cub.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. Journal of Neuroscience. 2007a;27(13):3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. Journal of Neuroscience. 2015;35(33):11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. Journal of Neuroscience. 2010;30(30):10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Reward or reinforcement: what’s the difference? Neurosci Biobehav Rev. 1989;13(2–3):181–186. doi: 10.1016/s0149-7634(89)80028-4. [DOI] [PubMed] [Google Scholar]
- White NM. In: Neurobiology of Sensation and Reward. Gottfried JA, editor. Chapter 3. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. [PubMed] [Google Scholar]
- White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Salinas JA. Mnemonic functions of the dorsal striatum and hippocampus in aversive conditioning. Behav Brain Research. 2003;142(1–2):99–107. doi: 10.1016/s0166-4328(02)00402-3. [DOI] [PubMed] [Google Scholar]
- Wilcox MV, Carlson VCC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA. Repeated Binge-Like Ethanol Drinking Alters Ethanol Drinking Patterns and Depresses Striatal GABAergic Transmission. Neuropsychopharmacology. 2014;39(3):579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Roles for nigrostriatal-not just mesocorticolimbic-dopamine in reward and addiction. Trends in neurosciences. 2009;32(10):517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. the development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JX, Li J, Zhou R, Zhang XH, Ge YB, YRu Yuan X. Alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcoholism, clinical and experimental research. 2006;30(5):819–824. doi: 10.1111/j.1530-0277.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Simson P, Moy S, Breese GR. Evidence for a selective effect of ethanol on N-methyl-d-aspartate responses: ethanol affects a subtype of the ifenprodil-sensitive N-methyl-d-aspartate receptors. The Journal of pharmacology and experimental therapeutics. 1996;278(1):114–124. [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. The European journal of neuroscience. 2005;22(2):513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behavioural brain research. 2006;166(2):189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. The European journal of neuroscience. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney V, Shippenberg T. Shift from Goal-Directed to Habitual Cocaine Seeking after Prolonged Experience in Rats. Journal of Neuroscience. 2010;30(46):15457. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Lee CR. Intrinsic and integrative properties of substantia nigra pars reticulata neurons. Neuroscience. 2011;198:69–94. doi: 10.1016/j.neuroscience.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]